Abstract
A 65-year-old woman who presented with a constellation of symptoms, including cough with haemoptysis, fever, chills and hypoxia along with weight loss, was found to have diffuse alveolar haemorrhage. After a myriad of investigations returned normal, an open lung biopsy was performed, which revealed the diagnosis to be subacute eosinophilic pneumonia. This is one of its kind of rare presentations where eosinophilic pneumonia presents as diffuse alveolar haemorrhage and has been reported only five times prior to this.
Keywords: pneumonia (respiratory medicine), respiratory medicine, pathology
Background
Eosinophilic pneumonia is a rare lung disease, which involves infiltration of eosinophils into the lungs. It is usually associated with tobacco smoking and infections. It frequently presents as a febrile illness with hypoxaemic respiratory failure, showing diffuse pulmonary opacities on imaging with eosinophilia on bronchoalveolar lavage fluid analysis.1 We present a rare case of eosinophilic pneumonia in a woman who presented with haemoptysis and hypoxaemic respiratory failure and found to have diffuse alveolar haemorrhage on evaluation. As per our literature review, diffuse alveolar haemorrhage associated with eosinophilic pneumonia has been reported only five other times.
Case presentation
A 65-year-old woman, who was a former healthcare worker presented with a report of cough, haemoptysis, and fever with chills and night sweats. She had a history of obstructive sleep apnoea on continuous positive airway pressure (CPAP) with 2 L/min of oxygen at night, gastro-oesophageal reflux disease, previous exposure to tuberculosis with positive purified protein derivative (PPD), and untreated and mild intermittent asthma. She was having a dry cough for over a month, but 1 week prior to the presentation, she started having haemoptysis along with fever, chills and drenching night sweats. She had also experienced an unintentional weight loss of 9 kg over the past 6 months. Social history was significant for alcohol use of 2–4 beers a month, daily marijuana smoking and no tobacco use. She denied any recent travel, sick contacts, history of rheumatological disorders, Raynaud’s phenomenon, joint pain or haematuria. For her hobby, she would paint silk and was not using respirator protection. She also reported exposure to Jacquard dye set concentration where she accidentally put the dye set in her CPAP machine in place of distilled water. On initial physical examination, she had a blood pressure of 165/103 mm Hg, heart rate of 110 beats/min, a 24-hour max temperature of 39.1°C, respiratory rate of 24 breaths/min and oxygen saturation of 99% on ambient air. The patient was tachycardic with regular rhythm and no murmur heard, She also had scattered crackles in all lung fields with no wheezing or decreased breath sounds heard.
Investigations
A CT of the chest revealed diffuse, irregularly shaped nodular opacities with ground-glass opacities and mediastinal lymphadenopathy (figures 1 and 2). Her complete blood count on admission revealed an absolute eosinophil count of 0.2×109/L and a white blood count of 21.1×109/L. Her absolute eosinophil count peaked at 0.8×10 9/L and then improved after treatment to 0.2×109/L. Due to previously positive PPD, the patient had three acid-fast smears which did not grow anything. An influenza swab, respiratory syncytial virus swab and respiratory viral PCR panel were all unremarkable. Her Streptococcus pneumoniae urine antigen, Bordetella pertussis PCR and Legionella urine antigen were also negative. An erythrocyte sedimentation rate was elevated at 86 mm/hour and C reactive protein was also elevated at 272.5 mg/L. Her cytoplasmic and perinuclear antineutrophilic cytoplasmic antibody, glomerular basement membrane antibodies, and C3 and C4 complements were all negative. Her antinuclear antibody titre was 1:40 in a speckled pattern. The imaging also revealed multiple enlarged mediastinal and right perihilar lymph nodes, likely secondary to an infectious or inflammatory cause. Subsequently, a bronchoscopy was done with multiple bronchoalveolar lavages, which revealed increasingly bloody aliquots (figure 3). It also showed a nucleated cell count of 792/μL. A cell differential was unable to be performed due to increased degradation of cells. Histoplasma antigen, aspergillus antigen, herpes simplex PCR, varicella PCR and bronchial culture with Gram stain were all negative, which helped rule out further infectious causes. An open lung biopsy with pathology was performed, which subsequently revealed acute fibrinous and organising pneumonia with increased eosinophils and debris-laden macrophages consistent with subacute eosinophilic pneumonia (figures 4 and 5).
Figure 1.
