Abstract
Glial cells are mechanosensitive, and thus, engineered systems have taken a step forward to design mechanotransduction platforms in order to impart diverse mechanical stresses to cells. Mechanical strain encountered in the central nervous system can arise from diverse mechanisms, such as tissue reorganization, fluid flow, and axon growth, as well as pathological events including axon swelling or mechanical trauma. Biomechanical relevance of the in vitro mechanical testing requires to be placed in line with the physiological and mechanical changes in central nervous tissues that occur during the progression of neurodegenerative diseases. Mechanotransduction signaling utilized by glial cells and the recent approaches intended to model altered microenvironment adapted to pathological context are discussed in this review. New insights in systems merging substrate's stiffness and topography should be considered for further glial mechanotransduction studies, while testing platforms for drug discoveries promise great advancements in pharmacotherapy. Potential leads and strategies for clinical outcomes are expected to be developed following the exploration of these glial mechanosensitive signaling pathways.
I. INTRODUCTION
Glial cells are largely involved in neural tissue remodeling throughout the physiological and pathological development of the nervous system. Glial cells also participate in the regenerative process after injury.1,2 These cells have the ability to perceive the mechanical signals driven by microenvironmental changes. Although neural diseases have multiple known origins (genetic defect, congenital disorder, tumor, autoimmunity, trauma, infection, environmental health, tissue mechanics, etc.), tissue mechanics is described as a major mechanism encountered and often driving pathogenesis.3,4 Particularly when the tissue integrity is affected, the homeostasis is dysregulated, and the mechanical changes are, therefore, among the main signal that cells are sensing. Since tissue damage or malformation leads to profound changes in the mechanical properties of the nervous tissue, it is essential to understand the response of these glial cells toward microenvironmental mechanical changes in order to restore tissue homeostasis and function. Recent discoveries concerning the mechanosensitivity of glial cells have contributed to our understanding of the mechanisms of action by which these cells probe and interact with their surrounding substrates and juxtaposed cells.
Specifically, glial cells adapt to the physiological or pathological context using mechanosensing capacity, through mechanotransduction machinery. In principle, mechanotransduction is the result of cell sensing, integration, and conversion of external mechanical cues into biochemical signals.5 The mechanical stimuli that are derived from cell substrate stiffness and surface tension affect the cell plasma membrane tension and result in ion influx and signaling pathways activation. On a side note, the underlying pathways (e.g., stretched-activated ion channel signaling,6 integrin signaling,7 actomyosin contractility,8 Hippo pathway,9 and the transcription factor Yap/Taz10) governing these mechanisms are often interconnected, depending on the nature of the mechanical signal. Thus, it is not surprising to find that glial cells are strongly involved in the pathogenesis of neurological diseases since physiological perturbations recorded in the central nervous system (CNS) distort tissue mechanical stiffness and homeostasis.3,11 Even slight changes in the properties of the brain extracellular matrix (ECM) or extracellular fluid pressure caused by disease progression may result in tissue stiffening and compression, which in turn lead to an alteration in the mechanical signaling. For instance, tissue stiffening is prevalent in traumatic injuries,12 dementia,13 and Alzheimer's disease (AD).14–16 On the other hand, soft mechanical signature of glial scars has been recorded in the CNS17 for multiple sclerosis (MS)18 and glioma.19
Therefore, emphasis has been placed on studying glial mechanobiology to understand the mechanotransduction signals that are involved in response to changes in microenvironment mechanical properties.4 The mechanobiology area has advanced in tools and techniques to reproduce as faithfully as possible the physiological constraints associated with disease development.
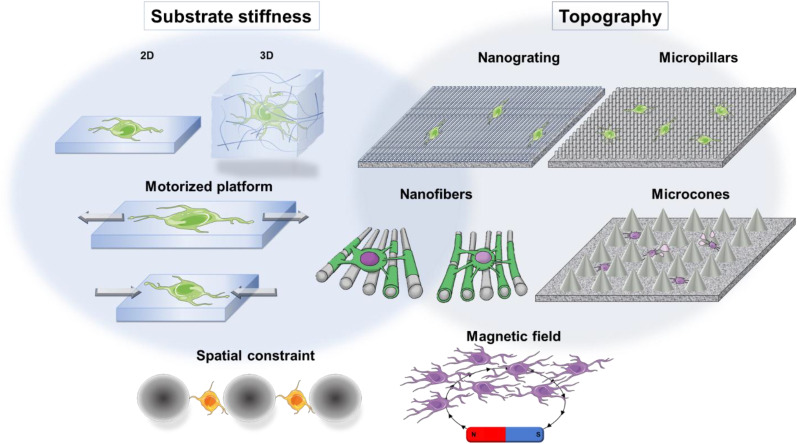
In this review, we emphasize the emerging focus on glial mechanotransduction with the development of biomimicking platforms to study the cell behavior in disease models through various mechanical stimuli and potential underlying findings in pharmacotherapy. Hence, we will elucidate the physiological and mechanical changes in CNS tissues that occur during the progression of neurodegenerative diseases. Then, we will discuss the current and recent advances in engineering systems that may be used to impart mechanical stresses (hydrogels, motorized forms, spatial constraints, cell-topography interaction systems, magnetic-induced traction, and micro/nanopatterning) to cells in the context of glial cells. The compilation of the latest works on mechanotransduction signaling utilized by glial cells and the recent approaches intended to model altered microenvironment adapted to pathological context by modulating substrate's stiffness and controlling cell responses will be developed. Finally, potential leads and strategies for clinical outcomes will be discussed as a perspective.
II. MECHANICAL STIFFNESS VARIATION IN THE DISEASED AND AGING CENTRAL NERVOUS SYSTEM
Besides the well-understood involvement of biomolecular signaling in disease progression, it is becoming clear that mechanotransduction may also be involved due to changes in tissue stiffness and cytoskeletal structures.11 This section summarizes the variations in mechanical stiffness and ECM modifications that are encountered within the CNS and associated pathologies and encompasses the limits of current methods, highlighting the precautions and parameters to be considered when studying a particular condition. We envision that this section can be read as a database to allow the rapid establishment of a system reproducing physiological conditions that are necessary for the most reliable study of the mechanotransduction pathways used in the chosen case. Data have, therefore, been compiled in Table I enclosed, while short physiopathological description can be found in Subsections II A–II G.
TABLE I.
Influence of the pathophysiology and measuring methods of brain stiffness in humans and animal models.
| Pathology | Condition | Species | Stiffness (Pa) | Method | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy CNS | Ageing | Human | Young adult | Ageing | Magnetic resonance elastography (MRE) | 27 | ||||
| 3.5–3.8 kPa | 2.5–2.7 kPa | |||||||||
| Human | 3.07 kPa | 2.37 kPa | MRE | 14 | ||||||
| Mouse | 25 kPa | NA | MRE | 37 | ||||||
| Human | Predicted stiffness at age 76 | MRE | 29 | |||||||
| Cerebrum | 2.6 ± 0.1 kPa | |||||||||
| Frontal lobes | 2.6 ± 0.1 kPa | |||||||||
| Occipital lobes | 2.8 ± 0.2 kPa | |||||||||
| Parietal lobes | 2.6 ± 0.2 kPa | |||||||||
| Temporal lobes | 2.7 ± 0.1 kPa | |||||||||
| Deep GM/WM | 3.0 ± 0.3 kPa | |||||||||
| Cerebellum | 2.2 ± 0.2 kPa | |||||||||
| Sensory motor | 2.8 ± 0.3 kPa | |||||||||
| Human | Predicted stiffness at age 41 | MRE | 28 | |||||||
| Cerebrum | 2.3545 ± 0.02 kPa | |||||||||
| Frontal lobes | 2.2326 ± 0.02 kPa | |||||||||
| Occipital lobes | 2.4487 ± 0.02 kPa | |||||||||
| Parietal lobes | 2.1414 ± 0.02 kPa | |||||||||
| Temporal lobes | 2.6175 ± 0.02 kPa | |||||||||
| Deep GM/WM | 2.2694 ± 0.02 kPa | |||||||||
| Cerebellum | 1.7972 ± 0.20 kPa | |||||||||
| Sensory motor | 2.1353 ± 0.02 kPa | |||||||||
| Frontotemporal | 2.4049 ± 0.02 kPa | |||||||||
| composite region | ||||||||||
| Rat | Neonatal | Young adult | Aged | Atomic force microscopy (AFM) | 59 | |||||
| ∼240 Pa | ∼390 Pa | ∼480 Pa | ||||||||
| None | Bovine | White matter | Gray matter | Nanoindentation | 152 | |||||
| 1.33 ± 0.63 kPa | 0.68 ± 0.20 kPa | |||||||||
| Adult Guinea pigs | Complex Young's Modulus of retinal cells | Scanning Force Miscroscope (SFM) | 22 | |||||||
| Cell type | Force | Stiffness | ||||||||
| Neurons soma | 30 Hz | 480 Pa | ||||||||
| (Hippocampus) | 200 Hz | 970 Pa | ||||||||
| Astrocyte soma | 30 Hz | 300 Pa | ||||||||
| (Hippocampus) | 200 Hz | 520 Pa | ||||||||
| Neurons soma | 30 Hz | 650 Pa | ||||||||
| (Retina) | 200 Hz | 1590 Pa | ||||||||
| Muller cells soma | 30 Hz | 260 Pa | ||||||||
| (Retina) | 200 Hz | 600 Pa | ||||||||
| Muller cells inner | 30 Hz | 130 Pa | ||||||||
| processes | 200 Hz | 160 Pa | ||||||||
| Muller cells outer | 30 Hz | 100 Pa | ||||||||
| processes | 200 Hz | 210 Pa | ||||||||
| Muller cells endfeet | 30 Hz | 220 Pa | ||||||||
| Muller cells endfeet | 200 Hz | 370 Pa | ||||||||
| Mouse | Converted shear modulus | Ferrule-top dynamic indentation | 151 | |||||||
| 0.5–0.8 ± 0.1 kPa | ||||||||||
| Cow | White matter | Gray matter | AFM | 20 | ||||||
| 1.895 ± 0.592 kPa | 1.389 ± 0.289 kPa | |||||||||
| Rat | All regions of the brain 150 – 300 Pa | Indentation with AFM 25-μm sphere at 1 Hz and 5% strain | 26 | |||||||
| Degenerative CNS diseases | Alexander's disease | Mouse | Wild-type | Alexander | Strain- controlled rotational rheometer Hippocampus 750 μm-thick brain sections | 11 | ||||
| 446.8 ± 20.95 Pa | 571.7 ± 34.74 Pa | |||||||||
| Alzheimer | Human | Unit = kPa, (mean ± SD) | MRE | 14 | ||||||
| ROI | CN | AD | ||||||||
| Global | 2.51 ± 0.09 | 2.40 ± 0.09 | ||||||||
| Frontal | 2.65 ± 0.15 | 2.47 ± 0.12 | ||||||||
| Occipital | 2.65 ± 0.13 | 2.68 ± 0.24 | ||||||||
| Parietal | 2.42 ± 0.10 | 2.33 ± 0.10 | ||||||||
| Temporal | 2.69 ± 0.11 | 2.58 ± 0.09 | ||||||||
| Deep GM/WM | 2.79 ± 0.25 | 2.63 ± 0.27 | ||||||||
| Cerebellum | 2.15 ± 0.11 | 2.11 ± 0.17 | ||||||||
| Sensory/Motor | 2.82 ± 0.29 | 2.62 ± 0.11 | ||||||||
| FPT | 2.63 ± 0.10 | 2.48 ± 0.09 | ||||||||
| Normal adults | Ageing | AD | Litterature-based MRE | 15 | ||||||
| 9.21 kPa | 7.11 kPa | 6.60 kPa | ||||||||
| Mouse | Wild type651 ± 138 Pa | AD 402 ± 97 Pa | AFM Hypoxia induced in mice | 36 | ||||||
| Wild type | AD | MRE | 37 | |||||||
| 25.0 ± 6.4 Pa | 19.3 ± 3.3 Pa | |||||||||
| Multiple sclerosis | Mouse | Unit = kPa, (mean ± SD) | AFM Fresh forebrain thick coronal sections Cryo-section for demyelinated tissue | 33 | ||||||
| Fresh forebrain 1.87 ± 0.87 kPa | ||||||||||
| Wild-type | Demyelination | |||||||||
| Remyelination | ||||||||||
| Corpus callosum (lysolecithin) | 12.01 ± 6.16 | 4.34 ± 2.55 | 7.15 ± 0.18 | |||||||
| Corpus callosum (cuprizone) | 12.07 ± 3.12 | 8.28 ± 3.49 | 13.3 ± 4.90 | |||||||
| Stiff lesions (MS) | NA | 3.81 ± 6.73 | NA | |||||||
| Soft lesions (MS) | NA | 1.14 ± 1.48 | NA | |||||||
| Mouse | Unit = Pa (mean ± SD) | AFM 20 μm bead; k = 13 – 37 mN/m | 30 | |||||||
| Young | Old | Hypomyelination | Demyelination | |||||||
| Cerebellum GM | 260.6 ± 36.1 | 273.1 ± 26.9 | 180.5 ± 49.8 | … | ||||||
| Cerebellum WM | 196.7 ± 22.0 | 221.9 ± 36.3 | 239.5 ± 34.1 | … | ||||||
| Cortex | 253.6 ± 81.9 | 307.6 ± 54.8 | 206.1 ± 34.7 | 271.3 ± 17.4 | ||||||
| Corpus Callosum | 216.5 ± 112.5 | 327.7 ± 86.3 | 229.5 ± 21.6 | 139.1 ± 16.5 | ||||||
| Striatum GM | 286.6 ± 39.0 | 312.2 ± 39.5 | 286.7 ± 82.7 | … | ||||||
| Striatum WM | 315.6 ± 58.8 | 504.1 ± 109.0 | 352.2 ± 75.8 | … | ||||||
| Substantia nigra | 222.9 ± 51.3 | 278.8 ± 64.4 | 172.7 ± 55.2 | |||||||
| pars compacta | ||||||||||
| Cingulum | … | … | … | 312.5 ± 68.5 | ||||||
| Human | Fold change | AFM Relative MS stiffness versus healthy | 33 | |||||||
| Stiff lesions (MS) | 3.81 ± 6.73 | |||||||||
| Soft lesions (MS) | 1.14 ± 1.48 | |||||||||
| Human | ND | MRE | 34 | |||||||
| Parkinson | Mouse | Control | MPTP | MRE MPTP-induced disease | 25 | |||||
| Hippocampal region | 4.608 ± 0.719 kPa | 6.958 ± 1.085 kPa | ||||||||
| Entire brain | 5.234 ± 0.564 kPa | 6.971 ± 1.019 kPa | ||||||||
| Trauma | CNS injury | Rat | Uninjured | Injured | AFM indentation 1,730 measurements of two brains | 17 | ||||
| Cortical tissue (mean) | 50–500 Pa | ∼60 Pa | ||||||||
| Cortical tissue (median) | 285 Pa | |||||||||
| Medial agranular cortex | 219 ± 65 Pa | |||||||||
| Lateral agranular cortex | 295 ± 72 Pa | |||||||||
| Anterior cingulate cortex | 318 ± 75 Pa | |||||||||
| Gray matter | 420 Pa | |||||||||
| White matter | 177 Pa | |||||||||
| Cancer | Gioblastoma | Human | ECM stiffness | Gliotic tissue 10–180 Pa | Lower grade Glioma 50–1400 Pa | GlioblastomaBM 70–13 500 Pa | AFM ECM stiffness fresh-frozen human brain biopsies | 41 | ||
| Drosophilia | Control | Induced Glioma | AFM | 19 | ||||||
| Apparent YM | ∼300 Pa | 500–1500 Pa | ||||||||
A. Regional CNS stiffness variation
The CNS comprises of a heterogeneous distribution of neural cells and their respective ECM, and the organization of cellular and ECM components vary across different regions.20–22 This unique tissue structure confers heterogeneous cell mechanobiology and mechanical properties. The mechanical behavior of the brain and spinal cord tissues is, therefore, an essential element in understanding biological responses in the case of trauma and pathologies. Studies have revealed variation in stiffness properties emanating cellular compliance.23 For instance, one of the functions of glial cells is to embed the neurons, which possess higher mechanical compliance.24 Glial cells provide neurons with physical protection against mechanical aggression and trauma.22 Hence, stiffness differences have been recorded between the white and gray matter. Specifically, the white matter contains bundled myelinated axons and is often presented with stiffer properties than the gray matter, where neuron somas are found. Therefore, the cell-type dependent intrinsic stiffness variation is thought to be involved in such a phenomenon. Rheological studies have described the CNS tissue characteristics similar to those of a non-linear viscoelastic material.24 Indeed, neural cytoskeleton and the ECM networks stiffen when they are increasingly deformed.24 Also, modifying the probed axis of the brain yields different outcomes. To explain this variation, it was suggested that the brain mechanical properties vary depending on the orientation and methodology chosen, which results from the characteristic anisotropic structure of the brain. Moreover, local stiffness is likely to change after a traumatic injury or brain disease. In particular, brain stiffness decreases in neurodegenerative disorders that are thought to be related to an impairment in neurogenesis.25
B. Aging
Over the course of time, many changes occur in the brain microenvironment, including loss of neuronal-glial cell connectivity and cell depletion accompanied by progressive alteration of the ECM. Thus, brain stiffness undergoes a continuous and linear decrease over aging, leading to brain atrophy.26 The inner brain is physiologically stiffer than cortical brain tissue, but the age-related brain atrophy is heterogeneous and results in regional brain softening due to the early shrinking of gray matter starting from the adolescence.27 The annual decline for the cerebrum stiffness was evaluated at −8 Pa per year for patients <60 years old28 and −11 Pa for patients >60 years old.29 The overall brain stiffness decline is estimated to be between −4.9 Pa and −13.6 Pa per year.28
Mechanical stiffnesses of the diverse brain region can be found in Table I. However, precautions are required when choosing the model which must be in line with the method used for an optimal definition of the corresponding model. Arani et al. utilized a mathematical model to predict the theoretical cerebrum stiffness of 2.56 ± 0.08 kPa for the age of 76 years old based on a 60–80 aged cohort,29 while Takamura et al. recently refined the model with an estimated cerebrum stiffness at 2.35 kPa at the age of 41 with a younger cohort comprising of 20–60-year-old patients.28 The difference in methodology could explain the softer measurement from the latter study.
