Point mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common cause of familial Parkinson’s disease (PD) and are implicated in a significant proportion of apparently sporadic PD cases. Clinically, LRRK2-driven PD is indistinguishable from sporadic PD, making it an attractive genetic model for the much more common sporadic PD.
KEYWORDS: LRRK2, Parkinson's disease, endolysosome, kinase, microtubule
ABSTRACT
Point mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common cause of familial Parkinson’s disease (PD) and are implicated in a significant proportion of apparently sporadic PD cases. Clinically, LRRK2-driven PD is indistinguishable from sporadic PD, making it an attractive genetic model for the much more common sporadic PD. In this review, we highlight recent advances in understanding LRRK2's subcellular functions using LRRK2-driven PD models, while also considering some of the limitations of these model systems. Recent developments of particular importance include new evidence of key LRRK2 functions in the endolysosomal system and LRRK2’s regulation of and by Rab GTPases. Additionally, LRRK2's interaction with the cytoskeleton allowed elucidation of the LRRK2 structure and appears relevant to LRRK2 protein degradation and LRRK2 inhibitor therapies. We further discuss how LRRK2's interactions with other PD-driving genes, such as the VPS35, GBA1, and SNCA genes, may highlight cellular pathways more broadly disrupted in PD.
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disorder affecting nearly 7 million individuals worldwide, including 1 to 2% of people over 60 years of age and up to 5% of those over 85 years of age (1). Tremor, rigidity, and bradykinesia are key clinical features. These symptoms are driven by neuronal dysfunction and death in a stereotyped anatomic distribution in which dopaminergic (DA) neurons in the substantia nigra of the midbrain are particularly devastated. Many additional PD symptoms clearly relate to neurodegeneration in other neuroanatomic sites (i.e., anosmia due to olfactory bulb involvement and autonomic symptoms due to involvement of the dorsal motor nucleus of the vagus nerve). Other symptoms, like sleep disturbances and abnormalities of mood, have less clear neuroanatomic causes (2). On a cellular level, surviving neurons in affected regions often contain Lewy bodies and Lewy neurites, cytoplasmic protein aggregates composed predominantly of aggregated α-synuclein (3).
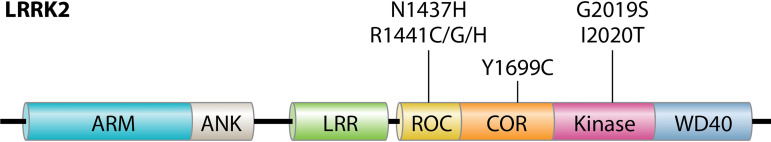
While most cases of PD are idiopathic, approximately 5 to 10% are familial, with clear-cut autosomal inheritance patterns (4, 5). Point mutations in the kinase leucine-rich repeat kinase 2 (LRRK2) (found in the Park8 locus on chromosome 12q12) are the most common cause of autosomally inherited PD. LRRK2 mutations drive up to 5% of familial cases worldwide and up to 40% of cases in certain populations, such as North African Berber and Ashkenazi Jewish patients (6). Seven point mutations (G2019S, I2020T, R1441C/G/H, Y1699C, and N1437H) in the LRRK2 enzymatic domains cause autosomal dominant PD (Fig. 1). LRRK2 G2019S is by far the most prevalent mutation and is common in LRRK2-driven PD (LRRK2-PD) patients with Berber and Ashkenazi Jewish heritage. This mutation is also common in patients of European descent but is rare in Asian patients (7, 8). The penetrance of LRRK2-PD is age dependent but incomplete, with estimates (ranging from 24% to nearly 100%) varying depending on the particular mutation and the particular study (9, 10). Certain LRRK2 point mutations such as I2020T are rare and affect limited numbers of patients. These mutations also appear to be highly penetrant. Conversely, LRRK2 G2019S is common but only moderately penetrant, which may help explain why LRRK2 point mutations underlie up to 1 to 2% of apparently idiopathic PD. Multiple risk loci for sporadic PD have been identified within the LRRK2 gene using population studies (11). LRRK2 variants G2385R and R1628P are associated with increased PD risk in Asian populations, while the N551K-R1398H haplotype appears protective against sporadic PD (12, 13). Importantly, genetic variants in LRRK2 are also risk factors for Crohn’s disease, leprosy, and tuberculosis, suggesting that the LRRK2 protein plays important roles outside the central nervous system (14–16).
FIG 1.
LRRK2 domain structure. LRRK2 is a multidomain kinase with two enzymatic domains, a Roc GTPase and a serine-threonine kinase of the TKL family, linked by a COR domain. LRRK2’s N and C termini are composed of protein-protein interaction domains, including N-terminal armadillo (ARM), ankyrin (ANK), and LRR domains and a C-terminal WD40 domain. Indicated at the top are the seven known LRRK2 point mutations that drive autosomal dominant PD.
CLINICAL FEATURES OF LRRK2-PD
We use the term LRRK2-PD to describe the group of patients with both clinical symptoms of PD and one of the seven known causative mutations. Clinical manifestations of LRRK2-PD largely phenocopy those of sporadic PD, with similar symptoms, age of onset (60s or 70s), and rate of clinical decline (17). This is in contrast to autosomal recessive causes of PD, such as PINK1, DJ1, and parkin, which frequently manifest with additional symptoms and at a younger age (reviewed by Gasser et al. [18]). The classic clinical symptoms of LRRK2-PD make it a compelling genetic model for idiopathic PD.
Neuropathologically, the majority of LRRK2-PD brains show α-synuclein-positive Lewy pathology pathognomonic for PD. This finding provides conclusive evidence that LRRK2 point mutations either directly or indirectly increase α-synuclein aggregation in humans. In 2015, a review of all LRRK2-PD cases with published neuropathology (54 cases with pathogenic LRRK2 mutations, with 37 described in detail) reported that essentially all showed nigral neuronal loss and about one-half showed Lewy pathology (19). However, the same LRRK2 point mutations that drive Lewy pathology can also manifest with a wide range of neuropathologies. Some LRRK2 cases with PD clinical features lacked Lewy pathology and had other, distinct neuropathologies, including tauopathy with features of progressive supranuclear palsy and the glial synucleinopathy multiple system atrophy. Others demonstrated pure striatonigral degeneration, with death of substantia nigra neurons but no clear protein aggregates (19). Interestingly, LRRK2 cases lacking Lewy pathology often arose in patients whose family members had Lewy pathology. The frequency of Lewy pathology appears related to the particular LRRK2 point mutation involved, with LRRK2 G2019S being most likely to display classic Lewy pathology and LRRK2 I2020T very rarely having Lewy pathology (19, 20). A more recent review of 55 cases of LRRK2-PD noted that about one-half had tau pathology, with most of this being mild to moderate Alzheimer’s disease changes (i.e., Braak stage III or lower) (21). A causative relationship between Alzheimer’s disease and LRRK2 mutations has not been shown.
LRRK2 EXPRESSION AND MODELS OF LRRK2-PD
LRRK2 protein is expressed at low levels throughout the body, with highest levels in kidney, lung, and immune cells and modest expression in the brain (22). In the brain, LRRK2 expression is highest in the putamen, a target of substantia nigra neurons, although it is also measurable in substantia nigra (13). A reproducible physical interaction between the LRRK2 and α-synuclein proteins has not been demonstrated (reviewed by O’Hara et al. [23]). LRRK2 mutant murine models (bacterial artificial chromosome [BAC] and cDNA transgenic and CRISPR knock-in models) do not fully recapitulate human PD. These models consistently fail to demonstrate age-dependent neurodegeneration and α-synuclein pathology, although more subtle neurophysiological and behavioral phenotypes have been observed in particular models (see Volta and Melrose for a comprehensive review [24]). Murine models genetically overexpressing both LRRK2 and α-synuclein have also yielded conflicting results (25, 26). Recent studies suggest that LRRK2 may augment aggregation and cell-to-cell transmission of α-synuclein in murine systems in which α-synuclein is introduced intracranially via injection (27–29). Intriguingly, very recent work used neuron-specific viral delivery of mutant LRRK2 to induce DA neuronal degeneration in rats without causing α-synuclein pathology (30). Additional studies will be required to understand how well these model systems reproduce human PD.
Murine knockout models of LRRK2 have no brain abnormalities but do show abnormalities of lung and kidney, two organs that normally express high levels of LRRK2. These include enlarged lamellar bodies (lysosome-related organelles) in lung alveolar type II cells and enlarged secondary lysosomes with accumulation of lipofuscin in renal proximal tubules (31–34). These changes support a role for LRRK2 in normal endolysosomal function. Lysosomal defects appear to cause α-synuclein aggregation in kidneys of LRRK2 knockout mice (31). A murine double knockout of LRRK2 and its homolog LRRK1 (which has not been genetically linked to PD) shows DA neurodegeneration, neuronal accumulation of α-synuclein, and impaired autophagy; however, the mechanism by which this occurs has not been worked out (35).
A significant body of research demonstrates LRRK2’s importance in diseases unrelated to PD, highlighting LRRK2’s role in regulating immune function. In response to gamma interferon (IFN-γ), LRRK2 protein levels and phosphorylation increase in both human induced pluripotent stem cell (iPSC)-derived cell lines and other macrophage cell lines. Multiple LRRK2 alleles (typically point mutations) augment risk for inflammatory bowel disease, particularly Crohn’s disease (16, 36). LRRK2 Crohn’s risk alleles reproducibly increase nuclear localization and activation of nuclear factor of activated T cells (NFAT). However, whether this occurs in a kinase-dependent manner is debated. The N2081D allele increases risk for both PD and Crohn’s disease and increases LRRK2 kinase activity (16). Early studies indicated that LRRK2 has a kinase-independent scaffolding function and traps inactive NFAT in the cytoplasm (36). The Crohn’s risk allele M2397 appears to decrease LRRK2 protein stability, allowing NFAT to translocate to the nucleus (36). However, later work suggested that direct phosphorylation by LRRK2 activates NFAT (37). In the past few months, Kim et al. demonstrated that, in response to neuron-released α-synuclein, LRRK2 activates microglia via direct phosphorylation of NFATc2 and causes NFATc2 nuclear translocation and neurotoxicity (38).
LRRK2 also mediates inflammatory responses to multiple infectious organisms, including mycobacteria and Salmonella strains. LRRK2 impedes Mycobacterium tuberculosis phagosome maturation in macrophages, with LRRK2-deficient mice showing decreased M. tuberculosis burdens early in infection (15). The LRRK2 R1628P variant, which is a risk allele for PD, causes excessive inflammation (termed type 1 reactions) in leprosy (which is caused by Mycobacterium leprae) (14, 39). LRRK2 also promotes inflammation in response to Salmonella infection through activation of NLRC4, with LRRK2-deficient mice having an impaired response to Salmonella infection (40). Further supporting its role in immune function, LRRK2 is highly expressed in the myeloid lineage, including macrophages, monocytes, and neutrophils (41). The importance of myeloid cells in the pathogenesis of LRRK2-PD is not clear; however, at minimum, the high level of LRRK2 in myeloid cells facilitates studies of LRRK2 function in patient-derived blood samples (42, 43).
