Abstract
Aspirin (ASA) is recommended for the prevention of cardiovascular disease; however, the compliance is low. Reported use may not reflect actual use. Serum thromboxane B2 (STxB2) measurement was evaluated to validate reported ASA use. Males aged 45 to 79 years and females aged 55 to 79 years completed a survey and STxB2 measurement (Thromboxane B2 EIA Kit; Cayman Chemical, Ann Arbor, Michigan). The 107 patients were grouped by use of ASA (56 ASA+ and 51 ASA−) and possible interfering medications (INT) such as nonsteroidal anti-inflammatory drugs. The STxB2 levels (ng/mL) were significantly lower in ASA users: ASA+ INT− 3.0 (0.7, 8.4), ASA+ INT+ 2.0 (0.8, 4.9), ASA− INT+ 176 (75, 390), and ASA− INT− 271 (199, 366). The INT use did not cause a significant difference in STxB2 levels. A STxB2 cut point of 25 ng/mL had high sensitivity (94.1%) and specificity (91.1%) for ASA use. The STxB2 was a reliable marker of ASA use and could be used to confirm ASA exposure in population-based health studies.
Keywords: aspirin, thromboxane B2, compliance, cardiovascular prevention, nonsteroidal anti-inflammatory drug, NSAID
Background
Aspirin (ASA) reduces the risk of cardiovascular ischemic events, including stroke and myocardial infarction.1–3 As a result regular ASA use is widely recommended for both primary and secondary prevention of cardiovascular events. In 2009, the US Preventive Services Task Force (USPSTF) recommended daily ASA use for the primary prevention of cardiovascular disease in men aged 45 to 79 years and ischemic stroke in women aged 55 to 79 years.4 The American Heart Association/American Stroke Association similarly recommends ASA to reduce the risk of recurrent stroke and other cardiovascular events in patients with noncardioembolic ischemic stroke or transient ischemic attack5 and for the primary prevention of cardiovascular disease events in persons whose potential benefit outweighs the risks associated with treatment.6 The American College of Cardiology Foundation and the American Heart Association recommend ASA for patients with obstructive or nonobstructive atherosclerosis of the extracranial carotid and/or vertebral arteries for the prevention of myocardial infarction and other ischemic cardiovascular events.7 Most recently, the 2012 American College of Chest Physician antithrombotic medication guideline recommended low-dose ASA therapy for persons aged 50 years or older without symptomatic cardiovascular disease for the primary prevention of cardiovascular events.8 Despite these recommendations and the ready availability of this inexpensive preventative therapy, compliance with these recommendations is low.9–16 As promotion of ASA use is now increasingly highlighted as a central component of cardiovascular public health campaigns (eg, the Million Hearts initiative sponsored by the Centers for Disease Control and Centers for Medicare and Medicaid Services17,18), there is increased need to more precisely measure actual ASA exposure in a population that self-reports ASA use.
Clinical investigations and public health interventions that evaluate rates of ASA use and associated outcomes must consider the rate of noncompliance in patients. Reported ASA use may be higher or lower than actual use. Cuisset et al19 tested 136 patients who were prescribed ASA therapy and reported ASA use 1 month after undergoing coronary stenting. Nineteen (14%) of the patients had high arachidonic acid-induced platelet aggregation consistent with a poor response with prescribed ASA therapy. Observed, controlled ASA administration confirmed that 18 patients were noncompliant, and only 1 patient was a nonresponder to ASA therapy. Schwartz et al20 and Cotter et al21 observed similar findings. Studies that have not accounted for noncompliance with medication use have thus likely overestimated the rate of ASA use and underestimated ASA benefit and risk. Laboratory confirmation of reported ASA use is essential to understand the true rate of use.
ASA irreversibly acetylates cyclooxygenase 1 and 2 (COX-1 and COX-2) that prevent the access of substrates to the catalytic site.22–26 At the lower doses used for primary and secondary prevention of cardiovascular events, ASA primarily inhibits COX-1.25,27–29 In contrast, other nonsteroidal anti-inflammatory drugs (NSAIDs) elicit varying degrees of inhibition of COX-1 and/or COX-2.30–33 ASA and other NSAIDs interact with the same active site on COX.26,34–38 Inhibition of COX results in decreased conversion of arachidonic acid to thromboxane A2.39 Thromboxane A2 is rapidly degraded into metabolites, including the stable thromboxane B2 (TxB2).40 Treatment with ASA thus results in decreased serum levels of TxB2. The current study evaluated the measurement of serum thromboxane B2 (STxB2) to validate self-reported ASA use
Research Design and Methods
Patients
Healthy men aged 45 to 79 years and women aged 55 to 79 years were eligible to participate in the study. These sex and age ranges are recommended by USPSTF for the primary prevention of heart attack and stroke.4
Patients were recruited via an e-mail announcement to faculty and staff in the academic health center, and recruitment flyers were posted throughout the institution. Patients were initially screened by a telephone survey. Patients were pre hoc stratified on enrollment into 4 groups based on self-reported ASA use and use of potential interfering medications (INT; defined as medications with a potential impact on the STxB2 level). All eligible patients were consecutively enrolled until the first of the 4 groups was filled, and then patients were enrolled to fill each other group. Patients were then scheduled for a study visit that included completion of a brief survey and blood collection.
The study was approved by our institutional review board. Each volunteer gave written consent for participation.
Survey
Patients completed a brief 6-question survey to assess self-reported exposure to ASA. The patients were asked whether they had taken ASA or any ASA-containing drugs; medications for pain such as generic and brand names of NSAIDs; and cold, flu, or allergy medications in the last 24 hours and in the last 7 days. These categories of drugs were queried to determine whether the patient was taking any ASA-containing products. ASA is found in many over the counter products. In addition, many medications may contain NSAIDs that may interfere with ASA action on COX.34–36 If patients answered “yes” to any of these questions, then the surveyor collected the drug name, dose, and administration in the prior 7 days. Prior to analysis, drug trade names were converted to generic drug names by one of the study investigators (NDZ). Patients were asked whether they had diabetes41,42 or smoked cigarettes43–45 because both have been associated with increased thromboxane A2 metabolites. In addition, patients were asked whether they regularly take ASA for the prevention of cardiovascular disease.