C. Demyelinating diseases—Multiple sclerosis
Demyelinating diseases result in a lack of myelin and are often associated with ECM modification.13,18,30 A particular disease associated with a local decrease in brain tissue stiffness is multiple sclerosis. The development of this inflammatory disease is characterized by the progressive destruction of myelin, leading to the loss of axonal myelination and the basement membrane. Additionally, an increase in fibrillar collagens, which results in perivascular fibrosis, is observed.17 In this case, the control of ECM expression by glial cells is disturbed. Subsequently, this leads to an increased proteoglycan production and hyaluronic acid (HA) secretion, which will accumulate in the vicinity of the demyelinated axon and impair remyelination.31,32 However, these changes in the glial microenvironment do not always follow the same process. Indeed, the tissue mechanical properties in demyelinating diseases depend on the severity and chronicity of the pathology. A recent study highlighted that acute demyelination could be reversed when followed by remyelination, resulting in reduced tissue stiffness.33,34 On the contrary, chronic demyelination, as in the case of multiple sclerosis, led to an increase in tissue rigidity.33 The mouse corpus callosum stiffness after induced demyelination was measured at 4 and 8 kPa for lysolecithin and cuprizone treatments as acute models and 16 kPa for cuprizone chronic model, respectively, while 12 kPa was recorded in the control group33 (Table I). Initially, the stiffness differences could be explained by the infiltration of macrophages and microglia activation in the acute lesion. Thereafter, astrogliosis was observed in the chronic lesion with glial cells expressing increased levels of cytoskeleton filaments [glial fibrillary acidic protein (GFAP) and vimentin] as well as ECM components (fibronectin, fibrillar collagen, biglycan, and decorin)35 in greater amount presenting the hallmark of an active lesion.
D. Alzheimer's disease
In dementia, and particularly in Alzheimer's disease (AD), the changes in ECM composition are associated with a loss of matrix molecules' content that are essential in sustaining progenitors and stem cell niches in the brain. The brain mechanical stiffness was found to be reduced mostly in the regions that are affected by the pathology, including frontal, parietal, and temporal lobes14,36 (Table I). In addition, this phenomenon intensifies along with the severity of the pathology. Also, Alzheimer's disease is characterized by the formation of amyloid plaques along with intracellular neurofibrillary tangles which results in the loss of neuronal network connectivity and functionality, followed by brain atrophy.15 The early amyloid fibrils deposition is prone to favor an increase in brain stiffness, while the progressive synaptic loss and neurodegeneration result in an overall stiffness loss of 22.5%.37
E. Spinal cord injury and CNS trauma
Glial cells are reported to be more compliant than neurons.24,38 However, the activation and accumulation of astrocytes and microglia in glial scar significantly increase the tissue stiffness after spinal cord injury.22 In acute brain injury, tenascin upregulation in sites around brain lesions stiffen the ECM,39 leading to long-term deleterious effects. Besides that, the remodeling of neurogenic niche after an injury can participate in long term issues in brain functionality, leading to chronic diseases.40 However, in a recent study, tissue softening was recorded after traumatic injury of neural tissue, which correlates with an increased expression of matrix molecules (Laminin, collagen IV) and cytoskeleton component (GFAP and vimentin) (Table I).17
F. Gliosis and glioma
The modulation of the ECM expression during gliosis and glioma progression leads to an increase in brain stiffness (Table I).3,41 Additionally, brain stiffness was found to increase by 10 fold, reaching E = 1000 Pa in gliobastoma.3 As previously stated, ECM is not the only cause of brain stiffness, but glial cells also play an important role in determining tissue rigidity because of their ability to modulate their intrinsic mechanical characteristics in response to chemical or mechanical signals. For instance, ischemia-induced gliosis resulted in the upregulation of GFAP expression in Müller glial cells, which in turn led to a global cell stiffening.42
Considerable changes in ECM composition, cell expression, and properties occur after brain injury and in neurodegenerative diseases. Since pathological progression is associated with changes in matrix stiffness, whether it is a decrease or an increase, then, maintaining mechanical homeostasis is one of the important aspects to target and solve in order to treat a pathological state of the brain.
G. Importance of matrix compliance throughout neurogenesis for in vitro models
Both ECM and intrinsic cell rigidity contribute to tissue stiffness and vary throughout neural development. During neurogenesis, neural stem cells sense the changes in their microenvironment driving the differentiation in neural lineage and following a sequence of changes. The differentiation in specific cell types is driven by preferential biomechanical cues. For instance, neuronal differentiation occurs first in brain development43 and neurites growth preferentially under very soft matrix stiffness conditions in vitro (elastic modulus E = 200 Pa), while astrocytes spread on the stiffer environment (E = 9000 Pa).44,45 Likewise, oligodendrocyte (OL) differentiation and maturation are triggered when the stiffness of the microenvironment is around E = 6500 Pa.46 Therefore, the cellular preference in the microenvironment rigidity demonstrates that an interplay occurs between intracellular contractile forces and extracellular attachment in neural cell lineage.38
III. ENGINEERED METHODS TO UNDERSTAND GLIAL RESPONSE TO MECHANOTRANSDUCTION
Understanding the mechanisms of mechanotransduction (conversion of mechanical signals into a cascade of biochemical phenomena)4 and their contribution to development, physiology, and cerebrospinal diseases represent a major challenge in glial mechanobiology. To unravel the cellular mechanisms involved in glial mechanotransduction, engineering cell culture systems are required to better understand the molecular interplays which are specific to mechanical stimuli encountered or leading to a pathological context. Notable advances have recently been published in the field of in vitro engineering systems. The platforms are expected to reproduce the CNS matrix with faithful physiological conformity, thereby allowing the study of a defined signaling pathway that may be involved in the biological phenomenon that is driven by a specific mechanical stimuli. Additionally, these platforms and culture systems were designed to evaluate the biomechanical characteristics that cells could detect in order to determine the signaling pathways and downstream elements involved in various cellular behaviors, such as cell activation, proliferation, spreading, differentiation, polarization, or myelination.
The current techniques and strategies to study mechanosensing in glial cells are discussed below.
A. Small scale techniques
Force-application techniques (AFM, optical tweezers) are standard methods to study mechanobiology and mechanical probing of mechanotransduction at the single cell level.24,47 AFM provide the lowest force (5–10 pN), while optical tweezers (0.1–100 pN), pipette aspiration (10 pM–1 nN), and magnetic tweezers (0.1–1 nN) can deliver higher force magnitude.48 These techniques remain, unfortunately, cell-selective, low throughput, and invasive methods, mimicking acute stress for cells by their possible insult to the plasma membrane and their cortical shell integrity.
B. 2D gels
In order to modulate the external forces that cells can sense, it is necessary to adjust their microenvironment. Several approaches can be considered to generate mechanical tension such as biophysical modulation by tunable mechanical stiffness, appropriate topography, extracellular matrix coating, and culture in dynamic conditions or in three-dimensions.49,50 Research in natural or synthetic materials has highlighted a wide range of possibilities to obtain the desired stiffness, viscosity, or topography to study mechanotransduction (Table II). Inert and artificial substrates have been developed to offer the opportunity to study cell behavior under precisely defined mechanical conditions on a two-dimensional (2D) hydrogel.
TABLE II.
Advantages and drawbacks of hydrogels with tunable mechanical stiffness.
| Substrate | Material | Surface coating | Stiffness and specific features | Cells tested | Biological outcomes | Limits | Reference |
|---|---|---|---|---|---|---|---|
| Synthetic hydrogel | Polyacrilamide (PAA) | PDL | Stiffness 0.1–70 kPa | Rat oligodendrocyte progenitors (OPC) | Compliance: OPCs (30–150 Pa) stiffen during differentiation (OL = 40–210 Pa) independently of substratum stiffness (0.1–0.4 kPa) OPC adhesion is independent of substratum stiffness but optimal at 1 kPa OPC survival and proliferation are optimal at 0.7–1 kPa OPC migration is optimal at 0.7 kPa OPC differentiation is enhanced on stiffer substrates (1–70 kPa) |
Elucidation of mechanotransduction mechanisms beyond the scope of the study Not suitable for complexes and specific morphological changes such as myelin sheath wrapping |
55 |
| PLL Fibronectine | Stiffness 0.5–7 kPa | Mouse Oli-neu | RGD-peptide treatment increases fluid-phase endocytosis Y27632 or blebbistatin increases cell surface area Blebbistatin abolishes the RGD-peptide effect on cell area Endocytosis increases with soft matrix Rho/ROCK and myosin II inhibition by C3 transferase, Y27632 or by blebbistatin restored cell surface expansion on soft matrices Inhibition of actomyosin contractility promotes spreading of myelin-membrane sheets on a non-permissive substrate |
Not suitable for complexes and specific morphological changes such as myelin sheath wrapping | 46 | ||
| PDL Fibronectin Laminin | Stiffness 0.362 ± 0.065 to 9.720 ± 1.352 kPa for PAA Larger than 100 μm | Rat glial precursor (CG-4) Rat neuroblastoma (B-104) Rat primary OPCs |
Low substrate stiffness and merosin enhances oligodendroglial differentiation and morphological complexity Blebbistatin promotes OL differentiation on compliant substrates in presence of merosin |
Only study OL differentiation in 2D environment Not suitable for complexes and specific morphological changes such as myelin sheath wrapping |
7 | ||
| PDL | Stiffness 0.01–230 kPa Nonfouling and anti-adhesive; Isotropic; Elastic; Biologically inert; Homogeneity in surface topography, mechanical properties, and coating density; thin and translucent | Astrocytes | Sharp transition from the compliant to the rigid astrocyte phenotype from 1 kPa | Not suitable for complexes and topography-directed morphological study | 53 | ||
| Laminin | Stiffness 0.1–75 kPa 70 μm nominal thickness | Rat NSCs | ECM Stiffness biases NSC differentiation Compliant substrates yields 60% neurons, 10% astrocytes, and 5% oligodendrocytes, while stiff substrate yields 30% neurons, 20% astrocytes, and 0% oligodendrocytes On stiff ECMs, mechanotransduction inhibitors restored neuronal differentiation for all NSC populations to levels found on compliant ECMs |
Not suitable for complexes and topography-directed morphological study | 63 | ||
| Fibronectin | Stiffness 0.08–119 kPa | Human glioma (U373-MG, U87-MG, and U251- MG) | Mechanical rigidity regulates the motility of glioma cells through actomyosin network | Not suitable for complexes and topography-directed morphological study | 57 | ||
| PDL Matrigel | 100 μm thick | Mouse primary spinal cord neuron | Substrate flexibility also had a significant eject on neurite branching and neural-glial differentiation | Not suitable for complexes and topography-directed morphological study | 56 | ||
| Modified PAA | Laminin | Independent control on ECM peptide tethering and substrate stiffness | Rat Cortical OPC | PIEZO1 as a key mediator of OPC mechanical signaling | Not suitable for complexes and topography-directed morphological study | 59 | |
| PDL | Stiffness 0.17–3.2 kPa | Hippocampal neurons | The suppression of F-actin cytoskeleton formation improved neuritogenesis | Not suitable for complexes and topography-directed morphological study | 54 | ||
| Synthetic hydrogel | Polydimethylsiloxane (PDMS) | PDL | Stiffness 1.7–1700 kPa | Brain cells (neuron, glia) | Glial cells cultured on a soft substrate obviously showed a less dense and more porous actin and GFAP mesh The viscoelasticity of both neurites and glia did not show a significant dependence on the substrates' stiffness |
Not suitable for complexes and topography-directed morphological study | 66 |
| PLL | Stiffness 0.2–8 kPa | Rat primary astrocytes | Astrocytes grown on soft substrates displayed a consistently more quiescent phenotype while those on stiff substrates displayed an astrogliosis-like morphology | Not suitable for complexes and topography-directed morphological study | 68 | ||
| PLL Fibronectin | Stiffness 17–173 kPa | Rat hippocampal neurons | Soft substrates provide a more optimal stiffness for hippocampal neurons | Not suitable for complexes and topography-directed morphological study | 71 | ||
| Fibronectin | Stiffness 12–750 kPa | Rat NSCs | Differentiation and maturation | Not suitable for complexes and topography-directed morphological study | 70 | ||
| Fibronectin Laminin | Stiffness 0.4–3.7 kPa and 750 kPa | Human NSPC (hNSPC) Rat adult hippocampal NSC (rahNSCs) |
Blebbistatin abolishes spontaneous Ca2+ transients Substrate stiffness triggers YAP nuclear localization while Piezo1 knockdown can override the mechanical cue for localizing Yap to the nucleus Substrate stiffness reversely modulates neural differentiation (MAP-2) according to cell origin |
Delicate system and does not comply to study 3D culture | 6 | ||
| PAA PDMS | Sulfo-SANPAH PLL Laminin 211 | 0.5 kPa to 40 kPa (PAA) 4 Mpa (PDMS) | Primary rat Schwann cells (SC) | YAP/Taz remains nuclear in low cell density and relocates in the cytoplasm under blebbistatin treatment YAP/Taz is nuclear on very stiff substrate but cytopasmic on more compliant ones in presence of laminin 211. YAP/Taz nuclear localization is promoted by mechanical stretching |
Not suitable for complexes and topography-directed morphological study Short term stimulation and culture |
9 | |
| PAA Fibrin | Laminin | Stiffness 0.2–9 kPa for PAA 0.25–2.1 kPa for fibrin | Rat primary neuronal and glial cells | Soft gels promotes neurites extension for neurons while astrocytes spreading is impaired with disorganized actin network | soluble factors in co-culture may hinder the mechanical stiffness effect strict observation | 44 | |
| Poly-ethylene-glycol (PEG) | Fibronectin Collagen I Collagen IV | Stiffness NC | Rat neural brain cells | Cell survival, proliferation and differentiation | Not suitable for complexes and topography-directed morphological study | 74 | |
| Poly-ornithine | Stiffness 3.4 kPa Small pores (50–150 Å) | Rat neural brain cells | Cell survival, proliferation and differentiation | Not suitable for complexes and topography-directed morphological study | 73 | ||
| Biological hydrogel | 3D Collagen I - Hyaluronic acid | None | Stiffness 1–10 kPa | MSC | Neuronal and Glial differentiation | 3D-matrix related issues (reproducibility, pore size control, difficult cell visualization and downstream analysis). | 79 |
| 3D Collagen I | None | Stiffness NC | Mouse cortical astrocyte | Cell morphology, proliferation, differentiation | 107 | ||
| 3D Collagen I | None | Stiffness NC | Rat primary cortical astrocyte | Proliferation and differentiation | 80 | ||
| 3D Collagen I | None | Stiffness NC | Human fetal cortical astrocytes | Proliferation and differentiation | 81 | ||
| 3D Alginate | Laminin | Stiffness 0.18–20 kPa | Rat NSCs | Cell proliferation, differentiation | 85 | ||
| 3D Alginate | Peptide | None | Neurons | Neurite outgrowth | 107 | ||
| Agarose | Chondroitin sulfate | 42.7–2006.8 dyne/cm2 | Dorsal root ganglia | Neurite extension from DRG is influenced by the combination of mechanical barrier and ECM coating | 87 | ||
| Fibrin | None | NC | Mouse spinal and cortical Neurons | ECM effect on neurite extension under compliant substrate condition | 83 | ||
| 3D Alginate | None | Stiffness 0.5–2.5 kPa | Rat astrocyte | Cell viability | 86 | ||
| HA | Collagen IV | Stiffness 0.15–0.3 kPa | Gliobastoma | ECM effect on mechanical stiffness-induced glioblastoma proliferation | 79 | ||
| Synthetic and Biological Hydrogel | PAA Hyaluronan (HA)-PEGDA (Glycosyl) | Collagen I Laminin | Stiffness 0.31 ± 0.03 to 14.08 ± 1.28 kPa for PAA 0.30 ± 0.03 kPa for HA | Human Glioma (LN229 and LN18) | Glial cells can bind HA through CD44 interaction Glioblastoma cells starts to spread from 1 kPa on PAA gels but spread on lower stiffnes on HA gels in a cell line-specific fashion Laminin reduces cell spreading on HA gels |
Incorporation of collagen or integrin ligand into HA crosslinked matrices can change the local stiffness by the self-assembly of fibrous structure or present epitopes otherwise seen only on stiff substrates | 58 |
| vmIPN gel PAA-PEG | RGD RGE Laminin 1 |
Stiffness 0.01–10 kPa 70 μm thick | Rat Adult Neural Stem Cells (NSC) |
Differentiation in neuron versus glial cells Neuron 500 Pa | Not suitable for complexes and topography-directed morphological study | 60 | |