LRRK2 DOMAIN ORGANIZATION AND ENZYMATIC FUNCTION
LRRK2 is a 286-kDa multidomain kinase that can form homodimers; dimerization is required for maximal LRRK2 kinase activity (44, 45). Two enzymatic domains, a Roc (Ras of complex) GTPase and a serine-threonine kinase of the tyrosine kinase-like (TKL) family, are linked by a COR (C terminus of Roc) domain (Fig. 1). LRRK2’s N and C termini are composed of protein-protein interaction domains, including N-terminal armadillo, ankyrin, and LRR domains and a C-terminal WD40 domain (46). LRRK2’s tandem Roc-COR domain structure qualifies it as a member of the ROCO protein superfamily and suggests that the COR domain may mediate interactions between LRRK2’s GTPase and kinase, something that has been validated in recent cryo-electron microscopy structures of LRRK2 (47, 48). A homologous protein, LRRK1, which has not been linked to PD, has a similar domain structure as well as 26% identity and 45% similarity to LRRK2 (49). LRRK2 and LRRK1 are both conserved in vertebrates, while the invertebrates Drosophila melanogaster and Caenorhabditis elegans have a single LRRK ortholog (50).
Several unique features of LRRK2’s enzymatic domains have been described. In LRRK2’s kinase domain, the activation loop contains a DYG motif rather than the typically conserved DFG motif of other kinases. The tyrosine hydroxyl of LRRK2’s DYG motif serves as a “molecular brake” on LRRK2 kinase activity, decreasing substrate accessibility to the activation loop and favoring the inactive kinase state (51). Accordingly, a Y2018F LRRK2 mutation that recapitulates the standard DFG motif increases LRRK2 kinase activity (51). LRRK2 has a preference for phosphorylating Thr over Ser residues, both in vitro and in vivo (52). LRRK2’s Roc GTPase domain utilizes a standard phosphate-binding P-loop motif to bind GTP and GDP with micromolar binding affinities (dissociation constant [Kd] values of 4.1 ± 0.3 μM and 1.2 ± 0.1 μM for GTP and GDP, respectively) (53). GTP binding appears necessary to activate LRRK2’s kinase function, as indicated by the lack of kinase activity for LRRK2 variants with point mutations that cannot bind GTP, such as T1348N (54). Crosstalk between the kinase and GTPase domain occurs both intramolecularly and via dimerization. In vitro, LRRK2 can dimerize both via homodimerization of the COR domain and via homodimerization of the C-terminal WD40 domain (48). Cryo-electron tomography of the catalytic half of LRRK2 revealed a J-like shape in which the COR and Roc domains turn back toward the kinase domain, allowing the kinase and GTPase domains to closely interact and to modulate one another (48).
Recent identification of bona fide in vivo LRRK2 substrates, namely, the LRRK2 autophosphorylation site S1292 (55) and a subset of Rab proteins discussed in more detail below (56, 57), demonstrates that all known PD-driving LRRK2 mutations increase LRRK2 kinase activity. The LRRK2 point mutations G2019S and I2020T lie in the kinase activation loop and may increase kinase activity through a mechanism similar to that of the Y2018F mutation. Four PD-driving point mutations (R1441G/C/H and N1437H) fall in LRRK2’s Roc GTPase domain, and a fifth (Y1699C) lies in the COR domain between the Roc and kinase domains. R1441C/G/H and Y1699C suppress GTP hydrolysis and increase LRRK2’s affinity for GTP (58), which leads to 3- to 4-fold activation of the kinase toward Rab substrates (55, 56). N1437H appears to allow LRRK2 to remain GTP bound by locking LRRK2 into highly stable dimers that have both decreased affinity for GTP and decreased GTPase activity (59). S1292 is positioned close to the kinase active site and also near the Crohn’s disease-related residue N2081, suggesting that this interface is critical in LRRK2’s kinase function (48). The G2385R PD risk allele in the WD40 domain disrupts dimerization of the WD40 domain and, like autosomal PD-driving point mutations, enhances LRRK2 activity in cells (60).
In addition to familial LRRK2-PD cases, there is some evidence that LRRK2 kinase activity may be elevated in idiopathic PD (61). Therefore, highly selective LRRK2 kinase inhibitors have been developed as possible therapeutics, and a number are in clinical trials (clinical trial no. NCT04056689; www.clinicaltrials.gov). However, treatment of animal models ranging from mice to nonhuman primates with LRRK2 kinase inhibitors causes phenotypic abnormalities identical to those of LRRK2 knockout models (31–34, 62). Kidney and lung abnormalities may be reversible once the inhibitor is removed, although these studies are based on short-term (a few weeks) drug administration (63).
All currently studied LRRK2 kinase inhibitors are type I inhibitors, occupying the ATP-bound pocket of the kinase in a “DFG-in” conformation (64, 65). At a cellular level, type I LRRK2 inhibitors drive overexpressed LRRK2 to the microtubule and cause LRRK2 ubiquitination and proteasomal degradation, reproducibly decreasing LRRK2 steady-state levels (66). Therefore, it remains unresolved whether systemic abnormalities following LRRK2 kinase inhibitor treatment are entirely due to kinase inhibition or are also related to decreased LRRK2 protein levels. Differentiation of these mechanisms is essential for understanding and circumventing possible toxicity of long-term treatment with LRRK2 inhibitors. Highly selective type 2 LRRK2 kinase inhibitors, which would stabilize LRRK2 in an open “DFG-out” conformation, have not been developed. However, studies of less selective type 2 LRRK2 kinase inhibitors indicate that these compounds do not cause the same changes in LRRK2 localization and degradation (48). Changes in LRRK2’s binding partners depending on its subcellular localization appear to be critical to its normal and disease-driving functions as well as its degradation.
LRRK2 SUBCELLULAR LOCALIZATION
Due to LRRK2’s low levels of expression, direct assessment of its endogenous subcellular localization is a significant technical challenge, and very few studies have conclusively visualized endogenous LRRK2 using microscopy. In overexpression systems, as well as biochemical fractionation studies of endogenous protein, LRRK2 appears predominantly cytoplasmic (67); however, increasing evidence suggests that key aspects of LRRK2 function and regulation occur at membranes as well as at the cytoskeleton. The remaining sections discuss known LRRK2 functions in different subcellular compartments (Fig. 2), as well as the cellular and genetic interactions of LRRK2 with other PD-driving genes (Fig. 3).
FIG 2.
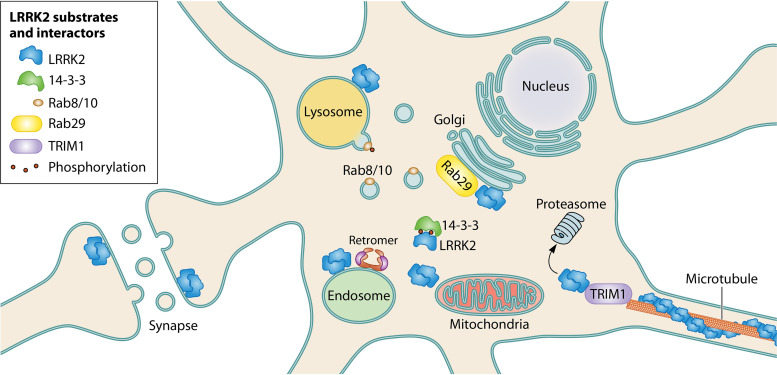
LRRK2’s subcellular localizations and key interacting partners. LRRK2 is found in the cytoplasm, where it binds to 14-3-3 proteins in a phosphorylation-dependent manner, and at cell membranes, where it forms a homodimer. LRRK2 function appears particularly important to the endolysosomal system and trans-Golgi network. Here, LRRK2 is activated by and phosphorylates Rab29 and also recruits and phosphorylates Rab8A/10 (as well as other Rab proteins) to initiate downstream pathways. In overexpression, LRRK2 binds the microtubule in an ordered helix, and we have found that LRRK2 proteasomal degradation is regulated by TRIM1, a microtubule-bound protein. LRRK2 also appears to have important roles in normal retromer and mitochondrial function.
FIG 3.
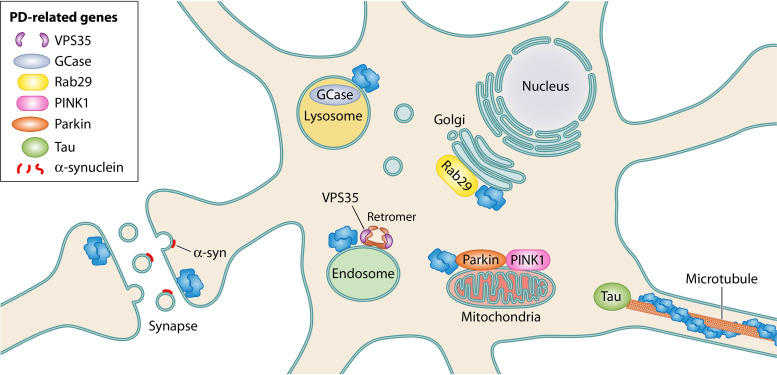
LRRK2’s interactions with key PD-related genes. LRRK2 has been shown to physically or genetically interact with a number of other PD genes. Rab29 and VPS35 appear to be upstream regulators of LRRK2, leading to LRRK2 endolysosomal recruitment and activation. GCase, a lysosomal enzyme whose dysfunction causes Gaucher’s disease, is likely to be regulated through LRRK2-mediated Rab10 activity. Parkin and PINK1 drive mitophagy, which appears to be disrupted by hyperphosphorylation of Rab10 by LRRK2. Mutant LRRK2 can cause synucleinopathies, with abnormal aggregation of α-synuclein, as well as tauopathies, with abnormal aggregation of the microtubule-associated protein tau. How LRRK2 mutations drive α-synuclein and tau aggregation requires further investigation.
(i) Cytoplasmic LRRK2.
The majority of overexpressed and endogenous LRRK2 is found in the cytoplasm (67, 68). In the cytoplasm, LRRK2 associates with homodimers of 14-3-3 proteins, a family of adaptor proteins that are highly expressed in the brain (69, 70). When 14-3-3 binding is disrupted, LRRK2 has been noted to aggregate into inclusion bodies, suggesting that 14-3-3 may stabilize proper LRRK2 folding in the cytoplasm (69, 70). LRRK2’s binding to 14-3-3 requires interaction between 14-3-3 and multiple phosphorylated residues of LRRK2; these include Ser910 and Ser935, which are part of a cluster of phosphoserines (Ser910, Ser935, Ser955, and Ser973) located between LRRK2’s ankyrin and LRR domains, as well as Ser1444, located in the Roc domain (67, 69–72). Phosphorylation of the serine cluster is indirectly related to LRRK2 kinase activity, and LRRK2 kinase inhibitors lead to dephosphorylation of these sites (66). PD-driving R1441C/G/H, Y1699C, and I2020T mutants show intrinsically decreased/absent phosphorylation of Ser910 and Ser935 and subsequent reduced affinity for 14-3-3 proteins (72).
