Abstract
The ability to monitor protein biomarkers continuously and in real-time would significantly advance the precision of medicine. Current protein-detection techniques, however, including ELISA and lateral flow assays, provide only time-delayed, single-time-point measurements, limiting their ability to guide prompt responses to rapidly evolving, life-threatening conditions. In response, here we present an electrochemical aptamer-based sensor (EAB) that supports high-frequency, real-time biomarker measurements. Specifically, we have developed an electrochemical, aptamer-based (EAB) sensor against Neutrophil Gelatinase-Associated Lipocalin (NGAL), a protein that, if present in urine at levels above a threshold value, is indicative of acute renal/kidney injury (AKI). When deployed inside a urinary catheter, the resulting reagentless, wash-free sensor supports real-time, high-frequency monitoring of clinically relevant NGAL concentrations over the course of hours. By providing an “early warning system”, the ability to measure levels of diagnostically relevant proteins such as NGAL in real-time could fundamentally change how we detect, monitor, and treat many important diseases.
Keywords: real-time protein monitoring, aptamer, electrochemical sensor, neutrophil gelatinase-associated lipocalin, acute kidney injury
Graphical Abstract
The ability to measure biomarkers at clinically relevant concentrations and in real-time would create a potentially powerful new approach to monitor health status.1-4 Such a technology, for example, could change the way we diagnose (e.g., monitoring of cardiac troponin to warn of an incipient heart attack)5 and treat (e.g., monitoring the levels of cytokines to optimize the antibiotic treatment of sepsis)6 diseases. All current methods for measuring protein biomarkers in clinical samples, however, either are batch processes ill-suited for high-frequency, real-time monitoring or are unable to cope with complex clinical samples. ELISAs, for example, are batch processes that typically take hours to return an answer and require specialized personnel running bulky, expensive equipment in a dedicated laboratory, precluding real-time monitoring.7-9 Lateral flow assays, in contrast, provide results in 10 to 15 min, but they also require multiple sampling/sensing steps and provide merely semiquantitative results, characteristics that preclude frequent, quantitative monitoring.10,11 In contrast, multiple biosensor approaches have been described (including surface plasmon resonance,12 biolayer interferometry,13 electrical impedance spectroscopy,14 field effect transistors15) that support the continuous, real-time measurement of specific proteins. However, because their signaling mechanisms are all predicated on monitoring adsorption (e.g., changes in the mass, charge, or optical properties of the material adsorbed to the sensor surface), none survive deployment in authentic, unmodified clinical samples.
The absence of a technology that supports high-frequency, real-time measurement of protein concentrations remains a critical void in our healthcare.16 Methods for the continuous, real-time measurement of small molecules, in contrast, are better established. The continuous glucose monitor, for example, has vastly improved the management of diabetes,17 but because it relies critically on the specific chemical reactivity of its target, this technology is not generalizable to the measurement of other molecules.18,19 In response, we have been developing electrochemical aptamer-based (EAB) sensors, a platform technology supporting continuous, high-frequency molecular monitoring irrespective of the chemical or enzymatic reactivity of the target. Specifically, we have demonstrated sensors in this class supporting the real-time measurement20 of multiple low-molecular-weight drugs21,22 and toxins23,24 in situ in the circulatory system and in situ in flowing streams of foodstuffs, respectively.
While the monitoring of small molecules using EAB sensors is established, the technology had not previously been adapted to the continuous, real-time measurement of protein biomarkers. Here, we explore this idea using the high-frequency, real-time monitoring of Neutrophil Gelatinase-Associated Lipocalin (NGAL), a protein that appears in urine upon acute renal injury (also referred to as acute kidney injury, or AKI).25,26
AKI is a life-threatening complication observed after major surgeries and even some minor procedures (e.g., contrast-induced nephropathy).27 The current gold standard for diagnosing AKI is the measurement of serum creatinine, whose level rises several hours after the onset of the renal failure. The slow time scale of this diagnostic, however, greatly reduces its value. Urinary NGAL, in contrast, rises quite rapidly after the onset of AKI,28 which would allow for diagnosis—and intervention—far earlier in the disease process. Unfortunately, however, NGAL is currently detected using ELISAs, which are slow and do not support real-time monitoring.29 It thus appears that, by supporting the prompt deployment of additional treatment before irreversible kidney damage occurs,30 the ability to monitor clinically relevant concentrations of NGAL (i.e., excursions above reported cutoffs lying between 2 and 32 nM) in real-time could significantly improve outcomes for patients suffering from acute renal injury.