| Modified biological hydrogel | Gelatin-hydroxyphenylpropionic acid | None | Stiffness 0.629–8172 kPa | Human MSC | The cells on a softer hydrogel (600 Pa) expressed more neurogenic protein markers, while cells on a stiffer hydrogel (12000 Pa) showed a higher up-regulation of myogenic protein markers | Not suitable for complexes and topography-directed morphological study | 82 |
| Methacrylamide chitosan | Laminin | Stiffness 1–30 kPa Porous network slightly varying across stiffness | Rat NSPC |
Importance of substrate stiffness in neural-glial lineage Optimal cell proliferation on 3.5 kPa surfaces Neuronal differentiation was favored on the softest surfaces <1 kPa, while OL differentiation was favored on stiffer scaffolds (>7 kPa) and astrocyte differentiation was only observed on <1 and 3.5 kPa |
Not suitable for complexes and topography-directed morphological study | 84 |
1. Polyacrylamide gels
Cyto-compatible materials such as polyacrylamide (PAA) hydrogel can be fabricated with a range of elasticities (shear modulus G′ = 0.01–230 kPa) simply by varying the amount of the polymer (acrylamide) and its crosslinker (bis-acrylamide). Increasing cross-linker concentration proportionally increases the PAA gel elastic modulus until reaching an inflection point which is changed according to the PAA initial concentration.51,52 These modifications are not inducing an additional biological stimulus since this is a biologically inert material that does not intrinsically support cell adhesion. Thus, stiffness can be tuned without biological concern.53 Polyacrylamide gels exhibit a strong homogeneity in surface topography, mechanical properties, and coating density—features which are crucial for reproducibility. The high biocompatibility and the magnitude of stiffness range available makes PAA hydrogels an ideal starting matrix to reproduce CNS physiological range of mechanical stiffness. Ideally, the gel surface can be functionalized by the addition of adhesive polymers (polylysine),53–55 matrigel,56 region-specific ECM proteins (collagen I, collagen IV, laminin and fibronectin),7,9,57–59 or ECM peptides (RGD, IKVAV, LRE)60,61 to the mixture when studying particular tissue environment. ECM protein or peptide density can be tailored to define the surface chemistry of the material precisely.62
These models are simple to develop and can easily demonstrate cellular signaling by selectively evaluating one single effect. For instance, neuronal differentiation can be promoted by soft platforms (0.1–0.5 kPa), while stiffer ones (1–10 kPa) favored the appearance of glial cells of astrocytic phenotype.60,63 In the same fashion, the survival and proliferation of oligodendrocyte progenitor cells (OPC) were modulated by substrate stiffness (0.1–70 kPa).46,55 PAA matrices reproducing CNS mechanical properties in native and traumatic contexts (1.5 and 30 kPa, respectively) were used to highlight that stiff matrix impaired the myosin activity by inhibiting OL branching and differentiation in contrast to Schwann cells which were not affected by the change in rigidity.64
One must consider that one of the major disadvantages of PAA gel is the change in porosity accompanied by the change in mechanical properties, leading to modified biological responses with regard to cell fate.65 Furthermore, challenges and troubleshooting for PAA gels include the findings of the correct set of parameters (e.g., UV intensity, light wavelength, exposure time, distance from UV lamp, initiator concentration, gel thickness, and acrylamide and bis-acrylamide concentration), the uneven gel attachment which is a particular issue encountered during gel-gradient fabrication, and heterogenous gel thickness. Advices in methodology prior to gel manufacturing can be found in the literature.51,52
2. Polydimethylsiloxane (PDMS) gels
Other studies used polydimethylsiloxane (PDMS) to explore mechanotransduction in neural and particularly glial cells (elastic modulus = 1 kPa–4 MPa).9,66,67 PDMS rigidity was tuned to study the effect of mechanical stiffness on glial proliferation, differentiation, and maturation.9,66,68–70 The good elastic property enables numerous applications for PDMS elastomers in micro-engineering (micropillars) or as a stretchable material to test the effects mechanical forces on cells. Details on the recent research will be specified in Sec. III E 1. However, the inert and nonfouling characteristics of these synthetic materials require additional modifications to ensure proper cell attachment and integrin signaling, including the adsorption of charge enhancers (polylysine, poly-ornithine)66,68 or the covalent binding of adhesive ECM proteins (laminin, fibronectin).6,71 The nature of the generated biological interactions and especially the coating density are critical parameters that must be tightly controlled while attempting to reproduce cell attachment model as these models may differ from the biological reality. Similarly to PAA gels, functionalization of PDMS gel with ECM protein or ECM-derived peptides can be carried out with a protein crosslinker agent. The latter are interesting for tissue engineering due to their ease of manipulation, incorporation into biomaterials and minimal impact on the mechanical characteristics of the gel.72 The optimal choice of the substrate-protein combination will depend on the biological relevance of the reproduced tissue microenvironment. When the complexity of the natural habitat of the cells is difficult to reproduce in vitro or when it is desired to decouple signaling integrins, a simplified coating model based only on the modification of the surface charge with a polylysine coating can be used to improve cell adhesion and cell spreading.7,53
As a result, hydrogels are often used as compliant growth substrates and can be fine-tuned to optimize their homogeneity in surface topography, mechanical properties, and coating density. For a study looking for compliant materials, it is advisable to start with PAA, while the search for a larger structural stiffness will prefer to work on a PDMS substrate.
3. Other synthetic and biohybrid gels
Other synthetic gels were less frequently used to assess glial mechanotransduction by tuning their mechanical stiffness. Polyethyleneglycol (PEG) based gel of relatively low stiffness (3.4 kPa) was utilized to study neural cell biology and behavior.73,74 Mixing natural-based materials with synthetic compounds to enhance biophysical properties and biocompatibility of the hydrogel is a developing strategy. Bioorthogonal polymer cross-linking, such as tetrazine-norbornene ligation, can be performed to obtain in situ hydrogels suitable for three-dimensional (3D) cell culture.75 Herein, the catalytic oxidation of the dihydrogen tetrazine using horseradish peroxidase enhanced the gelation time and grant gel stiffness modulation. By applying this method, PEG was added to gelatin to form tunable stiffness composite hydrogels (storage modulus = 1.2–3.8 kPa).76 The viability of encapsulated cells was enhanced and could be applied to glial and neuronal cell culture as an improved method of studying mechanosensitivity in a 3D model.
4. Hydrogels from natural materials
Biomaterials of natural origins have been used to culture glial cells, mainly for differentiation assays, and sometimes to assess mechanical stiffness. Among them, hyaluronic acid (HA),58,77–79 collagen I,80,81 gelatin,82 matrigel,83 fibrin,44 modified chitosan,84 alginate,85,86 and agarose87 have been used on astrocytes and neural progenitor cells (NPCs). These materials are mostly isolated from native surrounding ECM and basal lamina and contains adhesive sites for cells and, therefore, do not require additional functionalization nor surface modification to allow cell attachment. For instance, HA, which reproduces the native microenvironment that surrounds glial cells, incorporates CD-44 binding site that facilitates neural cell adhesion, while ECM polymeric proteins such as collagen, laminin, and fibronectin provides integrin binding sites. Other plant-based and non mammalian polymers would still require surface functionalization. Although substrate stiffness was not assessed for some of those hydrogels, these studies deserve to be mentioned in this section as tremendous effort in developing ECM-resembling microenvironment has been the made in the last few years. Notwithstanding, due to their natural origin, such materials suffer high variability in structure and composition with significant batch-to-batch changes in biomolecule composition and proportion. This heterogeneity added with higher structural complexity hinder proper experimental design to decouple biochemical from mechanical stimulus. For those reasons, synthetic-based hydrogels have been preferred for their bioinert properties, their well controlled content and their ease to modulate substrate stiffness. Finally, self-assembled nanopeptides have been demonstrated suitable for glial cell culture.88 Apparent physiological mechanical stiffness can be tuned though enzymatic addition or pH changes and hydrogel constructs have been designed to be incorporated with ECM components that are native to nerve tissues (e.g., heparan sulfate proteoglycan and laminin peptide IKVAV).88–90
5. Recently engineered gels used to study mechanotransduction for different cell types
Recent developments in biomaterial engineering techniques have significantly improved the manufacturing capabilities of systems for analyzing cellular mechanotransduction. Hydrogels have become intensely complex and have obtained interesting new properties. Stiffness gradient hydrogel is the direct evolution from the substrate stiffness assay using various hydrogels with low to high mechanical stiffness. Gradient hydrogel can be generated by differential diffusion distance of unreacted crosslinker and monomer into pre-polymerized gel. With such system, one can attest the effect of local stiffness variation on cell mechanosensivity in a controlled manner and at a small scale.91
The incorporation of magnetic nano or microparticle in hydrogel permit the fabrication of a magnetic sensitive biomaterial.92 Magnetic hydrogels are now envisioned as a therapeutic biomaterial for spinal cord regeneration.93 The magnetic field allow the control of the alignment of polymer fibers to generate a topography resembling the anisotropic architecture of spinal cord microenvironment.94,95 Correspondingly, dorsal root ganglion neurons that were cultured in native stiffness-mimicking magnetic hydrogels demonstrated activation of mechanosensitive ion channels, TRPV4 and Piezo2.96 This model could be transversally applied to glial cells study.
Others have reported the assessment a novel hydrogel with rapid beating properties. Small mechanical forces are exerted through near infrared light pulse under spatiotemporal control. The authors have designed a thin, soft, and patterned synthetic gel that comprised of acrylamide variants and gold nanorods (AuNRs) for photothermal responsiveness. This approach has opened new insights into mechanotransduction studies by providing forces at low magnitudes in a natural-mimicking cyclic stimulus, rather than constant strain that is associated with classic hydrogels. For instance, this technique could be very useful in looking at quick molecular events, such as cell signaling pathway activation, nuclear translocation of mechanosensors or cell membrane dynamics.97
The design of hydrogels has considerably complexified to give rise to new systems with fine and intricate properties, such as photoresponsive hydrogels,98 thermoresponsive hydrogels,92,99 stiffening hydrogels,100 Matrix metalloproteinase (MMP)-degradable hydrogel platforms101 or conductive hydrogels.102 Among these new hydrogels, many may have properties which could satisfy new demands in the study of mechanotransduction pathways.48,92,103–105
C. 3D gels
Tissue-engineered models in 3D are developing for CNS application.80,81,106,107 Reproducible 3D culture system based on alginate gel have been developed to monitor neurite outgrowth108 and to mimic astrogliosis.86 While tuning the material amount, mesh size evolves inversely proportional to alginate content to form the hydrogel. Alginate hydrogel has typical mechanical properties with a solid-like character, represented by storage modulus (G′), predominant over liquid-like viscous feature or loss modulus (G″). Also, PEG was used to construct a three-dimensional hydrogel and demonstrated the importance of mesh nets size along with storage modulus to modulate OPC proliferation and lineage commitment.109 The focus is now on developing 3D micropatterned biomaterial systems which enable the seamless integration with experimental cell mechanics in a controlled 3D microenvironment.110 For instance, synthetic fibrous collagen-wise material with tunable mechanics and user-defined architecture has been developed and could be applied for glial cell culture.111 Also, a biosynthetic elastin-like matrix was used to study neural progenitor cell (NPC) differentiation exposing cells to native brain tissue stiffness (elastic moduli ≈ 0.5–1.5 kPa).112
D. Microbeads and spatial constraints
Other types of mechanical stress can be generated by the addition of micro-objects restricting the interstitial space and exerting spatial constraints on the cells (Table III). Microspheres are used in culture to generate this spatial constraint in high cell density, reproducing spatial restriction encountered in brain diseases such as gliosis or after injury and fluid infiltration leading to tissue compression. Space reduction induced by plating microspheres enhances OPCs' differentiation and generation of myelinated fibers.113 This method is simple and potentially useful to reproduce space constraint. However, it does not mimic the normal physiological conditions and lack the matrix substrate interaction for studying the mechanotransduction involved in other phenomenon.114
TABLE III.
Advantages and drawbacks of systems using spatial constraint and magnetic particles to study glial mechanotransduction.
| Substrate | Material | Surface coating | Device/Mechanical stimulation | Stiffness and specific features | Cells tested | Biological outcomes | Limits | References |
|---|---|---|---|---|---|---|---|---|
| Biological hydrogel | Stretched silicon sheets | Matrigel | Compression–space restriction | Stiffness non communicated (NC) | Mouse oligodendrocyte progenitor cell (OPC) | Stimulation promotes OL differentiation by heterochromatin formation through Syne1 (LINC) mechanotransduction | Comparable to microsphere space constraint | 133 |
| Polystyrene Thick ACLAR 33 C film | Matrigel Collagen I | Custom-built mechanobioreactors with extension chamber | Stiffness NC | Rat astrocyte | Living scaffold emulating developmental conditions | Mimic radial glia | 137 | |
| Result robustness is coating-dependent | ||||||||
| Required astrocyte processes network with sufficient resilience and growth capacity | ||||||||
| More robust stretched processes at 12.5 μm/h | ||||||||
| The applied diplacement rate is different than stretched-injury models | Heterogeneous stretch within cultures with the most robust stretch seen near the corners of the towing membranes | |||||||
| Changes in astrocyte processes thickness underscore the heterogeneous effect of the mechanical tension | ||||||||
| Synthetic Hydrogel and substrate | PAA PDMS | Sulfo-SANPAH PLL Laminin 211 | Cell density | Stiffness 0.5 kPa to 40 kPa (PAA) 4 Mpa (PDMS) | Primary rat Schwann cells (SC) | YAP/Taz remains nuclear in low cell density and relocates in the cytoplasm under blebbistatin treatment | Not suitable for complexes and topography-directed morphological study | 9 |
| Short term stimulation and culture | ||||||||
| Biological hydrogel | Agarose | Chondroitin sulfate Laminin | Mechanical stiffness and interface hindering | Shear modulus 42.7–2006.8 dyne/cm2 | Dorsal root ganglia (DRG) | Neurite extension from DRG is influenced by the combination of mechanical barrier and ECM coating | Not suitable for complexes and topography-directed morphological study | 87 |
| Magnetic MNP | Superparamagnetic iron oxide nanoparticles | PLL | Mechanical tension through generation of magnetic force | Zeta potential of the PLL-SPIONs (∼+15 mV) at pH = 7.0 Saturation magnetization 351.6 kA/m | Schwann cells | Integrin-mediated migration of Schwann cell across astrocyte monolayer is enhanced by the presence of a magnetic field | Cell uptake of foreign body could alterate the signaling and behavior. The induction of magnetic field does not reproduce the nature of the forces encountered in vivo by the cells | 141 |
| Superparamagnetic iron oxide nanoparticles | PLL | Mechanical tension through generation of magnetic force | Zeta potential for naked-MNPs (–20 mV) and for PLL-MNPs (+10 mV) Saturation magnetization MS = 78 Am2/kg | Schwann cells | Integrin-mediated migration of Schwann cell assessed by the presence of magnetic field | 140 |
E. Nanotopography
Topographical cues have been demonstrated to play an important role in determining cell fate. Topographical interaction can be studied by specific patterns and designed culture substrate to mimic defined conditions encountered in CNS tissues (stem cell niche, topography-directed neurogenesis, demyelination, axon and neurite extension, etc.). Different methods exist to design a particular topography (Table IV). Among them, nanotopography can be used to design precise (lithography) or random surface features (nanotube, porous membrane, electrospinning, self-assembled nanofiber) that may be applicable to study glial cell behavior when designing scaffold for neural regeneration.115 These systems may be extended to understand glia mechanotransduction.