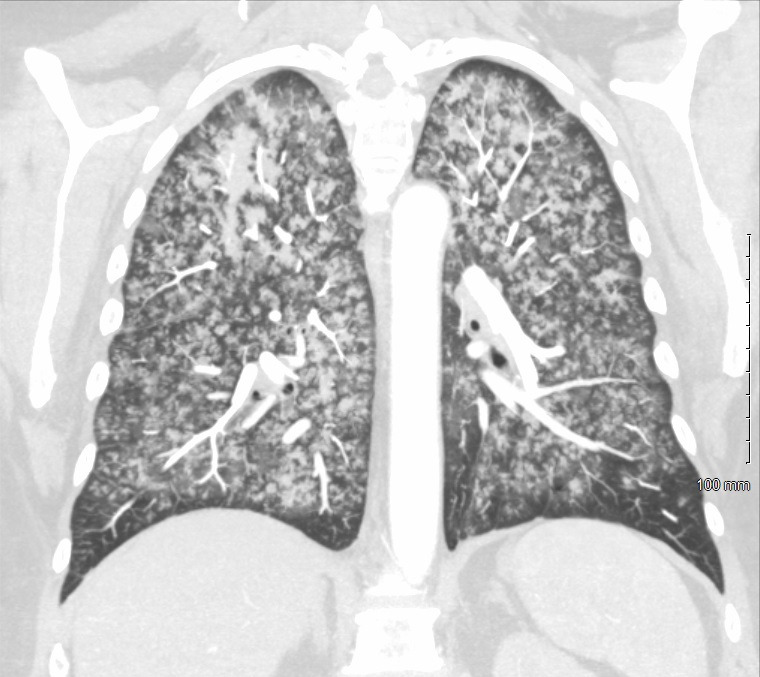
Coronal plane of CT of the chest. The image demonstrates diffuse ground-glass opacities involving both the lungs with a slight predominance of the upper lobes.
Figure 2.
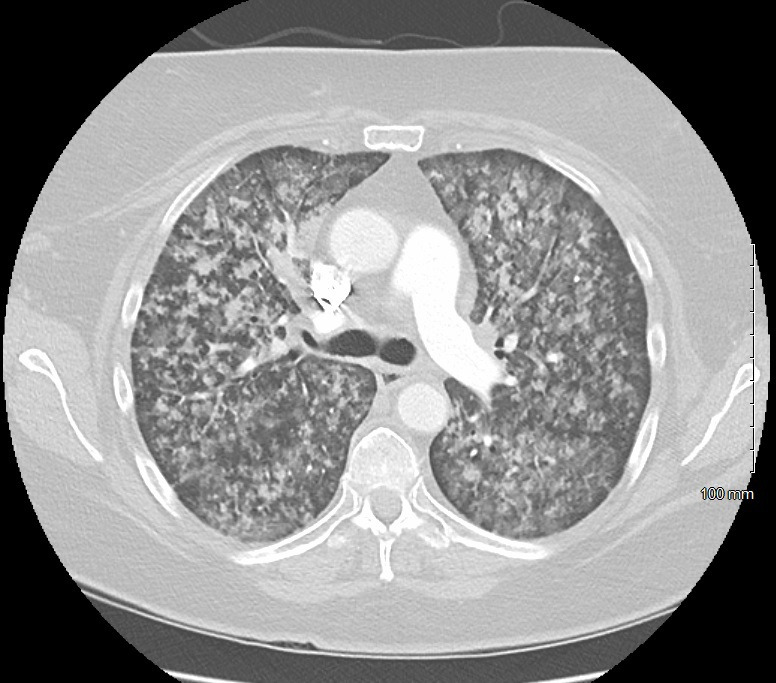
Axial plane CT of the chest. The image shows diffuse ground-glass opacities with no nodules or enlarged hilar adenopathy concerning for malignancy.
Figure 3.
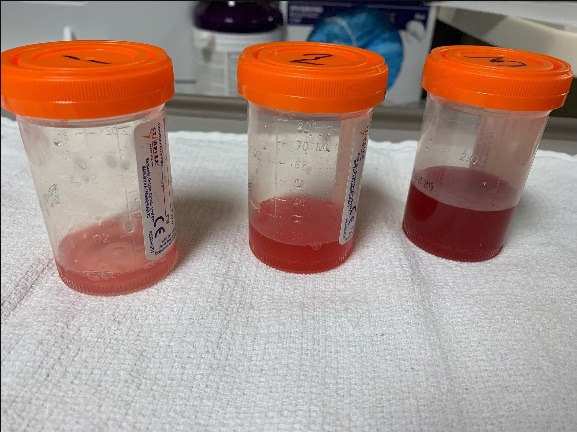
Bronchoalveolar lavage. Serial bronchoalveolar lavages showing increasingly bloody aliquots which is characteristic for diffuse alveolar haemorrhage.