(ii) Membrane-associated LRRK2.
A fraction (∼10 to 20%) of overexpressed LRRK2 forms membrane-associated dimers; dimerization increases the specific activity of LRRK2’s kinase (67, 73). Stimulation of Toll-like receptors (TLRs) (especially TLR4) in macrophage and microglial cell lines increases LRRK2 Ser935 phosphorylation and membrane association (73). TLR4 stimulation by lipopolysaccharide and initiation of autophagocytosis with rapamycin also result in membrane association of LRRK2, potentially implicating LRRK2 in autophagic processes within the immune system (73). Abundant evidence demonstrates that LRRK2 can associate with multiple membrane-bound organelles, including the trans-Golgi network and membrane-bound vesicles, where it phosphorylates a subset of Rab GTPases (56). Rabs were first identified as in vivo substrates of LRRK2 through phosphoproteomic screening in mouse embryonic fibroblasts (56). A family of over 60 small Ras-like GTPases, Rabs serve as critical regulators of membrane trafficking processes by cycling between GTP-bound/membrane-associated and GDP-bound/cytosolic states (74). LRRK2 phosphorylates a conserved Thr/Ser residue in the Rab switch II effector domain of 16 Rab proteins, including Rab8a, Rab10, and Rab29 (57). Phosphorylation disrupts Rab binding to Rab GDP dissociation inhibitors (GDIs), causing Rab accumulation at membranes.
In addition to being a LRRK2 kinase substrate, Rab29 serves, at least in overexpression systems, as a critical activator of LRRK2 kinase (68, 75). Rab29 (also called Rab7L1) is one of five genes located in the PARK16 locus (11); additional genetic studies suggest that LRRK2 and Rab29 function coordinately to increase risk for PD (76). In overexpression studies, Rab29 activates LRRK2 through its recruitment to the membranes of the trans-Golgi network (68). The mechanisms by which LRRK2 kinase is activated at membranes are only beginning to be worked out; however, using a mitochondrially anchored Rab29, Gomez et al. elegantly demonstrated that LRRK2 membrane activation does not depend on LRRK2 association with the trans-Golgi network per se but appears to depend only on LRRK2 membrane association (77). Phosphorylated substrates such as phospho-Rab10 accumulate at the same membrane at which LRRK2 becomes anchored (77).
(iii) LRRK2 and retromer.
The retromer complex recycles membrane proteins from the endosome to the trans-Golgi network or plasma membrane (78). Recent work suggests that it also plays a role in synaptic vesicle endocytosis and recycling (79). The retromer core is composed of a heterotrimer of cargo-binding vacuolar protein sorting-associated proteins (VPSs), VPS35, VPS29, and VPS26 (80). A D620N mutation in VPS35 (Park17 locus) causes late-onset autosomal dominant PD, indicating that retromer defects can drive PD (81, 82). Very recent studies of human iPSC-derived neurons carrying the VPS35 D620N mutation showed mitochondrial dysfunction, altered mitophagy, and decreased overall autophagic flux (83). An interesting connection between VPS35, LRRK2, and Rab29 is beginning to emerge. In fly and murine models, overexpression of wild-type VPS35 rescues retromer-mediated defects (lysosomal enlargement and loss of mannose-6-phosphate receptors) caused by LRRK2 G2019S overexpression or Rab29 knockdown (76). More recently, VPS35 D620N was shown to enhance LRRK2-mediated phosphorylation of Rab10 and Ser1292 autophosphorylation, suggesting that VPS35 may be an upstream regulator of LRRK2 capable of hyperactivating LRRK2 when mutated (84).
(iv) Endolysosomal LRRK2.
LRRK2 appears to play a regulatory role in lysosomal processes, including mitophagy and autophagy, although attempts to pinpoint LRRK2’s role in autophagy have yielded inconsistent results (see the work of Madureira et al. for a thorough review [86]). However, the enlarged, abnormal lysosomes and lysosome-related organelles found in LRRK2 knockouts and with LRRK2 kinase inhibition implicate LRRK2 kinase activity in proper lysosomal function. Recent mechanistic work supports a model in which LRRK2’s phosphorylation of Rab proteins at endolysosomal membranes is critical for cellular homeostasis, particularly under conditions of lysosomal stress.
Recently, multiple lysosome-disrupting agents have been used to study LRRK2’s response to lysosomal stress, including chloroquine, l-leucyl-l-leucine methyl ester (LLOME), monensin, and nigericin (75, 87, 88). In many systems, treatment with lysosomotropic drugs drives LRRK2 to endolysosomal membranes and increases LRRK2 phosphorylation of substrates such as Rab10 (75, 88). Rab10, Rab8a, and possibly other Rab proteins appear to activate numerous compensatory pathways, which are still being worked out, to restore cellular homeostasis. Iwatsubo and colleagues showed that prolonged chloroquine-induced lysosomal overload causes Rab29-assisted LRRK2 translocation and accumulation at enlarged lysosomes (87, 88). LRRK2-mediated phosphorylation of Rab8 and Rab10 at enlarged lysosomes suppresses lysosomal stress by increasing lysosomal secretion in an EH-domain-binding protein 1 (EHBP1)- and EHBP1L1-dependent manner (87). In primary astrocytes, LRRK2 localizes to LLOME-ruptured lysosomes, recruits Rab10 in a kinase-dependent manner, and leads to JIP4-mediated formation of lysosomal tubular structures that dynamically release cargo from stressed lysosomes (89). LLOME-induced translocation of LRRK2 and Rab8a to lysosomes is affected by intracellular calcium levels in macrophages. CHMP4B, a core subunit of the calcium-dependent ESCRT-III complex responsible for the lysosomal repair pathway, colocalizes with LRRK2 and Rab8a at lysosomes (90). Conversely, LRRK2 depletion reduces lysosomal CHMP4B localization and targets vesicles for degradation via the lysophagy pathway (90).
A number of unanswered questions remain regarding the role of LRRK2 in lysosomal homeostasis. First, it is debated whether Rab29 is absolutely required for LRRK2 lysosomal localization and substrate Rab phosphorylation. While Kuwahara et al. showed that knockdown of endogenous Rab29 in RAW264.7 macrophages following chloroquine treatment led to a robust reduction in Rab10 phosphorylation (88), Kalogeropulou et al. found that Rab29 knockout in mouse embryonic fibroblasts, under both lysosomal stress and nonstress conditions, had no effect on Rab10 or Rab12 phosphorylation (75). It is possible that endogenous Rab29 is required only in certain cell types or cellular contexts. Second, endolysosomal LRRK2 localization and function under nonstress conditions have not been completely elucidated. Liu et al. showed that, in the absence of lysosomal stress, Rab10 localizes to macropinocytic vesicles, where it is phosphorylated by LRRK2 (91). In this context, EHBP1L1’s activity in endosomal tubular recycling is blocked by LRRK2-mediated Rab10 phosphorylation. Thus, LRRK2 targets appear to function in multiple endolysosomal pathways. Finally, the mechanisms by which LRRK2-mediated lysosomal homeostasis could lead to LRRK2-PD and idiopathic PD are far from clear. Recent work showed early endosome accumulation in conjunction with late endosome and lysosome depletion in postmortem human nigral DA neurons of idiopathic PD patients (92). Similarly, in a rotenone rat model of PD, rotenone-induced cytopathologies, including endolysosomal stress, were reversed by treatment with the LRRK2 kinase inhibitor PF-360 (92).
(v) LRRK2 and GBA1.
Mutation of the GBA1 gene is one of the most common risk factors associated with PD (93, 94). GBA1 encodes β-glucocerebrosidase 1 (GCase), a lysosomal enzyme that hydrolyzes glucosylceramide to glucose and ceramide. Homozygous GBA1 mutations lead to loss of GCase activity and result in the lysosomal disorder with glucosylceramide accumulation known as Gaucher’s disease. Both homozygous and heterozygous GBA1 mutations increase risk for PD (94, 95). iPSC-derived neurons from LRRK2-PD patients show reduced GCase activity that is rescued by treatment with LRRK2 kinase inhibitor (96). GCase activity appears to be dependent on Rab10 protein levels, suggesting that GCase may be regulated by LRRK2-mediated Rab10 phosphorylation. LRRK2 inhibition leads to restoration of GCase activity in neurons and prevents lysosomal disruption and accumulation of α-synuclein (92, 96, 97).
(vi) LRRK2 and mitochondria.
Mitochondrial dysfunction is important in both genetic and sporadic PD, with the familial PD PINK and parkin genes being critical to proper mitochondrial quality control. Early reports indicated that a small fraction of overexpressed LRRK2 is found at mitochondrial membranes (98, 99). The full role of LRRK2 in mitochondrial function is unknown, but compelling evidence suggests it has kinase-dependent roles in mitophagy and mitochondrial tethering to the endoplasmic reticulum (ER). LRRK2 functions in multiple pathways to regulate mitophagy. LRRK2 interacts with the mitochondrial outer membrane protein Miro, which is degraded to stop mitochondrial motility prior to the initiation of mitophagy (85). Mutant LRRK2 G2019S prevents proteasomal degradation of Miro, leading to delayed mitophagy (85). Recently, it was shown that PINK1 and parkin drive Rab10 accumulation onto depolarized mitochondria, which leads to recruitment of the autophagy receptor optineurin and subsequent mitophagy (100). Increased phosphorylation of Rab10 by PD mutant LRRK2 G2019S or R1441C decreases Rab10-mediated mitophagy, which is rescued by LRRK2 kinase inhibition (100).
There are conflicting results regarding the effects of LRRK2 PD mutations on mitochondrial network dynamics (see the report by Singh et al. for a recent review [101]). However, multiple groups have found that LRRK2 interacts with the mitochondrial fission protein Drp1 (102, 103). Drp1-dependent mitochondrial fission occurs early in mitophagy to sequester damaged mitochondria from the healthy network (104). Recent evidence showed that mitochondrial stress, including Drp1-dependent mitochondrial fragmentation, drives altered innate immune responses in LRRK2 KO macrophages (105). LRRK2 also impairs interactions between both parkin and Drp1 and certain mitochondrial targets in a kinase-dependent manner (106). LRRK2 binding to parkin (as well as to E3 ligases MARCH5 and MULAN) was shown to regulate mitochondrial tethering to the ER (107). The hyperactive kinase G2019S LRRK2 more readily dissociates from these ligases, triggering their PERK-mediated phosphorylation and activation, thus driving degradation of ER mitochondrial tethering proteins (107). It is interesting that defects in mitophagy due to LRRK2 kinase hyperactivity parallel those due to PINK1 or parkin knockout (i.e., Miro degradation or mitochondrial Rab10 accumulation). In contrast, hyperactive kinase LRRK2 G2019S appears to augment parkin’s E3 ligase function as it relates to ER mitochondrial tethering. The relevance of these LRRK2 phenotypes to PD remains to be further investigated.
(vii) LRRK2’s regulation of ciliogenesis and centrosomal cohesion.