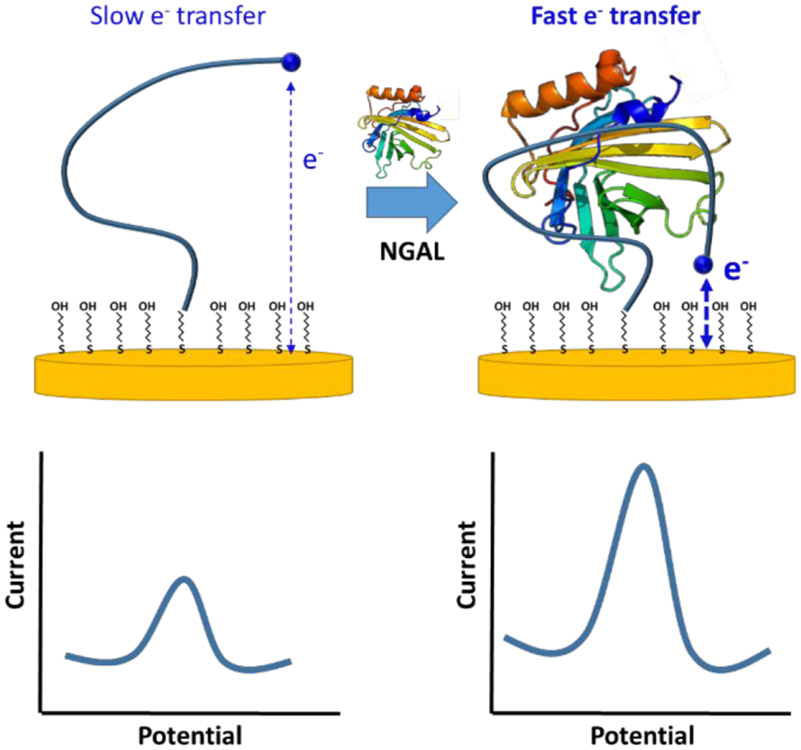
EAB sensors employ electrochemistry to interrogate a binding-induced conformational change in an electrode-bound aptamer (Figure 1). In order to achieve this, we modify one end of the aptamer with the redox reporter methylene blue (to generate a signal) and the other with a thiol (for attachment to a gold electrode). Any binding-induced change in the conformation of the aptamer thus changes in turn the rate of electron transfer from the methylene blue, generating a measurable electrochemical signal that monotonically increases with target concentration. Here, we have used an NGAL-binding aptamer previously shown to support optical detection of this biomarker.31 To introduce the necessary binding-induced conformational change, we truncated the parent sequence, thus destabilizing it, under the argument that aptamer folding is sufficiently cooperative,32 that such destabilization produces binding-induced folding.33 This couples recognition with folding, i.e., with the largest possible conformational change. Specifically, we created seven sequential truncation variants (details in Figure 1S in Supporting Information) from which we selected a 26-base variant as representing the optimal trade-off between high signal gain and high affinity.34
Figure 1.
EAB sensors employ the binding-induced folding of a redox-reporter-modified aptamer to generate an easily measurable, rapidly reversible electrochemical signal (the changes in conformation affects the rate of electron transfer thus changing the intensity of the electrochemical signal) without the need for exogenous reagents or wash steps, thus enabling continuous, real-time molecular monitoring. The platform’s binding-induced folding signal transduction mechanism is highly selective, allowing EAB sensors to perform even when challenged in complex sample streams.
