Abstract
Background
Vaccines comprising recombinant subunit proteins are well-suited to low-cost and high-volume production for global use. The design of manufacturing processes to produce subunit vaccines depends, however, on the inherent biophysical traits presented by an individual antigen of interest. New candidate antigens typically require developing custom processes for each one and may require unique steps to ensure sufficient yields without product-related variants.
Results
We describe a holistic approach for the molecular design of recombinant protein antigens—considering both their manufacturability and antigenicity—informed by bioinformatic analyses such as RNA-seq, ribosome profiling, and sequence-based prediction tools. We demonstrate this approach by engineering the product sequences of a trivalent non-replicating rotavirus vaccine (NRRV) candidate to improve titers and mitigate product variants caused by N-terminal truncation, hypermannosylation, and aggregation. The three engineered NRRV antigens retained their original antigenicity and immunogenicity, while their improved manufacturability enabled concomitant production and purification of all three serotypes in a single, end-to-end perfusion-based process using the biotechnical yeast Komagataella phaffii.
Conclusions
This study demonstrates that molecular engineering of subunit antigens using advanced genomic methods can facilitate their manufacturing in continuous production. Such capabilities have potential to lower the cost and volumetric requirements in manufacturing vaccines based on recombinant protein subunits.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12934-021-01583-6.
Keywords: Pichia pastoris, Subunit vaccine, Quality by design, Biomanufacturing
Background
The global demand for vaccines is on pace to exceed current capacities for manufacturing of both existing and new vaccines [1]. Increasing instances of epidemics and emerging diseases, such as COVID-19, also require constant development of new vaccines [2–4]. Despite this need, both the cost of development and the risk of failure for any given vaccine candidate are high [5]. New approaches to facilitate efficient development and manufacturing of vaccines are essential to address current and future global needs.
Subunit vaccines are increasingly prevalent due to their safety and efficacy [6–8], Examples include recombinant subunits for influenza and varicella (shingles) as well as virus-like particles (VLPs) used for hepatitis B (HBV) and human papillomavirus (HPV) [9, 10]. Despite recent research on improving designs for subunit vaccines [11, 12], antigens are primarily selected first for their ability to invoke appropriate immunogenic responses [13]. Further consideration of the suitability of an antigen for objectives like low-cost production during translational development could facilitate timely translation of new designs and ultimately improved global access for these products.
In contrast to subunit vaccines, the development and manufacturing of new monoclonal antibodies (mAbs) as biopharmaceuticals is highly efficient. This advantage is realized from similar processes for production for each new molecule [14]. MAbs are often amenable to efficient production using mammalian host cells, owing to high titers of secreted protein and standardized purification processes allowing for streamlined recovery. Without these features, non-mAbs require bespoke processes to produce and present distinct technical challenges. For example, many subunit vaccines are produced in intracellular compartments, like inclusion bodies in bacterial cells, which must be recovered by cellular lysis, multiple stages of chromatography, and often solubilization or refolding to achieve the desired conformation for immunogenicity. Reducing the number of steps in production, along with increasing the volumetric productivity for a given antigen, could maximize efficiency—and thus lower costs, of manufacturing [15, 16].
Here, we present an approach informed by host cell biology to engineer subunit vaccine candidates for efficient manufacturing while retaining their immunogenicity. We engineered a trivalent subunit vaccine candidate for rotavirus to enable an improved process for production that uses 50% fewer steps (relative to a reference process based on intracellular bacterial expression and recovery) [17]. We minimally modified the sequences of the three candidate protein antigens to promote secretion by a yeast host using a combination of genomic sequencing methods to inform specific changes for each antigen. These targeted changes improved the secreted titer and quality for each serotype antigen, addressing molecular variants affecting the quality attributes of these proteins, including aggregation, unwanted glycosylation, and N-terminal truncation. We showed all three antigens were compatible with concomitant expression and co-purification in a single campaign. This study provides a proof-of-concept for integrated manufacturing of a multi-valent subunit vaccine while retaining quality and relative quantities of the antigens.
Results
Assessment of expression of trivalent rotavirus antigens in yeast
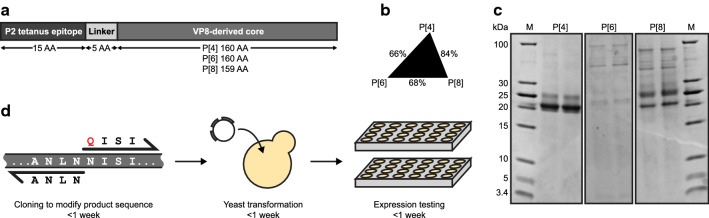
Rotavirus is a leading cause of mortality in children under five globally [18]. Three viral serotypes are most prevalent (P[4], P[6], and P[8]) [19]. While live viral vaccines are available for rotavirus, low-cost subunit vaccines could facilitate improved global coverage [17, 20, 21]. A trivalent non-replicating rotavirus vaccine (NRRV) designed at the NIH is currently advancing in clinical development. This candidate vaccine comprises three recombinant fusion-protein antigens based on VP8 truncations of the serotypes P[4], P[6], and P[8] [22]. Each VP8 truncation is genetically fused at the N-terminus to the P2 epitope of tetanus toxin (Fig. 1a). The current manufacturing strategy for these three serotypes involves fermentation in Escherichia coli, and recovery by multiple steps of centrifugation, cell lysis, and chromatography, followed by blending of the three antigens and formulation with adjuvant [23]. While the three serotypes have largely homologous amino acid sequences (Fig. 1b), they are different enough to present unique manufacturing challenges, making this subunit vaccine candidate an ideal case study for molecular engineering to improve its production.
Fig. 1.
Differences in product titer and quality among three non-replicating rotavirus vaccine (NRRV) serotypes provide case studies for sequence engineering. a Diagram of the three NRRV subunit vaccine antigens. AA = amino acid. b Percent identity of amino acid sequences between the three subunit antigens. c SDS-PAGE of the initial expression of each NRRV antigen. Expression from two clones is shown for each antigen. d Diagram of a design cycle for sequence engineering
Secretion of recombinant heterologous proteins by a host cell can simplify its recovery by reducing the number of non-chromatographic steps like centrifugation, lysis and refolding often required for intracellularly expressed proteins. Secretion-based strategies for production are also conducive for continuous operations that can increase the volumetric productivity in manufacturing [24]. For these reasons, we sought to enable secretion of the three antigens in the yeast Komagataella phaffii. We tested the secreted expression of each NRRV serotype independently in K. phaffii using the reported amino acid sequences expressed in Escherichia coli [23]. To adapt the sequences of each of the three antigens for this purpose, we optimized the codons of the genes for each serotype for expression in K. phaffii, added a signal peptide for secretion into the culture supernatant, and then assessed protein titers and quality following small-scale batch cultivation (Fig. 1c). Each serotype presented a markedly different set of molecular variants when recovered. The P[4] antigen was observed with a titer of ~ 50 mg/L in plate-scale culture (Additional file 1: Fig. S1A), but exhibited a high fraction of low-molecular weight variants. The P[6] antigen was barely detected in culture supernatant or intracellularly (Additional file 1: Fig. S1B). Secreted P[8] was extensively hypermannosylated and we detected product-related high-molecular weight variants (HMWV) (Additional file 1: Fig. S2A-B). These variations in antigen expression and quality presented challenges in developing a robust process for manufacturing, and did not suggest that a process using common steps or operations likely would be possible. Separation of product-related variants such as those observed with P[4] and P[8] typically require additional customized steps. The poor secreted titers observed with P[6] might also require extensive development of fermentation conditions and media or intracellular expression and subsequent recovery to achieve sufficient productivity for cost-effective manufacturing.
We sought to engineer each serotype to improve its manufacturability and quality attributes, specifically to address the product-related variants identified (glycosylation, sequence variants, HMWV), without impacting the immunogenicity of the reference antigens. In our approach, we adopted a strategy similar to the guidance for biopharmaceuticals for Quality by Design [25]. For each antigen, we identified the key attributes for each that influenced their secretion by host cells and the complexity of a purification process required for recovery. Informed by these heuristics and data collected during characterization of the host cell biology experienced during protein secretion, we designed and tested a limited number of conservative sequence modifications for their impact on protein titers and observed quality attributes. Each design cycle comprised modification of an antigen sequence, generation of a strain, and evaluation of secreted protein titer and product-related variants. Iterative cycles were executed in less than 3 weeks in K. phaffii (Fig. 1d), enabling the rapid assessment of how variations in sequence motifs impacted several key molecular attributes in the secreted proteins. We describe below the approach used to modify each serotype.