Cilia are microtubule-based cell surface appendages that are crucial for processes such as protein trafficking and signal transduction (108, 109). Hyperactive kinase mutants of LRRK2 interfere with ciliogenesis. Mouse embryonic fibroblasts expressing LRRK2 R1441G, cholinergic neurons in the striatum of LRRK2 R1441C mice, and LRRK2 G2019S human iPSCs show decreased ciliation (55, 110). LRRK2’s phosphorylation of Rab proteins, particularly Rab8A and Rab10, modulates ciliogenesis in a complex manner that requires Rab-interacting lysosomal protein-like 1 (RILPL1) (57, 110). Whereas phosphorylation of Rab8A activates ciliogenesis, phosphorylation of Rab10 enhances Rab10 binding to RILPL1 and strengthens Rab10’s ability to block ciliogenesis (110). Reduced ciliation due to mutant LRRK2 expression impairs sonic hedgehog signaling; it has been hypothesized that this may disrupt neuroprotective circuits for DA neurons (110). PD-driving LRRK2 mutations also decrease centrosomal cohesion (111). This is in keeping with LRRK2’s function in ciliogenesis, since centrioles (radially organized arrays of triplet microtubules) form the centrosomes and serve as the foundation for the cilial basal body (108). Centrosomal cohesion defects appear to be mediated by LRRK2’s phosphorylation of Rab8 and Rab10, with RILPL1 also being involved, and can be reversed with LRRK2 kinase inhibitor treatment (111, 112). Because centrosomal cohesion defects are easily measured in blood samples, centrosomal cohesion assays are being evaluated as a possible biomarker for PD (113).
(viii) LRRK2 at microtubules.
In addition to localizing to the cytoplasm and membrane-bound vesicles and organelles, certain pathogenic LRRK2 mutants, namely, the Roc-COR mutants R1441C/G and Y1699C as well as the kinase mutant I2020T, appear to localize as filaments around microtubule networks when overexpressed (51, 114, 115). Wild-type LRRK2 and G2019S LRRK2, which normally do not form these filaments, will do so when treated with type I kinase inhibitors (51, 116).
A recent 14-Å in situ cryo-electron tomography structure of LRRK2 I2020T in complex with microtubules showed that LRRK2 oligomerizes as a right-handed helix around left-handed microtubules, with the Roc domain facing the microtubule and the kinase domain exposed to the cytoplasm (117). Related cryo-electron microscopy structural modeling of the catalytic portion of LRRK2 showed that a closed kinase conformation is required for microtubule association (48). Consistently, in vitro microtubule motor protein motility assays suggest that treatment with type I LRRK2 kinase inhibitors, which stabilize the closed conformation, promotes microtubule association, while treatment with type II kinase inhibitors, which stabilize the open conformation, prevents microtubule association (48). Microtubule association of pathogenic or type I kinase-inhibited LRRK2 is disrupted by GTP-binding inhibitors and is restored when GTP binding is stabilized, demonstrating that the Roc domain regulates microtubule association as well as kinase domain conformation (116). LRRK2’s Roc domain can also mediate direct interaction of LRRK2 with β-tubulin, selectively interacting with specific tubulin isoforms, including TUBB, TUBB4, and TUBB6 (118). LRRK2 may block microtubule acetylation, since its interaction with β-tubulin occurs through the K362 residue, which in α/β-tubulin heterodimers is very close to the K40 acetylation (118).
The functional relevance of the LRRK2-microtubule association remains elusive since these findings occurred largely in the context of LRRK2 overexpression. Nevertheless, this association is intriguing since disruption of microtubule dynamics, particularly the cellular machinery associated with axonal transport, is linked to neurological disease (119). LRRK2 blocks motility of kinesin and dynein motor proteins in vitro, implicating LRRK2 in cargo transport disruption (48). In vivo overexpressed LRRK2 Roc-COR mutants (R1441C and Y1699C) disrupt axonal transport (48), which can be restored by increasing microtubule acetylation (120). This leads to a model in which Roc-COR mutants interfere with axonal trafficking by forming filaments around deacetylated microtubules (120).
Recently, we discovered that the microtubule-associated E3 ligase TRIM1 (also called MID2) appears to be critically involved in LRRK2 degradation (121). TRIM1 interacts with LRRK2 at the microtubule to cause LRRK2 ubiquitination and proteasomal degradation. Knockdown of TRIM1 increases endogenous LRRK2 levels, and TRIM1 can rescue neurite outgrowth deficits caused by LRRK2 G2019S. The localization of LRRK2 to the microtubule prior to ubiquitination and degradation is consistent with observations that type I kinase inhibitors also induce LRRK2 microtubule localization, ubiquitination, and proteasomal degradation. These findings may point to the microtubule as an important subcellular site in at least some LRRK2 degradation pathways.
(ix) LRRK2 and tau.
LRRK2’s possible physical association with the microtubule cytoskeleton is intriguing, given its clinical effects on tau, a microtubule-associated protein. In patients, PD-driving LRRK2 mutations can lead to tau aggregation, as evidenced by rare LRRK2 mutation carriers who develop pure tauopathies without α-synucleinopathy (19). LRRK2 can increase tau aggregation and phosphorylation in a transgenic LRRK2/Tau P301L mutant mouse model, and phosphorylation of tau is increased in some R1441G and G2019S LRRK2 transgenic mice (122–124). However, only limited evidence suggests tau as a physiological LRRK2 substrate; a larger body of research suggests that, if LRRK2 mediates tau phosphorylation, the pathway is indirect (125, 126). In Drosophila, either increased or decreased levels of the LRRK2 homolog Lrrk enhance tau neurotoxicity by promoting excess stabilization of filamentous actin and mislocalization of the mitochondrial fission protein Drp1 (127). Interestingly, murine models of PD mutant VPS35 D620N, which enhances LRRK2 kinase function, show striking tau neuropathology, again connecting hyperactive LRRK2 to tau aggregation (128).
(x) LRRK2 at synapses.
LRRK2 is particularly challenging to study in neurons, where it is expressed at very low levels. Consistent with its function in endolysosomal trafficking, LRRK2 may be involved in synaptic vesicle trafficking. Some evidence suggests that LRRK2 regulates synaptic vesicle fusion through interactions with components of the SNARE complex (129). Recent studies suggest LRRK2’s regulation of synaptic vesicle endocytosis may be especially important in disease. Three proteins involved in synaptic vesicle endocytosis, namely, endophilin A, synaptojanin, and auxilin, can be phosphorylated by LRRK2 in model systems (46, 130, 131). LRRK2 G2019S-mediated phosphorylation of endophilin A impedes synaptic vesicle endocytosis in a fly model (131). Human iPSC models of LRRK2 mutant PD show abnormal synaptic vesicle endocytosis, with reduced synaptic vesicle density (130). This appears to cause accumulation of oxidized dopamine, potentially mediating the selective neurotoxicity in PD (130).
LRRK2’S ROLE IN DA NEURON DEGENERATION
How LRRK2 mutations cause circumscribed degeneration of DA neuronal circuitry is unknown. However, the specific cellular requirements for DA neuron regulation and upkeep suggest that proper mitochondrial, endolysosomal, and cytoskeletal functions are critical. Supporting the relevance of these pathways, single-gene mutations causing DA neurodegeneration include those in parkin and PINK (mitochondrial), VPS35 and GBA1 (endolysosomal), and tau (cytoskeleton) genes. As discussed throughout this review, LRRK2 not only functions in these three pathways but also interacts genetically or physically with each of the aforementioned genes.
DA neurons are notable for their high energy requirements and their widely branched, long, and unmyelinated axons (132). These characteristics make them susceptible to neurodegeneration if their energy demands are not met (133). For this reason, oxidative stress and other stresses that disrupt mitochondrial activity and subsequent energy production appear to be particularly damaging to substantia nigra neurons (134, 135). LRRK2’s normal role in maintaining mitochondrial function may be critical in DA neuronal health. PD mutant LRRK2 delays mitophagy through multiple mechanisms, hindering mitochondrial turnover and proper energy production.
The highly branched DA neuronal networks facilitate continuous communication with neighboring neurons. However, this extensive neuronal signaling also causes protein homeostatic stress that requires robust lysosomal activity to maintain proteostasis (136, 137). Endolysosomal dysfunction is increasingly implicated in PD (136). Compelling recent evidence indicates that LRRK2 is important in Rab-dependent and independent pathways that maintain endolysosomal homeostasis and that hyperactive kinase mutations in LRRK2 disrupt this function.
DA neurons appear to have widely fluctuating calcium ion levels and poor calcium-buffering capacity, factors that activate proteases, including calpains and phosphatases like calcineurin (138). Activated calpains promote α-synuclein aggregation and also appear to target cytoskeletal proteins (139, 140). We found that the E3 ligase TRIM1 functions at the cytoskeleton to ubiquitinate LRRK2 for proteasomal degradation. If cytoskeletal disruption leads to decreased LRRK2 degradation, then DA neurons harboring hyperactive LRRK2 mutations could be particularly susceptible to injury. Lastly, neuroinflammation, particularly in the form of hyperactivated microglia, may contribute to DA neurodegeneration. Recent evidence suggests an important role for LRRK2 in neuroinflammatory pathways with microglial responses altered by PD mutant LRRK2 in an NFAT-dependent manner. The relative importance of these pathways to DA neurodegeneration has not been fully worked out, and critical questions remain, as discussed in more detail below.
FUTURE DIRECTIONS FOR LRRK2 RESEARCH
While the highlighted research demonstrates significant progress in understanding LRRK2’s various subcellular functions, more work is needed to determine the relevance of these functions to PD pathogenesis. Growing evidence in recent years shows that the LRRK2-Rab GTPase interaction is a key component of LRRK2 biology. Phosphorylation of Rab8 and Rab10 substrates affects ciliogenesis, endolysosomal dynamics, and centrosomal cohesion, all of which raise interesting hypotheses about the mechanism of LRRK2 pathology. However, the roles of a majority of LRRK2’s Rab GTPase substrates are still inadequately characterized. Further work is needed to investigate how mutant LRRK2’s effects on Rab proteins and other substrates could disrupt DA neuron homeostasis and ultimately lead to neurodegeneration. Additionally, the intersection of LRRK2 biology with that of other PD-driving genes, particularly VPS35, Rab29, parkin, and PINK genes, is clearly important but remains to be fully delineated.
Type 1 LRRK2 inhibitors induce endolysosome-related lung and kidney toxicity; however, it is unclear whether this effect is entirely due to direct kinase inhibition or is also mediated by inhibitor-induced ubiquitination and degradation of LRRK2. Such considerations are key to the development of LRRK2 kinase inhibitors as therapeutics, as clearly defining drug mechanisms may suggest modifications that can decrease human side effects.