The NGAL-detecting EAB sensor provides the sensitivity and specificity necessary to achieve clinically relevant monitoring for potential kidney injury. To demonstrate this, we first challenged the sensor against its target spiked into either artificial or authentic urine (the picomolar basal level of the target in urine of healthy subjects does not interfere; Figure 2S of Supporting Information) seeing in both scenarios ~20% signal change at 32 nM NGAL (Figure 2A; and see Figure 1S and 4S for full calibration curves). As indicated by the near-identical sensor performance in synthetic (i.e., protein-free) and authentic urine, the sensor does not detectably respond to any of the 1500 proteins in urine at their endogenous levels.35 Likewise, it does not respond any of three other model proteins tested even at much higher concentration (Figure 3S). Based on these in vitro titrations, we estimate detection limits (at the 3σ confidence level) of 2 and 3.5 nM, respectively, in artificial and authentic urine. Given the 2 to 32 nM thresholds for NGAL indicative of kidney damage reported in the prior literature,30 these limits of detection appear sufficient to support clinically relevant monitoring.
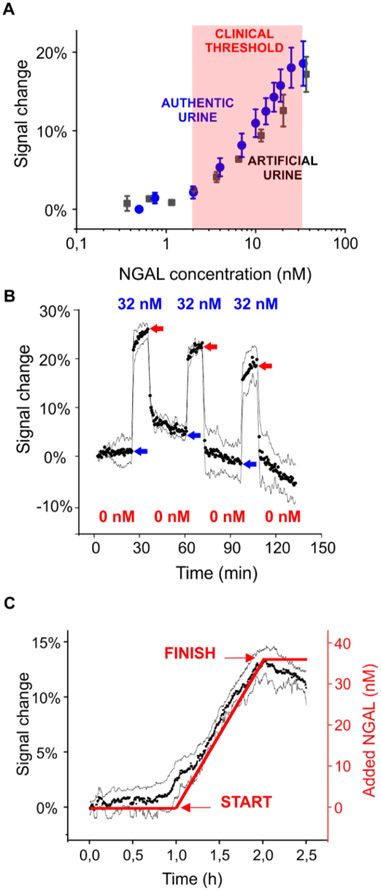
Figure 2.
Real-time monitoring of clinically relevant threshold levels of NGAL in urine. (A) Threshold NGAL level indicative of diseases has variously been reported30 to be from 2 to 32 nM. The useful dynamic range of our sensor spans these concentrations in both artificial urine and authentic human urine. The data represent the average signals and the standard deviations of at least three independently fabricated sensors, illustrating the excellent sensor-to-sensor reproducibility we achieve even with these hand-held devices. (B) The sensor responds promptly to the addition (blue arrows) and removal (red arrows) of NGAL. Shown are three cycles of addition (32 nM) and removal of NGAL over 2 h. (C) The sensor easily monitors a continuous rise in (spiked) NGAL that mimics the profile expected for acute renal injury.
To perform continuous clinical monitoring, a sensor must equilibrate rapidly relative to the time course of the disease being monitored and remain stable relative to its duration. In addition, while the rate with which NGAL levels change in clinical scenarios is not yet known (precisely because of the inadequacy of current techniques), it is clear that minutes of time resolution over the course of a few hours during and immediately after surgery is more than adequate for clinical needs.26 The optimized EAB sensor achieves these attributes. For example, upon a challenge with 32 nM NGAL, the sensor equilibration time constant is on the order of 1 min (Figure 2B), which provides the time resolution necessary to support prompt intervention by healthcare personnel. Likewise, the removal of NGAL from the solution induces the recovery of the baseline value also on the order of 1 min. To model a more realistic clinical scenario, we challenged our sensor by steadily adding NGAL to a urine stream over the course of 1 h to a final concentration of 32 nM, the highest clinically indicative threshold reported in the literature. As expected, the electrochemical signal rose with the start and stopped rising with the termination of this continuous injection (Figure 2C).