Group Allocation
Patients were recruited into 4 groups based on their use of ASA and any other INT: ASA and interfering medication use (ASA+ INT+), ASA without interfering medication use (ASA+ INT−), interfering medication without ASA use (ASA − INT+), and neither ASA or interfering medication use (ASA− INT−). The surveys were reviewed by 2 clinician investigators (NDZ and ATH) to assure patients were correctly assigned to their groups. Adjudication was performed after completion of the survey and blood collection but prior to performance of STxB2 testing. Use of acetaminophen or narcotic pain medications was associated with an assignment to the no interfering medication category. If a patient answered yes they had consumed a drug in the last 24 hours, but answered no to consuming in the last 7 days, the answer was adjudicated to yes in the last 7 days.
Blood Collection
Blood was collected from an antecubital vein using standard blood collection techniques. A tourniquet was applied 3 to 4 inches above the selected venipuncture site. The skin was prepared with 70% isopropyl alcohol. Blood was obtained using a 21-gauge needle. Blood was collected into 10 mL nonanticoagulated, nonsiliconized glass vacutainer tubes (Becton Dickinson, Franklin Lakes, New Jersey). The specimen was inverted several times to mix and placed in a 37°C nonagitating water bath for 1 hour. The specimen was centrifuged at 2600g for 10 minutes at 4°C. The serum was aliquoted into 2.0 mL plastic-graduated centrifuge tubes (Fisher Scientific, Pittsburgh, Pennsylvania) and frozen immediately at −80°C.
Serum Thromboxane B2
The STxB2 was measured using the Thromboxane B2 EIA Kit (Cayman Chemical, Ann Arbor, Michigan). This is a competitive enzyme immunoassay in which the TxB2 in the patient’s specimen competes with reagent TxB2–acetylcholinesterase conjugate for binding to rabbit anti-TxB2 monoclonal antibody.46 The antibody complex is immobilized by binding to mouse anti-rabbit IgG bound to a 96-well plate. The amount of TxB2–acetylcholinesterase conjugate bound is detected using a substrate for acetylcholinesterase and is inversely proportional to the amount of TxB2 in the patient’s specimen. The color of the reaction was read at 412 nm using a SpectraMax 190 (Molecular Devices, Sunnyvale, California) spectrophotometer. Specimens were serially diluted to achieve results such that the percentage of binding over maximal binding (%B/B0) in the assay was between 20% and 80%. Dilutions were tested in duplicate. The initial dilution range of the specimen for testing was determined by the reported ASA use of the patient, based on the prestudy validation data (data not shown). The method has an interassay coefficient of variation (CV) of 14.7%, based on a pooled serum control run on 5 different days, and an intra-assay CV of 8.8%, based on duplicate testing of specimens.
Statistical Analysis
Data are presented as frequency and percentage or median and interquartile range. Tests of differences between independent groups were conducted using chi-square or Fisher exact tests for categorical variables and Mann-Whitney tests for continuous variables. Nonparametric receiver–operating characteristic (ROC) analysis was used to calculate the discriminative ability via the c-statistic. A 2-sided P value of <.05 was considered statistically significant. Data analyses were performed using Stata/IC software version 12 (Stata, Inc, College Station, Texas).
Results
Research patients
A total of 120 individuals were screened for study inclusion using a telephone survey. Ten eligible patients did not complete the follow-up survey and blood draw. Two patients who completed the initial telephone survey were subsequently deemed ineligible on the day of the follow-up visit. One patient reported no ASA use and was tested using predetermined dilutions. Duplicate results did not fall in the measurement linear range. As an accurate level was not determined, this patient was excluded from all analyses. Data were thus available for 107 patients (Table 1). Two patients did not complete the entire survey as they did not answer questions on the back of the survey sheet. Data regarding the use of ASA for the prevention of cardiovascular disease, diabetes, and cigarette smoking are missing for these 2 patients. The 4 groups were similar with respect to age, sex, and prevalence of diabetes and smoking (Table 1). There was a higher rate of males in the INT− groups; however, this difference was not significantly different within the ASA+ and ASA− groups.
Table 1.
Patient Demographics and Responses to Survey Questions.
| ASA+ INT− | ASA+ INT+ | p | ASA− INT+ | ASA− INT− | p | |
|---|---|---|---|---|---|---|
| n | 31 | 25 | 24 | 27 | ||
| Male, n (%) | 11 (35) | 7 (28) | .55 | 8 (33) | 14 (52) | .18 |
| Age, mean ± SD | 62 ± 4.3 | 61 ± 7.2 | .51 | 58 ± 4.1 | 59 ± 6.6 | .34 |
| Diabetes, n (%) | 2 (6) | 3 (12) | .65 | 0 (0) | 1 (4) | 1.00 |
| Smoking, n (%) | 1 (3) | 1 (4) | 1.00 | 1 (4) | 1 (4) | 1.00 |
| Use aspirin regularly for the prevention of cardiovascular disease, n (%) | 29 (94) | 19 (76) | .12 | 0 (0) | 1 (4) | 1.00 |
| Used aspirin | ||||||
| In the past 24 hours, n (%) | 29 (94) | 22 (88) | .65 | 0 (0) | 0 (0) | NA |
| In the past 7 days, n (%) | 31 (100) | 25 (100) | NA | 0 (0) | 0 (0) | NA |
| Used pain medication | ||||||
| In the past 24 hours, n (%) | 1 (3) | 13 (52) | <.0001 | 9 (38) | 1 (4) | .004 |
| In the past 7 days, n (%) | 3 (10) | 22 (88) | <.0001 | 21 (88) | 1 (4) | <.0001 |
| Used cold, flu, or allergy medication | ||||||
| In the past 24 hours, n (%) | 0 (0) | 11 (44) | <.0001 | 7 (29) | 0 (0) | .003 |
| In the past 7 days, n (%) | 0 (0) | 11 (44) | <.0001 | 12 (50) | 0 (0) | <.0001 |
| Used NSAID | ||||||
| In the past 24 hours, n (%) | 0 (0) | 7 (28) | .002 | 5 (21) | 0 (0) | .018 |
| In the past 7 days, n (%) | 0 (0) | 21 (84) | <.0001 | 17 (71) | 0 (0) | <.0001 |
Abbreviations: ASA, aspirin; INT, possible interfering medication; NSAID, nonsteroidal anti-inflammatory drug; n, number; SD, standard deviation; NA, not applicable.