It is also important to note that much of our understanding of LRRK2 biology is based on biological systems that may insufficiently mimic true physiological conditions. Due to the technical challenge of visualizing an endogenous protein expressed at low levels, LRRK2’s activities have been characterized primarily in the context of overexpression. This raises the question of whether these activities are replicated at endogenous levels. For example, Rab29-mediated LRRK2 activation in cellulo has not reproduced in transgenic mice overexpressing Rab29, and Rab29 knockout has no effect on LRRK2 activity. Although robust LRRK2 localization and oligomerization around microtubules due to PD-driving mutations or following kinase inhibition occur with overexpressed proteins, this effect has not yet been shown with endogenous LRRK2. Our studies of TRIM1 ubiquitin ligase have begun to reveal a possible pathway for LRRK2 microtubule localization related to degradation. However, most of our work was also done in the context of overexpression. Therefore, it is of great interest to develop novel techniques and tools to detect and to visualize LRRK2 at endogenous levels.
Finally, our current understanding of LRRK2 biology and PD mutant LRRK2 dysfunction does not account for human LRRK2 pathology. In particular, no studies thus far provide a parsimonious rationale for the fact that human patients with LRRK2 mutations can develop α-synuclein pathology, tau pathology, or even pure nigral degeneration. This information is essential to keep in mind when choosing LRRK2 model systems and constructing hypotheses about how LRRK2 may drive PD.
ACKNOWLEDGMENTS
We thank R. Jeremy Nichols and Hannah Ahrendt for their critical reading of the manuscript.
REFERENCES
- 1.de Lau LM, Breteler MM. 2006. Epidemiology of Parkinson's disease. Lancet Neurol 5:525–535. 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Schapira AHV, Chaudhuri KR, Jenner P. 2017. Non-motor features of Parkinson disease. Nat Rev Neurosci 18:435–450. 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 3.Schulz-Schaeffer WJ. 2010. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia. Acta Neuropathol 120:131–143. 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein C, Westenberger A. 2012. Genetics of Parkinson's disease. Cold Spring Harb Perspect Med 2:a008888. 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corti O, Lesage S, Brice A. 2011. What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev 91:1161–1218. 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- 6.Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, Singleton A, Lees AJ, Shaw K, Bhatia KP, Bonifati V, Quinn NP, Lynch J, Healy DG, Holton JL, Revesz T, Wood NW. 2005. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet 365:415–416. 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 7.Shu L, Zhang Y, Sun Q, Pan H, Tang B. 2019. A comprehensive analysis of population differences in LRRK2 variant distribution in Parkinson's disease. Front Aging Neurosci 11:13. 10.3389/fnagi.2019.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan EK, Shen H, Tan LC, Farrer M, Yew K, Chua E, Jamora RD, Puvan K, Puong KY, Zhao Y, Pavanni R, Wong MC, Yih Y, Skipper L, Liu JJ. 2005. The G2019S LRRK2 mutation is uncommon in an Asian cohort of Parkinson's disease patients. Neurosci Lett 384:327–329. 10.1016/j.neulet.2005.04.103. [DOI] [PubMed] [Google Scholar]
- 9.Marder K, Wang Y, Alcalay RN, Mejia-Santana H, Tang MX, Lee A, Raymond D, Mirelman A, Saunders-Pullman R, Clark L, Ozelius L, Orr-Urtreger A, Giladi N, Bressman S. 2015. Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology 85:89–95. 10.1212/WNL.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. 2008. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol 7:583–590. 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. 2009. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet 41:1308–1312. 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross OA, Soto-Ortolaza AI, Heckman MG, Aasly JO, Abahuni N, Annesi G, Bacon JA, Bardien S, Bozi M, Brice A, Brighina L, Van Broeckhoven C, Carr J, Chartier-Harlin MC, Dardiotis E, Dickson DW, Diehl NN, Elbaz A, Ferrarese C, Ferraris A, Fiske B, Gibson JM, Gibson R, Hadjigeorgiou GM, Hattori N, Ioannidis JP, Jasinska-Myga B, Jeon BS, Kim YJ, Klein C, Kruger R, Kyratzi E, Lesage S, Lin CH, Lynch T, Maraganore DM, Mellick GD, Mutez E, Nilsson C, Opala G, Park SS, Puschmann A, Quattrone A, Sharma M, Silburn PA, Sohn YH, Stefanis L, Tadic V, Theuns J, Tomiyama H, Uitti RJ, Valente EM, van de Loo S, Vassilatis DK, Vilariño-Güell C, White LR, Wirdefeldt K, Wszolek ZK, Wu RM, Farrer MJ. 2011. Association of LRRK2 exonic variants with susceptibility to Parkinson's disease: a case-control study. Lancet Neurol 10:898–908. 10.1016/S1474-4422(11)70175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. 2004. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44:601–607. 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Fava VM, Xu YZ, Lettre G, Van Thuc N, Orlova M, Thai VH, Tao S, Croteau N, Eldeeb MA, MacDougall EJ, Cambri G, Lahiri R, Adams L, Fon EA, Trempe JF, Cobat A, Alcais A, Abel L, Schurr E. 2019. Pleiotropic effects for parkin and LRRK2 in leprosy type-1 reactions and Parkinson's disease. Proc Natl Acad Sci U S A 116:15616–15624. 10.1073/pnas.1901805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartlova A, Herbst S, Peltier J, Rodgers A, Bilkei-Gorzo O, Fearns A, Dill BD, Lee H, Flynn R, Cowley SA, Davies P, Lewis PA, Ganley IG, Martinez J, Alessi DR, Reith AD, Trost M, Gutierrez MG. 2018. LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages. EMBO J 37:e98694. 10.15252/embj.201798694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui KY, Fernandez-Hernandez H, Hu J, Schaffner A, Pankratz N, Hsu NY, Chuang LS, Carmi S, Villaverde N, Li X, Rivas M, Levine AP, Bao X, Labrias PR, Haritunians T, Ruane D, Gettler K, Chen E, Li D, Schiff ER, Pontikos N, Barzilai N, Brant SR, Bressman S, Cheifetz AS, Clark LN, Daly MJ, Desnick RJ, Duerr RH, Katz S, Lencz T, Myers RH, Ostrer H, Ozelius L, Payami H, Peter Y, Rioux JD, Segal AW, Scott WK, Silverberg MS, Vance JM, Ubarretxena-Belandia I, Foroud T, Atzmon G, Pe'er I, Ioannou Y, McGovern DPB, Yue Z, Schadt EE, Cho JH, Peter I. 2018. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn's disease and Parkinson's disease. Sci Transl Med 10:eaai7795. 10.1126/scitranslmed.aai7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kluss JH, Mamais A, Cookson MR. 2019. LRRK2 links genetic and sporadic Parkinson's disease. Biochem Soc Trans 47:651–661. 10.1042/BST20180462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasser T, Hardy J, Mizuno Y. 2011. Milestones in PD genetics. Mov Disord 26:1042–1048. 10.1002/mds.23637. [DOI] [PubMed] [Google Scholar]
- 19.Kalia LV, Lang AE, Hazrati LN, Fujioka S, Wszolek ZK, Dickson DW, Ross OA, Van Deerlin VM, Trojanowski JQ, Hurtig HI, Alcalay RN, Marder KS, Clark LN, Gaig C, Tolosa E, Ruiz-Martinez J, Marti-Masso JF, Ferrer I, Lopez de Munain A, Goldman SM, Schule B, Langston JW, Aasly JO, Giordana MT, Bonifati V, Puschmann A, Canesi M, Pezzoli G, Maues De Paula A, Hasegawa K, Duyckaerts C, Brice A, Stoessl AJ, Marras C. 2015. Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol 72:100–105. 10.1001/jamaneurol.2014.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Perera G, Takahashi-Fujigasaki J, Mash DC, Vonsattel JPG, Uchino A, Hasegawa K, Nichols RJ, Holton JL, Murayama S, Dzamko N, Halliday GM. 2018. Reduced LRRK2 in association with retromer dysfunction in post-mortem brain tissue from LRRK2 mutation carriers. Brain 141:486–495. 10.1093/brain/awx344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider SA, Alcalay RN. 2017. Neuropathology of genetic synucleinopathies with parkinsonism: review of the literature. Mov Disord 32:1504–1523. 10.1002/mds.27193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM. 2007. Parkinson's disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet 16:223–232. 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 23.O'Hara DM, Pawar G, Kalia SK, Kalia LV. 2020. LRRK2 and α-synuclein: distinct or synergistic players in Parkinson's disease? Front Neurosci 14:577. 10.3389/fnins.2020.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volta M, Melrose H. 2017. LRRK2 mouse models: dissecting the behavior, striatal neurochemistry and neurophysiology of PD pathogenesis. Biochem Soc Trans 45:113–122. 10.1042/BST20160238. [DOI] [PubMed] [Google Scholar]
- 25.Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, Xie C, Long CX, Yang WJ, Ding J, Chen ZZ, Gallant PE, Tao-Cheng JH, Rudow G, Troncoso JC, Liu Z, Li Z, Cai H. 2009. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant α-synuclein. Neuron 64:807–827. 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daher JP, Pletnikova O, Biskup S, Musso A, Gellhaar S, Galter D, Troncoso JC, Lee MK, Dawson TM, Dawson VL, Moore DJ. 2012. Neurodegenerative phenotypes in an A53T α-synuclein transgenic mouse model are independent of LRRK2. Hum Mol Genet 21:2420–2431. 10.1093/hmg/dds057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novello S, Arcuri L, Dovero S, Dutheil N, Shimshek DR, Bezard E, Morari M. 2018. G2019S LRRK2 mutation facilitates α-synuclein neuropathology in aged mice. Neurobiol Dis 120:21–33. 10.1016/j.nbd.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Bieri G, Brahic M, Bousset L, Couthouis J, Kramer NJ, Ma R, Nakayama L, Monbureau M, Defensor E, Schule B, Shamloo M, Melki R, Gitler AD. 2019. LRRK2 modifies α-syn pathology and spread in mouse models and human neurons. Acta Neuropathol 137:961–980. 10.1007/s00401-019-01995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daher JP, Volpicelli-Daley LA, Blackburn JP, Moehle MS, West AB. 2014. Abrogation of α-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc Natl Acad Sci U S A 111:9289–9294. 10.1073/pnas.1403215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen APT, Tsika E, Kelly K, Levine N, Chen X, West AB, Boularand S, Barneoud P, Moore DJ. 2020. Dopaminergic neurodegeneration induced by Parkinson's disease-linked G2019S LRRK2 is dependent on kinase and GTPase activity. Proc Natl Acad Sci U S A 117:17296–17307. 10.1073/pnas.1922184117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ, III, Shen J. 2010. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of α-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A 107:9879–9884. 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzig MC, Kolly C, Persohn E, Theil D, Schweizer T, Hafner T, Stemmelen C, Troxler TJ, Schmid P, Danner S, Schnell CR, Mueller M, Kinzel B, Grevot A, Bolognani F, Stirn M, Kuhn RR, Kaupmann K, van der Putten PH, Rovelli G, Shimshek DR. 2011. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum Mol Genet 20:4209–4223. 10.1093/hmg/ddr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuji RN, Flagella M, Baca M, Baptista MA, Brodbeck J, Chan BK, Fiske BK, Honigberg L, Jubb AM, Katavolos P, Lee DW, Lewin-Koh SC, Lin T, Liu X, Liu S, Lyssikatos JP, O'Mahony J, Reichelt M, Roose-Girma M, Sheng Z, Sherer T, Smith A, Solon M, Sweeney ZK, Tarrant J, Urkowitz A, Warming S, Yaylaoglu M, Zhang S, Zhu H, Estrada AA, Watts RJ. 2015. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci Transl Med 7:273ra215. 10.1126/scitranslmed.aaa3634. [DOI] [PubMed] [Google Scholar]