Modification of amino acid sequence eliminates product-related variants
Based on our analysis of the secreted P[8] antigen, we identified unwanted glycosylation and the formation of dimers as key molecular attributes to address; these variants can both complicate separation by chromatographic methods. N-linked glycosylation and dimers resulted from specific known amino acid motifs (N-X-S/T) and an unpaired cysteine, respectively. Four sites in P[8] were predicted by the NetNGlyc tool [26] to have potential for N-linked glycosylation during protein folding and export (Additional file 1: Table S1). We altered the P[8] sequence, therefore, to remove the most confidently predicted glycosylation sites using a conservative, targeted change (NQ). After elimination of the two most likely glycosylation sites (N85 and N151), no hypermannosylation was detected on P[8] by SDS-PAGE or LC–MS (Fig. 2, Additional file 1: Fig. S3).
Fig. 2.
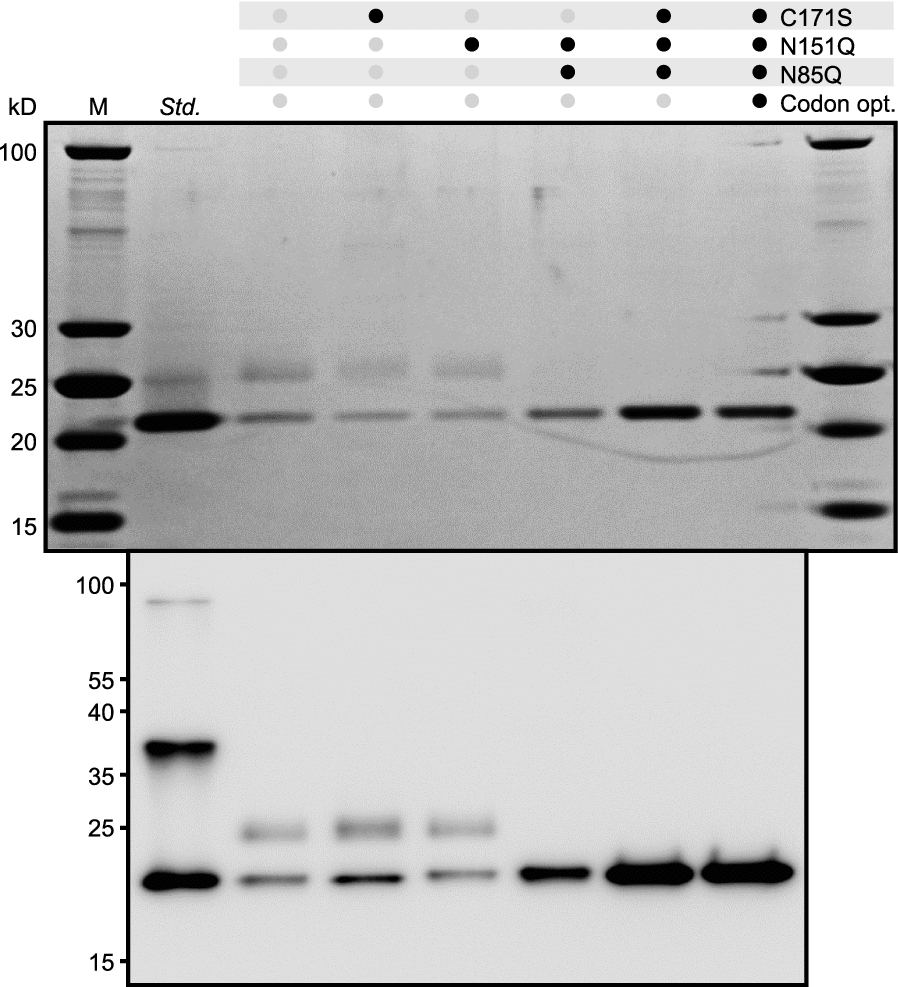
Sequence changes to remove N-linked glycosylation and Cys dimerization improve the quality and titer of P[8]. SDS-PAGE (top) and Western blot (bottom) of variants of P[8]
In parallel, we generated CS modifications of the free cysteine present in the P[8] antigen (C171) and observed nearly complete elimination of HMWV (Fig. 2). When combined, these changes at C171, N85, and N151 also increased the secreted titer of aglycosylated, monomeric P[8] from ~ 26 to ~ 87 mg/L in plate-scale batch cultivation. These data affirmed that specific, targeted changes can simultaneously improve multiple quality attributes of the antigen and the titers of secreted protein.
Product nucleic acid sequence influences N-terminal fidelity and folding
We then applied the same principles to address the observed molecular variants in the P[4] antigen. Initial expression of P[4] revealed multiple product-related variants with lower molecular weights by SDS-PAGE; these were confirmed by intact MS as four discrete N-terminal truncations (Fig. 3a). Interestingly, P[8] exhibited less truncation than P[4] despite having an identical N-terminal amino acid sequence (the P2 tetanus epitope). We theorized, therefore, that alternative translation start sites or ribosome skipping did not induce the observed differences in truncation [27, 28].
Fig. 3.
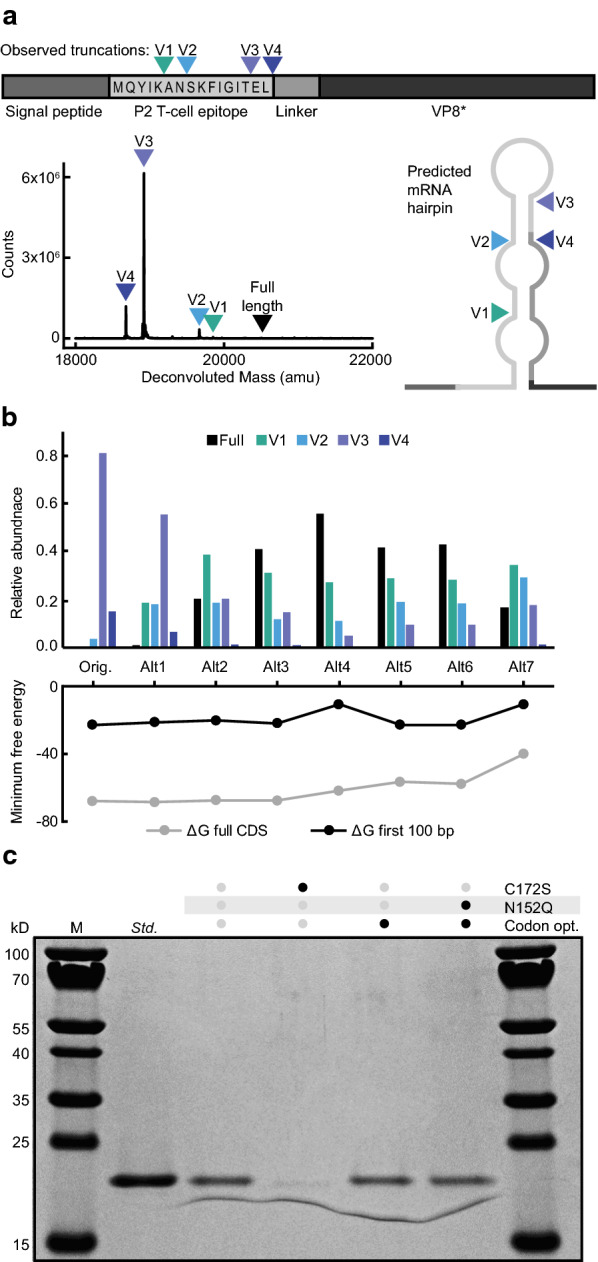
Codon optimization reduces the N-terminal truncation of P[4]. a Truncation variants of the original P[4] sequence by LC–MS. The N-terminus of each truncation variant is indicated on the antigen diagram and predicted RNA hairpin structure. b Relative abundance within the sample of truncated P[4] variants identified by LC–MS (top). Free energies of RNA hairpin formation after codon optimization for each variant (bottom). c SDS-PAGE of variants of P[4]
Alternatively, it has been noted that undesired RNA structures, such as hairpins in nascent mRNA, can cause ribosomal stalls and truncations [29]. We hypothesized that secondary structures in the transcript could impact the variants manifest in the recovered polypeptides. Using RNA structure [30], we identified an energetically favorable mRNA structure predicted to form near the sites of truncation in P[4] (Fig. 3a). We modified the codon usage of the P2 tetanus epitope to reduce the strength of the predicted RNA secondary structure while preserving the amino acid sequence (Fig. 3b). With increasing mitigation of the predicted RNA secondary structure from − 22.8 to − 10.7 kcal/mol, the fraction of full length P[4] in culture supernatant increased from 0% to nearly 60%, while the fraction of the longest truncated variant (V1) increased from 0% to nearly 30%. Overall, the percentage of the antigen that was full-length or nearly full-length (V1) increased from 0% to nearly 90% through improved codon usage alone, confirming that RNA sequence was at least partially responsible for the observed N-terminal truncation.