TABLE IV.
Advantages and drawbacks of microengineered scaffolds to study glial mechanotransduction.
| Type | Material | Surface coating | Device/Mechanical stimulation | Stiffness and specific features | Cells tested | Biological outcomes | Limits | References |
|---|---|---|---|---|---|---|---|---|
| Nanograting | PDMS | Laminin | Nanotopography | Stiffness NC | Human embryonic stem cell (H1) | High actomyosin contractility induced by a nano-grating topography is crucial for neuronal maturation | Cells adhesion is restricted to the topography and limits cell spreading and migration behavior. | 119 |
| Blebbistatin and ML-7 reduces the expression level of microtubule-associated protein 2 | ||||||||
| PDMS | Poly-L-ornithine Laminin | Micro- and nanotopography | Stiffness NC | Mouse primary neural progenitor | Glial differentiation is enhanced on isotropic 2 μ m holes and 1 μ m pillars in contrast to neuron differentiation which is enhanced on anisotropic gratings and isotropic 1 μm pillars | 117 | ||
| PDMS | Fibronectin | Nanotopography | Stiffness NC | hMSC | FAK phosphorylation was required for topography-induced neural differentiation while FAK overexpression overruled the topographical cues in determining cell lineage bias | 115 | ||
| Micropatterning | Fused silica | PLL | Microtopography | 25 μm height and 50 μm diameter | Rat and mouse OPC | Hightroughput method identified a cluster of antimuscarinic compounds that enhance oligodendrocyte differentiation and remyelination | 118 | |
| Micropatterning and electrospinning | Gelatin PLA | None | Suspended microfiber | Stiffness ≈20 Mpa | Human glioblastoma cells | The low apparent stiffness of the fibers is biomimetic of fibril components of the extracellular matrix, facilitating adequate cell−cell and cell−substrate interactions for the cell aggregates to remodel the fiber network | 120 | |
| Electrospun artificial axons | PLA | PDL, laminin | Topography ECM interaction | ND | Rat cortical OPC | Differentiation Myelin sheath formation | 122 | |
| PCL PLA Gelatin | PDL | Topography substrate stiffness | Intrisic material stiffness Gelatin: 2–4 MPa PCL: 0.5–1 × 103 Mpa PLA: 2–3 × 103 MPa | Rat cortical OPC | Differentiation Myelin sheath formation | High intrisic and mechanical stiffness values | 124 | |
| Mechanical stiffness PCL: 0.014–0.050 N m−1 | ||||||||
| Polystirene | PLL | Topography | Stiffness NC | Rat cortical OPC | Differentiation Myelin sheath formation | 121 | ||
| 3D Printing artificial axons | PDMS pHEMA poly(HDDA-co-starPEG | PDL Laminin Fibronectin | Topography | Stiffness Fibers 0.1–10 000 kPa PDMS ink E = 976 kPa pHEMA ink E = 88–333 kPa poly(HDDA-co-starPEG) ink E = 0.42–140 kPa | Rat cortical OPC | Differentiation Myelin sheath formation | Large fibers diameter | 127 |
1. Nanolithography obtained patterns
PDMS is a suitable starter biomaterial to design well-defined patterns. Customizable multi-architecture chip (MARC) array based on PDMS was used to build distinct topographies of various architectural complexities, including both isotropic and anisotropic features, in nano- to micrometer dimensions, with different aspect ratios and hierarchical structures.116,117 The cost-effective feature of micropillars make this method suitable for high throughput screening assays for glial cell behavior to topographical cues.118 This method could be effectively applied to study glial mechanotransduction in an attempt to reproduce the particular brain or spinal topography. Anisotropically grating patterned substrates are used to study glial cell differentiation.117 In particular, Ankam et al. used this technique to elucidate the underlying mechanisms of topography-induced differentiation of human embryonic stem cells (hESCs) toward neuronal lineages.119 In addition, suspended microfibers were recently fabricated via low-voltage 3D micropatterning.120 Nonetheless, the restriction of cell anchoring sites is also the main disadvantage of this technique, which considerably limits the mechanisms of cell spreading and migration that can generate signaling biases in mechanotransduction pathways.
2. Artificial axons
Henceforth, growing interest in establishing the mechanosensing capacity of myelinating cells by modulating microenvironmental and biomechanical characteristics in vitro is arising. Although the link between mechanotransduction and myelination is not fully determined at the molecular level, the activation of the mechanotransduction pathways is thought to be essential for the quality of myelination.38 A focus on developing synthetic neuronal axons displaying the biochemistry, morphology, and carrying biophysical characteristics of its biological analog has been increasing over the past recent years. Electrospinning can produce nano- to microfibers mimicking the axon biophysical cues including fiber diameter (0.5–2 μm), alignment and density. Therefore, electrospun artificial axons had been proposed as a model for studying myelination since the geometry of the substrate could facilitate cell surface interaction, spreading, and wrapping in the absence of neural factors.121 Under controlled biochemical cues of soluble factors determining their differentiation and maturation, OLs can extend their plasma membrane and generate a simulacrum of myelin sheath around the artificial axons.122 The direct visualization and quantification of myelin formation offered by these biomimetic platforms is thought to be an optimal system for pharmacological agent screening and testing.123,124 In this sense, artificial fibers can be used to assess stiffness changes in the microenvironment by modulating the nature, length and diameter of the fibers.125 Suspended fibers are thought to be an optimized model that overcome the influence of the support (glass generally) to better control the structural stiffness of the fiber mesh.124 The intrinsic and mechanical stiffness of these suspended fibers can be tuned to study mechanotransduction pathways in OL differentiation and myelination.125 Several biomaterials have been tested as a fiber substrate, including polystyrene, polylactic acid (PLA), polycaprolactone (PCL), and gelatin, and showed the possibility of modulating myelination in defined conditions.122,125 The stiffness range of such model is the closest to native condition, as compared to other topography study system (micropillars) or fiber fabrication systems.
Another aspect that compels the high interest in fiber-based myelination platform is the possibility to test out drugs and discover potential target for therapeutic treatment. Inert fibers are able to receive different types of coating to functionalize their surface in order to sustainably deliver non-viral genes (microRNA) and protein drugs.123 Classical myelination assays, which consists ofOL-neuron co-culture, have very low throughput and are time-consuming. Thus, this can be considered as a good developing model for high throughput screening system for drug testing to target myelination process under defined mechanical conditions.125 Furthermore, current platforms for high throughput in vitro assays have been designed to assesses myelination of living axons. This type of assay is optimal for screening large compound libraries to identify new targets and drugs that stimulate myelination.126 Although, this system is advantageous on non-biological substrate which does not incorporate the complex cellular neural-glial interactions, it lacks the mechanical aspect of it. Engineering systems with tunable mechanical properties could be therefore developed for co-culture study.
Although effort have been put to develop 3D culture for glia, electrospun fibers are material-dependent regarding their intrinsic stiffness. Hence, structural and intrinsic stiffnesses remain vastly far from native CNS and axon stiffness respectively.111,125 Nonetheless, there is no available platform to date that better mimics both topography and CNS stiffness to study the behavior of glial cells in response to stiffness changes.
Additionally, recent findings have demonstrated the fabrication of 3D-printed engineered artificial axons.127 The authors described their product as minimally supported aligned fibers in mechanically compliant range (0.1–1000 kPa) and with a relatively small diameter (5–20 μm). The appeal of this platform lies in the fact that those features can be independently modulated to reproduce specific physio-pathological states arousing interest to study myelination process under defined conditions encountered by OLs in vivo. Their work showed that myelin production and wrapping is dependent on fiber diameter, stiffness, and surface ligand interaction. The application of this model to mechanical stretching platform can be considered as a challenge. Improvements are required to develop a universal and reproducible system that could be scaled up. The production of artificial fibers by 3D printing technology could be an alternative but the resolution is not able to produce axon-like diameters under 10 μm at the moment. The ability to manufacture an axon-like material with a 3D printer is a promising technology that is expected to develop further in the future. Notwithstanding, 3D-printing machine definition is emerging and constantly upgrading, granting expectations to improve on the resolution matter soon.127
F. Externally applied forces (motorized platform)
Advanced platforms can be compatible with dynamic systems that can add a new dimension of mechanical stress related to tissue deformation (Table V). Two types of motorized devices are found in literature, the tensile strain and stretching platforms. Dynamic cell culture systems are often used to apply mechanical stimulus to reproduce physiological constraints and forces perceived by cells. Especially in the case of glial cells, dynamic platforms can mimic cell elongation, such as axonal growth, during brain development to assess glial response to the generated forces. In addition, stretching stresses reproduce the deformation of CNS tissues following trauma.128–130 Thus, a compliant and flexible matrix is necessary to obtain a deformable cell substrate. For instance, elastic polymeric gels and thin crosslinked silicone films following traction or stretched force are used as culture models to measure the effect of substrate rigidity on cell mechanistics. As previously stated, the matrix elastic properties can be modified by changing the ratio of monomer to crosslinker in polyacrylamide gels.131 In biomechanics, the force-velocity relationship between the matrix compliance and the ability of cells to be mechanosensitive can be explained by the two-spring model based on the linear elasticity of hydrogels. In summary, soft substrates increase the force needed to maintain the stability of an adhesion, while on rigid surfaces this force is reduced when the actomyosin system is already fully mobilized to stabilize the focal adhesions.132 Therefore, the optimal situation for a cell would be to have a surrounding matrix stiffness of the same magnitude as that of cell compliance.
TABLE V.
Advantages and drawbacks of motorized platforms to study glial mechanotransduction.
| Substrate | Material | Surface coating | Device/Mechanical stimulation | Stiffness and specific features | Cells tested | Biological outcomes | Limits | References |
|---|---|---|---|---|---|---|---|---|
| Biological hydrogel | Stretched silicon sheets | Matrigel | Cell-shortening device/Compression–space restriction | Stiffness non communicated (NC) | Mouse oligodendrocyte progenitor cell (OPC) | Stimulation promotes OL differentiation by heterochromatin formation through Syne1 (LINC) mechanotransduction | Comparable to microsphere space constraint | 133 |
| Polystyrene Thick ACLAR 33 C film | Matrigel Collagen I | Custom-built mechanobioreactors with extension chamber/Stretch-growth Long process outgrowth | Stiffness NC | Rat astrocyte | Living scaffold emulating developmental conditions | Mimic radial glia | 137 | |
| More robust stretched processes at 12.5 μm/h | Result robustness is coating-dependent | |||||||
| The applied displacement rate is different than stretched-injury models | Required astrocyte processes network with sufficient resilience and growth capacity | |||||||
| Heterogeneous stretch within cultures with the most robust stretch seen near the corners of the towing membranes Changes in astrocyte processes thickness underscore the heterogeenous effect of the mechanical tension | ||||||||
| Synthetic hydrogel | PDMS plates fabricated from Sylgard 184 silicone | Fibonectin | Tensile strain device/10% static tensile strain for 48h | Stiffness NC | Rat primary OPC | Early differentiation in OL investigated under mechanical stimulus shows reduction in cell migration and microtubule network reorganization | Only tested tensile strain, which may not encompass the complexity of mechanical stresses encountered in vivo | 67 |
| PDL Laminin Fibonectin | Tensile strain device (1) Biaxial static tensile strain of 15% for 24 h (Proliferation) (2) 10% static tensile strain for 3–5 Day (Differentiation) | Stiffness NC | Rat primary OPC | Sustained tensile strain inhibits OPC proliferation and promoted OL differentiation through chromatine reorganization and nucleus shape changes | Only tested tensile strain, which may not encompass the complexity of mechanical stresses encountered in vivo | 134 | ||
| Synthetic substrate | Teflon disk with silicon membrane | Laminin Fibronectin | Tensile Strain/10% static equibiaxial stretch | Stiffness Unstretched 10 kPa Stretched 1.6 Mpa | Mouse cortical neural stem/progenitor cell (NSPC) | Stretch impacts NSPC differentiation into OL, but not neurons or astrocytes, and is dependent on ECM-integrin linkages | The stiffness range is high and does not mimic a physiological range | 135 |
| Rat hippocampal NSPC | Generation of OL decreased on laminin | |||||||
| Synthetic substrate ? | Silicon chamber | Laminin | Cell stretching Shear stress (1) Computer-controlled stepping motor machine | Stiffness NC | Rat OPC | YAP regulates OL morphology and interactions with neuronal axons Mechanical stretching induces | Suitable only for early differentiation step but does not encompass topographical cues to study OL maturation and myelination | 10 |
| (2) Shear stress by flask rotation | ||||||||
| nuclear YAP translocation and focal adhesion assembly | ||||||||
| Shear stress decreased the number of OL processes | ||||||||
| Synthetic Hydrogel and substrate | PAA PDMS Silicone sheets | Sulfo-SANPAH PLL Laminin 211 | Cell density Substrate stiffness Uniaxial stretching | 0.5 kPa to 40 kPa (PAA) 4 Mpa (PDMS) | Primary rat Schwann cells (SC) | YAP/Taz remains nuclear in low cell density and relocates in the cytoplasm under blebbistatin treatment | Not suitable for complexes and topography-directed morphological study Short term stimulation and | 9 |
| YAP/Taz is nuclear on very stiff substrate but cytopasmic on more compliant ones in presence of laminin 211. YAP/Taz nuclear localization is promoted by mechanical stretching | ||||||||
| culture | ||||||||
| Modified biological hydrogel | Methacrylamide chitosan | Laminin | Mach 1 micromechanical testing system/Uniaxial stress-relaxation Substrate stiffness | Stiffness 1–30 kPa | Rat NSPC | Used for mechanical testing of the gels, not assessed for cells | Not suitable for complexes and topography-directed morphological study | 84 |
| Porous network slightly varying across stiffness | ||||||||
| Synthetic substrate | Bioflex Plates | Collagen I | biaxial stretch | Stiffness NC | Adult astrocytes | Mechanical stress activates stretched-activated ion channels and regulates the expression of endothelin and endothelin receptors in astrocytes | Not suitable for complexes and topography-directed morphological study | 136 |
In practice, a Matrigel-coated pre-stretched silicon substrate was used as a matrix to directly address the effect of mechanical forces on nuclear heterochromatin organization in OPCs. The platform was mounted in a device that generates uniaxial cell deformation upon mechanical release of the substrate.133 Such assay resumes mechanical compression by spatial restriction and exhibits comparable stimulus generated by microsphere space constraint. Thus, the authors identified SYNE1 as a key mechanotransducer in the nuclear envelope complex (LINC) and transmits the mechanical stress to the nucleoskeleton, subsequently leading to the formation of heterochromatin, a main step in the OL differentiation. Also, the application of tensile strain to cells plated on elastomeric PDMS plates is a model developed recently.67,134 Mechanical stretching was used to demonstrate the differential cell commitment to glial lineage along with the importance of ECM nature to direct oligodendrocyte differentiation,135 or the regulation of the astrocytic endothelin secretion through ion channel activation.136 Glial morphological changes and nuclear translocation of mechanotransducer Yes-Associated Protein (YAP) could be verified by the dynamic stimulation.9,10 Katiyar et al. used a stretching platform coated with Matrigel or collagen I to engineer a “living scaffold” based on long astrocytes processes.137 Such model could open the way to explore mechanotransduction in co-culture model on motorized platforms. Also, a novel cell-stretching array platform was designed to obtain defined cellular alignment in vitro,138 which is an interesting feature that can be easily applied for glial cell culture.