- 34.Baptista MA, Dave KD, Frasier MA, Sherer TB, Greeley M, Beck MJ, Varsho JS, Parker GA, Moore C, Churchill MJ, Meshul CK, Fiske BK. 2013. Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PLoS One 8:e80705. 10.1371/journal.pone.0080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giaime E, Tong Y, Wagner LK, Yuan Y, Huang G, Shen J. 2017. Age-dependent dopaminergic neurodegeneration and impairment of the autophagy-lysosomal pathway in LRRK-deficient mice. Neuron 96:796–807.e6. 10.1016/j.neuron.2017.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, Lee J, Krummey S, Lu W, Cai H, Lenardo MJ. 2011. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol 12:1063–1070. 10.1038/ni.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takagawa T, Kitani A, Fuss I, Levine B, Brant SR, Peter I, Tajima M, Nakamura S, Strober W. 2018. An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis. Sci Transl Med 10:eaan8162. 10.1126/scitranslmed.aan8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim C, Beilina A, Smith N, Li Y, Kim M, Kumaran R, Kaganovich A, Mamais A, Adame A, Iba M, Kwon S, Lee WJ, Shin SJ, Rissman RA, You S, Lee SJ, Singleton AB, Cookson MR, Masliah E. 2020. LRRK2 mediates microglial neurotoxicity via NFATc2 in rodent models of synucleinopathies. Sci Transl Med 12:eaay0399. 10.1126/scitranslmed.aay0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fava VM, Manry J, Cobat A, Orlova M, Van Thuc N, Ba NN, Thai VH, Abel L, Alcais A, Schurr E. 2016. A missense LRRK2 variant is a risk factor for excessive inflammatory responses in leprosy. PLoS Negl Trop Dis 10:e0004412. 10.1371/journal.pntd.0004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W, Liu X, Li Y, Zhao J, Liu Z, Hu Z, Wang Y, Yao Y, Miller AW, Su B, Cookson MR, Li X, Kang Z. 2017. LRRK2 promotes the activation of NLRC4 inflammasome during Salmonella Typhimurium infection. J Exp Med 214:3051–3066. 10.1084/jem.20170014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallings RL, Herrick MK, Tansey MG. 2020. LRRK2 at the interface between peripheral and central immune function in Parkinson's. Front Neurosci 14:443. 10.3389/fnins.2020.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan Y, Howden AJM, Sarhan AR, Lis P, Ito G, Martinez TN, Brockmann K, Gasser T, Alessi DR, Sammler EM. 2018. Interrogating Parkinson's disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils. Biochem J 475:23–44. 10.1042/BCJ20170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atashrazm F, Hammond D, Perera G, Bolliger MF, Matar E, Halliday GM, Schule B, Lewis SJG, Nichols RJ, Dzamko N. 2019. LRRK2-mediated Rab10 phosphorylation in immune cells from Parkinson's disease patients. Mov Disord 34:406–415. 10.1002/mds.27601. [DOI] [PubMed] [Google Scholar]
- 44.Greggio E, Zambrano I, Kaganovich A, Beilina A, Taymans JM, Daniels V, Lewis P, Jain S, Ding J, Syed A, Thomas KJ, Baekelandt V, Cookson MR. 2008. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem 283:16906–16914. 10.1074/jbc.M708718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guaitoli G, Raimondi F, Gilsbach BK, Gomez-Llorente Y, Deyaert E, Renzi F, Li X, Schaffner A, Jagtap PK, Boldt K, von Zweydorf F, Gotthardt K, Lorimer DD, Yue Z, Burgin A, Janjic N, Sattler M, Versees W, Ueffing M, Ubarretxena-Belandia I, Kortholt A, Gloeckner CJ. 2016. Structural model of the dimeric Parkinson's protein LRRK2 reveals a compact architecture involving distant interdomain contacts. Proc Natl Acad Sci U S A 113:E4357–E4366. 10.1073/pnas.1523708113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Islam MS, Moore DJ. 2017. Mechanisms of LRRK2-dependent neurodegeneration: role of enzymatic activity and protein aggregation. Biochem Soc Trans 45:163–172. 10.1042/BST20160264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Civiero L, Vancraenenbroeck R, Belluzzi E, Beilina A, Lobbestael E, Reyniers L, Gao F, Micetic I, De Maeyer M, Bubacco L, Baekelandt V, Cookson MR, Greggio E, Taymans JM. 2012. Biochemical characterization of highly purified leucine-rich repeat kinases 1 and 2 demonstrates formation of homodimers. PLoS One 7:e43472. 10.1371/journal.pone.0043472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deniston CK, Salogiannis J, Mathea S, Snead DM, Lahiri I, Matyszewski M, Donosa O, Watanabe R, Bohning J, Shiau AK, Knapp S, Villa E, Reck-Peterson SL, Leschziner AE. 2020. Structure of LRRK2 in Parkinson's disease and model for microtubule interaction. Nature 588:344–349. 10.1038/s41586-020-2673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greggio E, Lewis PA, van der Brug MP, Ahmad R, Kaganovich A, Ding J, Beilina A, Baker AK, Cookson MR. 2007. Mutations in LRRK2/dardarin associated with Parkinson disease are more toxic than equivalent mutations in the homologous kinase LRRK1. J Neurochem 102:93–102. 10.1111/j.1471-4159.2007.04523.x. [DOI] [PubMed] [Google Scholar]
- 50.Langston RG, Rudenko IN, Cookson MR. 2016. The function of orthologues of the human Parkinson's disease gene LRRK2 across species: implications for disease modelling in preclinical research. Biochem J 473:221–232. 10.1042/BJ20150985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt SH, Knape MJ, Boassa D, Mumdey N, Kornev AP, Ellisman MH, Taylor SS, Herberg FW. 2019. The dynamic switch mechanism that leads to activation of LRRK2 is embedded in the DFGψ motif in the kinase domain. Proc Natl Acad Sci U S A 116:14979–14988. 10.1073/pnas.1900289116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nichols RJ, Dzamko N, Hutti JE, Cantley LC, Deak M, Moran J, Bamborough P, Reith AD, Alessi DR. 2009. Substrate specificity and inhibitors of LRRK2, a protein kinase mutated in Parkinson's disease. Biochem J 424:47–60. 10.1042/BJ20091035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu CX, Liao J, Park Y, Reed X, Engel VA, Hoang NC, Takagi Y, Johnson SM, Wang M, Federici M, Nichols RJ, Sanishvili R, Cookson MR, Hoang QQ. 2019. Parkinson's disease-associated mutations in the GTPase domain of LRRK2 impair its nucleotide-dependent conformational dynamics. J Biol Chem 294:5907–5913. 10.1074/jbc.RA119.007631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ito G, Okai T, Fujino G, Takeda K, Ichijo H, Katada T, Iwatsubo T. 2007. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson's disease. Biochemistry 46:1380–1388. 10.1021/bi061960m. [DOI] [PubMed] [Google Scholar]
- 55.Sheng Z, Zhang S, Bustos D, Kleinheinz T, Le Pichon CE, Dominguez SL, Solanoy HO, Drummond J, Zhang X, Ding X, Cai F, Song Q, Li X, Yue Z, van der Brug MP, Burdick DJ, Gunzner-Toste J, Chen H, Liu X, Estrada AA, Sweeney ZK, Scearce-Levie K, Moffat JG, Kirkpatrick DS, Zhu H. 2012. Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci Transl Med 4:164ra161. 10.1126/scitranslmed.3004485. [DOI] [PubMed] [Google Scholar]
- 56.Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, Baptista MA, Fiske BK, Fell MJ, Morrow JA, Reith AD, Alessi DR, Mann M. 2016. Phosphoproteomics reveals that Parkinson's disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5:e12813. 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steger M, Diez F, Dhekne HS, Lis P, Nirujogi RS, Karayel O, Tonelli F, Martinez TN, Lorentzen E, Pfeffer SR, Alessi DR, Mann M. 2017. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife 6:e31012. 10.7554/eLife.31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao J, Wu CX, Burlak C, Zhang S, Sahm H, Wang M, Zhang ZY, Vogel KW, Federici M, Riddle SM, Nichols RJ, Liu D, Cookson MR, Stone TA, Hoang QQ. 2014. Parkinson disease-associated mutation R1441H in LRRK2 prolongs the “active state” of its GTPase domain. Proc Natl Acad Sci U S A 111:4055–4060. 10.1073/pnas.1323285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang X, Wu C, Park Y, Long X, Hoang QQ, Liao J. 2019. The Parkinson's disease-associated mutation N1437H impairs conformational dynamics in the G domain of LRRK2. FASEB J 33:4814–4823. 10.1096/fj.201802031R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang P, Fan Y, Ru H, Wang L, Magupalli VG, Taylor SS, Alessi DR, Wu H. 2019. Crystal structure of the WD40 domain dimer of LRRK2. Proc Natl Acad Sci U S A 116:1579–1584. 10.1073/pnas.1817889116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Maio R, Hoffman EK, Rocha EM, Keeney MT, Sanders LH, De Miranda BR, Zharikov A, Van Laar A, Stepan AF, Lanz TA, Kofler JK, Burton EA, Alessi DR, Hastings TG, Greenamyre JT. 2018. LRRK2 activation in idiopathic Parkinson's disease. Sci Transl Med 10:eaar5429. 10.1126/scitranslmed.aar5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, Pothos EN, Shen J. 2009. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A 106:14622–14627. 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baptista MAS, Merchant K, Barrett T, Bhargava S, Bryce DK, Ellis JM, Estrada AA, Fell MJ, Fiske BK, Fuji RN, Galatsis P, Henry AG, Hill S, Hirst W, Houle C, Kennedy ME, Liu X, Maddess ML, Markgraf C, Mei H, Meier WA, Needle E, Ploch S, Royer C, Rudolph K, Sharma AK, Stepan A, Steyn S, Trost C, Yin Z, Yu H, Wang X, Sherer TB. 2020. LRRK2 inhibitors induce reversible changes in nonhuman primate lungs without measurable pulmonary deficits. Sci Transl Med 12:eaav0820. 10.1126/scitranslmed.aav0820. [DOI] [PubMed] [Google Scholar]