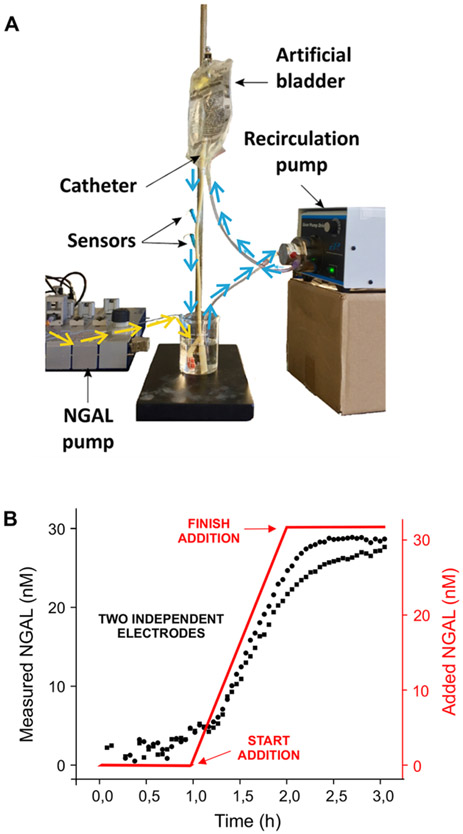
To perform the real-time monitoring of kidney health in patients suffering from acute kidney damage, the sensing platform must access urinary NGAL without the intervention of healthcare personnel. To model this, we fabricated sensors on microelectrodes (the calibration curve we obtained using these is available in Figure 4S of Supporting Information),36 two of which we then placed into a 14fr Foley (i.e., urinary) catheter via an IV needle. We simulated a clinical scenario by inserting the modified catheter into an IV bag (representing the bladder) and using two pumps: one for recirculating the urine and one for injecting NGAL (Figure 3A). We then monitored NGAL concentrations over 3 h, during which we added NGAL (from 0 to 32 nM) to the urine sample stream over the course of 1 h (Figure 3B). As expected, the sensor again responded quantitatively to the increasing NGAL concentration.
Figure 3.
EAB sensors can be used to monitor NGAL levels in situ in a urinary catheter. (A) In order to mimic a real-life situation, we created a system that passes artificial urine through a 14fr Foley catheter that contains two micron-scale EAB sensors. In particular, using a pump we increased the level of NGAL in the reservoir at the bottom (yellow lines), while a second pump circulates the artificial urine through an IV bag (representing an artificial bladder) and then through the catheter (blue lines). Using this, we monitored NGAL concentrations with 3 min resolution over the course of 3 h. (B) At 1 h, we started introducing NGAL, linearly increasing its concentration over the course of 1 h to 32 nM. As shown, both sensors responded to the addition in real-time and with closely similar responses. The few minutes of lag time between the addition of NGAL and the first sensor response is associated with the time required for NGAL to pass through the pump and the artificial bladder before reaching the sensors.