Similar to P[8], we observed hypermannosylation in the P[4] antigen and we identified sites predicted to be glycosylated. Modification of a single N-linked glycosylation site (N152Q) resulted in comparable titer and product quality as observed for P[8] (Fig. 3c). Surprisingly, despite strong similarity between P[8] and P[4], removal of the free cysteine residue (C172S) drastically reduced the secreted expression of P[4] and was not included in the selected optimized sequence.
Genome-wide host cell characterization enables alleviation of translation stall sites
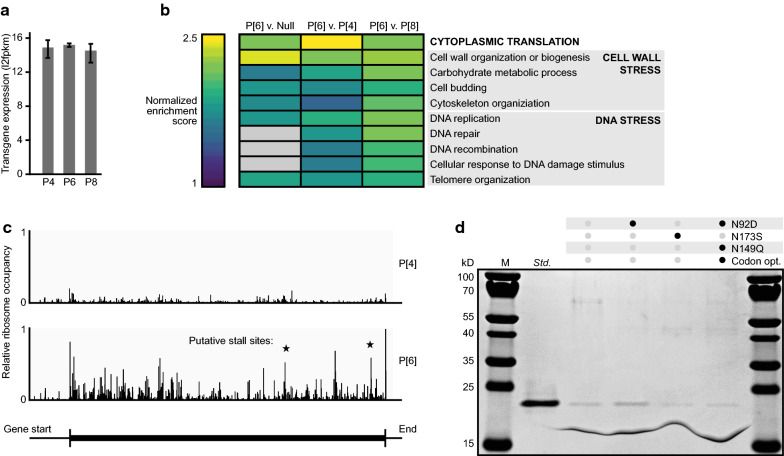
During initial expression, the P[6] variant strain yielded ~ eightfold lower titers of secreted protein than either the P[4] or P[8]-expressing strains, making titer a key process-related parameter to optimize for this serotype. Productive secretion of a recombinant protein requires proper mRNA transcription, translation, protein translocation and folding, and vesicular export. This complex, multi-step process makes diagnosis of a particular bottleneck a difficult task [31]. We used transcriptomics to survey differentially expressed genes in the strain expressing the P[6] antigen compared to strains expressing either P[4] and P[8]. The transcripts of P[6], P[4], and P[8] each were expressed at comparable levels by their respective strains, indicating transcription is not the rate-limiting step for P[6] (Fig. 4a). Of all gene sets that were differentially expressed in P[6]-producing cells (Additional file 1: Table S2), cytoplasmic translation had the highest enrichment score, followed by gene sets related to cell wall integrity and DNA repair, which are reported signals of stress in K. phaffii (Fig. 4b) [32]. We hypothesized that disrupted translation may preclude both adequate production of P[6] and proper cell growth and maintenance. We therefore focused our efforts on identifying sequence motifs that could impact protein translation.
Fig. 4.
Transcriptomics and ribosome profiling reveal a translational stall site that prevents expression of P[6]. a Expression levels of the recombinant gene in strains expressing each NRRV antigen. b Gene set enrichment analysis of the P[6] strain compared to strains expressing P[4], P[8], or no recombinant protein (Null). Gene sets shown exhibit high enrichment scores and significant adjusted p-values (< 0.05) in at least one comparison. c Relative ribosome occupancy across the recombinant transcript in strains expressing P[4] or P[6]. The magnitude of occupancy is normalized to the occupancy on all transcripts in each strain. Putative stall sites represent peaks that are not reflected in the P[4] transcript, and are located near a sequence discrepancy between P[4] and P[6]. d SDS-PAGE of variants of P[6]
Similar to our approach with P[4], we first examined the nucleic acid sequence of P[6] for the presence of energetically favorable RNA secondary structures using in silico tools for prediction. We observed a predicted RNA hairpin structure like that in P[4], but analogous mitigation of this structure did not improve production of P[6]. Upon inspection of the amino acid sequence of P[6], we noticed several highly hydrophobic amino acid motifs, which have been previously implicated in ribosomal stalling [33]. We altered amino acids near and within hydrophobic regions to reduce local hydrophobicity, but also did not observe increased expression of P[6] in any of these variants (Additional file 1: Fig. S4).
To systematically identify candidate sites of translational stalling, we used ribosome profiling, a technique in which ribosome-associated fragments of mRNA are sequenced to determine regions of transcripts that are enriched with ribosomes [34]. We visualized ribosome occupancy at each codon of the recombinant transcript in strains expressing either P[6] or P[4] for comparison (Fig. 4c). Ribosome occupancy was determined from the number of ribosome footprints that mapped to a particular transcript, after adjusting for both the abundance of the transcript and that of footprints mapping to the native transcriptome. We observed over fourfold higher ribosome occupancy overall on the transcript of P[6] relative to P[4], which supported our hypothesis of sequence-based translational stall sites in this serotype. We also observed sites with high ribosome occupancy at several positions of P[6] that were not similarly detected at those same positions in P[4]. Most of these positions coincided with local sequence motifs that differed in P[6] from the P[4] and P[8] consensus sequences. We nominated five of these motifs with the largest potential for ribosomal occupancy as likely sites of translational stalling and modified the amino acid sequence of each site to match that of a consensus of P[4] and P[8] sequences. Given the productive secreted expression of P[4] and P[8], we hypothesized that changing these sites in P[6] to match the P[4] and P[8] sequence could mitigate a translational stall while preserving the structure and antigenicity of the protein. One of these modifications (N92D) resulted in a ~ 2 × improvement in the expression of P[6] (Fig. 4d). The combination of RNA-Seq and ribosome profiling, therefore, enabled us to test a reasonable number of hypotheses and increase the secreted expression of P[6] to levels readily detected by SDS-PAGE with a single substitution of an amino acid.
Engineered subunits retain structure and function
The modifications to the primary sequences of the three subunits, though modest, could alter the structural integrity and antigenicity of the proteins. We performed bio-layer interferometry as a measure of the secondary structure of each serotype using monoclonal antibodies specific to each (Fig. 5a). All but one of the altered sequences had a minimal effect on binding affinity to the antibodies, suggesting that the modified variants retained antigenicity comparable to the reference sequences. Interestingly, the removal of the free cysteine residue of P[4] significantly altered the binding affinity of this variant, in addition to reducing expression (Fig. 3c, Fig. S1) as noted previously. This observation underscores that modifications to the amino acid sequence of a protein can interfere with the structure of a protein, and that alterations need to balance both objectives for antigenicity and manufacturability.
Fig. 5.