- 64.Scott JD, DeMong DE, Greshock TJ, Basu K, Dai X, Harris J, Hruza A, Li SW, Lin SI, Liu H, Macala MK, Hu Z, Mei H, Zhang H, Walsh P, Poirier M, Shi ZC, Xiao L, Agnihotri G, Baptista MA, Columbus J, Fell MJ, Hyde LA, Kuvelkar R, Lin Y, Mirescu C, Morrow JA, Yin Z, Zhang X, Zhou X, Chang RK, Embrey MW, Sanders JM, Tiscia HE, Drolet RE, Kern JT, Sur SM, Renger JJ, Bilodeau MT, Kennedy ME, Parker EM, Stamford AW, Nargund R, McCauley JA, Miller MW. 2017. Discovery of a 3-(4-pyrimidinyl) indazole (MLi-2), an orally available and selective leucine-rich repeat kinase 2 (LRRK2) inhibitor that reduces brain kinase activity. J Med Chem 60:2983–2992. 10.1021/acs.jmedchem.7b00045. [DOI] [PubMed] [Google Scholar]
- 65.Gilsbach BK, Messias AC, Ito G, Sattler M, Alessi DR, Wittinghofer A, Kortholt A. 2015. Structural characterization of LRRK2 inhibitors. J Med Chem 58:3751–3756. 10.1021/jm5018779. [DOI] [PubMed] [Google Scholar]
- 66.Zhao J, Molitor TP, Langston JW, Nichols RJ. 2015. LRRK2 dephosphorylation increases its ubiquitination. Biochem J 469:107–120. 10.1042/BJ20141305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berger Z, Smith KA, Lavoie MJ. 2010. Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemistry 49:5511–5523. 10.1021/bi100157u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Purlyte E, Dhekne HS, Sarhan AR, Gomez R, Lis P, Wightman M, Martinez TN, Tonelli F, Pfeffer SR, Alessi DR. 2018. Rab29 activation of the Parkinson's disease-associated LRRK2 kinase. EMBO J 37:1–18. 10.15252/embj.201798099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nichols RJ, Dzamko N, Morrice NA, Campbell DG, Deak M, Ordureau A, Macartney T, Tong Y, Shen J, Prescott AR, Alessi DR. 2010. 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson's disease-associated mutations and regulates cytoplasmic localization. Biochem J 430:393–404. 10.1042/BJ20100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dzamko N, Deak M, Hentati F, Reith AD, Prescott AR, Alessi DR, Nichols RJ. 2010. Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser910/Ser935, disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem J 430:405–413. 10.1042/BJ20100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevers LM, de Vries RM, Doveston RG, Milroy LG, Brunsveld L, Ottmann C. 2017. Structural interface between LRRK2 and 14-3-3 protein. Biochem J 474:1273–1287. 10.1042/BCJ20161078. [DOI] [PubMed] [Google Scholar]
- 72.Muda K, Bertinetti D, Gesellchen F, Hermann JS, von Zweydorf F, Geerlof A, Jacob A, Ueffing M, Gloeckner CJ, Herberg FW. 2014. Parkinson-related LRRK2 mutation R1441C/G/H impairs PKA phosphorylation of LRRK2 and disrupts its interaction with 14-3-3. Proc Natl Acad Sci U S A 111:E34–E43. 10.1073/pnas.1312701111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schapansky J, Nardozzi JD, Felizia F, LaVoie MJ. 2014. Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum Mol Genet 23:4201–4214. 10.1093/hmg/ddu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hutagalung AH, Novick PJ. 2011. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91:119–149. 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalogeropulou A, Freemantle J, Lis P, Vides E, Polinski N, Alessi D. 2020. Endogenous Rab29 does not impact basal or nigericin and monensin stimulated LRRK2 pathway activity. BioRxiv 2020.06.08.139675. 10.1101/2020.06.08.139675. [DOI] [PMC free article] [PubMed]
- 76.MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, MacCabe BD, Marder KS, Honig LS, Clark LN, Small SA, Abeliovich A. 2013. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron 77:425–439. 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomez RC, Wawro P, Lis P, Alessi DR, Pfeffer SR. 2019. Membrane association but not identity is required for LRRK2 activation and phosphorylation of Rab GTPases. J Cell Biol 218:4157–4170. 10.1083/jcb.201902184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lucas M, Hierro A. 2017. Retromer. Curr Biol 27:R687–R689. 10.1016/j.cub.2017.05.072. [DOI] [PubMed] [Google Scholar]
- 79.Ye H, Ojelade SA, Li-Kroeger D, Zuo Z, Wang L, Li Y, Gu JY, Tepass U, Rodal AA, Bellen HJ, Shulman JM. 2020. Retromer subunit, VPS29, regulates synaptic transmission and is required for endolysosomal function in the aging brain. Elife 9:e51977. 10.7554/eLife.51977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burd C, Cullen PJ. 2014. Retromer: a master conductor of endosome sorting. Cold Spring Harb Perspect Biol 6:a016774. 10.1101/cshperspect.a016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C, Reinthaler E, Harutyunyan A, Kralovics R, Peters A, Zimprich F, Brucke T, Poewe W, Auff E, Trenkwalder C, Rost B, Ransmayr G, Winkelmann J, Meitinger T, Strom TM. 2011. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet 89:168–175. 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, Behrouz B, Melrose HL, Hentati E, Puschmann A, Evans DM, Conibear E, Wasserman WW, Aasly JO, Burkhard PR, Djaldetti R, Ghika J, Hentati F, Krygowska-Wajs A, Lynch T, Melamed E, Rajput A, Rajput AH, Solida A, Wu RM, Uitti RJ, Wszolek ZK, Vingerhoets F, Farrer MJ. 2011. VPS35 mutations in Parkinson disease. Am J Hum Genet 89:162–167. 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanss Z, Larsen SB, Antony P, Mencke P, Massart F, Jarazo J, Schwamborn JC, Barbuti PA, Mellick GD, Kruger R. 2020. Mitochondrial and clearance impairment in p.D620N VPS35 patient-derived neurons. Mov Disord 10.1002/mds.28365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mir R, Tonelli F, Lis P, Macartney T, Polinski NK, Martinez TN, Chou MY, Howden AJM, Konig T, Hotzy C, Milenkovic I, Brucke T, Zimprich A, Sammler E, Alessi DR. 2018. The Parkinson's disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem J 475:1861–1883. 10.1042/BCJ20180248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsieh CH, Shaltouki A, Gonzalez AE, Bettencourt da Cruz A, Burbulla LF, St Lawrence E, Schule B, Krainc D, Palmer TD, Wang X. 2016. Functional impairment in Miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson's disease. Cell Stem Cell 19:709–724. 10.1016/j.stem.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madureira M, Connor-Robson N, Wade-Martins R. 2020. LRRK2: autophagy and lysosomal activity. Front Neurosci 14:498. 10.3389/fnins.2020.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eguchi T, Kuwahara T, Sakurai M, Komori T, Fujimoto T, Ito G, Yoshimura SI, Harada A, Fukuda M, Koike M, Iwatsubo T. 2018. LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc Natl Acad Sci U S A 115:E9115–E9124. 10.1073/pnas.1812196115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuwahara T, Funakawa K, Komori T, Sakurai M, Yoshii G, Eguchi T, Fukuda M, Iwatsubo T. 2020. Roles of lysosomotropic agents on LRRK2 activation and Rab10 phosphorylation. Neurobiol Dis 145:105081. 10.1016/j.nbd.2020.105081. [DOI] [PubMed] [Google Scholar]
- 89.Bonet-Ponce L, Beilina A, Williamson CD, Lindberg E, Kluss JH, Saez-Atienzar S, Landeck N, Kumaran R, Mamais A, Bleck CKE, Li Y, Cookson MR. 2020. LRRK2 mediates tubulation and vesicle sorting from lysosomes. Sci Adv 6:eabb2454. 10.1126/sciadv.abb2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herbst S, Campbell P, Harvey J, Bernard EM, Papayannopoulos V, Wood NW, Morris HR, Gutierrez MG. 2020. LRRK2 activation controls the repair of damaged endomembranes in macrophages. EMBO J 39:e104494. 10.15252/embj.2020104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Z, Xu E, Zhao HT, Cole T, West AB. 2020. LRRK2 and Rab10 coordinate macropinocytosis to mediate immunological responses in phagocytes. EMBO J 39:e104862. 10.15252/embj.2020104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rocha EM, De Miranda BR, Castro S, Drolet R, Hatcher NG, Yao L, Smith SM, Keeney MT, Di Maio R, Kofler J, Hastings TG, Greenamyre JT. 2020. LRRK2 inhibition prevents endolysosomal deficits seen in human Parkinson's disease. Neurobiol Dis 134:104626. 10.1016/j.nbd.2019.104626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Do J, McKinney C, Sharma P, Sidransky E. 2019. Glucocerebrosidase and its relevance to Parkinson disease. Mol Neurodegener 14:36. 10.1186/s13024-019-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sidransky E, Samaddar T, Tayebi N. 2009. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology 73:1424–1426. 10.1212/WNL.0b013e3181b28601. [DOI] [PubMed] [Google Scholar]
- 95.Bultron G, Kacena K, Pearson D, Boxer M, Yang R, Sathe S, Pastores G, Mistry PK. 2010. The risk of Parkinson's disease in type 1 Gaucher disease. J Inherit Metab Dis 33:167–173. 10.1007/s10545-010-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ysselstein D, Nguyen M, Young TJ, Severino A, Schwake M, Merchant K, Krainc D. 2019. LRRK2 kinase activity regulates lysosomal glucocerebrosidase in neurons derived from Parkinson's disease patients. Nat Commun 10:5570. 10.1038/s41467-019-13413-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanyal A, DeAndrade MP, Novis HS, Lin S, Chang J, Lengacher N, Tomlinson JJ, Tansey MG, LaVoie MJ. 2020. Lysosome and inflammatory defects in GBA1-mutant astrocytes are normalized by LRRK2 inhibition. Mov Disord 35:760–773. 10.1002/mds.27994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. 2006. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol 60:557–569. 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 99.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. 2005. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A 102:16842–16847. 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wauters F, Cornelissen T, Imberechts D, Martin S, Koentjoro B, Sue C, Vangheluwe P, Vandenberghe W. 2020. LRRK2 mutations impair depolarization-induced mitophagy through inhibition of mitochondrial accumulation of RAB10. Autophagy 16:203–222. 10.1080/15548627.2019.1603548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh A, Zhi L, Zhang H. 2019. LRRK2 and mitochondria: recent advances and current views. Brain Res 1702:96–104. 10.1016/j.brainres.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stafa K, Tsika E, Moser R, Musso A, Glauser L, Jones A, Biskup S, Xiong Y, Bandopadhyay R, Dawson VL, Dawson TM, Moore DJ. 2014. Functional interaction of Parkinson's disease-associated LRRK2 with members of the dynamin GTPase superfamily. Hum Mol Genet 23:2055–2077. 10.1093/hmg/ddt600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, Perry G, Casadesus G, Zhu X. 2012. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet 21:1931–1944. 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. 2008. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433–446. 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weindel CG, Bell SL, Vail KJ, West KO, Patrick KL, Watson RO. 2020. LRRK2 maintains mitochondrial homeostasis and regulates innate immune responses to Mycobacterium tuberculosis. Elife 9:e51071. 10.7554/eLife.51071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bonello F, Hassoun SM, Mouton-Liger F, Shin YS, Muscat A, Tesson C, Lesage S, Beart PM, Brice A, Krupp J, Corvol JC, Corti O. 2019. LRRK2 impairs PINK1/parkin-dependent mitophagy via its kinase activity: pathologic insights into Parkinson's disease. Hum Mol Genet 28:1645–1660. 10.1093/hmg/ddz004. [DOI] [PubMed] [Google Scholar]