Figure 4.
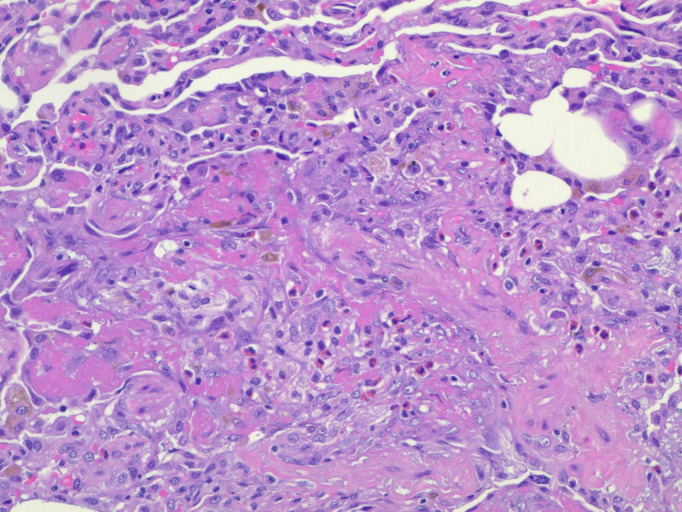
Open lung biopsy. H&E stain of the lung at 200× magnification. The histopathological image shows acute fibrinous and organising pneumonia with increased eosinophils and debris-laden macrophages.
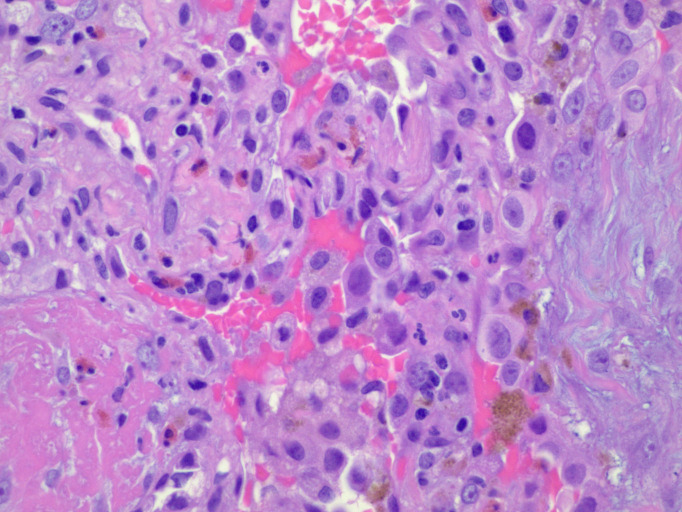
Figure 5.
Open lung biopsy. H&E stain at 400× magnification. The image shows areas of organising pneumonia with eosinophils in abundance.
Differential diagnosis
Cough with haemoptysis in the setting of systemic symptoms can be multifactorial. Given the hypoxaemia on presentation, pulmonary embolism, heart failure and pulmonary arteriovenous malformation are frequent causes to consider. But in our case, a normal cardiovascular examination along with CT of the chest showing no pulmonary embolism essentially ruled out these causes.
Mycobacterial infections, fungal infections and community-acquired pneumonia were also considered, but with negative acid-fast bacillus smears, negative QuantiFERON gold test and other respiratory cultures, common infectious causes were excluded. Airway diseases like bronchiectasis, broncholith, cystic fibrosis and a foreign body is associated with haemoptysis. In our case, these causes were ruled out early in the disease course with unremarkable history and negative imaging findings for these etiologies.
Malignancies, such as bronchoalveolar carcinoma, carcinoid tumour and metastatic tumours have been found to be a frequent cause of haemoptysis, especially in patients with underlying risk factors, such as smoking and age. Although, our patient did lose weight unintentionally, but given the fact that she was not a smoker and her CT scan revealed bilateral ground-glass opacities, malignant causes were essentially low on the differential.