Yet, these platforms still lack a long response time and are yet limited in generating homogeneous uniaxial or bidirectional forces regardless of the spatial stimulation. Recent advancement in engineering three-motorized stage system allowing imaging during the two phases of the cyclic stretch could be investigated to design multiparametric stimuli.139 Nonetheless, those dynamic platforms are using hydrogel-based substrates to assess material deformation. Therefore, the recommendation to wisely choose the appropriate material and stiffness range apply herein. The type and nature of polymer can modify the cell response and adhesion properties and may require additional coating to ensure proper cell anchorage and focal adhesion formation.
G. Magnetic particle (MNP) to reproduce axon-traction force
In a different field, iron oxide (Fe3O4) magnetic nanoparticles (MNPs) are able to produce mechanical tension provoking axon elongation and growth.140,141 Their use was proposed to potentially improve nerve regeneration and to implement guidance for regenerating axons through cell magnetic actuation.142 In order to develop novel functional nanotools, the MNPs could be used as an in vitro system assay to promote axonal elongation/growth by exploiting the mechanical forces that act on MNP-neurons and thus study remyelination in co-culture platform with OL.
IV. GLIAL MECHANOSENSORS
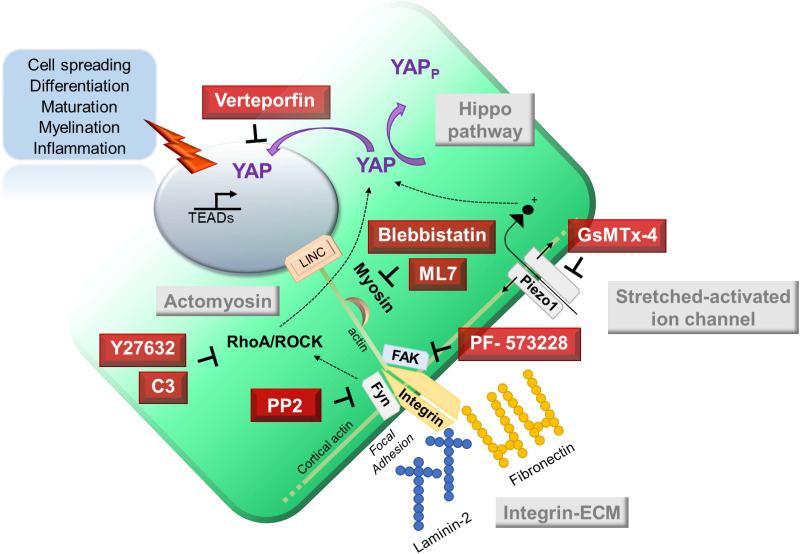
Physical changes in the cell microenvironment, including ECM architecture, compression strain, shear stress or osmotic pressure, trigger cell adaptation according to the nature and magnitude of the mechanical signals. The integration, conversion and amplification of these physical signals into biochemical signals are performed by the mechanotransduction process. Hence, mechanosensory systems are distributed over the cell membrane at the interface with the substrate (integrin complexes) or the extracellular fluidic milieu (stretched-activated ion channels). These sensors work closely with the cytoskeletal network (actomyosin), which are in turn connected with adaptor proteins that relay intracellular and nuclear signaling (YAP) and ultimately result in cell architecture changes and morphological adaptation.58,143 Cell surface-ligand signaling (integrin-ECM) and the Hippo signaling pathway are well studied although many effectors are still to be confirmed in their sequence of action and partners. Stretched-activated ion channels (Piezo1) are the new kids on the block of glial mechanotransduction. Intraglial variations in preferred pathways have been demonstrated by recent works on Schwan cells9 and oligodendrocytes.10,59 RNA-seq transcriptome study recently showed that although the Hippo pathways effectors [Large Tumor Suppressor Kinase (LATS), Mammalian Ste20-like Kinase (MST)] are well preserved, YAP and TAZ expression highly varies across the glial cell types.144 The identified glial mechanosensors and the underlying mechanotransduction pathways are described in this schematic representation (Fig. 1). Pharmacological inhibitors targeting key molecular actor in mechanotransduction pathway have been used in glial cells, including blebbistatin,145–147 verteporfin,9,112,125 GsMTX-4,19,136,148–150 Y-27632, PP2, PF-573228, C3, and ML-7. The pharmacological inhibitors used to study specific signaling encountered in glia are summarized in Table VI.
FIG 1.
Glial mechanotransduction pathways and pharmacological inhibitors.
TABLE VI.
Pharmacological inhibitors of cell mechanotransduction used in glia.
| Name | Target | References |
|---|---|---|
| Blebbistatin | Myosin II | 6–10, 46, 47, 54, 57, 63, 119, 145–147, and 59 |
| Verteporfin | YAP-TEAD interaction | 9, 112, and 124 |
| YAP 14–3-3 interaction | ||
| YAP nuclear translocation | ||
| GsMTX-4 | Piezo-1 activity | 6, 19, 136, 148, 149, and 150 |
| Y-27632 | ROCK | 46, 57, 63, 64, 119, 145, 146, and 147 |
| PP2 | Src kinases (Fyn) | 63, 145, and 122 |
| PF-573228 | FAK | 63 |
| C3 | RhoA/B/C | 63 |
| ML-7 | Myosin light chain (MLCK) | 63 and 119 |
V. CURRENT LIMITATIONS
A. CNS stiffness measurement
In mechanobiology, the brain tissue stiffness is represented by the elastic moduli or the storage Young's modulus, E, and can be measured by indentation through atomic force microscopy (AFM),18,26,151 or by using magnetic resonance elastography (MRE).14 The latter is noninvasive and often used to assess human brain degeneration and mechanical alteration during aging.27 Corrspondingly, the young adult brain displays a storage modulus of approximately 3.5 kPa, while the aged brain possesses lower stiffness ∼2.5 kPa. However, the resolution obtained by MRE is not always defined enough to distinguish atrophic changes in brain geometry and may be subjected to mathematical errors. While AFM measurements are more accurate and, therefore, more widely used in testing animal brain stiffness, both methods must be put in perspective to optimize the comparison between studies.24 Murine brain stiffness described in the literature exhibits a value approximately 20 times higher when measured by AFM36 compared to the MRE technique.37 Regional brain stiffness variation can reach a substantial amplitude. For example, whole rat brain stiffness measured with an indenter set with 25 μm diameter sphere at 1 Hz and 5% strain was found to be between 150 and 300 Pa,26 whereas the median stiffness of rat cortical sections was measured in a range between 50 and 500 Pa by using 89.3 μm beads with a force of 20–30 nN.17 Therefore, the selection of the tissue sampling method is an important approach to consider when establishing a study model. Particularly, gray and white matters have different permeability and fluid volume content, which modifies their mechanical signature and can alter the stiffness measurement depending on the method employed. For example, CNS sections used for AFM studies do not allow fluid leakage while whole tissue is prone to fluid escape during testing which is one of the main reason thought to explain the differences in brain stiffness measurements.152 In addition, the relevance of measuring cerebral stiffness in vivo rather than ex vivo has just been demonstrated by observing postmortem stiffening of CNS tissues by fluidic and metabolic changes.153
Further comparative studies could in future correct the variations in data attributed to specific study methods. Those studies could focus on brain stiffness calibration and direct comparison in vivo and ex vivo by using MRE and AFM could provide further insights to establish regional tissue maps of the variations in mechanical rigidity within the CNS.
B. 2D vs 3D engineered methods
Although commonly used, 2D cell culture platforms have several disadvantages when it comes to encompassing physiological forces. To illustrate the lack of natural and physiological representation, the cellular interaction model is greatly restricted by side-by-side linear contact, which sometimes results in cell flattening presenting an altered morphology in contrast to their native behavior. Also, it is noteworthy that cells cultured in 2D will face a poor relevant cell-ECM interaction and that could lead to altered gene expression. Certainly one of the major drawbacks of classic 2D culture systems is the triggered glial immunoreactivity due to dysregulated homeostasis generated by abnormal environment, especially for microglia and astrocytes.105 Overall, the use of 2D models should be made for approaches that would target a specific type of interaction to be investigated in order to focus on the expected cellular responses and avoid false positives due to artificially grown culture conditions in order to minimize the risk of having a lack of predictive ability for in vivo events. In particular, when myelinating cells are cultured on 2D platforms, the cell body will generate mostly cell processes and spreading but will not achieve an entire membrane wrapping resulting in complete myelination that can be observed around axons.
In contrast, the 3D modeling is particularly attractive because it reproduces more accurately the mechanical, but also structural and geometrical conditions that cells encounter in tissue. The matrix proteins deposited evenly on experimental substrates do not constitute an ideal reproduction of living organisms. Indeed, in humans, matrix fibers exist at many scales of length, which is difficult to model by 2D surface-based substrates. Finally, when a cell applies a tensile force on a compliant substrate attached to stiffer support, the resulting physical deformations are strongly localized and decrease exponentially with the distance from the point of application of the force. The range of deformations is related to the thickness of the substrate.154 On the other hand, in the context of a 3D substrate, when a cell contracts on or within the matrix, the deformations extend over relatively long distances and are approximately proportional to the inverse square of the distance from the cell.132 In the absence of cross-linking, the tension applied to fiber is all along its length resulting from the nonlinear rheological properties of the matrix.155–157 Nevertheless, 3D platforms are not exempted from limitations. At the technical level, 3D culture systems have a higher degree of complexity and thus generate higher costs and a higher demand for expertise and specialized equipment (i.e., bioreactor). Another major problem to consider is the difficulty of visualizing cells in a thick matrix with the usual microscopy techniques. Also, the difficulty to retrieve cells for further downstream analysis can be impaired by the nature and the structure of the system. Laser capture microdissection could be a response to this issue but remains marginally used125 due to the difficulty to engineer a suitable system for specific cell collection. Therefore, the matrix features including opacity, biodegradability, pore size or stiffness have to be controlled. In particular, it is common that 3D models are prone to reproducibility defects and exposing great variability within their structure. It is therefore important to consider the most homogeneous distribution possible of the structure to allow an equal distribution of nutrients and biological factors to the cells in order to avoid areas of cellular necrosis.
C. Glial cell culture model
The observation of cellular phenomena is often carried out over relatively short periods (days or weeks) which could not correlate with the lengthy development of a pathology (months up to years). Although, pathogenesis indeed arises over a long-term period, nonetheless, mechanical changes materialize during a short time. Mechanotransduction is a rapid mechanism translating quick cell behavior change that trigger microenvironmental modifications possibly leading to significant tissue alteration over a longer period. Hence, short-term studies based on single-cell or co-culture are still relevant to unravel specific cell mechanisms but one must remain cautious in extrapolating data toward more complex models of pathologies encompassing diverse topographies, matrix component, cell types and phenotypes.
Glial cell cultures for in vitro studies are also limited to the availability of cell type. While sampling mature glia from a living organism is ethically and technically limited, alternatives can be sought in studying organotypic culture on varying mechanical stimuli.158,159 The CNS organoid development and the associated malformations can be observed in detail that comes closer to living conditions. However, the level of modeling of mechanical constraints is still sketchy and requires technological advances. Since all of the mechanotransduction platforms without exception have used animal cells, it would be now advisable to consider performing the same experiments with human cells in order to be in line with a development of a therapeutic strategy. Other research would be likely to use gene engineering such as induced pluripotent stem cell (iPSC) technology to induce glial cells-like phenotype from healthy and diseased available patient biopsy (skin or fat mainly). This field is promising and has demonstrated that functional glial cells could be obtained.160–162 Human glia could be obtained at any level of commitment or maturation and similar mechanical constraints addressed with animal cells could be eventually assessed. For instance, we can envision that myelination ability of iPSC-derived OLs obtained from healthy and diseases patients (for instance MS) could compared on tunable stiffness artificial axon platform for drug discovery.
VI. CONCLUSIONS AND PERSPECTIVES
Glial cells are mechanosensitive and their biological responses depend highly on extracellular mechanical features including the nature and stiffness of their substrate, as well as the applied stresses or strains.38,143 Mechanical strain in the CNS can arise from several physiological or developmental mechanisms, such as tissue reorganization, fluid flow, and axon growth, as well as pathological events including axon swelling or mechanical trauma. During development, CNS tissue stiffness changes induced by the microenvironment determine the cell morphology and lineage specification. Mechanotransduction studies on cell-substrate interactions can aid design of neuro-glial micro-organs and tissue constructs (Fig. 2). Hence, glial commitment demonstrates sub cell type preferences for substrate stiffness. The mechanisms leading to cell differentiation and maturation are directly influenced by cell shape and morphology. Consequently, the interplay between biochemical and topographical cues is thus driving neuronal and glial cell differentiation. Therefore, the use of testing platforms for drug discoveries promises great advancements in pharmacotherapy of CNS disorders, specifically in the cases of remyelination strategies or astrogliosis prevention. Novel therapeutic targets and biomarkers are expected to be identified following the exploration of these mechanocircuits. As a result, the understanding of the signaling crosstalk between ECM mechanics, fluid flow and mechanosensitive ion channels and their synchronization with paracrine factors to control glial, and more largely, neural cell lineage and behavior would aid in the medicine approaches against neurodegenerative disorders. Therefore, a better understanding of the mechanotransduction pathways involved can potentially identify critical biomolecules for controlling cell fate. Focusing on mechanotransduction signaling to identify specific key molecules as therapeutic target to modulate the glial behavior in disease condition is part of the drug discovery strategy that can arise from those studies. Evidence, which reveals that the effects of drugs acting on the glial system can be influenced by the context of mechanical stiffness established by the disease, is beginning to emerge. By using tunable stiffness platform conjointly with verteporfin treatment, Ong et al. highlighted that oligodendrocyte differentiation and maturation may be two mechanisms independently and diversely regulated through the mechanosensory YAP signaling.125 Thus, a number of molecules possess a therapeutic potential for the nervous system. Among these molecules, clemastine has shown potential in promoting the differentiation of oligodendrocytes and in aiding the remyelination in defective nervous fibers in preclinical and clinical studies.163–165 However, the results are not conclusive over the long term and the pharmacology has yet to be deepened. It is crucial that such molecules with therapeutic potential be tested in models taking into account the mechanical dimensions to evaluate their mode of action and optimize their beneficial activities in order to design a future therapy. In addition, mechanotransduction platforms should also be designed into high throughput devices to help identify new molecules, and this will require manufacturing standardization procedures.
FIG. 2.
Glial mechanotransduction platforms and assays.
Specifically, no regenerative therapy is effective for the CNS now. In the past decades, several strategies have been used; among them are the cell therapies including the local injection of pluripotent stem cells or mature cells. Nonetheless, cell therapies have often been disappointing due to the poor cell viability. The design of biomaterials to promote healing and regeneration in the nervous system via transplantation of glial progenitors or the implantation of tissue scaffolds is justified.64 Biomaterials can physically reproduce the CNS tissue and offer permissive environment for cell survival, growth, and differentiation. Promising results were obtained after the implantation of soft hydrogels at the injury site of CNS tissue which prevent glial scar formation and enabled neurite outgrowth.131 However, serious improvements in biomaterial properties are required to extend cell survival and tissue integration. Particularly, the stiffness of the implant can trigger gliosis and inflammation.166 Also, preserving a mechanical homeostasis is one of the greatest challenges of the current CNS cell therapy strategies. The knowledge of cell substrate interactions will aid biomaterials design for directing the fate of endogeneous glial cells and exogenous transplanted cells.
ACKNOWLEDGMENTS
Partial financial support was received from the Ministry of Education Tier 1 Grant (Nos. RG38/19 and RG37/20).