Aspirin Use in Patients
A total of 56 patients who reported ASA use in the last 7 days and 51 who had not enrolled in the study (Table 1). ASA was consumed within the last day by 51 (91%) of these patients with a similar proportion in both INT− and INT+ groups (P = .65). Low-dose (81 mg) ASA was more commonly used than the standard higher dose (325 mg; 62% vs 18%). ASA was used regularly for the prevention of cardiovascular disease by 48 (86%) of these ASA-using patients.
Impact of Possible Interfering Medications
Possible interfering medications were identified as pain, cold, flu, and allergy medications (Table 2). The reported drugs were further classified as NSAIDs by the study investigators. The most common group of drugs reported in these 3 categories was NSAIDs that were used by 78% of the patients in the INT+ groups. The most frequent NSAID reported was ibuprofen (34 patients), which was used by 76% of ASA+ INT+ patients and 62% of ASA− INT+ patients. Other commonly used medications were various allergy symptom medications (18 patients) and acetaminophen (11 patients).
Table 2.
Medications Reported by Patients Grouped by Aspirin (ASA) and Possible Interfering Medication (INT) Use.
| ASA+ INT− |
| Acetaminophen (2) |
| Acetaminophen/hydrocodone |
| ASA+ INT+ |
| Acetaminophen + ibuprofen |
| Acetaminophen + loratadine |
| Acetaminophen + acetaminophen/caffeine/aspirin + ibuprofen |
| Cetirizine |
| Cetirizine + ibuprofen |
| Cyproheptadine + fluticasone nasal spray + ibuprofen |
| Fexofenadine/pseudoephedrine + ibuprofen |
| Fluticasone propionate |
| Ibuprofen (11) |
| Ibuprofen + naproxen |
| Ibuprofen + unknown cold medication |
| Ibuprofen + unknown nasal spray |
| Loratadine + meloxicam + tylenol/codeine |
| Naproxen |
| Pseudoephedrine |
| ASA− INT+ |
| Acetaminophen + azelastine nasal spray |
| Acetaminophen + cetirizine |
| Acetaminophen + ibuprofen |
| Acetaminophen + unknown allergy medication |
| Celecoxib |
| Cetirizine + ibuprofen |
| Chlorpheniramine + ibuprofen + loratadine |
| Chlorpheniramine + ibuprofen + pseudoephedrine |
| Diphenhydramine |
| Diphenhydramine + ibuprofen |
| Eletriptan + oxycodone + unknown decongestant |
| Fexofenadine/pseudoephedrine |
| Ibuprofen (10) |
| Naproxen |
| Oxymetazoline hydrochloride + zincum aceticum/zincum gluconicum |
| ASA− INT− |