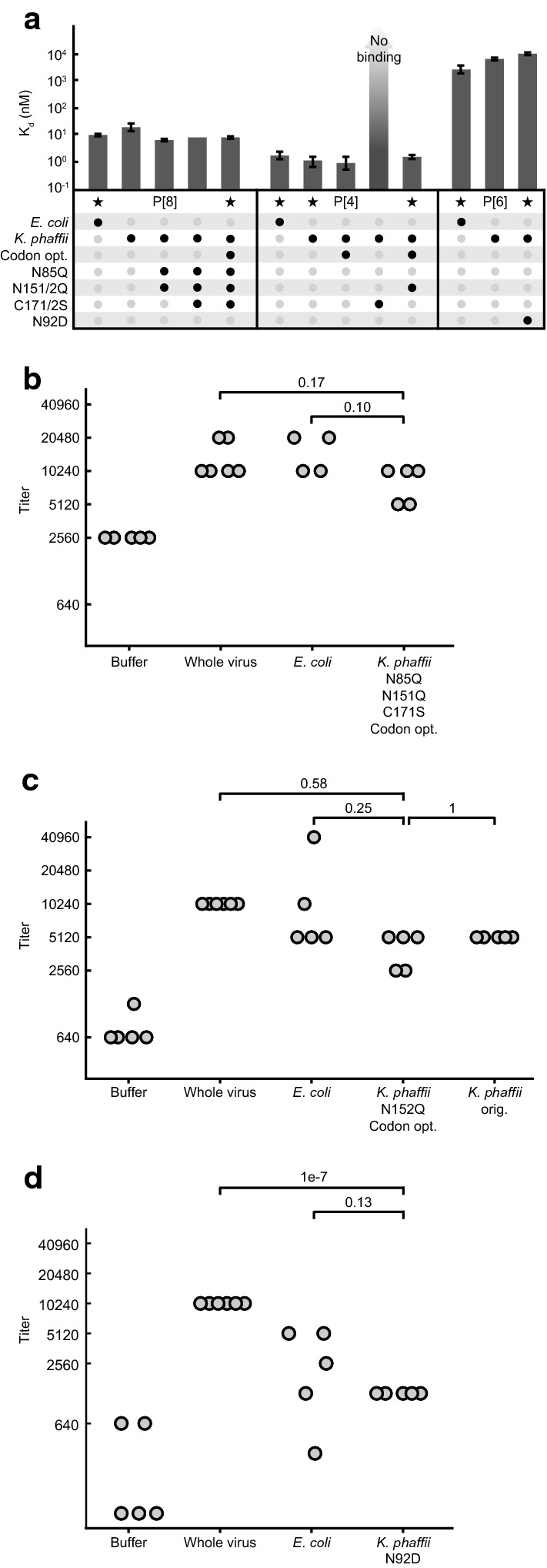
Engineered NRRV antigens produced in yeast are comparable to original antigens produced in bacteria. a Mean binding affinity of antigen variants measured by biolayer interferometry. Variants labeled with a star were further evaluated in animals. Error bars represent the maximum and minimum binding affinity of three technical replicates. b–d Titer of neutralizing antibodies raised against b P[8], c P[4], or d P[6], measured by fluorescence-based virus neutralization assay. Each data point represents the serum of one animal, as measured 35 days after the first injection, or 7 days after the final injection. Antigens were mixed with alhydrogel before injection. Treatment groups within each serotype were compared by ANOVA, followed by post hoc comparison of means using Tukey’s HSD test according to best practices [35]. ANOVA results were not significant for any serotype (p > 0.05), but adjusted p-values from Tukey’s test are noted
We then evaluated whether the selected engineered antigens maintained their immunogenicity, since the modified sequences may disrupt key linear or conformational epitopes that promote immune responses. We compared the ability of the engineered antigens for each serotype to induce the production of neutralizing antibodies in guinea pigs relative to the original sequences produced in bacteria. We selected one engineered antigen per serotype having the best observed titer and quality (Fig. 5a). We produced these three engineered antigens, alongside a control having the original P[4] sequence, by secreted expression in K. phaffii and purified the proteins to assess their immunogenicity in adjuvanted doses. Groups of guinea pigs were vaccinated with the engineered antigens produced in K. phaffii, and compared directly to groups that were vaccinated with antigens produced in E. coli having the original sequences [20]. We measured the titers of neutralizing antibodies present in serum collected from all animals in our study after three administered doses. The measured titers of neutralizing antibodies raised in response to our engineered antigens were not significantly different from titers raised by the original antigens for all three serotypes (Fig. 5b–d). Interestingly, we observed cross neutralization among serotypes for both engineered and original antigens (Additional file 1: Fig. S5A-C), evident by the strong neutralizing activity against P[4] virus using sera from groups vaccinated with P[6]. This cross neutralization was also evident in antigen-specific ELISAs, which confirmed general cross reactivity among the serotypes (Additional file 1: Fig. S5D). Cross reactivity is expected given the high homologies and biophysical similarities among serotypes, and may contribute to the broadly neutralizing responses achieved by the trivalent vaccine [17]. These results suggested that the engineering changes to the three antigens implemented to improve manufacturability did not significantly alter their immunogenicity in this model.
Reduction of product-related variants enables a common purification process
Given the overall similarity in sequence among all three antigens and the reduction in product-related variants after molecular engineering of each antigen, we hypothesized that all three antigens could be co-expressed and purified in a single cycle of production. Multi-valent subunit vaccines, in general, are strong candidates for co-purification because antigen serotypes often have similar biophysical characteristics, and integrated production could reduce the total number of manufacturing campaigns required. We previously demonstrated an integrated, continuous manufacturing system for producing biopharmaceutical proteins (InSCyT) [36]. Two core elements—microbial perfusion-based fermentation and integrated chromatographic operations [37, 38]—made it possible to rapidly and simultaneously develop processes for production and recovery on this system. We first developed a single process for perfusion-based fermentation based on our prior experience producing similar molecules, and executed it to produce each antigen individually (Fig. S6A-C). Starting from processes reported previously [36], we then developed a process for purification comprising a multimodal cation exchange capture resin for the removal of host-cell proteins followed by an anion exchange resin for the removal of host-cell DNA. The same two-step process was sufficient to recover each of the three individual antigens and remove residual host-cell proteins to below 500 ppm and DNA below the limit of detection of our assay (Fig. S6D-G). Product-related variants were already sufficiently minimized by sequence engineering such that no additional purification steps were required.
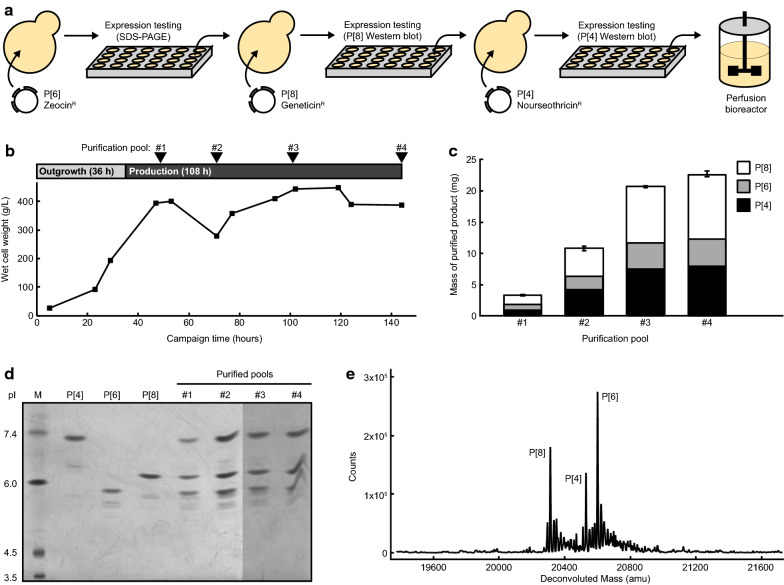
Co-expression and co-purification enable manufacturing of three antigen serotypes in a single campaign
We proceeded to produce all three antigens in a single integrated, end-to-end manufacturing process. We created a K. phaffii strain that expressed all three engineered antigens required for the trivalent vaccine candidate. Each transgene was introduced in series using three orthogonal selection markers, beginning with the serotype that demonstrated the lowest level of secreted expression by our host organism (P[6], P[4], then P[8]) (Fig. 6a). After each transformation of a new transgene, secreted expression of the antigen was verified by SDS-PAGE (Additional file 1: Fig. S7A–B). We then used this strain producing all three antigens in the InSCyT system (Fig. 6b). Cells were cultivated in perfusion fermentation, and the perfusate was subsequently purified in two steps by multimodal cation exchange chromatography followed by anion exchange chromatography, as performed before for the individual antigens. Production of all three serotypes was sustained over 108 h, and the relative ratios of each serotype were largely preserved throughout the campaign (Fig. 6c). Successful co-expression and co-purification of all three serotypes were confirmed by IEF (Fig. 6d) and LC–MS (Fig. 6e). Process-related impurities including host-cell proteins and host-cell DNA were reduced in the chromatographic operations below typical regulatory guidelines (Additional file 1: Fig. S7C,D). Overall, our approach of molecular design informed by host cell biology combined with an integrated, end-to-end manufacturing system enabled production of 200 mass-equivalent doses (limited by P[6]) of high-quality trivalent antigens in just six days on a single subliter benchtop system.
Fig. 6.