- 107.Toyofuku T, Okamoto Y, Ishikawa T, Sasawatari S, Kumanogoh A. 2020. LRRK2 regulates endoplasmic reticulum-mitochondrial tethering through the PERK-mediated ubiquitination pathway. EMBO J 39:e100875. 10.15252/embj.2018100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumar D, Reiter J. 2020. How the centriole builds its cilium: of mothers, daughters, and the acquisition of appendages. Curr Opin Struct Biol 66:41–48. 10.1016/j.sbi.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 109.Plotnikova OV, Pugacheva EN, Golemis EA. 2009. Primary cilia and the cell cycle. Methods Cell Biol 94:137–160. 10.1016/S0091-679X(08)94007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dhekne HS, Yanatori I, Gomez RC, Tonelli F, Diez F, Schule B, Steger M, Alessi DR, Pfeffer SR. 2018. A pathway for Parkinson's disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. Elife 7:e40202. 10.7554/eLife.40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Madero-Perez J, Fdez E, Fernandez B, Lara Ordonez AJ, Blanca Ramirez M, Gomez-Suaga P, Waschbusch D, Lobbestael E, Baekelandt V, Nairn AC, Ruiz-Martinez J, Aiastui A, Lopez de Munain A, Lis P, Comptdaer T, Taymans JM, Chartier-Harlin MC, Beilina A, Gonnelli A, Cookson MR, Greggio E, Hilfiker S. 2018. Parkinson disease-associated mutations in LRRK2 cause centrosomal defects via Rab8a phosphorylation. Mol Neurodegener 13:3. 10.1186/s13024-018-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lara Ordonez AJ, Fernandez B, Fdez E, Romo-Lozano M, Madero-Perez J, Lobbestael E, Baekelandt V, Aiastui A, Lopez de Munain A, Melrose HL, Civiero L, Hilfiker S. 2019. RAB8, RAB10 and RILPL1 contribute to both LRRK2 kinase-mediated centrosomal cohesion and ciliogenesis deficits. Hum Mol Genet 28:3552–3568. 10.1093/hmg/ddz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fernandez B, Lara Ordonez AJ, Fdez E, Mutez E, Comptdaer T, Leghay C, Kreisler A, Simonin C, Vandewynckel L, Defebvre L, Destee A, Bleuse S, Taymans JM, Chartier-Harlin MC, Hilfiker S. 2019. Centrosomal cohesion deficits as cellular biomarker in lymphoblastoid cell lines from LRRK2 Parkinson's disease patients. Biochem J 476:2797–2813. 10.1042/BCJ20190315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kett LR, Boassa D, Ho CC, Rideout HJ, Hu J, Terada M, Ellisman M, Dauer WT. 2012. LRRK2 Parkinson disease mutations enhance its microtubule association. Hum Mol Genet 21:890–899. 10.1093/hmg/ddr526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Caesar M, Felk S, Aasly JO, Gillardon F. 2015. Changes in actin dynamics and F-actin structure both in synaptoneurosomes of LRRK2(R1441G) mutant mice and in primary human fibroblasts of LRRK2(G2019S) mutation carriers. Neuroscience 284:311–324. 10.1016/j.neuroscience.2014.09.070. [DOI] [PubMed] [Google Scholar]
- 116.Blanca Ramirez M, Lara Ordonez AJ, Fdez E, Madero-Perez J, Gonnelli A, Drouyer M, Chartier-Harlin MC, Taymans JM, Bubacco L, Greggio E, Hilfiker S. 2017. GTP binding regulates cellular localization of Parkinson's disease-associated LRRK2. Hum Mol Genet 26:2747–2767. 10.1093/hmg/ddx161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Watanabe R, Buschauer R, Böhning J, Audagnotto M, Lasker K, Lu T-W, Boassa D, Taylor S, Villa E. 2020. The in situ structure of Parkinson’s disease-linked LRRK2. Cell 10.1016/j.cell.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Law BM, Spain VA, Leinster VH, Chia R, Beilina A, Cho HJ, Taymans JM, Urban MK, Sancho RM, Blanca Ramirez M, Biskup S, Baekelandt V, Cai H, Cookson MR, Berwick DC, Harvey K. 2014. A direct interaction between leucine-rich repeat kinase 2 and specific β-tubulin isoforms regulates tubulin acetylation. J Biol Chem 289:895–908. 10.1074/jbc.M113.507913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guedes-Dias P, Holzbaur ELF. 2019. Axonal transport: driving synaptic function. Science 366:eaaw9997. 10.1126/science.aaw9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Godena VK, Brookes-Hocking N, Moller A, Shaw G, Oswald M, Sancho RM, Miller CC, Whitworth AJ, De Vos KJ. 2014. Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat Commun 5:5245. 10.1038/ncomms6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stormo AED, FitzGibbon M, Shavarebi F, Earley EM, Lum LS, Verschueren E, Swaney DL, Skibinski G, Ravisankar A, van Haren J, Davis EJ, Johnson JR, Von Dollen J, Mirescu C, Iaccarino C, Dauer WT, Nichols RJ, Wittmann T, Cox TC, Finkbeiner S, Krogan NJ, Oakes SA, Hiniker A. 2020. The E3 ligase TRIM1 ubiquitinates LRRK2 and controls its localization, degradation, and toxicity. BioRxiv 10.1101/2020.10.21.336578. [DOI] [PMC free article] [PubMed]
- 122.Bailey RM, Covy JP, Melrose HL, Rousseau L, Watkinson R, Knight J, Miles S, Farrer MJ, Dickson DW, Giasson BI, Lewis J. 2013. LRRK2 phosphorylates novel tau epitopes and promotes tauopathy. Acta Neuropathol 126:809–827. 10.1007/s00401-013-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Melrose HL, Dachsel JC, Behrouz B, Lincoln SJ, Yue M, Hinkle KM, Kent CB, Korvatska E, Taylor JP, Witten L, Liang YQ, Beevers JE, Boules M, Dugger BN, Serna VA, Gaukhman A, Yu X, Castanedes-Casey M, Braithwaite AT, Ogholikhan S, Yu N, Bass D, Tyndall G, Schellenberg GD, Dickson DW, Janus C, Farrer MJ. 2010. Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol Dis 40:503–517. 10.1016/j.nbd.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, Burke RE, Li C. 2009. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci 12:826–828. 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kawakami F, Shimada N, Ohta E, Kagiya G, Kawashima R, Maekawa T, Maruyama H, Ichikawa T. 2014. Leucine-rich repeat kinase 2 regulates tau phosphorylation through direct activation of glycogen synthase kinase-3β. FEBS J 281:3–13. 10.1111/febs.12579. [DOI] [PubMed] [Google Scholar]
- 126.Shanley MR, Hawley D, Leung S, Zaidi NF, Dave R, Schlosser KA, Bandopadhyay R, Gerber SA, Liu M. 2015. LRRK2 facilitates tau phosphorylation through strong interaction with tau and cdk5. Biochemistry 54:5198–5208. 10.1021/acs.biochem.5b00326. [DOI] [PubMed] [Google Scholar]
- 127.Bardai FH, Ordonez DG, Bailey RM, Hamm M, Lewis J, Feany MB. 2018. Lrrk promotes tau neurotoxicity through dysregulation of actin and mitochondrial dynamics. PLoS Biol 16:e2006265. 10.1371/journal.pbio.2006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen X, Kordich JK, Williams ET, Levine N, Cole-Strauss A, Marshall L, Labrie V, Ma J, Lipton JW, Moore DJ. 2019. Parkinson's disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A 116:5765–5774. 10.1073/pnas.1814909116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Belluzzi E, Gonnelli A, Cirnaru MD, Marte A, Plotegher N, Russo I, Civiero L, Cogo S, Carrion MP, Franchin C, Arrigoni G, Beltramini M, Bubacco L, Onofri F, Piccoli G, Greggio E. 2016. LRRK2 phosphorylates pre-synaptic N-ethylmaleimide sensitive fusion (NSF) protein enhancing its ATPase activity and SNARE complex disassembling rate. Mol Neurodegener 11:1. 10.1186/s13024-015-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nguyen M, Krainc D. 2018. LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson's disease. Proc Natl Acad Sci U S A 115:5576–5581. 10.1073/pnas.1717590115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matta S, Van Kolen K, da Cunha R, van den Bogaart G, Mandemakers W, Miskiewicz K, De Bock P-J, Morais VA, Vilain S, Haddad D, Delbroek L, Swerts J, Chávez-Gutiérrez L, Esposito G, Daneels G, Karran E, Holt M, Gevaert K, Moechars DW, De Strooper B, Verstreken P. 2012. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron 75:1008–1021. 10.1016/j.neuron.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 132.Surmeier DJ, Obeso JA, Halliday GM. 2017. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113. 10.1038/nrn.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bolam JP, Pissadaki EK. 2012. Living on the edge with too many mouths to feed: why dopamine neurons die. Mov Disord 27:1478–1483. 10.1002/mds.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Surmeier DJ, Guzman JN, Sanchez J, Schumacker PT. 2012. Physiological phenotype and vulnerability in Parkinson's disease. Cold Spring Harb Perspect Med 2:a009290. 10.1101/cshperspect.a009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Misgeld T, Schwarz TL. 2017. Mitostasis in neurons: maintaining mitochondria in an extended cellular architecture. Neuron 96:651–666. 10.1016/j.neuron.2017.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vidyadhara DJ, Lee JE, Chandra SS. 2019. Role of the endolysosomal system in Parkinson's disease. J Neurochem 150:487–506. 10.1111/jnc.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heo S, Diering GH, Na CH, Nirujogi RS, Bachman JL, Pandey A, Huganir RL. 2018. Identification of long-lived synaptic proteins by proteomic analysis of synaptosome protein turnover. Proc Natl Acad Sci U S A 115:E3827–E3836. 10.1073/pnas.1720956115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu J, Liu MC, Wang KK. 2008. Calpain in the CNS: from synaptic function to neurotoxicity. Sci Signal 1:re1. 10.1126/stke.114re1. [DOI] [PubMed] [Google Scholar]
- 139.Diepenbroek M, Casadei N, Esmer H, Saido TC, Takano J, Kahle PJ, Nixon RA, Rao MV, Melki R, Pieri L, Helling S, Marcus K, Krueger R, Masliah E, Riess O, Nuber S. 2014. Overexpression of the calpain-specific inhibitor calpastatin reduces human alpha-synuclein processing, aggregation and synaptic impairment in [A30P]αSyn transgenic mice. Hum Mol Genet 23:3975–3989. 10.1093/hmg/ddu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Posmantur R, Kampfl A, Siman R, Liu J, Zhao X, Clifton GL, Hayes RL. 1997. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience 77:875–888. 10.1016/s0306-4522(96)00483-6. [DOI] [PubMed] [Google Scholar]