| Acetaminophen |
Abbreviations: ASA, aspirin; INT, possible interfering medication.
Serum Thromboxane B2
The STxB2 levels were significantly lower in individuals taking ASA compared to no ASA use (Figure 1). The STxB2 levels in the 4 groups were 3.0 (0.7, 8.4) ng/mL in the ASA+ INT− group, 2.0 (0.8, 4.9) ng/mL in the ASA+ INT+ group, 176 (75, 390) ng/mL in the ASA− INT+ group, and 271 (199, 366) ng/mL in the ASA− INT− group. The STxB2 levels between individuals who were and were not taking a possible interfering substance were not significantly different (ASA+ INT+ vs ASA+ INT−, P = .47 and ASA− INT+ vs ASA− INT−, P = .13). There was no significant difference in STxB2 levels between males and females.
Figure 1.
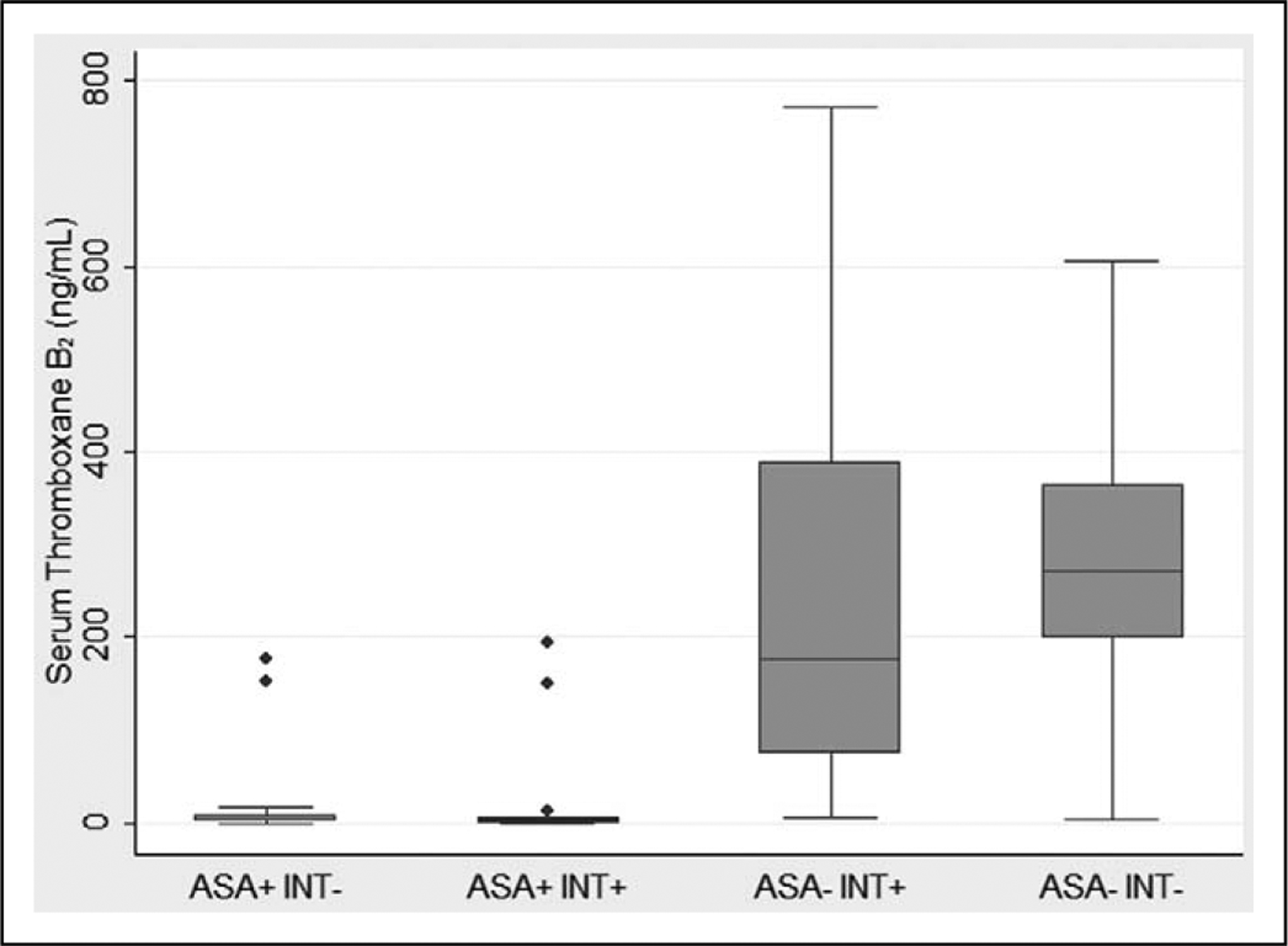
Comparison of serum thromboxane B2 levels in patients based on aspirin and possible interfering medication use. The boxplot shows the median (line), the 25th and 75th percentiles (box), and the upper and lower values within 1.5 times the interquartile range beyond the 25th and 75th percentile (whiskers). The dots represent outliers. ASA, aspirin; INT, possible interfering medication.
The STxB2 level can discriminate ASA use. The ROC curve is shown in Figure 2 with a c-statistic of 0.97. A STxB2 cut point of 25 ng/mL has a sensitivity of 94.1% and a specificity of 91.1% for ASA use. Four patients reporting ASA use had STxB2 levels above 25 ng/mL. Two were in the ASA+ INT− group with levels of 152.4 ng/mL and 177.4 ng/mL. Two were in the ASA+ INT+ group with levels of 149.4 ng/mL and 194.8 ng/mL. Both of these had taken ibuprofen plus fexofenadine/pseudoephedrine and an unknown nasal spray, respectively. In all, 3 of these 4 patients took only 1 recent dose of ASA: 325 mg 6 days prior to collection, 650 mg 6 days prior to collection, and 81 mg on the day of collection. Both the dose of ASA and time since last dose are known to influence the STxB2 levels.47–51 Two patients in the no ASA groups had low STxB2 levels of 5.7 ng/mL in the ASA− INT+ group and 3.0 ng/mL in the ASA− INT− group. There is no obvious explanation from the data gathered as to the cause of these low outliers.
Figure 2.
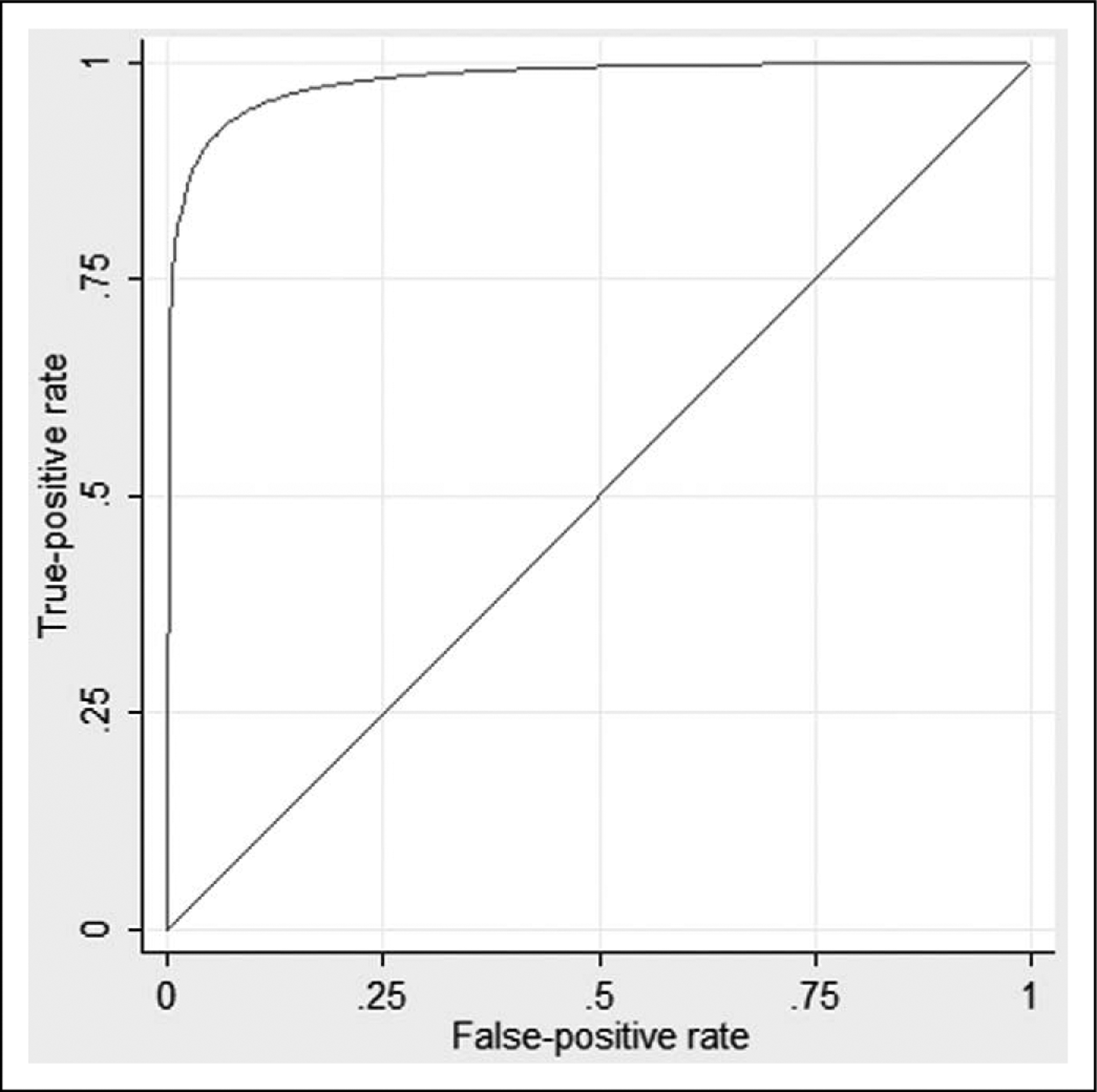
Receiver–operating characteristic curve of serum thromboxane B2 levels and aspirin use. The c-statistic is 0.97.