Note: This paper is part of the special issue on Functional Biomaterials.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Kim S. Y., Porter B. E., Friedman A., and Kaufer D., “ A potential role for glia-derived extracellular matrix remodeling in postinjury epilepsy,” J. Neurosci. Res. 94, 794–803 (2016). 10.1002/jnr.23758 [DOI] [PubMed] [Google Scholar]
- 2. Song I. and Dityatev A., “ Crosstalk between glia, extracellular matrix and neurons,” Brain Res. Bull. 136, 101–108 (2018). 10.1016/j.brainresbull.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 3. Barnes J. M., Przybyla L., and Weaver V. M., “ Tissue mechanics regulate brain development, homeostasis and disease,” J. Cell Sci. 130, 71–82 (2017). 10.1242/jcs.191742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Humphrey J. D., Dufresne E. R., and Schwartz M. A., “ Mechanotransduction and extracellular matrix homeostasis,” Nat. Rev. Mol. Cell Biol. 15, 802–812 (2014). 10.1038/nrm3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hemphill M. A., Dauth S., Yu C. J., Dabiri B. E., and Parker K. K., “ Traumatic brain injury and the neuronal microenvironment: A potential role for neuropathological mechanotransduction,” Neuron 85, 1177–1192 (2015). 10.1016/j.neuron.2015.02.041 [DOI] [PubMed] [Google Scholar]
- 6. Pathak M. M., Nourse J. L., Tran T., Hwe J., Arulmoli J., Le D. T. T., Bernardis E., Flanagan L. A., and Tombola F., “ Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells,” Proc. Natl. Acad. Sci. U. S. A. 111, 16148–16153 (2014). 10.1073/pnas.1409802111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lourenço T., Paes De Faria J., Bippes C. A., Maia J., Lopes-Da-Silva J. A., Relvas J. B., and Graõs M., “ Modulation of oligodendrocyte differentiation and maturation by combined biochemical and mechanical cues,” Sci. Rep. 6, 1–17 (2016). 10.1038/srep21563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang H., Tewari A., Einheber S., Salzer J. L., and Melendez-Vasquez C. V., “ Myosin II has distinct functions in PNS and CNS myelin sheath formation,” J. Cell Biol. 182, 1171–1184 (2008). 10.1083/jcb.200802091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poitelon Y., Lopez-Anido C., Catignas K., Berti C., Palmisano M., Williamson C., Ameroso D., Abiko K., Hwang Y., Gregorieff A., Wrana J. L., Asmani M., Zhao R., Sim F. J., Wrabetz L., Svaren J., and Feltri M. L., “ YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells,” Nat. Neurosci. 19, 879–887 (2016). 10.1038/nn.4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimizu T., Osanai Y., Tanaka K. F., Abe M., Natsume R., Sakimura K., and Ikenaka K., “ YAP functions as a mechanotransducer in oligodendrocyte morphogenesis and maturation,” Glia 65, 360–374 (2017). 10.1002/glia.23096 [DOI] [PubMed] [Google Scholar]
- 11. Wang L., Xia J., Li J., Hagemann T. L., Jones J. R., Fraenkel E., Weitz D. A., Zhang S., Messing A., and Feany M. B., “ Tissue and cellular rigidity and mechanosensitive signaling activation in Alexander disease,” Nat. Commun. 9, 1899 (2018). 10.1038/s41467-018-04269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolf J. A., Stys P. K., Lusardi T., Meaney D., and Smith D. H., “ Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels,” J. Neurosci. 21, 1923–1930 (2001). 10.1523/JNEUROSCI.21-06-01923.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith D. H., Johnson V. E., and Stewart W., “ Chronic neuropathologies of single and repetitive TBI: substrates of dementia?,” Nat. Rev. Neurol. 9, 211–221 (2013). 10.1038/nrneurol.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murphy M. C., Jones D. T., Jack C. R., Glaser K. J., Senjem M. L., Manduca A., Felmlee J. P., Carter R. E., Ehman R. L., and Huston J., “ Regional brain stiffness changes across the Alzheimer's disease spectrum,” NeuroImage Clin. 10, 283–290 (2016). 10.1016/j.nicl.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nogueira M. L., Lafitte O., Steyaert J. M., Bakardjian H., Dubois B., Hampel H., and Schwartz L., “ Mechanical stress related to brain atrophy in Alzheimer's disease,” Alzheimer's Dementia 12, 11–20 (2016). 10.1016/j.jalz.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 16. Nogueira M. L., Hamraz M., Abolhassani M., Bigan E., Lafitte O., Steyaert J. M., Dubois B., and Schwartz L., “ Mechanical stress increases brain amyloid β, tau, and α-synuclein concentrations in wild-type mice,” Alzheimer's Dementia 14, 444–453 (2018). 10.1016/j.jalz.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 17. Moeendarbary E., Weber I. P., Sheridan G. K., Koser D. E., Soleman S., Haenzi B., Bradbury E. J., Fawcett J., and Franze K., “ The soft mechanical signature of glial scars in the central nervous system,” Nat. Commun. 8, 1–11 (2017). 10.1038/ncomms14787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weickenmeier J., de Rooij R., Budday S., Steinmann P., Ovaert T. C., and Kuhl E., “ Brain stiffness increases with myelin content,” Acta Biomater. 42, 265–272 (2016). 10.1016/j.actbio.2016.07.040 [DOI] [PubMed] [Google Scholar]
- 19. Chen X., Wanggou S., Bodalia A., Zhu M., Dong W., Fan J. J., Yin W. C., Min H. K., Hu M., Draghici D., Dou W., Li F., Coutinho F. J., Whetstone H., Kushida M. M., Dirks P. B., Song Y., chung Hui C., Sun Y., Wang L. Y., Li X., and Huang X., and “ A Feedforward mechanism mediated by mechanosensitive ion channel PIEZO1 and tissue mechanics promotes glioma aggression,” Neuron 100, 799–815.e7 (2018). 10.1016/j.neuron.2018.09.046 [DOI] [PubMed] [Google Scholar]
- 20. Budday S., Nay R., de Rooij R., Steinmann P., Wyrobek T., Ovaert T. C., and Kuhl E., “ Mechanical properties of gray and white matter brain tissue by indentation,” J. Mech. Behav. Biomed. Mater. 46, 318–330 (2015). 10.1016/j.jmbbm.2015.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Budday S., Sommer G., Birkl C., Langkammer C., Haybaeck J., Kohnert J., Bauer M., Paulsen F., Steinmann P., Kuhl E., and Holzapfel G. A., “ Mechanical characterization of human brain tissue,” Acta Biomater. 48, 319–340 (2017). 10.1016/j.actbio.2016.10.036 [DOI] [PubMed] [Google Scholar]
- 22. Lu Y. B., Franze K., Seifert G., Steinhäuser C., Kirchhoff F., Wolburg H., Guck J., Janmey P., Wei E. Q., Käs J., and Reichenbach A., “ Viscoelastic properties of individual glial cells and neurons in the CNS,” Proc. Natl. Acad. Sci. U. S. A. 103, 17759–17764 (2006). 10.1073/pnas.0606150103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernick K. B., Prevost T. P., Suresh S., and Socrate S., “ Biomechanics of single cortical neurons,” Acta Biomater. 7, 1210–1219 (2011). 10.1016/j.actbio.2010.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franze K., Janmey P. A., and Guck J., “ Mechanics in neuronal development and repair,” Annu. Rev. Biomed. Eng. 15, 227–251 (2013). 10.1146/annurev-bioeng-071811-150045 [DOI] [PubMed] [Google Scholar]
- 25. Klein C., Hain E. G., Braun J., Riek K., Mueller S., Steiner B., and Sack I., “ Enhanced adult neurogenesis increases brain stiffness: In vivo magnetic resonance elastography in a mouse model of dopamine depletion,” PLoS One 9, e92582 (2014). 10.1371/journal.pone.0092582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elkin B. S., Ilankovan A., and Morrison B., “ Age-dependent regional mechanical properties of the rat hippocampus and cortex,” J. Biomech. Eng. 132, 1–10 (2010). 10.1115/1.4000164 [DOI] [PubMed] [Google Scholar]
- 27. Sack I., Streitberger K. J., Krefting D., Paul F., and Braun J., “ The influence of physiological aging and atrophy on brain viscoelastic properties in humans,” PLoS One 6, e23451 (2011). 10.1371/journal.pone.0023451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takamura T., Motosugi U., Sasaki Y., Kakegawa T., Sato K., Glaser K. J., Ehman R. L., and Onishi H., “ Influence of age on global and regional brain stiffness in young and middle‐aged adults,” J. Magn. Reson. Imaging 51, 727 (2020). 10.1002/jmri.26881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arani A., Murphy M. C., Glaser K. J., Manduca A., Lake D. S., Kruse S. A., Jack C. R., Ehman R. L., and Huston J., “ Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults,” Neuroimage 111, 59–64 (2015). 10.1016/j.neuroimage.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eberle D., Fodelianaki G., Kurth T., Jagielska A., Möllmert S., Ulbricht E., Wagner K., Taubenberger A. V., Träber N., Escolano J.-C., Franklin R., Van Vliet K. J., and Guck J., “ Acute but not inherited demyelination in mouse models leads to brain tissue stiffness changes,” BioRxiv 449603.
- 31. Back S. A., Tuohy T. M. F., Chen H., Wallingford N., Craig A., Struve J., Ning L. L., Banine F., Liu Y., Chang A., Trapp B. D., Bebo B. F., Rao M. S., and Sherman L. S., “ Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation,” Nat. Med. 11, 966–972 (2005). 10.1038/nm1279 [DOI] [PubMed] [Google Scholar]
- 32. Yiu G. and He Z., “ Glial inhibition of CNS axon regeneration,” Nat. Rev. Neurosci. 7, 617–627 (2006). 10.1038/nrn1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Urbanski M. M., Brendel M. B., and Melendez-Vasquez C. V., “ Acute and chronic demyelinated CNS lesions exhibit opposite elastic properties,” Sci. Rep. 9, 1–13 (2019). 10.1038/s41598-018-37745-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schregel K., Née Tysiak E. W., Garteiser P., Gemeinhardt I., Prozorovski T., Aktas O., Merz H., Petersen D., Wuerfel J., and Sinkus R., “ Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography,” Proc. Natl. Acad. Sci. U. S. A. 109, 6650–6655 (2012). 10.1073/pnas.1200151109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohan H., Krumbholz M., Sharma R., Eisele S., Junker A., Sixt M., Newcombe J., Wekerle H., Hohlfeld R., Lassmann H., and Meinl E., “ Extracellular matrix in multiple sclerosis lesions: Fibrillar collagens, biglycan and decorin are upregulated and associated with infiltrating immune cells,” Brain Pathol. 20, 966–975 (2010). 10.1111/j.1750-3639.2010.00399.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Menal M. J., Jorba I., Torres M., Montserrat J. M., Gozal D., Colell A., Piñol-Ripoll G., Navajas D., Almendros I., and Farré R., “ Alzheimer's disease mutant mice exhibit reduced brain tissue stiffness compared to wild-type mice in both normoxia and following intermittent hypoxia mimicking sleep apnea,” Front. Neurol. 9, 1 (2018). 10.3389/fneur.2018.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy M. C., Curran G. L., Glaser K. J., Rossman P. J., Huston J., Poduslo J. F., Jack C. R., Felmlee J. P., and Ehman R. L., “ Magnetic resonance elastography of the brain in a mouse model of Alzheimer's disease: Initial results,” Magn. Reson. Imaging 30, 535–539 (2012). 10.1016/j.mri.2011.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bauer N. G. and C. ffrench-Constant, “Physical forces in myelination and repair: A question of balance?,” J. Biol. 8, 78 (2009). 10.1186/jbiol169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laywell D., Dorriest U., Bartscht U. D. O., Faissnert A., Schachnert M., and Steindler D. A., “ Enhanced expression of the developmentally regulated extracellular matrix molecule tenascin following adult brain injury,” Neurobiology 89, 2634–2638 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang E. H., Adorjan I., Mundim M. V., Sun B., Dizon M. L. V., and Szele F. G., “ Traumatic brain injury activation of the adult subventricular zone neurogenic niche,” Front. Neurosci. 10, 332 (2016). 10.3389/fnins.2016.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miroshnikova Y. A., Mouw J. K., Barnes J. M., Pickup M. W., Lakins J. N., Kim Y., Lobo K., Persson A. I., Reis G. F., McKnigh T. R., Holland E. C., Phillips J. J., and Weaver V. M., “ Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression,” Nat. Cell Biol. 18, 1336–1345 (2016). 10.1038/ncb3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu Y. B., Iandiev I., Hollborn M., Körber N., Ulbricht E., Hirrlinger P. G., Pannicke T., Wei E. Q., Bringmann A., Wolburg H., Wilhelmsson U., Pekny M., Wiedemann P., Reichenbach A., and Käs J. A., “ Reactive glial cells: Increased stiffness correlates with increased intermediate filament expression,” FASEB J. 25, 624–631 (2011). 10.1096/fj.10-163790 [DOI] [PubMed] [Google Scholar]
- 43. Morrow T., Song M. R., and Ghosh A., “ Sequential specification of neurons and glia by developmentally regulated extracellular factors,” Development 128, 3585–3594 (2001). [DOI] [PubMed] [Google Scholar]
- 44. Georges P. C., Miller W. J., Meaney D. F., Sawyer E. S., and Janmey P. A., “ Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures,” Biophys. J. 90, 3012–3018 (2006). 10.1529/biophysj.105.073114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller W. J., Leventhal I., Scarsella D., Haydon P. G., Janmey P., and Meaney D. F., “ Mechanically induced reactive gliosis causes ATP-mediated alterations in astrocyte stiffness,” J. Neurotrauma 26, 789–797 (2009). 10.1089/neu.2008.0727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kippert A., Fitzner D., Helenius J., and Simons M., “ Actomyosin contractility controls cell surface area of oligodendrocytes,” BMC Cell Biol. 10, 71 (2009). 10.1186/1471-2121-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spedden E., White J. D., Naumova E. N., Kaplan D. L., and Staii C., “ Elasticity maps of living neurons measured by combined fluorescence and atomic force microscopy,” Biophys. J. 103, 868–877 (2012). 10.1016/j.bpj.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mohammed D., Versaevel M., Bruyère C., Alaimo L., Luciano M., Vercruysse E., Procès A., and Gabriele S., “ Innovative tools for mechanobiology: Unraveling outside-in and inside-out mechanotransduction,” Front. Bioeng. Biotechnol. 7, 162 (2019). 10.3389/fbioe.2019.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim H. N. and Choi N., “ Consideration of the mechanical properties of hydrogels for brain tissue engineering and brain-on-a-chip,” Biochip J. 13, 8–19 (2019). 10.1007/s13206-018-3101-7 [DOI] [Google Scholar]
- 50. George J., Hsu C. C., Nguyen L. T. B., Ye H., and Cui Z., “ Neural tissue engineering with structured hydrogels in CNS models and therapies,” Biotechnol. Adv. 42, 107370 (2020). 10.1016/j.biotechadv.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 51. Tse J. R. and Engler A. J., “ Preparation of hydrogel substrates with tunable mechanical properties,” Curr. Protoc. Cell Biol. 47, 1–16 (2010). 10.1002/0471143030.cb1016s47 [DOI] [PubMed] [Google Scholar]
- 52. Denisin A. K. and Pruitt B. L., “ Tuning the range of polyacrylamide gel stiffness for mechanobiology applications,” ACS Appl. Mater. Interfaces 8, 21893–21902 (2016). 10.1021/acsami.5b09344 [DOI] [PubMed] [Google Scholar]
- 53. Moshayedi P., Da F Costa L., Christ A., Lacour S. P., Fawcett J., Guck J., and Franze K., “ Mechanosensitivity of astrocytes on optimized polyacrylamide gels analyzed by quantitative morphometry,” J. Phys.: Condens. Matter 22, 194114 (2010). 10.1088/0953-8984/22/19/194114 [DOI] [PubMed] [Google Scholar]