Sequence engineering enables sustained production of a trivalent NRRV vaccine from a single strain in a single manufacturing campaign. a Construction and testing of a strain that expresses all three NRRV antigens. b Cell density across the manufacturing campaign. Periods of outgrowth on glycerol, induction of protein expression on methanol, and points of product pooling and purification are shown. c Yield of NRRV antigens from the campaign. Concentrations were determined by spectrophotometric absorbance at 280 nm. Ratios of antigens were determined by reverse-phase liquid chromatography. d Isoelectric focusing gel of each purified pool of product. e Product masses of each antigen after purification identified by LC–MS
Discussion
Here, we have shown that conservative targeted molecular engineering for a subunit vaccine candidate can improve product titers and quality while preserving antigenicity and immunogenicity. This approach relies on rational analysis using a combination of natural variations of the sequence and genome-scale analysis of the host for expression. This proof-of-concept study demonstrates that disparate antigen candidates can be intentionally designed to conform to a common process for manufacturing, like those operated on the InSCyT manufacturing system here. Our approach to address molecular variants impacting the quality of the antigens at the amino acid sequence level was demonstrated by simple mitigation of N-linked glycosylation and dimerization in P[8]. Identification of a small number of critical amino acid residues allowed rapid screening of exhaustive sequence variations, yielding a strongly expressed antigen with no major product-related variants. Free cysteine residues in model proteins have been shown to covalently bind to pharmaceutical additives such as thimerosal, a commonly used preservative in multi-dose formulations [39]. We confirmed subsequently (and described elsewhere) that removing the free cysteine residue from P[8] abrogated the formation of a mercury adduct that can limit stability of the NRRV vaccine [40, 41].
To improve the product quality of the P[4] antigen, we also tested alternative codon optimization as a tool for product engineering and observed the thermodynamic stability of the P[4] mRNA structure impacted the N-terminal fidelity. The N-terminal truncations observed when expressing the original P[4] sequence are characteristic of promiscuous serine proteases; several of which are conserved in yeasts [42]. Based on our results, we hypothesized that mRNA secondary structures may slow translation and translocation, allowing increased cytoplasmic protease activity. Silent mutations have previously been demonstrated to impact product quality in biopharmaceutical manufacturing [43], and this potential mechanism merits further consideration in future studies. The connection between mRNA structure and protein truncation, nevertheless, demonstrates a key relationship through which truncated variants may be mitigated.
To increase the titer of P[6], we characterized the host cells themselves to generate a testable number of sequence changes. RNAseq revealed that translation was likely a critical cellular process inhibiting the secreted expression of P[6]. We did not identify a canonical ribosomal stall site, as such sites can be specific to the organism, and translation of exogenous genes in most biomanufacturing host organisms is not well understood [44]. Ribosome profiling as a technique, however, is agnostic to the mechanism of translational failure, and enabled us to identify a non-canonical site of translational stalling. Ribosome profiling captures data regarding all abundant transcripts in a single initial experiment, so future efforts could be focused to identify, and ultimately predict, translational preferences and stall sites that are specific to the host [45]. In this case, a modest substitution from the P[4] and P[8] sequence likely restored efficient translation of the P[6] transcript, though we were unable to identify the exact mechanism of failure.
Changes to the primary structure of a subunit vaccine, even conservative amino acid substitutions, could alter its antigenicity. Pathogens mutate T-cell epitopes or sequence-specific protease sites to facilitate evolutionary escape from antigen presentation to CD4+ T-cells [46]. Primary sequence changes may also alter secondary and tertiary structures that create discontinuous or conformational epitopes [47, 48]. We recently demonstrated that physicochemical properties of the candidate antigens here are sensitive to single “weak spot” residues that may also impact antigenicity [49]. For these reasons, and as demonstrated here, conservative, isolated amino acid mutations are preferable to sequence-wide screening because they reduce the probability of disrupting unknown antigenic epitopes. The ability of our engineered antigens to induce neutralizing antibodies in guinea pigs validates the viability of our approach, and suggests it could apply to vaccine candidates still in the early stages of discovery to facilitate future translational development.
The reduction of product-related variants and increase in observed titers for all three antigens realized here enabled a uniform and simple purification of each antigen. Ultimately this simplicity aided the concomitant manufacturing of all three antigens required for a trivalent vaccine candidate. Specifically, reducing product-related variants by molecular engineering up front reduced the purification process to just two steps. Concomitant manufacturing of multiple serotypes, if performed consistently, could eliminate further steps in manufacturing like blending of antigens. Concomitant manufacturing could also significantly reduce the cost of manufacturing by minimizing the required facility time and space, as well as the number of tests and reviews needed for quality assessments and assurances [50].
Conclusion
In this study, we have demonstrated that conservative molecular engineering can increase volumetric productivity, reduce unit operations, and enable concomitant manufacturing, all of which could reduce the cost of vaccine manufacturing [51]. A second implication of this study is that simple changes to a product sequence early in development can have a profound impact on the simplicity and scalability of the manufacturing process, potentially impacting further advancement of the product into the clinic and beyond. Separately, the accessibility of -omics data is exceptional for yeasts and other eukaryotic hosts, which enables further understanding of host biology for identifying rational host-dependent changes for product sequences [52]. This accessibility paired with the speed of cultivation and screening for expression suggests that yeast like K. phaffii are uniquely suited for the development of subunit vaccines. We believe our approach to molecular engineering demonstrates that subunit vaccines can be intentionally designed to conform to platform-like processes, achieving the same benefits in standardization seen with the manufacturing of monoclonal antibodies.
Materials and methods
Strains and cultivations
Wild-type Komagataella phaffii (NRRL Y-11430) was modified to express variants of P[4], P[6], or P[8] under control of the AOX1 promoter using a commercial vector (pPICZ A, Thermo Fisher Scientific). Modified vectors containing markers for selection on G418 (Thermo Fisher Scientific) or Nourseothricin (Gold Biotechnology) were also used to generate a single strain expressing all three serotypes.
Strains for initial characterization were grown in 3 mL culture in 24-well deep well plates (25 °C, 600 rpm) using complex medium (BMGY-Buffered Glycerol Complex Medium, Teknova) supplemented to 4% (v/v) glycerol. After 24 h of biomass accumulation, cells were pelleted and resuspended in BMMY (Buffered Methanol Complex Medium, Teknova) containing 1.5% (v/v) methanol. Samples for RNA-seq and ribosome profiling were collected after 16 h growth in BMMY.
Strains were additionally cultivated in 200 mL culture in 1 L shake flasks to generate material for non-clinical studies and in InSCyT [36] bioreactors for demonstration of end-to-end manufacturing. At both scales, cells were grown in rich defined medium [53] supplemented to 4% (v/v) glycerol for biomass accumulation or 5% (v/v) methanol for production. In the bioreactor, temperature, pH, and dissolved oxygen were maintained at 25 °C, 6.5, and 25%, respectively.
Transcriptome analysis
RNA was extracted and purified according to the Qiagen RNeasy kit (cat #74104) and RNA quality was analyzed to ensure RNA Quality Number > 7. RNA libraries were prepared using the Roche KAPA HyperPrep kit and sequenced on an Illumina NextSeq to generate 40-nt paired-end reads.
Sequenced reads were aligned and quantified using STAR v2.5.3a [54] and RSEM v1.3.0 [55]. Expression was visualized using log2(Fragments per Kilobase of Transcript per Million Mapped Reads + 1) values. Differential gene expression was analyzed using the DESeq2 package in R starting from gene integer counts and including log-fold-change shrinkage. Gene set enrichment analysis (GSEA) was performed in R using the fgsea package [56]. Gene expression data have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE159325.
Ribosome footprinting
Cell samples from cultures expressing P[4] or P[6] were transferred to a shake-flask containing 500 mL of pre-warmed fresh BMMY media to achieve an OD600 = 0.5. Cell harvest, cell lysis, and recovery of ribosome footprints were performed as described previously [57]. Footprints were dephosphorylated and cDNA libraries were prepared using the SMARTer smRNA-Seq Kit (Clontech), with an additional rRNA depletion step prior to PCR amplification, all as described previously [57].
Libraries were sequenced on an Illumina NextSeq to generate 75-nt paired-end reads. The reverse reads were of low quality as expected from the library preparation kit and were not used in analysis. For the forward reads, the first 3 nucleotides and poly(A) sequences were removed from raw FASTQ reads using Cutadapt v.1.16 [58] with options –u 3 -a A{100} –m 12. The trimmed reads were then aligned to a target consisting of K. phaffii rRNA sequences with bwa v0.7.16a. Unaligned reads were extracted with samtools v1.5 and FASTQ files were regenerated with bedtools v.2.26.0 for further analysis. Reads were then aligned to the wild-type K. phaffii genome plus the P[4] or P[6] expression cassettes using STAR v.2.5.3a [54] with the –alignIntronMax 600 option. Secondary alignments were removed using samtools v.1.5 and the primary alignment bam files were used as input to plastid v.0.4.8 [59]. Plastid was run according to the author’s documentation and the commands used are available in the file plastid_processing.sh, which can be found in the Github repository jrbrady / PlastidCommands. The read sizes considered ranged between 26 and 31 nt, and an offset of 13 nt was used for all lengths except 26 nt, for which a 12 nt offset was used. Ribosome profiling data have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE159336.