NSAIDs and STxB2 levels
NSAIDs may compete with ASA for interaction with COX-1 in platelets.34–36 Reported studies conflict on the impact of concurrent use of NSAIDs and ASA on STxB2 levels and cardiovascular events.35,51–56 Secondary analysis was performed to look at the influence of NSAIDs on STxB2 levels. Patients not taking a possible interfering substance (ASA+ INT− and ASA− INT−) were compared to patients reporting NSAID use (ASA+ NSAID+ and ASA− NSAID+). The STxB2 levels were lower in the ASA+ groups; however, there were no significant differences between the NSAID− and NSAID+ groups: ASA+ NSAID− 3.0 (0.7, 8.4) ng/mL versus ASA+ NSAID+ 2.0 (0.8, 3.3) ng/mL, P = 0.42 and ASA− NSAID− 271 (199, 366) ng/mL versus ASA− NSAID+ 169 (43, 380) ng/mL, P = .17.
As possible interfering drugs and NSAIDS did not result in significant differences, further analysis was performed in all patients reporting ASA use (ASA+) and no ASA use (ASA−). The ASA+ patients were older than the ASA− patients (62 ± 5.8 years vs 59 ± 5.6, P = .01); however, other demographic variables were similar between the 2 groups. The STxB2 level was significantly lower in ASA users (2.1 [0.8, 5.3] ng/mL vs 252 [169, 380] ng/mL, P < .0001).
Discussion
ASA is recommended for the primary and secondary prevention of cardiovascular ischemic events; however, the drug is underutilized.9–16 Furthermore, use of ASA may be overreported by patients.19–21 Future studies examining the rates of ASA use and the impact of public and health professional interventions to increase ASA use must consider the rate of noncompliance. Laboratory confirmation of ASA exposure is therefore desirable. ASA irreversibly acetylates COX-1 and COX-2 resulting in decreased conversion of arachidonic acid to thromboxane A2 and subsequently decreased the levels of the more stable metabolite TxB2. This study demonstrates STxB2 can be used to confirm reported ASA use in men ≥45 years and women ≥55 years, the target population for current cardiovascular primary prevention ASA guidelines.
The STxB2 levels observed in this study were significantly lower in ASA users (2.1 [0.8, 5.3] ng/mL) compared to patients not using ASA (252 [169, 380] ng/mL). These levels were similar to those reported in previous studies.57–60 Also consistent with other studies is the wide variability of levels between individuals as noted by the large standard deviation of results. Based on the current data set, a STxB2 cut point of 25 ng/mL has high sensitivity and specificity for ASA use.
Patients who participated in this study were asked to report the use of pain, cold, flu, and allergy medications. ASA is found in common over the counter medications, and the STxB2 level can be decreased from any source of ASA. However, STxB2 levels were not different in patients using or not using these medications.
Reports conflict on the impact of taking concurrent ASA and non-ASA NSAIDS as these drugs compete for the same site on COX35,52–56 NSAIDs were reported by 78% of the patients in the INT+ groups. In this study, the use of NSAIDs did not have a significant influence on the STxB2 level. This study captured only a single point in time to examine the utility of using STxB2 as a screening measure of ASA exposure. The lack of an effect of NSAIDs on STxB2 levels in this study may be due to the timing of the NSAID use relative to the ASA, the specific NSAIDs taken by the patient, and the timing of the blood collection relative to the patient taking the drugs. Both ASA and NSAIDs may inhibit STxB2 production. Catella-Lawson et al35 demonstrated that ASA-induced inhibition of STxB2 production was blocked by taking ibuprofen prior to the ASA but not after. At 2 hours after administration, both the ibuprofen before ASA and ASA before ibuprofen groups demonstrated more than 97% inhibition; however, by 24 hours, the inhibition of STxB2 production was only 53% in the ibuprofen before ASA group. Two other NSAIDS, diclofenac and rofecoxib, did not inhibit the ASA effect on STxB2. Rao et al34 also demonstrated the reversible inhibition of STxB2 production by ibuprofen. When either ASA, ibuprofen, or ASA plus ibuprofen were ingested, the ex vivo production of thromboxane was decreased at 1.5 hours, but at 24 hours the production returned to control levels with ibuprofen alone or with ASA, but remained inhibited in patients who took only ASA. Schuijt et al53 observed that when ibuprofen or diclofenac was used 3 times per day for 8 days and daily ASA was started on the second day, the effect of ASA on STxB2 was reduced with ibuprofen but not diclofenac. Other studies have also demonstrated inhibition of the ASA effect by NSAIDs.36,37 In contrast, randomized studies demonstrated that the inhibitory effect of ASA on STxB2 production was maintained when ibuprofen56 or naproxen52,55 was given after several days of daily ASA use.