- 54. Tanaka A., Fujii Y., Kasai N., Okajima T., and Nakashima H., “ Regulation of neuritogenesis in hippocampal neurons using stiffness of extracellular microenvironment,” PLoS One 13, e0191928 (2018). 10.1371/journal.pone.0191928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jagielska A., Norman A. L., Whyte G., Van Vliet K. J., Guck J., and Franklin R. J. M., “ Mechanical environment modulates biological properties of oligodendrocyte progenitor cells,” Stem Cells Dev. 21, 2905–2914 (2012). 10.1089/scd.2012.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Flanagan L. A., Ju Y. E., Marg B., Osterfield M., and Janmey P. A., “ Neurite branching on deformable substrates,” Neuroreport 13, 2411–2415 (2002). 10.1097/00001756-200212200-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ulrich T. A., De Juan Pardo E. M., and Kumar S., “ The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells,” Cancer Res. 69, 4167–4174 (2009). 10.1158/0008-5472.CAN-08-4859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pogoda K., Bucki R., Byfield F. J., Cruz K., Lee T., Marcinkiewicz C., and Janmey P. A., “ Soft substrates containing hyaluronan mimic the effects of increased stiffness on morphology, motility, and proliferation of glioma cells,” Biomacromolecules 18, 3040–3051 (2017). 10.1021/acs.biomac.7b00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Segel M., Neumann B., Hill M. F. E., Weber I. P., Viscomi C., Zhao C., Young A., Agley C. C., Thompson A. J., Gonzalez G. A., Sharma A., Holmqvist S., Rowitch D. H., Franze K., Franklin R. J. M., and Chalut K. J., “ Niche stiffness underlies the ageing of central nervous system progenitor cells,” Nature 573, 130 (2019). 10.1038/s41586-019-1484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saha K., Keung A. J., Irwin E. F., Li Y., Little L., Schaffer D. V., and Healy K. E., “ Substrate modulus directs neural stem cell behavior,” Biophys. J. 95, 4426–4438 (2008). 10.1529/biophysj.108.132217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perera T. H., Howell S. M., and Smith Callahan L. A., “ Manipulation of extracellular matrix remodeling and neurite extension by mouse embryonic stem cells using IKVAV and LRE peptide tethering in hyaluronic acid matrices,” Biomacromolecules 20, 3009–3020 (2019). 10.1021/acs.biomac.9b00578 [DOI] [PubMed] [Google Scholar]
- 62. Grevesse T., Versaevel M., and Gabriele S., “ Preparation of hydroxy-PAAm hydrogels for decoupling the effects of mechanotransduction cues,” J. Vis. Exp. 90, 1–8 (2014). 10.3791/51010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Keung A. J., De Juan-Pardo E. M., Schaffer D. V., and Kumar S., “ Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells,” Stem Cells 29, 1886–1897 (2011). 10.1002/stem.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Urbanski M. M., Kingsbury L., Moussouros D., Kassim I., Mehjabeen S., Paknejad N., and Melendez-Vasquez C. V., “ Myelinating glia differentiation is regulated by extracellular matrix elasticity,” Sci. Rep. 6, 1–12 (2016). 10.1038/srep33751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Trappmann B., Gautrot J. E., Connelly J. T., Strange D. G. T., Li Y., Oyen M. L., Cohen Stuart M. A., Boehm H., Li B., Vogel V., Spatz J. P., Watt F. M., and Huck W. T. S., “ Extracellular-matrix tethering regulates stem-cell fate,” Nat. Mater. 11, 642–649 (2012). 10.1038/nmat3339 [DOI] [PubMed] [Google Scholar]
- 66. Chen L., Li W., Maybeck V., Offenhäusser A., and Krause H. J., “ Statistical study of biomechanics of living brain cells during growth and maturation on artificial substrates,” Biomaterials 106, 240–249 (2016). 10.1016/j.biomaterials.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 67. Makhija E., Jagielska A., Zhu L., Bost A. C., Ong W., Chew S. Y., Shivashankar G. V., and Van Vliet K. J., “ Mechanical strain alters cellular and nuclear dynamics at early stages of oligodendrocyte differentiation,” Front. Cell. Neurosci. 12, 1–12 (2018). 10.3389/fncel.2018.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wilson C. L., Hayward S. L., and Kidambi S., “ Astrogliosis in a dish: Substrate stiffness induces astrogliosis in primary rat astrocytes,” RSC Adv. 6, 34447–34457 (2016). 10.1039/C5RA25916A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mijailovic A. S., Qing B., Fortunato D., and Van Vliet K. J., “ Characterizing viscoelastic mechanical properties of highly compliant polymers and biological tissues using impact indentation,” Acta Biomater. 71, 388–397 (2018). 10.1016/j.actbio.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 70. Teixeira A. I., Ilkhanizadeh S., Wigenius J. A., Duckworth J. K., Inganäs O., and Hermanson O., “ The promotion of neuronal maturation on soft substrates,” Biomaterials 30, 4567–4572 (2009). 10.1016/j.biomaterials.2009.05.013 [DOI] [PubMed] [Google Scholar]
- 71. Chen W. H., Cheng S. J., Tzen J. T. C., Cheng C. M., and Lin Y. W., “ Probing relevant molecules in modulating the neurite outgrowth of hippocampal neurons on substrates of different stiffness,” PLoS One 8, e83394 (2013). 10.1371/journal.pone.0083394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hersel U., Dahmen C., and Kessler H., “ RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond,” Biomaterials 24, 4385–4415 (2003). 10.1016/S0142-9612(03)00343-0 [DOI] [PubMed] [Google Scholar]
- 73. Lampe K. J., Mooney R. G., Bjugstad K. B., and Mahoney M. J., “ Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture,” J. Biomed. Mater. Res., Part A 94A, 1162–1171 (2010). 10.1002/jbm.a.32787 [DOI] [PubMed] [Google Scholar]
- 74. Mooney R., Haeger S., Lawal R., Mason M., Shrestha N., Laperle A., Bjugstad K., and Mahoney M., “ Control of neural cell composition in poly(ethylene glycol) hydrogel culture with soluble factors,” Tissue Eng., Part A 17, 2805–2815 (2011). 10.1089/ten.tea.2010.0654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhan H., de Jong H., and Löwik D. W. P. M., “ Comparison of bioorthogonally cross-linked hydrogels for in situ cell encapsulation,” ACS Appl. Bio Mater. 2, 2862–2871 (2019). 10.1021/acsabm.9b00253 [DOI] [PubMed] [Google Scholar]
- 76. Carthew J., Frith J. E., Forsythe J. S., and Truong V. X., “ Polyethylene glycol–gelatin hydrogels with tuneable stiffness prepared by horseradish peroxidase-activated tetrazine–norbornene ligation,” J. Mater. Chem. B 6, 1394–1401 (2018). 10.1039/C7TB02764H [DOI] [PubMed] [Google Scholar]
- 77. Seidlits S. K., Khaing Z. Z., Petersen R. R., Nickels J. D., Vanscoy J. E., Shear J. B., and Schmidt C. E., “ The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation,” Biomaterials 31, 3930–3940 (2010). 10.1016/j.biomaterials.2010.01.125 [DOI] [PubMed] [Google Scholar]
- 78. Her G. J., Wu H. C., Chen M. H., Chen M. Y., Chang S. C., and Wang T. W., “ Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages,” Acta Biomater. 9, 5170–5180 (2013). 10.1016/j.actbio.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 79. Bonnesœur S., Morin-Grognet S., Thoumire O., Cerf D. L., Boyer O., Vannier J. P., and Labat B., “ Hyaluronan-based hydrogels as versatile tumor-like models: Tunable ECM and stiffness with genipin-crosslinking,” J. Biomed. Mater. Res., Part A 108, 1256–1268 (2020). 10.1002/jbm.a.36899 [DOI] [PubMed] [Google Scholar]
- 80. East E., Golding J. P., and Phillips J. B., “ A versatile 3D culture model facilitates monitoring of astrocytes undergoing reactive gliosis,” J. Tissue Eng. Regener. Med. 3, 634–646 (2009). 10.1002/term.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sreekanthreddy P., Gromnicova R., Davies H., Phillips J., Romero I. A., and Male D., “ A three-dimensional model of the human blood-brain barrier to analyse the transport of nanoparticles and astrocyte/endothelial interactions [version 1; referees: 2 approved with reservations],” F1000Research 4, 1279 (2015). 10.12688/f1000research.7142.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang L. S., Boulaire J., Chan P. P. Y., Chung J. E., and Kurisawa M., “ The role of stiffness of gelatin-hydroxyphenylpropionic acid hydrogels formed by enzyme-mediated crosslinking on the differentiation of human mesenchymal stem cell,” Biomaterials 31, 8608–8616 (2010). 10.1016/j.biomaterials.2010.07.075 [DOI] [PubMed] [Google Scholar]
- 83. El Ju Y., Janmey P. A., McCormick M. E., Sawyer E. S., and Flanagan L. A., “ Enhanced neurite growth from mammalian neurons in three-dimensional salmon fibrin gels,” Biomaterials 28, 2097–2108 (2007). 10.1016/j.biomaterials.2007.101.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Leipzig N. D. and Shoichet M. S., “ The effect of substrate stiffness on adult neural stem cell behavior,” Biomaterials 30, 6867–6878 (2009). 10.1016/j.biomaterials.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 85. Banerjee A., Arha M., Choudhary S., Ashton R. S., Bhatia S. R., Schaffer D. V., and Kane R. S., “ The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells,” Biomaterials 30, 4695–4699 (2009). 10.1016/j.biomaterials.2009.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rocha D. N., Ferraz-Nogueira J. P., Barrias C. C., Relvas J. B., and Pêgo A. P., “ Extracellular environment contribution to astrogliosis—Lessons learned from a tissue engineered 3D model of the glial scar,” Front. Cell. Neurosci. 9, 1–14 (2015). 10.3389/fncel.2015.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yu X. and Bellamkonda R. V., “ Dorsal root ganglia neurite extension is inhibited by mechanical and chondroitin sulfate-rich interfaces,” J. Neurosci. Res. 66, 303–310 (2001). 10.1002/jnr.1225 [DOI] [PubMed] [Google Scholar]
- 88. Cheng T. Y., Chen M. H., Chang W. H., Huang M. Y., and Wang T. W., “ Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering,” Biomaterials 34, 2005–2016 (2013). 10.1016/j.biomaterials.2012.11.043 [DOI] [PubMed] [Google Scholar]
- 89. Huang L. C., Wang H. C., Chen L. H., Ho C. Y., Hsieh P. H., Huang M. Y., Wu H. C., and Wang T. W., “ Bioinspired self-assembling peptide hydrogel with proteoglycan-assisted growth factor delivery for therapeutic angiogenesis,” Theranostics 9, 7072–7087 (2019). 10.7150/thno.35803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang T. W., Chang K. C., Chen L. H., Liao S. Y., Yeh C. W., and Chuang Y. J., “ Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system,” Nanoscale 9, 16281–16292 (2017). 10.1039/C7NR06528K [DOI] [PubMed] [Google Scholar]
- 91. Hadden W. J., Young J. L., Holle A. W., McFetridge M. L., Kim D. Y., Wijesinghe P., Taylor-Weiner H., Wen J. H., Lee A. R., Bieback K., Vo B. N., Sampson D. D., Kennedy B. F., Spatz J. P., Engler A. J., and Cho Y. S., “ Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels,” Proc. Natl. Acad. Sci. U. S. A. 114, 5647–5652 (2017). 10.1073/pnas.1618239114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang X., Liu M., Li Y., Dong Y., Pingguan-Murphy B., Lu T. J., and Xu F., “ Engineering cell microenvironment using novel functional hydrogels,” Eur. Polym. J. 72, 590–601 (2015). 10.1016/j.eurpolymj.2015.03.019 [DOI] [Google Scholar]
- 93. Liu Z., Liu J., Cui X., Wang X., Zhang L., and Tang P., “ Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering,” Front. Chem. 8, 124 (2020). 10.3389/fchem.2020.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Antman-Passig M. and Shefi O., “ Remote magnetic orientation of 3D collagen hydrogels for directed neuronal regeneration,” Nano Lett. 16, 2567–2573 (2016). 10.1021/acs.nanolett.6b00131 [DOI] [PubMed] [Google Scholar]
- 95. Omidinia-Anarkoli A., Boesveld S., Tuvshindorj U., Rose J. C., Haraszti T., and Laporte L. D., “ An injectable hybrid hydrogel with oriented short fibers induces unidirectional growth of functional nerve cells,” Small 13, 1702207–1702208 (2017). 10.1002/smll.201702207 [DOI] [PubMed] [Google Scholar]
- 96. Tay A., Sohrabi A., Poole K., Seidlits S., and Carlo D. D., “ A 3D magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons,” Adv. Mater. 30, 1800927–1800928 (2018). 10.1002/adma.201800927 [DOI] [PubMed] [Google Scholar]
- 97. Chandorkar Y., Castro Nava A., Schweizerhof S., Van Dongen M., Haraszti T., Köhler J., Zhang H., Windoffer R., Mourran A., Möller M., and Laporte L. D., “ Cellular responses to beating hydrogels to investigate mechanotransduction,” Nat. Commun. 10, 1–13 (2019). 10.1038/s41467-019-11475-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kloxin A. M., Kasko A. M., Salinas C. N., and Anseth K. S., “ Photodegradable hydrogels for dynamic tuning of physical and chemical properties,” Science 324, 59–63 (2009). 10.1126/science.1169494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Suntornnond R., An J., and Chua C. K., “ Bioprinting of thermoresponsive Hydrogels for next generation tissue engineering: A review,” Macromol. Mater. Eng. 302, 1600266–1600215 (2017). 10.1002/mame.201600266 [DOI] [Google Scholar]
- 100. Guvendiren M. and Burdick J. A., “ Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics,” Nat. Commun. 3, 792 (2012). 10.1038/ncomms1792 [DOI] [PubMed] [Google Scholar]
- 101. Lueckgen A., Garske D. S., Ellinghaus A., Desai R. M., Stafford A. G., Mooney D. J., Duda G. N., and Cipitria A., “ Hydrolytically-degradable click-crosslinked alginate hydrogels,” Biomaterials 181, 189–198 (2018). 10.1016/j.biomaterials.2018.07.031 [DOI] [PubMed] [Google Scholar]
- 102. Hur J., Im K., Kim S. W., Kim J., Chung D. Y., Kim T. H., Jo K. H., Hahn J. H., Bao Z., Hwang S., and Park N., “ Polypyrrole/agarose-based electronically conductive and reversibly restorable hydrogel,” ACS Nano 8, 10066–10076 (2014). 10.1021/nn502704g [DOI] [PubMed] [Google Scholar]
- 103. McMurray R. J., Dalby M. J., and Tsimbouri P. M., “ Using biomaterials to study stem cell mechanotransduction, growth and differentiation,” J. Tissue Eng. Regener. Med. 9, 528–539 (2015). 10.1002/term.1957 [DOI] [PubMed] [Google Scholar]
- 104. Rosales A. M. and Anseth K. S., “ The design of reversible hydrogels to capture extracellular matrix dynamics,” Nat. Rev. Mater. 1, 1–15 (2016). 10.1038/natrevmats.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Watson P. M. D., Kavanagh E., Allenby G., and Vassey M., “ Bioengineered 3D glial cell culture systems and applications for neurodegeneration and neuroinflammation,” SLAS Discovery 22, 583–601 (2017). 10.1177/2472555217691450 [DOI] [PubMed] [Google Scholar]
- 106. Hopkins A. M., Desimone E., Chwalek K., and Kaplan D. L., “ Progress in neurobiology 3D in vitro modeling of the central nervous system,” Prog. Neurobiol. 125, 1–25 (2015). 10.1016/j.pneurobio.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Balasubramanian S., Packard J. A., Leach J. B., and Powell E. M., “ Three-dimensional environment sustains morphological heterogeneity and promotes phenotypic progression during astrocyte development,” Tissue Eng., Part A 22, 885–898 (2016). 10.1089/ten.tea.2016.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dhoot N. O., Tobias C. A., Fischer I., and Wheatley M. A., “ Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth,” J. Biomed. Mater. Res., Part A 71A, 191–200 (2004). 10.1002/jbm.a.30103 [DOI] [PubMed] [Google Scholar]
- 109. Russell L. N. and Lampe K. J., “ Oligodendrocyte precursor cell viability, proliferation, and morphology is dependent on mesh size and storage modulus in 3D poly(ethylene glycol)-based hydrogels,” ACS Biomater. Sci. Eng. 3, 3459–3468 (2017). 10.1021/acsbiomaterials.7b00374 [DOI] [PubMed] [Google Scholar]