Autoimmune causes like Goodpasture syndrome, granulomatosis with polyangiitis, Behcet’s disease and systemic lupus erythematosus have been implicated in patients with fever and haemoptysis and long-standing cough. These were excluded with negative double-stranded DNA and other multiple autoimmune panels.
Treatment
The patient was started on community-acquired pneumonia treatment, which included ceftriaxone and doxycycline. She then underwent an open lung biopsy, and pathology confirmed eosinophilic pneumonia in a subacute setting. Subsequently, she was started on 60 mg prednisone for 3 weeks, and then 40 mg for a week, and then further tapered down 5 mg each week until done. She had complete resolution of her symptoms following initiation of prednisone.
Outcome and follow-up
The patient had complete resolution of her symptoms and did not experience any more haemoptysis after starting steroids. Her shortness of breath had resolved over her hospital stay, and she tolerated the steroid taper well. A high-resolution CT scan was ordered for 6 months following her admission, to follow any possible lung fibrosis, which revealed near-complete resolution of previously seen extensive solid and ground-glass nodular opacities, which was suggestive of resolving infection or inflammation.
Discussion
Eosinophilic pneumonia is a rare lung disease that involves activation of alveolar macrophages, which release cytokines that recruit eosinophils to the lung parenchyma.1 Patients commonly present with fever, dry cough and dyspnoea.1 It can present as acute, subacute or chronic, and is generally brought on by an offending agent, such as smoking or an infection.1 It often requires an open lung biopsy for confirmation. Rarely, eosinophilic pneumonia can cause direct damage and inflammation to the alveoli themselves, which may cause alveolar haemorrhage.2 3
Eosinophilic pneumonia is difficult to diagnose process. Its pathogenesis is not fully understood, but has been thought of as a type 1 hypersensitivity reaction with a foreign substance being exposed to alveolar macrophages.1 In order to diagnose, one must have an acute respiratory illness of less than or equal to 1 month, pulmonary infiltrates seen on CT or chest X-ray, pulmonary eosinophilia on bronchoalveolar lavage of greater than 25%, or lung biopsy demonstrating pathology for eosinophilic pneumonia without a lung biopsy. The patient must also be absent from other pulmonary eosinophilic disease states, such as eosinophilic granulomatosis with polyangiitis.1 Imaging is often the first clue to an acute lung parenchymal process. A CT of the chest often shows bilateral airspace opacities, interlobular septal thickening, and bilateral ground-glass opacities. Bilateral pleural effusions are also commonly seen.4 In the COVID-19 era, the finding of bilateral pleural effusions in a patient with diffuse ground-glass opacities is helpful as patients with COVID-19 rarely demonstrate bilateral pleural effusions on CT.5
In our patient, there were two potential causes of subacute eosinophilic pneumonia, the patient’s daily marijuana smoking along with her silk painting and exposure of Jacquard dye set in her CPAP machine. The Jacquard dye set contains both formaldehyde and methanol that could have certainly been irritants to the alveoli.6–8 Recreational marijuana smoking, although rare, has been known to cause eosinophilic pneumonia.9 10 Interestingly, the patient had been a recreational user of marijuana for an extended period of time and had been using marijuana from the same source during that time. The cited cases for marijuana causing eosinophilic pneumonia show instances where marijuana was smoked for the first time, or a new source of marijuana was used for by an established marijuana smoker, but never in an established marijuana smoker with the same source during that time. This leads us to believe that the incident of using Jacquard dye set in her CPAP machine may have been the cause.
Our patient’s case is rare in that eosinophilic pneumonia was the cause of her haemoptysis and diffuse alveolar haemorrhage. To date, there are only five cited cases of eosinophilic pneumonia causing diffuse alveolar haemorrhage in the literature, and this would be the sixth.11–15 The pathophysiology in this is not well understood. It has been proposed that the eosinophils invade pulmonary basement membranes and vasculature directly, causing damage to the alveoli in the process.2 3 This process may be similar to the pathology of eosinophilic granulomatosis with polyangiitis in that eosinophils from eosinophilic pneumonia damage the vasculature.16
Learning points.
Eosinophilic pneumonia is a rare lung disease often brought on by infection or smoking.
The causes of diffuse alveolar haemorrhage are broad, and eosinophilic pneumonia causing it has only occurred five other times.
Open lung biopsy is often required for the diagnosis of eosinophilic pneumonia.
Acknowledgments
We would like to extend our gratitude to ATL and AM for extensive literature review and manuscript writing. We would also like to thank our pathology department, especially SMS for her help in biopsy image acquisition.