In addition to noncompliance, studies investigating ASA use must also consider resistance to ASA therapy. ASA resistance is variably defined as a failure to respond appropriately to ASA therapy based on clinical or laboratory findings. Several studies have shown ASA resistance is associated with poorer outcomes.61,62 ASA effects on platelets can be measured by platelet function assays or levels of intermediates in the arachidonic acid pathway. Functional methods include light transmittance and whole blood multiple electrode platelet aggregometry, VerifyNow (Accumetrics, San Diego, California), thromboelastography with platelet mapping (Haemonetics Corporation, Braintree, Massachusetts), PlateletWorks (Helena Laboratories, Beaumont, Texas), and PFA-100 (Siemens Healthcare Diagnostics, Deerfield, Illinois). These methods require immediate testing of the specimen due to platelet instability and therefore may be unsuitable to multicenter studies where centralized testing is preferred. Furthermore, the sensitivity of these assays for the detection of ASA response varies widely. Lordkipanidzé et al63 tested 201 patients with stable coronary artery disease by multiple methods and found the rate of ASA resistance, a failure to show adequate response to ASA therapy, was in the range of 4% with light transmission aggregometry, 6.75% with VerifyNow ASA, and 59.5% with PFA-100. In a second study, Grove et al60 tested platelet function by multiple methods including light transmittance aggregometry, multiple electrode aggregometry, PFA-100, and VerifyNow ASA and the metabolites of ASA use STxB2 and urinary 11-dehydro-TxB2 in both healthy patients and patients with coronary artery disease. Grove et al found poor correlation between the functional assays and thromboxane metabolites. Other studies have also found similar discordance between functional assays for measuring ASA use.64–66 The discordance among assays likely reflects the impact of factors other than ASA on platelet function. Therefore, assessment of metabolites such as STxB2 has been proposed as the best marker of compliance with ASA therapy and has been used by other investigators for this purpose.57,60,67 Correlation of a platelet function assay and a compliance assay such as STxB2 may identify patients who are compliant with ASA therapy but resistance to ASA functional benefits.
This pilot study does have limitations. The study was small and did not have sufficient power to evaluate all parameters including the influence of ASA dose and timing of last ASA dose on STxB2 levels. This study permitted any dose of ASA with patients reporting a range of 81 to 975 mg per dose and a varying number of doses. Patients were also considered ASA+ if they took at least 1 dose of ASA within the 7 days prior to blood collection. Both the dose of ASA and time since last dose are known to influence the STxB2 levels.47–51 Patients were considered INT+ if they had taken an NSAID at any point within the 7 days prior to blood collection. As discussed above, the specific NSAID taken and the relative timing of the NSAID dose to the ASA dose influence the inhibition of STxB2. Furthermore, this study did not standardize the timing between the administration of ASA and possible interfering drugs and the blood collection. The study was conducted primarily from healthy participants, and thus exposure to other medications may affect platelet function, such as clopidogrel, dipyridamole, abciximab, and newer direct thromboxane inhibitors were not obtained. It is possible that other drugs that may influence the STxB2 taken by the patient but were not captured by our survey. This is a possible explanation for the 2 patients not taking ASA but who had low levels of STxB2. Finally, use of ASA was not confirmed by a method other than STxB2; however, as described above, the functional platelet assays may not correlate with STxB2 levels. Due to the rapid metabolism of ASA, direct measurement of the drug is difficult. Observed pill ingestion can also be used to eliminate the effect of noncompliance and allow for accurate timing of ASA ingestion.
This study demonstrates that STxB2 can be used as a reliable marker of ASA use. Use of NSAIDs in this study population did not cause a significant difference in results. A key advantage of STxB2 is the ability to collect samples from patients at multiple sites, send to a central laboratory, and batch test to allow for consistency in testing. As such, STxB2 levels could be used to confirm ASA exposure in population-based health studies.
Acknowledgments
The authors thank Anne Pralle for assistance with subject recruitment; Laura Dillon, Deborah Eck, and Seth Rowley for assistance with survey administration and blood collection; and Valerie Arends and Naomi Hanson for assistance with laboratory testing.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this research study was provided by the Lillehei Heart Institute, Cardiovascular Division, University of Minnesota Medical School, Minneapolis, MN.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:Nicole Dodge Zantek has a financial interest in Endo Health Solutions, Inc, a company which may commercially benefit from the results of this research. This interest has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies. The other authors declare no conflict of interest with respect to authorship and/or publication of this article.