- 110. Zhang Y., Liao K., Li C., Lai A. C. K., Foo J., and Chan V., “ Progress in integrative biomaterial systems to approach three-dimensional cell mechanotransduction,” Bioengineering 4, 72–20 (2017). 10.3390/bioengineering4030072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Baker B. M., Trappmann B., Wang W. Y., Sakar M. S., Kim I. L., Shenoy V. B., Burdick J. A., and Chen C. S., “ Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments,” Nat. Mater. 14, 1262 (2015). 10.1038/nmat4444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Madl C. M., LeSavage B. L., Dewi R. E., Lampe K. J., and Heilshorn S. C., “ Matrix remodeling enhances the differentiation capacity of neural progenitor cells in 3D hydrogels,” Adv. Sci. 6, 1801716 (2019). 10.1002/advs.201801716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rosenberg S. S., Kelland E. E., Tokar E., De La Torre A. R., and Chan J. R., “ The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation,” Proc. Natl. Acad. Sci. U. S. A. 105, 14662–14667 (2008). 10.1073/pnas.0805640105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kojic N. and Tschumperlin D. J., “ Mechanotransduction through local autocrine signaling,” in Cellular Mechanotrsanduction: Diverse Perspectives from Molecules to Tissues ( Cambridge University Press, 2009), Vol. 9780521895231, pp. 339–359. 10.1017/CBO9781139195874.015 [DOI] [Google Scholar]
- 115. Teo B. K. K., Ankam S., Chan L. Y., and Yim E. K. F., “ Nanotopography/mechanical induction of stem-cell differentiation,” Meth. Cell Biol. 98(C), 241–294 (2010). 10.1016/S0091-679X(10)98011-4 [DOI] [PubMed] [Google Scholar]
- 116. Schoen I., Hu W., Klotzsch E., and Vogel V., “ Probing cellular traction forces by micropillar arrays: Contribution of substrate warping to pillar deflection,” Nano Lett. 10, 1823–1830 (2010). 10.1021/nl100533c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Moe A. A. K., Suryana M., Marcy G., Lim S. K., Ankam S., Goh J. Z. W., Jin J., Teo B. K. K., Law J. B. K., Low H. Y., Goh E. L. K., Sheetz M. P., and Yim E. K. F., “ Microarray with micro- and nano-topographies enables identification of the optimal topography for directing the differentiation of primary murine neural progenitor cells,” Small 8, 3050–3061 (2012). 10.1002/smll.201200490 [DOI] [PubMed] [Google Scholar]
- 118. Mei F., Fancy S. P. J., Shen Y. A. A., Niu J., Zhao C., Presley B., Miao E., Lee S., Mayoral S. R., Redmond S. A., Etxeberria A., Xiao L., Franklin R. J. M., Green A., Hauser S. L., and Chan J. R., “ Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis,” Nat. Med. 20, 954–960 (2014). 10.1038/nm.3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ankam S., Lim C. K., and Yim E. K. F., “ Actomyosin contractility plays a role in MAP2 expression during nanotopography-directed neuronal differentiation of human embryonic stem cells,” Biomaterials 47, 20–28 (2015). 10.1016/j.biomaterials.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 120. Gill E. L., Willis S., Gerigk M., Cohen P., Zhang D., Li X., and Huang Y. Y. S., “ Fabrication of designable and suspended microfibers via low-voltage 3D micropatterning,” ACS Appl. Mater. Interfaces 11, 19679–19690 (2019). 10.1021/acsami.9b01258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee S., Chong S. Y. C., Tuck S. J., Corey J. M., and Chan J. R., “ A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers,” Nat. Protoc. 8, 771–782 (2013). 10.1038/nprot.2013.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bechler M. E., Byrne L., and Ffrench-Constant C., “ CNS Myelin Sheath lengths are an intrinsic property of oligodendrocytes,” Curr. Biol. 25, 2411–2416 (2015). 10.1016/j.cub.2015.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Diao H. J., Low W. C., Lu Q. R., and Chew S. Y., “ Topographical effects on fiber-mediated microRNA delivery to control oligodendroglial precursor cells development,” Biomaterials 70, 105–114 (2015). 10.1016/j.biomaterials.2015.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ong W., Lin J., Bechler M. E., Wang K., Wang M., Ffrench-Constant C., and Chew S. Y., “ Microfiber drug/gene delivery platform for study of myelination,” Acta Biomater. 75, 152–160 (2018). 10.1016/j.actbio.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 125. Ong W., Marinval N., Lin J., Nai M. H., Chong Y. S., Pinese C., Sajikumar S., Lim C. T., Ffrench-Constant C., Bechler M. E., and Chew S. Y., “ Biomimicking fiber platform with tunable stiffness to study mechanotransduction reveals stiffness enhances oligodendrocyte differentiation but impedes myelination through YAP-dependent regulation,” Small 16, 2003656–2003613 (2020). 10.1002/smll.202003656 [DOI] [PubMed] [Google Scholar]
- 126. Lariosa-Willingham K. D., Rosler E. S., Tung J. S., Dugas J. C., Collins T. L., and Leonoudakis D., “ Development of a central nervous system axonal myelination assay for high throughput screening,” BMC Neurosci. 17, 1–13 (2016). 10.1186/s12868-016-0250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Espinosa-Hoyos D., Jagielska A., Homan K. A., Du H., Busbee T., Anderson D. G., Fang N. X., Lewis J. A., and Van Vliet K. J., “ Engineered 3D-printed artificial axons,” Sci. Rep. 8, 1–13 (2018). 10.1038/s41598-017-18744-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Rashid B., Destrade M., and Gilchrist M. D., “ Mechanical characterization of brain tissue in compression at dynamic strain rates,” J. Mech. Behav. Biomed. Mater. 10, 23–38 (2012). 10.1016/j.jmbbm.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 129. Love J. M., Bober B. G., Orozco E., White A. T., Bremner S. N., Lovering R. M., Schenk S., and Shah S. B., “ mTOR regulates peripheral nerve response to tensile strain,” J. Neurophysiol. 117, 2075–2084 (2017). 10.1152/jn.00257.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tricaud N., “ Myelinating Schwann Cell Polarity and Mechanically-Driven Myelin Sheath Elongation,” Front. Cell. Neurosci. 11, 1–12 (2018). 10.3389/fncel.2017.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pelham R. J. and Wang Y.-L., “ Cell locomotion and focal adhesions are regulated by substrate flexibility,” Proc. Natl. Acad. Sci. 94, 13661–13665 (1997). 10.1073/pnas.94.25.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schwarz U. S. and Safran S. A., “ Physics of adherent cells,” Rev. Mod. Phys. 85, 1327–1381 (2013). 10.1103/RevModPhys.85.1327 [DOI] [Google Scholar]
- 133. Hernandez M., Patzig J., Mayoral S. R., Costa K. D., Chan J. R., and Casaccia P., “ Mechanostimulation promotes nuclear and epigenetic changes in oligodendrocytes,” J. Neurosci. 36, 806–813 (2016). 10.1523/JNEUROSCI.2873-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jagielska A., Lowe A. L., Makhija E., Wroblewska L., Guck J., Franklin R. J. M., Shivashankar G. V., and Van Vliet K. J., “ Mechanical strain promotes oligodendrocyte differentiation by global changes of gene expression,” Front. Cell. Neurosci. 11, 1–16 (2017). 10.3389/fncel.2017.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Arulmoli J., Pathak M. M., McDonnell L. P., Nourse J. L., Tombola F., Earthman J. C., and Flanagan L. A., “ Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner,” Sci. Rep. 5, 1–8 (2015). 10.1038/srep08499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ostrow L. W., Suchyna T. M., and Sachs F., “ Stretch induced endothelin-1 secretion by adult rat astrocytes involves calcium influx via stretch-activated ion channels (SACs),” Biochem. Biophys. Res. Commun. 410, 81–86 (2011). 10.1016/j.bbrc.2011.05.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Katiyar K. S., Winter C. C., Struzyna L. A., Harris J. P., and Cullen D. K., “ Mechanical elongation of astrocyte processes to create living scaffolds for nervous system regeneration,” J. Tissue Eng. Regener. Med. 11, 2737–2751 (2017). 10.1002/term.2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kamble H., Vadivelu R., Barton M., Shiddiky M. J. A., and Nguyen N.-T., “ Pneumatically actuated cell-stretching array platform for engineering cell patterns in vitro,” Lab Chip 18, 765–774 (2018). 10.1039/C7LC01316G [DOI] [PubMed] [Google Scholar]
- 139. Huang W., Zhang S., Ahmad B., and Kawahara T., “ Three-motorized-stage cyclic stretching system for cell monitoring based on chamber local displacement waveforms,” Appl. Sci. 9, 1560 (2019). 10.3390/app9081560 [DOI] [Google Scholar]
- 140. Riggio C., Calatayud M. P., Hoskins C., Pinkernelle J., Sanz B., Torres T. E., Ibarra M. R., Wang L., Keilhoff G., Goya G. F., Raffa V., and Cuschieri A., “ Poly-l-lysine-coated magnetic nanoparticles as intracellular actuators for neural guidance,” Int. J. Nanomed. 7, 3155–3166 (2012). 10.2147/ijn.s28460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Huang L., Xia B., Liu Z., Cao Q., Huang J., and Luo Z., “ Superparamagnetic iron oxide nanoparticle-mediated forces enhance the migration of Schwann cells across the astrocyte-Schwann cell boundary in vitro,” Front. Cell. Neurosci. 11, 1–14 (2017). 10.3389/fncel.2017.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shen Y., Cheng Y., Uyeda T. Q. P., and Plaza G. R., “ Cell mechanosensors and the possibilities of using magnetic nanoparticles to study them and to modify cell fate,” Ann. Biomed. Eng. 45, 2475–2486 (2017). 10.1007/s10439-017-1884-7 [DOI] [PubMed] [Google Scholar]
- 143. Stukel J. M. and Willits R. K., “ Mechanotransduction of neural cells through cell-substrate interactions,” Tissue Eng., Part B 22, 173–182 (2016). 10.1089/ten.teb.2015.0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cheng J., Wang S., Dong Y., and Yuan Z., “ The role and regulatory mechanism of hippo signaling components in the neuronal system,” Front. Immunol. 11, 1–7 (2020). 10.3389/fimmu.2020.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wang H., Rusielewicz T., Tewari A., Leitman E. M., Einheber S., and Melendez-Vasquez C. V., “ Myosin II is a negative regulator of oligodendrocyte morphological differentiation,” J. Neurosci. Res. 90, 1547–1556 (2012). 10.1002/jnr.23036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Nawaz S., Sánchez P., Schmitt S., Snaidero N., Mitkovski M., Velte C., Brückner B. R., Alexopoulos I., Czopka T., Jung S. Y., Rhee J. S., Janshoff A., Witke W., Schaap I. A. T., Lyons D. A., and Simons M., “ Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system,” Dev. Cell 34, 139–151 (2015). 10.1016/j.devcel.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Das A., Fischer R. S., Pan D., and Waterman C. M., “ YAP nuclear localization in the absence of cell-cell contact is mediated by a filamentous actin-dependent, Myosin II and phospho-YAP-independent pathway during extracellular matrix mechanosensing,” J. Biol. Chem. 291, 6096–6110 (2016). 10.1074/jbc.M115.708313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Koser D. E., Thompson A. J., Foster S. K., Dwivedy A., Pillai E. K., Sheridan G. K., Svoboda H., Viana M., Costa L. D. F., Guck J., Holt C. E., and Franze K., “ Mechanosensing is critical for axon growth in the developing brain,” Nat. Neurosci. 19, 1592–1598 (2016). 10.1038/nn.4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Velasco-Estevez M., Gadalla K. K. E., Liñan-Barba N., Cobb S., Dev K. K., and Sheridan G. K., “ Inhibition of Piezo1 attenuates demyelination in the central nervous system,” Glia 68, 356–375 (2020). 10.1002/glia.23722 [DOI] [PubMed] [Google Scholar]
- 150. Velasco-Estevez M., Rolle S. O., Mampay M., Dev K. K., and Sheridan G. K., “ Piezo1 regulates calcium oscillations and cytokine release from astrocytes,” Glia 68, 145–160 (2020). 10.1002/glia.23709 [DOI] [PubMed] [Google Scholar]
- 151. Antonovaite N., Beekmans S. V., Hol E. M., Wadman W. J., and Iannuzzi D., “ Structure-stiffness relation of live mouse brain tissue determined by depth-controlled indentation mapping,” arXiv:1802.02245. [DOI] [PMC free article] [PubMed]
- 152. Weickenmeier J., de Rooij R., Budday S., Ovaert T. C., and Kuhl E., “ The mechanical importance of myelination in the central nervous system,” J. Mech. Behav. Biomed. Mater. 76, 119–124 (2017). 10.1016/j.jmbbm.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 153. Weickenmeier J., Kurt M., Ozkaya E., de Rooij R., Ovaert T. C., Ehman R. L., Butts Pauly K., and Kuhl E., “ Brain stiffens post mortem,” J. Mech. Behav. Biomed. Mater. 84, 88–98 (2018). 10.1016/j.jmbbm.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Digabel J. L., Forcato M., Bicciato S., Elvassore N., and Piccolo S., “ Role of YAP/TAZ in mechanotransduction,” Nature 474, 179–183 (2011). 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 155. Xie J., Bao M., Bruekers M. C., and Huck W. T. S., “ Collagen gels with different fibrillar microarchitectures elicit different cellular responses,” ACS Appl. Mater. Interface 9, 19630–19637 (2017). 10.1021/acsami.7b03883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Sawhney R. K. and Howard J., “ Slow local movements of collagen fibers by fibroblasts drive the rapid global self-organization of collagen gels,” J. Cell Biol. 157, 1083–1091 (2002). 10.1083/jcb.200203069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Winer J. P., Oake S., and Janmey P. A., “ Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation,” PLoS One 4, e6382 (2009). 10.1371/journal.pone.0006382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Karzbrun E., Kshirsagar A., Cohen S. R., Hanna J. H., and Reiner O., “ Human brain organoids on a chip reveal the physics of folding,” Nat. Phys. 14, 515–522 (2018). 10.1038/s41567-018-0046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Abuwarda H. and Pathak M. M., “ Mechanobiology of neural development,” Curr. Opin. Cell Biol. 66, 104–111 (2020). 10.1016/j.ceb.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Biswas S., Chung S. H., Jiang P., and Dehghan S., “ Development of glial restricted human neural stem cells for oligodendrocyte differentiation in vitro and in vivo,” Sci. Rep. 9, 1–14 (2019). 10.1038/s41598-019-45247-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Madhavan M., Nevin Z. S., Shick H. E., Garrison E., Clarkson-Paredes C., Karl M., Clayton B. L. L., Factor D. C., Allan K. C., Barbar L., Jain T., Douvaras P., Fossati V., Miller R. H., and Tesar P. J., “ Induction of myelinating oligodendrocytes in human cortical spheroids,” Nat. Methods 15, 700–706 (2018). 10.1038/s41592-018-0081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Centeno E. G. Z., Cimarosti H., and Bithell A., “ 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling,” Mol. Neurodegener. 13, 1–16 (2018). 10.1186/s13024-018-0258-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Liu J., Dupree J. L., Gacias M., Frawley R., Sikder T., Naik P., and Casaccia P., “ Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice,” J. Neurosci. 36, 957–962 (2016). 10.1523/JNEUROSCI.3608-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Green A. J., Gelfand J. M., Cree B. A., Bevan C., Boscardin W. J., Mei F., Inman J., Arnow S., Devereux M., Abounasr A., Nobuta H., Zhu A., Friessen M., Gerona R., von Büdingen H. C., Henry R. G., Hauser S. L., and Chan J. R., “ Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): A randomised, controlled, double-blind, crossover trial,” Lancet 390, 2481–2489 (2017). 10.1016/S0140-6736(17)32346-2 [DOI] [PubMed] [Google Scholar]
- 165. Cree B. A. C., Niu J., Hoi K. K., Zhao C., Caganap S. D., Henry R. G., Dao D. Q., Zollinger D. R., Mei F., Shen Y. A. A., Franklin R. J. M., Ullian E. M., Xiao L., Chan J. R., and Fancy S. P. J., “ Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury,” Brain 141, 85–98 (2018). 10.1093/brain/awx312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Moshayedi P., Ng G., Kwok J. C. F., Yeo G. S. H., Bryant C. E., Fawcett J. W., Franze K., and Guck J., “ The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system,” Biomaterials 35, 3919–3925 (2014). 10.1016/j.biomaterials.2014.01.038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.