Footnotes
Contributors: ATL, AM and BJD were involved in the diagnosis and management of the patient while inpatient. ATL and AM wrote the initial draft which was proofread and approved by BJD and SMS. SMS was involved in the pathological diagnosis and image acquisition. BJD saw the patient at the 6-month follow-up. Consent was obtained by ATL. The final manuscript was agreed upon by all the authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.De Giacomi F, Vassallo R, ES Y. Causes, diagnosis, and management. Am J Respir Crit Care Med 2018;197:728–36. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol 2006;24:147–74. 10.1146/annurev.immunol.24.021605.090720 [DOI] [PubMed] [Google Scholar]
- 3.Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy 2008;38:709–50. 10.1111/j.1365-2222.2008.02958.x [DOI] [PubMed] [Google Scholar]
- 4.King MA, Pope-Harman AL, Allen JN, et al. Acute eosinophilic pneumonia: radiologic and clinical features. Radiology 1997;203:715–9. 10.1148/radiology.203.3.9169693 [DOI] [PubMed] [Google Scholar]
- 5.Plesner LL, Dyrberg E, Hansen IV, et al. [Diagnostic imaging findings in COVID-19]. Ugeskr Laeger 2020;182. [Epub ahead of print: 06 Apr 2020]. [PubMed] [Google Scholar]
- 6.Maejima K, Suzuki T, Numata H, et al. Recovery from changes in the blood and nasal cavity and/or lungs of rats caused by exposure to methanol-fueled engine exhaust. J Toxicol Environ Health 1993;39:323–40. 10.1080/15287399309531755 [DOI] [PubMed] [Google Scholar]
- 7.Persoz C, Achard S, Momas I, et al. Inflammatory response modulation of airway epithelial cells exposed to formaldehyde. Toxicol Lett 2012;211:159–63. 10.1016/j.toxlet.2012.03.799 [DOI] [PubMed] [Google Scholar]
- 8.Sandikci M, Seyrek K, Aksit H, et al. Inhalation of formaldehyde and xylene induces apoptotic cell death in the lung tissue. Toxicol Ind Health 2009;25:455–61. 10.1177/0748233709106824 [DOI] [PubMed] [Google Scholar]
- 9.Liebling PD, Siu S. A novel cause of eosinophilic pneumonia: recreational marijuana exposure. J Bronchology Interv Pulmonol 2013;20:183–5. 10.1097/LBR.0b013e31828caa0d [DOI] [PubMed] [Google Scholar]
- 10.Natarajan A, Shah P, Mirrakhimov AE, et al. Eosinophilic pneumonia associated with concomitant cigarette and marijuana smoking. BMJ Case Rep 2013;2013:bcr2013009001. 10.1136/bcr-2013-009001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida Y, Yamashita N, Ota T, et al. [A case of eosinophilic pneumonia presenting as hemoptysis, with epithelioid cell granuloma on lung biopsy]. Nihon Kyobu Shikkan Gakkai Zasshi 1995;33:422–8. [PubMed] [Google Scholar]
- 12.Sasaki S, Kawanami R, Motizuki Y, et al. [Serrapeptase-induced lung injury manifesting as acute eosiniphilic pneumonia]. Nihon Kokyuki Gakkai Zasshi 2000;38:540–4. [PubMed] [Google Scholar]
- 13.Sakamoto S, Kaburaki K, Gochyo K, et al. [Azithromycin-induced diffuse alveolar hemorrhage]. Nihon Kokyuki Gakkai Zasshi 2009;47:337–41. [PubMed] [Google Scholar]
- 14.Acosta-Miranda D, Rodriguez-Cintron W. Acute eosinophilic pneumonia presenting as alveolar hemorrhage. Chest 2013;144:457A. 10.1378/chest.1704695 [DOI] [Google Scholar]
- 15.Hara A, Mukae H, Hara S, et al. Drug-Induced eosinophilic pneumonia with pulmonary alveolar hemorrhage caused by benzbromarone. Intern Med 2010;49:435–8. 10.2169/internalmedicine.49.2830 [DOI] [PubMed] [Google Scholar]
- 16.Casal A, Díaz-Garel J, Pereiro T, et al. Pulmonary vasculitis. J Thorac Dis 2018;10:5560–75. 10.21037/jtd.2018.08.117 [DOI] [PMC free article] [PubMed] [Google Scholar]