References
- 1.Antithrombotic Trialists’ Colloboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrono C, Coller B, Dalen JE, et al. Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest. 2001;119(suppl 1):39S–63S. [DOI] [PubMed] [Google Scholar]
- 3.Weisman SM, Graham DY. Evaluation of the benefits and risks of low-dose aspirin in the secondary prevention of cardiovascular and cerebrovascular events. Arch Intern Med. 2002;162(19):2197–2202. [DOI] [PubMed] [Google Scholar]
- 4.US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150(6):396–404. [DOI] [PubMed] [Google Scholar]
- 5.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(1): 227–276. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. [DOI] [PubMed] [Google Scholar]
- 7.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease: Executive Summary A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery Developed in Collaboration With the American Academy of Neurology and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2011;57(8):1002–1044. [DOI] [PubMed] [Google Scholar]
- 8.Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e637S–e668S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera CM, Song J, Copeland L, Buirge C, Ory M, McNeal CJ. Underuse of aspirin for primary and secondary prevention of cardiovascular disease events in women. J Womens Health (Larchmt). 2012;21(4):379–387. [DOI] [PubMed] [Google Scholar]
- 10.Krumholz HM, Radford MJ, Ellerbeck EF, et al. Aspirin for secondary prevention after acute myocardial infarction in the elderly: prescribed use and outcomes. Ann Intern Med. 1996; 124(3):292–298. [DOI] [PubMed] [Google Scholar]
- 11.Shahar E, Folsom AR, Romm FJ, et al. Patterns of aspirin use in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1996;131(5):915–922. [DOI] [PubMed] [Google Scholar]
- 12.Stafford RS. Aspirin use is low among United States outpatients with coronary artery disease. Circulation. 2000;101(10): 1097–1101. [DOI] [PubMed] [Google Scholar]
- 13.Cheng EM, Cohen SN, Lee ML, Vassar SD, Chen AY. Use of antithrombotic agents among U.S. stroke survivors, 2000–2006. Am J Prev Med. 2010;38(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim C, Beckles GL. Cardiovascular disease risk reduction in the Behavioral Risk Factor Surveillance System. Am J Prev Med. 2004;27(1):1–7. [DOI] [PubMed] [Google Scholar]
- 15.Rolka DB, Fagot-Campagna A, Narayan KM. Aspirin use among adults with diabetes: estimates from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2001;24(2):197–201. [DOI] [PubMed] [Google Scholar]
- 16.Stafford RS, Monti V, Ma J. Underutilization of aspirin persists in US ambulatory care for the secondary and primary prevention of cardiovascular disease. PLoS Med. 2005;2(12):e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.New public-private sector initiative aims to prevent 1 million heart attacks and strokes in five years [press release]. Washington, DC: HHS News, U.S. Department of Health and Human Services. http://www.hhs.gov/news/press/2011pres/09/20110913a.html. Accessed September 13, 2011. [Google Scholar]
- 18.Frieden TR, Berwick DM. The “Million Hearts” initiative—preventing heart attacks and strokes. N Engl J Med. 2011;365(13):e27. [DOI] [PubMed] [Google Scholar]
- 19.Cuisset T, Frere C, Quilici J, et al. Aspirin noncompliance is the major cause of “aspirin resistance” in patients undergoing coronary stenting. Am Heart J. 2009;157(5):889–893. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz KA, Schwartz DE, Barber K, Reeves M, De Franco AC. Non-compliance is the predominant cause of aspirin resistance in chronic coronary arterial disease patients. J Transl Med. 2008;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotter G, Shemesh E, Zehavi M, et al. Lack of aspirin effect: aspirin resistance or resistance to taking aspirin? Am Heart J. 2004;147(2):293–300. [DOI] [PubMed] [Google Scholar]
- 22.Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J Clin Invest. 1975;56(3):624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burch JW, Stanford N, Majerus PW. Inhibition of platelet prostaglandin synthetase by oral aspirin. J Clin Invest. 1978;61(2):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funk CD, Funk LB, Kennedy ME, Pong AS, Fitzgerald GA. Human platelet/erythroleukemia cell prostaglandin G/H synthase: cDNA cloning, expression, and gene chromosomal assignment. FASEB J. 1991;5(9):2304–2312. [PubMed] [Google Scholar]
- 25.Patrono C, Ciabattoni G, Patrignani P, et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation. 1985; 72(6):1177–1184. [DOI] [PubMed] [Google Scholar]
- 26.Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol. 1995;2(8):637–643. [DOI] [PubMed] [Google Scholar]
- 27.Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest. 1982;69(6):1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipollone F, Patrignani P, Greco A, et al. Differential suppression of thromboxane biosynthesis by indobufen and aspirin in patients with unstable angina. Circulation. 1997;96(4):1109–1116. [DOI] [PubMed] [Google Scholar]
- 29.FitzGerald GA, Oates JA, Hawiger J, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983; 71(3):676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brideau C, Kargman S, Liu S, et al. A human whole blood assay for clinical evaluation of biochemical efficacy of cyclooxygenase inhibitors. Inflamm Res. 1996;45(2):68–74. [DOI] [PubMed] [Google Scholar]
- 31.Dannhardt G, Kiefer W. Cyclooxygenase inhibitors—current status and future prospects. Eur J Med Chem. 2001;36(2):109–126. [DOI] [PubMed] [Google Scholar]
- 32.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268(9):6610–6614. [PubMed] [Google Scholar]
- 33.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993;90(24):11693–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao GH, Johnson GG, Reddy KR, White JG. Ibuprofen protects platelet cyclooxygenase from irreversible inhibition by aspirin. Arteriosclerosis. 1983;3(4):383–388. [DOI] [PubMed] [Google Scholar]
- 35.Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345(25):1809–1817. [DOI] [PubMed] [Google Scholar]
- 36.Livio M, Del Maschio A, Cerletti C, de Gaetano G. Indomethacin prevents the long-lasting inhibitory effect of aspirin on human platelet cyclo-oxygenase activity. Prostaglandins. 1982;23(6):787–796. [DOI] [PubMed] [Google Scholar]
- 37.Capone ML, Sciulli MG, Tacconelli S, et al. Pharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjects. J Am Coll Cardiol. 2005;45(8):1295–1301. [DOI] [PubMed] [Google Scholar]