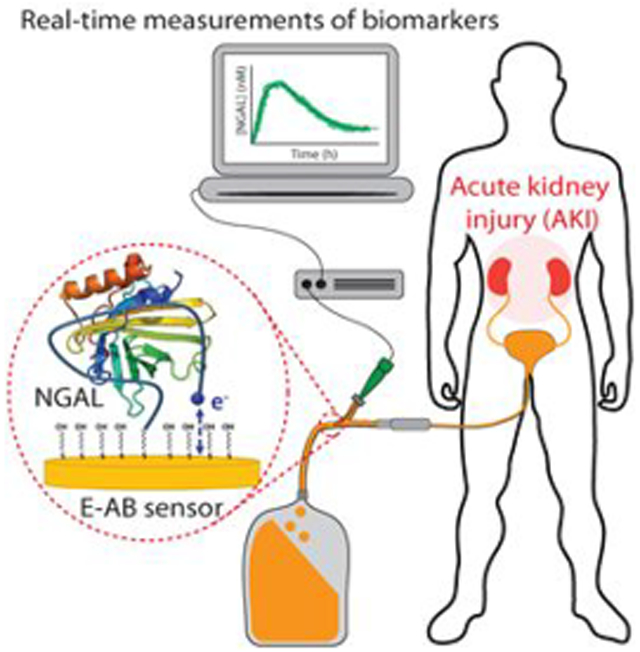
Here, we have achieved the first high-frequency, real-time monitoring of a protein biomarker in a simulated clinical scenario via an approach that does not require any sample processing or any other active intervention from healthcare personnel. Specifically, we have demonstrated the ability of an EAB sensor to monitor urinary NGAL in authentic urine samples and inside a urinary catheter with subminute time resolution and at concentrations corresponding to the threshold levels indicative of acute renal injury. At the immediate level, the work presented here illustrates a potentially powerful new tool to monitoring the occurrence of surgery-related acute renal injury, providing an opportunity for clinicians to intervene before permanent kidney damage occurs. More broadly, when taken with the modular architecture of the platform, and its good shelf (currently over 1 month)37 and operational (up to 12 h in blood)38 lifetimes, the work presented here suggests that EAB sensors may prove a powerful means of monitoring protein biomarkers in general, an advance that would enable many early warning systems in health care.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by Grant EB022015 from the National Institutes of Health. C.P. thanks the Generalitat de Catalunya for the Beatriu de Pino’s fellowship (2014 BP_A 00068).
Footnotes
The authors declare the following competing financial interest(s): Q. Yang, B.S. Ferguson and J. Wang are employees and share-holders of Aptitude Medical Systems, Inc.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssensors.0c01085.
Materials and methods section, optimization of the electrochemical sensing parameters, results obtained using the commercial ELISA test against patient samples, specificity studies, and calibration obtained using gold microelectrodes (PDF)
Contributor Information
Claudio Parolo, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, California 93106, United States.
Andrea Idili, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, California 93106, United States.
Gabriel Ortega, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, California 93106, United States.
Andrew Csordas, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, California 93106, United States.
Alex Hsu, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, California 93106, United States.
Netzahualcóyotl Arroyo-Currás, Department of Pharmacology and Molecular Sciences, Johns Hopkins School of Medicine, Baltimore, Maryland 21205, United States.
Qin Yang, Aptitude Medical Systems, Inc., Santa Barbara, California 93105, United States.
Brian Scott Ferguson, Aptitude Medical Systems, Inc., Santa Barbara, California 93105, United States.
Jinpeng Wang, Aptitude Medical Systems, Inc., Santa Barbara, California 93105, United States.
Kevin W. Plaxco, Department of Chemistry and Biochemistry and Interdepartmental Program in Biomolecular Science and Engineering, University of California, Santa Barbara, Santa Barbara, California 93106, United States.