Experimental design
The goal of the animal study was to compare engineered antigens manufactured in K. phaffii to the original antigens manufactured in E. coli. We studied nine groups of five animals each, including a group for each of the original antigens (3), a group for each of the engineered antigens (3), one group of the original P[4] antigen manufactured in K. phaffii, one group receiving a placebo (adjuvant + buffer), and one group receiving trivalent rotavirus vaccine (Additional file 1: Table S3).
Immunization of guinea pigs
Groups of 5 guinea pigs (Hartley, Sisi Al strain) weighing 400–500 g (National Institute of Agricultural Technology, INTA, laboratory animal production facility) were immunized intramuscularly three times at 2-week intervals with 200 µL of aqueous vaccine containing 12 µg of the recombinant antigen (Additional file 1: Table S3) adjuvanted with 2% Alhydrogel (Brenntag Biosector, Frederikssund, Denmark) (aluminum content 100 µg/dose). A group of animals immunized with a trivalent RVA vaccine formulated with a mix of HRVA Wa, DS1 and ST3 at 107 FFU/dose in Alhydrogel was used as positive control. A group of 5 animals receiving buffer in Alhydrogel was used as negative control. Blood samples were collected prior to each immunization as well as at 7, 14, and 28 days after the third immunization. All guinea pig experiments were conducted in compliance with the guidelines of the Institutional Animal Welfare Committee of the National Institute of Agricultural Technology, INTA, CICUAE protocol # 55.2018.
Neutralization and protein specific ELISA assays
Human rotavirus (HRVA) Wa GP[8], DS1 G2P[4] and ST3 G4P[6] adapted for cell culture were grown in MA104 cells using standard procedures. Viral titer was determined by cell culture immuno-fluorescence (CCIF) assay and expressed as FFU/mL as previously described [60].
Virus neutralizing antibody titers (VNtAb) in serum samples were determined by a modified fluorescence focus reduction neutralization assay [61]. Serial two-fold dilutions of each serum sample were mixed with an equal volume of virus to a final concentration of 100 FFU. Serum-virus mixes were incubated at 37 °C, 5% CO2 for 1 h, and then transferred onto confluent monolayers of MA-104 cells in 96 well plates. Plates were incubated at 37 °C, 5% CO2 for 48 h. The monolayers were fixed with 70% acetone and the presence of residual virus in each well was evidenced by immuno-staining using a 2KD1 nanobody to rotavirus VP6 protein labeled with Alexafluor 488 [62]. VNtAb of each serum sample is expressed as the inverse of the maximal dilution showing 60% fluorescence focus reduction.
Homotypic and heterotypic IgG antibody responses to vaccination were monitored using standard P2-VP8 antigen ELISA techniques. Individual serum samples were evaluated against the homologous antigen while the heterologous response was evaluated in serum pooled at every time point (T0 to T6). Thermo Fisher Maxisorp® high- binding 96-well ELISA plates were coated with each antigen diluted in carbonate buffer pH 9.6 for 24 h at 4 °C. Plates were washed with wash buffer (PBS with 0.05% TweenTM 20), blocked with 200 μL of blocking buffer (PBS with 10% Skim milk), and incubated for one to four hours at 37 °C. Serum samples were diluted in serial four-fold dilutions, starting at 1:160, and 100 μL of each dilution was transferred to the assay plate, incubated for 1 h at 37 °C and washed as described above. Plates were incubated for 1 h at 37 °C with peroxidase labeled goat anti-guinea pig IgG antibody (Jackson) diluted 1:3000 in wash buffer. After four washes, 100 μL per well of detection reagent (H2O2, ABTS in citrate buffer, pH 5) was allowed to develop for 15 min in the dark at room temperature, and stopped with 50 μL of 5% SDS. Absorbance was measured at 405 nm with a SpectraMax® M2 microplate reader (Molecular Devices, LLC). The VNtAb of each serum sample was expressed as the inverse of the maximal dilution showing 60% fluorescence focus reduction. Serum that yielded no signal for any technique at 1:160 dilution was assigned a titer of 1:80.
Protein purification
Protein purification for non-clinical studies and end-to-end manufacturing was carried out on the purification module of the InSCyT system as described previously [36]. All columns were equilibrated in the appropriate buffer prior to each run. Product-containing supernatant was adjusted to pH 4.5 using 100 mM citric acid. The adjusted supernatant was loaded into a pre-packed CMM HyperCel column (1-mL or 5-mL) (Pall Corporation, Port Washington, NY), re-equilibrated with 20 mM sodium citrate pH 4.5, washed with 20 mM sodium phosphate pH 6.0, and eluted with 20 mM sodium phosphate pH 7.0, 100 mM NaCl. Eluate from column 1 above 15 mAU was flowed through a 1-mL pre-packed HyperCel STAR AX column (Pall Corporation, Port Washington, NY). Flow-through from column 2 above 15 mAU was collected.
Analytical assays for product characterization
Biolayer interferometry was performed using the Octet Red96 with streptavidin (SA) biosensors (Pall ForteBio, Fremont, CA), which were hydrated for 15 min in kinetics buffer prior to each run. Kinetics buffer consisting of 1X PBS pH 7.2, 0.5% BSA, and 0.05% tween 20 was used for all dilutions, baseline, and disassociation steps. Monoclonal antibodies specific to P[4], P[6], and P[8], respectively, were biotinylated using the EZ-Link Sulfo-NHS-LC-biotinylation kit according to manufacturer instructions (Thermo Fisher Scientific) and used in the assay at concentrations of 1.25, 1.7, and 1 µg/mL, respectively. Triplicate samples were loaded in a 96-well black microplate (Greiner Bio-One) at starting concentrations of 2, 14, or 5 µg/mL, respectively. Seven 1:1 serial dilutions and a reference well of kinetics buffer were analyzed for each sample. Association and disassociation were measured at 1000 rpm for 300 and 600 s, respectively. Binding affinity was calculated using the Octet Data Analysis software v10.0 (Pall ForteBio), using reference subtraction, baseline alignment, inter-step correction, Savitzky-Golay filtering, and a global 1:1 binding model.
Wet cell weight (WCW) was determined as described previously [36]. Sample concentrations were determined by absorbance at A280 nm. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out under reducing and non-reducing conditions using Novex 12% Tris–Glycine Gels or Novex 16% Tricine Gels (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s recommended protocol and stained using Instant Blue Protein Stain (Thermo Fisher Scientific, Waltham, MA). Isoelectric focusing was carried out as described previously [36]. Samples were analyzed for host cell-protein content using the Pichia pastoris 1st generation HCP ELISA kit from Cygnus Technologies (Southport, NC) according to the manufacturer’s recommended protocol. Samples were analyzed for residual host-cell DNA using the Quant-iT dsDNA High-Sensitivity Assay Kit (Invitrogen) according to the manufacturer’s protocol except the standard curve was reduced to 0–20 ng.
High performance liquid chromatography (HPLC) analysis was performed on an Agilent 1260 HPLC system equipped with a diode array detector and controlled using OpenLab CDS software (Agilent Technologies, Santa Clara, CA). Antigen concentration was determined using a PLRP-S column (2.1 × 150 mm, 300 Å, 3 µm) operated at 0.5 mL/min and 60°C (Agilent Technologies, Santa Clara, CA). Buffer A was 0.1% (v/v) TFA in water and buffer B was 0.1% (v/v) TFA, 0.5% (v/v) water in ACN. A gradient of 37–42% B was performed over 30 min; total method run time was 45 min. Sample injection volumes were 10µL. Data analysis was completed using OpenLab CDS Data Analysis (Agilent Technologies, Santa Clara, CA).