- 38.Loll PJ, Picot D, Ekabo O, Garavito RM. Synthesis and use of iodinated nonsteroidal antiinflammatory drug analogs as crystallographic probes of the prostaglandin H2 synthase cyclooxygenase active site. Biochemistry. 1996;35(23):7330–7340. [DOI] [PubMed] [Google Scholar]
- 39.Majerus PW. Arachidonate metabolism in vascular disorders. J Clin Invest. 1983;72(5):1521–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975;72(8):2994–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davi G, Catalano I, Averna M, et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med. 1990;322(25):1769–1774. [DOI] [PubMed] [Google Scholar]
- 42.Mortensen SB, Larsen SB, Grove EL, Kristensen SD, Hvas AM. Reduced platelet response to aspirin in patients with coronary artery disease and type 2 diabetes mellitus. Thromb Res. 2010; 126(4):e318–e322. [DOI] [PubMed] [Google Scholar]
- 43.Schmid P, Karanikas G, Kritz H, et al. Passive smoking and platelet thromboxane. Thromb Res. 1996;81(4):451–460. [DOI] [PubMed] [Google Scholar]
- 44.Rangemark C, Benthin G, Granstrom EF, Persson L, Winell S, Wennmalm A. Tobacco use and urinary excretion of thromboxane A2 and prostacyclin metabolites in women stratified by age. Circulation. 1992;86(5):1495–1500. [DOI] [PubMed] [Google Scholar]
- 45.Wennmalm A, Benthin G, Granstrom EF, Persson L, Petersson AS, Winell S. Relation between tobacco use and urinary excretion of thromboxane A2 and prostacyclin metabolites in young men. Circulation. 1991;83(5):1698–1704. [DOI] [PubMed] [Google Scholar]
- 46.Thromboxane B2 EIA Kit [package insert]. Ann Arbor, MI: Cayman Chemical Company; 2012. [Google Scholar]
- 47.Feldman M, Cryer B, Rushin K, Betancourt J. A comparison of every-third-day versus daily low-dose aspirin therapy on serum thromboxane concentrations in healthy men and women. Clin Appl Thromb Hemost. 2001;7(1):53–57. [DOI] [PubMed] [Google Scholar]
- 48.Feldman M, Jialal I, Devaraj S, Cryer B. Effects of low-dose aspirin on serum C-reactive protein and thromboxane B2 concentrations: a placebo-controlled study using a highly sensitive C-reactive protein assay. J Am Coll Cardiol. 2001;37(8):2036–2041. [DOI] [PubMed] [Google Scholar]
- 49.Tohgi H, Konno S, Tamura K, Kimura B, Kawano K. Effects of low-to-high doses of aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin. Stroke. 1992;23(10):1400–1403. [DOI] [PubMed] [Google Scholar]
- 50.Brambilla M, Parolari A, Camera M, et al. Effectoftwodosesofaspirin on thromboxane biosynthesis and platelet function in patients undergoing coronary surgery. Thromb Haemost. 2010;103(3):516–524. [DOI] [PubMed] [Google Scholar]
- 51.Hart RG, Leonard AD, Talbert RL, et al. Aspirin dosage and thromboxane synthesis in patients with vascular disease. Pharmacotherapy. 2003;23(5):579–584. [DOI] [PubMed] [Google Scholar]
- 52.Angiolillo DJ, Hwang C, Datto C, Desai B, Sostek M. Impact of a fixed-dose combination of naproxen and esomeprazole magnesium on serum thromboxane B2 inhibition by low-dose aspirin over 5 days in healthy adults: a phase I, randomized, double-blind, placebo-controlled, noninferiority trial. Clin Ther. 2011;33(12):1883–1893. [DOI] [PubMed] [Google Scholar]
- 53.Schuijt MP, Huntjens-Fleuren HW, de Metz M, Vollaard EJ. The interaction of ibuprofen and diclofenac with aspirin in healthy volunteers. Br J Pharmacol. 2009;157(6):931–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackenzie IS, Coughtrie MW, MacDonald TM, Wei L. Antiplatelet drug interactions. J Intern Med. 2010;268(6):516–529. [DOI] [PubMed] [Google Scholar]
- 55.Oldenhof J, Hochberg M, Schiff M, Brune K. Effect of maximum OTC doses of naproxen sodium or acetaminophen on low-dose aspirin inhibition of serum thromboxane B2. Curr Med Res Opin. 2010;26(6):1497–1504. [DOI] [PubMed] [Google Scholar]
- 56.Cryer B, Berlin RG, Cooper SA, Hsu C, Wason S. Double-blind, randomized, parallel, placebo-controlled study of ibuprofen effects on thromboxane B2 concentrations in aspirin-treated healthy adult volunteers. Clin Ther. 2005;27(2):185–191. [DOI] [PubMed] [Google Scholar]
- 57.Hedegaard SS, Hvas AM, Grove EL, et al. Optical platelet aggregation versus thromboxane metabolites in healthy individuals and patients with stable coronary artery disease after low-dose aspirin administration. Thromb Res. 2009;124(1):96–100. [DOI] [PubMed] [Google Scholar]
- 58.Frelinger AL 3rd, Furman MI, Linden MD, et al. Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1- and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance. Circulation. 2006;113(25):2888–2896. [DOI] [PubMed] [Google Scholar]
- 59.Cox D, Maree AO, Dooley M, Conroy R, Byrne MF, Fitzgerald DJ. Effect of enteric coating on antiplatelet activity of low-dose aspirin in healthy volunteers. Stroke. 2006;37(8):2153–2158. [DOI] [PubMed] [Google Scholar]
- 60.Grove EL, Hvas AM, Johnsen HL, et al. A comparison of platelet function tests and thromboxane metabolites to evaluate aspirin response in healthy individuals and patients with coronary artery disease. Thromb Haemost. 2010;103(6):1245–1253. [DOI] [PubMed] [Google Scholar]
- 61.Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008;336(7637):195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sofi F, Marcucci R, Gori AM, Abbate R, Gensini GF. Residual platelet reactivity on aspirin therapy and recurrent cardiovascular events—a meta-analysis. Int J Cardiol. 2008;128(2):166–171. [DOI] [PubMed] [Google Scholar]
- 63.Lordkipanidzé M, Pharand C, Schampaert E, Turgeon J, Palisaitis DA, Diodati JG. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J. 2007;28(14): 1702–1708. [DOI] [PubMed] [Google Scholar]
- 64.Gurbel PA, Bliden KP, DiChiara J, et al. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation. 2007;115(25):3156–3164. [DOI] [PubMed] [Google Scholar]
- 65.Blais N, Pharand C, Lordkipanidze M, Sia YK, Merhi Y, Diodati JG. Response to aspirin in healthy individuals. Cross-comparison of light transmission aggregometry, VerifyNow system, platelet count drop, thromboelastography (TEG) and urinary 11-dehydrothromboxane B(2). Thromb Haemost. 2009;102(2):404–411. [DOI] [PubMed] [Google Scholar]
- 66.Santilli F, Rocca B, De Cristofaro R, et al. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: implications for aspirin “resistance”. J Am Coll Cardiol. 2009;53(8):667–677. [DOI] [PubMed] [Google Scholar]
- 67.Kuliczkowski W, Witkowski A, Polonski L, et al. Interindividual variability in the response to oral antiplatelet drugs: a position paper of the Working Group on antiplatelet drugs resistance appointed by the Section of Cardiovascular Interventions of the Polish Cardiac Society, endorsed by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2009;30(4):426–435. [DOI] [PubMed] [Google Scholar]