REFERENCES
- (1).ten Kate CA; Tibboel D; Kraemer US B-Type Natriuretic Peptide as a Parameter for Pulmonary Hypertension in Children. A Systematic Review. Eur. J. Pediatr 2015, 174 (10), 1267–1275. [DOI] [PubMed] [Google Scholar]
- (2).Kyle RA; Therneau TM; Rajkumar SV; Offord JR; Larson DR; Plevak MF; Melton LJ A Long-Term Study of Prognosis in Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med 2002, 346 (8), 564–569. [DOI] [PubMed] [Google Scholar]
- (3).Kim D-H; Cho I-H; Park J-N; Paek S-H; Cho H-M; Paek S-H Semi-Continuous, Real-Time Monitoring of Protein Biomarker Using a Recyclable Surface Plasmon Resonance Sensor. Biosens. Bioelectron 2017, 88, 232–239. [DOI] [PubMed] [Google Scholar]
- (4).Visser EWA; Yan J; van IJzendoorn LJ; Prins MWJ Continuous Biomarker Monitoring by Particle Mobility Sensing with Single Molecule Resolution. Nat. Commun 2018, 9 (1), 2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Garg P; Morris P; Fazlanie AL; Vijayan S; Dancso B; Dastidar AG; Plein S; Mueller C; Haaf P Cardiac Biomarkers of Acute Coronary Syndrome: From History to High-Sensitivity Cardiac Troponin. Int. Emerg. Med 2017, 12 (2), 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Faix JD Biomarkers of Sepsis. Crit. Rev. Clin. Lab. Sci 2013, 50 (1), 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Tighe PJ; Ryder RR; Todd I; Fairclough LC ELISA in the Multiplex Era: Potentials and Pitfalls. Proteomics: Clin. Appl 2015, 9 (3–4), 406–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Dong J; Ueda H ELISA-Type Assays of Trace Biomarkers Using Microfluidic Methods. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 2017, 9 (5), No. e1457. [DOI] [PubMed] [Google Scholar]
- (9).Iha K; Inada M; Kawada N; Nakaishi K; Watabe S; Tan YH; Shen C; Ke L-Y; Yoshimura T; Ito E Ultrasensitive ELISA Developed for Diagnosis. Diagnostics 2019, 9 (3), 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).de Puig H; Bosch I; Gehrke L; Hamad-Schifferli K Challenges of the Nano-Bio Interface in Lateral Flow and Dipstick Immunoassays. Trends Biotechnol 2017, 35 (12), 1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Parolo C; Merkoçi A Paper-Based Nanobiosensors for Diagnostics. Chem. Soc. Rev 2013, 42 (2), 450–457. [DOI] [PubMed] [Google Scholar]
- (12).Liu C; Lei T; Ino K; Matsue T; Tao N; Li C-Z Real-Time Monitoring Biomarker Expression of Carcinoma Cells by Surface Plasmon Resonance Biosensors. Chem. Commun 2012, 48 (84), 10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Sultana A; Lee JE Measuring Protein-Protein and Protein-Nucleic Acid Interactions by Biolayer Interferometry. Curr. Protoc. Protein Sci 2015, 79 (1), 1–26. [DOI] [PubMed] [Google Scholar]
- (14).Katz E; Willner I Probing Biomolecular Interactions at Conductive and Semiconductive Surfaces by Impedance Spectroscopy: Routes to Impedimetric Immunosensors, DNA-Sensors, and Enzyme Biosensors. Electroanalysis 2003, 15 (11), 913–947. [Google Scholar]
- (15).Angione MD; Pilolli R; Cotrone S; Magliulo M; Mallardi A; Palazzo G; Sabbatini L; Fine D; Dodabalapur A; Cioffi N; et al. Carbon Based Materials for Electronic Bio-Sensing. Mater. Today 2011, 14 (9), 424–433. [Google Scholar]
- (16).Scida K; Plaxco KW; Jamieson BG High Frequency, Real-Time Neurochemical and Neuropharmacological Measurements in Situ in the Living Body. Transl Res. 2019, 213, 50–66. [DOI] [PubMed] [Google Scholar]
- (17).Rigla M; Hernando ME; Gómez EJ; Brugués E; García-Sáez G; Capel I; Pons B; de Leiva A Real-Time Continuous Glucose Monitoring Together with Telemedical Assistance Improves Glycemic Control and Glucose Stability in Pump-Treated Patients. Diabetes Technol Ther. 2008, 10 (3), 194–199. [DOI] [PubMed] [Google Scholar]