Intact mass analysis was performed on a 6230B time of flight (TOF) LC–MS with a 1220 series HPLC (Agilent Technologies, Santa Clara, CA). Mobile phase A consisted of water with 0.1% formic acid, and mobile phase B was acetonitrile with 0.1% formic acid. About 20 pmol of each sample was injected, bound to a ZORBAX 300SB C3 column (Agilent Technologies), desalted, and subjected to electrospray ionization. The LC gradient consisted of 20–70% B over 1 min at a flow rate of 1.5 mL/min. Elution of proteins was monitored using the absorbance signal at 214 nm. 50 µL of isopropanol was injected after each sample to control sample carry-over. The typical electrospray ionization parameters consisted of: 290 °C gas temperature, 4000 V Vcap, 2000 V nozzle, and 275 V fragmentor voltage. Mass spectra were collected from 700 to 2800 m/z at a scan rate of 1 spectra/sec. MS spectra were processed using MassHunter Qualitative Analysis software (v B.07.00, Agilent Technologies) with deconvolution range of 10–50 kDa, using 1 Da mass step.
Supplementary Information
Additional file 1. Additional Tables and Figures.
Acknowledgements
The authors thank Danielle Camp for program support and alignment, the Koch Institute Integrated Genomics and Bioinformatics Core for technical support, James Taggart for support with ribosome profiling, Nishant Sawant for assistance in sample preparation for mass spec analysis, Lucia Rocha for help with ELISAs, cell culture, and VN assays, Vanesa Franco, Jose Vallejo, and Ezequiel Rivarola for care of guinea pigs, and Andres Wigdorovitz for helpful conversation. The authors acknowledge PATH for providing reference NRRV antigens and related immunological mAb reagents for this study.
Authors' contributions
NCD, JRB, KRL, and JCL conceived and planned experiments. NCD, JRB, MKT, and DLK generated and characterized yeast strains. JRB and CAW conducted transcriptomic and ribosome profiling experiments. LEC designed and performed protein purifications. AMB and AB operated bioreactors. SRA performed HPLC assays. KK and JMH performed analytical characterization. MB, CV, and VP performed animal studies. NCD, JRB, KRL, and JCL wrote the manuscript. JCL, KRL, VP, DBV, SBJ, and TM designed the experimental strategy and supervised analysis. All authors read and approved the final manuscript.
Funding
This work was funded by the Bill and Melinda Gates Foundation (Investment ID OPP1154682) (INV-008547). This study was also supported in part by the Koch Institute Support (core) grant P30‐CA14051 from the National Cancer Institute. J.R.B. and N.C.D were partially supported by a NIGMS/MIT Biotechnology Training Program Fellowship (NIH contract no. 2T32GM008334–26). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI, NIH, or the Bill & Melinda Gates Foundation.
Availability of data and materials
Sequencing data have been deposited in NCBI’s Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE159338.
Declarations
Ethics approval and consent to participate
Animal studies were reviewed and approved by The Institutional Committee for the Care and Use of Experimental Animals at INTA CICVyA, Argentina, evaluation request number 55/2018, “Immunogenic quality control of subunit vaccines for human rotavirus in guinea pigs.”
Consent for publication
Not applicable.
Competing interests
L.E.C., K.R.L., and J.C.L. have filed patents related to the InSCyT system and methods. K.R.L. and J.C.L. are co-founders and consultants to Sunflower Therapeutics PBC.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Neil C. Dalvie and Joseph R. Brady contributed equally to this work
References
- 1.Ford AQ, Touchette N, Fenton Hall B, Hwang A, Hombach J. Meeting report: global vaccine and immunization research forum. Vaccine. 2018;36:915–20. doi: 10.1016/j.vaccine.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Barrett ADT. Developing Zika vaccines: the lessons for disease X. Genome Med. 2018;10:47. doi: 10.1186/s13073-018-0561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poland GA, Kennedy RB, Ovsyannikova IG, Palacios R, Ho PL, Kalil J. Development of vaccines against Zika virus. Lancet Infect Dis. 2018;18:e211–9. doi: 10.1016/S1473-3099(18)30063-X. [DOI] [PubMed] [Google Scholar]
- 4.Gates B. Responding to Covid-19 - a once-in-a-century pandemic? N Engl J Med. 2020;380:1–3. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 5.Gouglas D, Le Thanh T, Henderson K, Kaloudis A, Danielsen T, Hammersland NC, et al. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob Heal. 2018;6:e1386–96. doi: 10.1016/S2214-109X(18)30346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baay M, Bollaerts K, Verstraeten T. A systematic review and meta-analysis on the safety of newly adjuvanted vaccines among older adults. Vaccine. 2018;36:4207–4214. doi: 10.1016/j.vaccine.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Rappuoli R. Reverse vaccinology. Curr Opin Microbiol. 2000;3:445–50. doi: 10.1016/s1369-5274(00)00119-3. [DOI] [PubMed] [Google Scholar]
- 8.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–96. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 9.McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307:178–80. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- 10.Josefsberg JO, Buckland B. Vaccine process technology. Biotechnol Bioeng. 2012;109:1443–60. doi: 10.1002/bit.24493. [DOI] [PubMed] [Google Scholar]
- 11.Hsia Y, Bale JB, Gonen S, Shi D, Sheffler W, Fong KK, et al. Design of a hyperstable 60-subunit protein icosahedron. Nature. 2016;535:136–9. doi: 10.1038/nature18010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Sagaseta J, Malito E, Rappuoli R, Bottomley MJ. Self-assembling protein nanoparticles in the design of vaccines. Comput Struct Biotechnol J. 2016;14:58–68. doi: 10.1016/j.csbj.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sette A, Rappuoli R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity. 2010;33:530–41. doi: 10.1016/j.immuni.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley B. Industrialization of mAb production technology: The bioprocessing industry at a crossroads [Internet]. MAbs. Taylor & Francis; 2009 [cited 2017 Oct 27]. p. 440–9. Available from: http://www.tandfonline.com/doi/abs/10.4161/mabs.1.5.9448 [DOI] [PMC free article] [PubMed]
- 15.Chilima TP, Crowell L, Love JC, Mukhopadhyay T, Farid SS. Manufacturing strategies for sustainable supply of ultra-low cost vaccines for global health. Vaccine Technol VII [Internet]. 2018 https://dc.engconfintl.org/vt_vii/119. Accessed 27 Sep 2019.
- 16.Shukla AA, Thömmes J. Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol. 2010;28:253–61. doi: 10.1016/j.tibtech.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Fix AD, Harro C, McNeal M, Dally L, Flores J, Robertson G, et al. Safety and immunogenicity of a parenterally administered rotavirus VP8 subunit vaccine in healthy adults. Vaccine. 2015;33:3766–72. doi: 10.1016/j.vaccine.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Steele AD, Victor JC, Carey ME, Tate JE, Atherly DE, Pecenka C, et al. Experiences with rotavirus vaccines: can we improve rotavirus vaccine impact in developing countries? Hum Vaccin Immunother. 2019;15:1215–27. doi: 10.1080/21645515.2018.1553593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 20.Groome MJ, Koen A, Fix A, Page N, Jose L, Madhi SA, et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis Elsevier. 2017;17:843–853. doi: 10.1016/S1473-3099(17)30242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnett E, Parashar UD, Tate JE. Real-world effectiveness of rotavirus vaccines, 2006–19: a literature review and meta-analysis. Lancet Glob Heal. 2020;8:e1195–e1202. doi: 10.1016/S2214-109X(20)30262-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen X, Cao D, Jones RW, Li J, Szu S, Hoshino Y. Construction and characterization of human rotavirus recombinant VP8* subunit parenteral vaccine candidates. Vaccine. 2012;30:6121–6. doi: 10.1016/j.vaccine.2012.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen X, Wen K, Cao D, Li G, Jones RW, Li J, et al. Inclusion of a universal tetanus toxoid CD4+ T cell epitope P2 significantly enhanced the immunogenicity of recombinant rotavirus ΔVP8* subunit parenteral vaccines. Vaccine. 2014;32:4420–7. doi: 10.1016/j.vaccine.2014.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karst DJ, Steinebach F, Morbidelli M. Continuous integrated manufacturing of therapeutic proteins. Curr. Opin. Biotechnol. 2018;53:76–84. doi: 10.1016/j.copbio.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Rathore AS (Anurag S, Mhatre R. Quality by design for biopharmaceuticals : principles and case studies. https://books.google.com/books?hl=en&lr=&id=0a2k_03a4Q4C&oi=fnd&pg=PT7&dq=quality+by+design+biopharmaceuticals&ots=4_62Io99nv&sig=h3jJqE29kxa8H3IKTLMW1IOovw8#v=onepage&q=qualitybydesignbiopharmaceuticals&f=false. Accessed 30 Sep 2019.
- 26.Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478–88. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortelny N, Pavlidis P, Overall CM. The path of no return-Truncated protein N-termini and current ignorance of their genesis. Proteomics. 2015;15:2547–52. doi: 10.1002/pmic.201500043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joazeiro CAP. Ribosomal stalling during translation: providing substrates for ribosome-associated protein quality control. Annu Rev. 2017 doi: 10.1146/annurev-cellbio-111315-125249. [DOI] [PubMed] [Google Scholar]
- 29.Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989;9:5134–42. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuter JS, Mathews DH. RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinform. 2010;11:129. doi: 10.1186/1471-2105-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delic M, Valli M, Graf AB, Pfeffer M, Mattanovich D, Gasser B. The secretory pathway: exploring yeast diversity. FEMS Microbiol Rev. 2013;37(6):872–914. doi: 10.1111/1574-6976.12020. [DOI] [PubMed] [Google Scholar]
- 32.Brady JR, Whittaker CA, Tan MC, Kristensen DL, Ma D, Dalvie NC, et al. Comparative genome-scale analysis of Pichia pastoris variants informs selection of an optimal base strain. Biotechnol Bioeng. 2020;117:543–55. doi: 10.1002/bit.27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson DN, Beckmann R. The ribosomal tunnel as a functional environment for nascent polypeptide folding and translational stalling. Curr Opin Struct Biol. 2011;21:274–82. doi: 10.1016/j.sbi.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Ingolia NT. Ribosome footprint profiling of translation throughout the genome. Cell. 2016;165:22–33. doi: 10.1016/j.cell.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Festing MFW. Guidelines for the design and statistical analysis of experiments in papers submitted to ATLA. ATLA Altern to Lab Anim. 2001;29:427–446. doi: 10.1177/026119290102900409. [DOI] [PubMed] [Google Scholar]
- 36.Crowell LE, Lu AE, Love KR, Stockdale A, Timmick SM, Wu D, et al. On-demand manufacturing of clinical-quality biopharmaceuticals. Nat Biotechnol. 2018;36:988. doi: 10.1038/nbt.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timmick SM, Vecchiarello N, Goodwine C, Crowell LE, Love KR, Love JC, et al. An impurity characterization based approach for the rapid development of integrated downstream purification processes. Biotechnol Bioeng. 2018;115:2048–60. doi: 10.1002/bit.26718. [DOI] [PubMed] [Google Scholar]
- 38.Vecchiarello N, Goodwine C, Crowell LE, Love KR, Cramer SM, Love JC. A combined screening and in-silico strategy for rapidly designing orthogonally selective integrated downstream processes for removing process and product-related impurities. (Manuscript in preparation). 2018; [DOI] [PubMed]
- 39.Trümpler S, Lohmann W, Meermann B, Buscher W, Sperling M, Karst U. Interaction of thimerosal with proteins - Ethylmercury adduct formation of human serum albumin and β-lactoglobulin A. Metallomics. 2009;1:87–91. [Google Scholar]
- 40.Sawant N, Kaur K, Holland DA, Hickey JM, Agarwal S, Brady JR, et al. Rapid developability assessments to formulate recombinant protein antigens as stable, low-cost, multi-dose vaccine candidates: Case-study with non-replicating rotavirus (NRRV) vaccine antigens. J Pharm Sci. 2021;110(3):1042–1053. doi: 10.1016/j.xphs.2020.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur K, Xiong J, Sawant N, Agarwal S, Hickey JM, Holland DA, et al. Mechanism of thimerosal-induced structural destabilization of a recombinant rotavirus P[4] protein antigen formulated as a multi-dose vaccine. J Pharm Sci. 2021;110(3):1054–1066. doi: 10.1016/j.xphs.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsalek L, Gruber C, Altmann F, Aleschko M, Mattanovich D, Gasser B, et al. Disruption of genes involved in CORVET complex leads to enhanced secretion of heterologous carboxylesterase only in protease deficient Pichia pastoris. Biotechnol J. 2017;12:1600584. doi: 10.1002/biot.201600584. [DOI] [PubMed] [Google Scholar]
- 43.Improved Coding Sequence For Human G-CSF. 2016 https://patents.google.com/patent/US20190071481A1/en. Accessed 30 Sep 2019.
- 44.Artieri CG, Fraser HB. Accounting for biases in riboprofiling data indicates a major role for proline in stalling translation. Genome Res. 2014;24:2011–21. doi: 10.1101/gr.175893.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tunney R, McGlincy NJ, Graham ME, Naddaf N, Pachter L, Lareau LF. Accurate design of translational output by a neural network model of ribosome distribution. Nat Struct Mol Biol. 2018;25:577–82. doi: 10.1038/s41594-018-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosa DS, Ribeiro SP, Cunha-Neto E. CD4+ T cell epitope discovery and rational vaccine design. Arch Immunol Ther Exp. 2010;58:121–30. doi: 10.1007/s00005-010-0067-0. [DOI] [PubMed] [Google Scholar]
- 47.Van Regenmortel MH. Which structural features determine protein antigenicity? Trends Biochem Sci. 1986;11:36–9. [Google Scholar]
- 48.Thomas JC, O’Hara JM, Hu L, Gao FP, Joshi SB, Volkin DB, et al. Effect of single-point mutations on the stability and immunogenicity of a recombinant ricin A chain subunit vaccine antigen. Hum Vaccin Immunother. 2013;9:744–52. doi: 10.4161/hv.22998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agarwal S, Hickey JM, Sahni N, Toth RT, Robertson GA, Sitrin R, et al. Recombinant subunit rotavirus trivalent vaccine candidate: physicochemical comparisons and stability evaluations of three protein antigens. J Pharm Sci. 2019;109:380–393. doi: 10.1016/j.xphs.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher AC, Kamga M-H, Agarabi C, Brorson K, Lee SL, Yoon S. The Current scientific and regulatory landscape in advancing integrated continuous biopharmaceutical manufacturing. Trends Biotechnol. 2019;37:253–67. doi: 10.1016/j.tibtech.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Munira SL, Hendriks JT, Atmosukarto II, Friede MH, Carter LM, Butler JRG, et al. A cost analysis of producing vaccines in developing countries. Vaccine Elsevier Ltd. 2019;37:1245–1251. doi: 10.1016/j.vaccine.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 52.Love KR, Dalvie NC, Love JC. The yeast stands alone: the future of protein biologic production. Curr Opin Biotechnol. 2018;53:50–8. doi: 10.1016/j.copbio.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Matthews CB, Kuo A, Love KR, Love JC. Development of a general defined medium for Pichia pastoris. Biotechnol Bioeng. 2017;115:103–13. doi: 10.1002/bit.26440. [DOI] [PubMed] [Google Scholar]
- 54.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sergushichev AA. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. bioRxiv. 2016 [Google Scholar]
- 57.Taggart JC, Li GW. Production of protein-complex components is stoichiometric and lacks general feedback regulation in eukaryotes. Cell Syst. 2018;7:580–589. doi: 10.1016/j.cels.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10. [Google Scholar]
- 59.Dunn JG, Weissman JS. Plastid: nucleotide-resolution analysis of next-generation sequencing and genomics data. BMC Genomics BioMed Central Ltd. 2016;17:1–12. doi: 10.1186/s12864-016-3278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parreño V, Marcoppido G, Vega C, Garaicoechea L, Rodriguez D, Saif L, et al. Milk supplemented with immune colostrum: protection against rotavirus diarrhea and modulatory effect on the systemic and mucosal antibody responses in calves experimentally challenged with bovine rotavirus. Vet Immunol Immunopathol. 2010;136:12–27. doi: 10.1016/j.vetimm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Saif LJ, Yuan L, Ward LA. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Gen Virol. Microbiol Soc. 1998;79:2661–72. doi: 10.1099/0022-1317-79-11-2661. [DOI] [PubMed] [Google Scholar]
- 62.Garaicoechea L, Olichon A, Marcoppido G, Wigdorovitz A, Mozgovoj M, Saif L, et al. Llama-derived single-chain antibody fragments directed to rotavirus VP6 protein possess broad neutralizing activity in vitro and confer protection against diarrhea in mice. J Virol Am Soc Microbiol. 2008;82:9753–9764. doi: 10.1128/JVI.00436-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional Tables and Figures.
Data Availability Statement
Sequencing data have been deposited in NCBI’s Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE159338.