- (18).Ascaso FJ; Huerva V Noninvasive Continuous Monitoring of Tear Glucose Using Glucose-Sensing Contact Lenses. Optom. Vis. Sci 2016, 93 (4), 426–434. [DOI] [PubMed] [Google Scholar]
- (19).Beck RW; Bergenstal RM; Laffel LM; Pickup JC Advances in Technology for Management of Type 1 Diabetes. Lancet 2019, 394 (10205), 1265–1273. [DOI] [PubMed] [Google Scholar]
- (20).Curtis SD; Ploense KL; Kurnik M; Ortega G; Parolo C; Kippin TE; Plaxco KW; Arroyo-Currás N Open Source Software for the Real-Time Control, Processing, and Visualization of High-Volume Electrochemical Data. Anal. Chem 2019, 91 (19), 12321–12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Idili A; Arroyo-Currás N; Ploense KL; Csordas AT; Kuwahara M; Kippin TE; Plaxco KW Seconds-Resolved Pharmacokinetic Measurements of the Chemotherapeutic Irinotecan in Situ in the Living Body. Chem. Sci 2019, 10 (35), 8164–8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Arroyo-Currás N; Somerson J; Vieira PA; Ploense KL; Kippin TE; Plaxco KW Real-Time Measurement of Small Molecules Directly in Awake, Ambulatory Animals. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (4), 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Somerson J; Plaxco KW Electrochemical Aptamer-Based Sensors for Rapid Point-of-Use Monitoring of the Mycotoxin Ochratoxin A Directly in a Food Stream. Molecules 2018, 23 (4), 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Li H; Somerson J; Xia F; Plaxco KW Electrochemical DNA-Based Sensors for Molecular Quality Control: Continuous, Real-Time Melamine Detection in Flowing Whole Milk. Anal. Chem 2018, 90 (18), 10641–10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Azzalini L; Spagnoli V; Ly HQ Contrast-Induced Nephropathy: From Pathophysiology to Preventive Strategies. Can. J. Cardiol 2016, 32 (2), 247–255. [DOI] [PubMed] [Google Scholar]
- (26).Almendarez M; Gurm HS; Mariani J; Montorfano M; Brilakis ES; Mehran R; Azzalini L Procedural Strategies to Reduce the Incidence of Contrast-Induced Acute Kidney Injury During Percutaneous Coronary Intervention. JACC Cardiovasc. Interv 2019, 12 (19), 1877–1888. [DOI] [PubMed] [Google Scholar]
- (27).Azzalini L; Spagnoli V; Ly HQ Contrast-Induced Nephropathy: From Pathophysiology to Preventive Strategies. Can. J. Cardiol 2016, 32 (2), 247–255. [DOI] [PubMed] [Google Scholar]
- (28).Makris K; Markou N; Evodia E; Dimopoulou E; Drakopoulos I; Ntetsika K; Rizos D; Baltopoulos G; Haliassos A Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) as an Early Marker of Acute Kidney Injury in Critically Ill Multiple Trauma Patients. Clin. Chem. Lab. Med 2009, 47 (1), 79–82. [DOI] [PubMed] [Google Scholar]
- (29).Krzeminska E; Wyczalkowska-Tomasik A; Korytowska N; Paczek L Comparison of Two Methods for Determination of NGAL Levels in Urine: ELISA and CMIA. J. Clin. Lab. Anal 2016, 30 (6), 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Soni SS; Cruz D; Bobek I; Chionh CY; Nalesso F; Lentini P; de Cal M; Corradi V; Virzi G; Ronco C NGAL: A Biomarker of Acute Kidney Injury and Other Systemic Conditions. Int. Urol. Nephrol 2010, 42 (1), 141–150. [DOI] [PubMed] [Google Scholar]
- (31).Wang J; Yu J; Yang Q; McDermott J; Scott A; Vukovich M; Lagrois R; Gong Q; Greenleaf W; Eisenstein M; et al. Multiparameter Particle Display (MPPD): A Quantitative Screening Method for the Discovery of Highly Specific Aptamers. Angew. Chem., Int. Ed 2017, 56 (3), 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lawrence C; Vallée-Bélisle A; Pfeil SH; de Mornay D; Lipman EA; Plaxco KW A Comparison of the Folding Kinetics of a Small, Artificially Selected DNA Aptamer with Those of Equivalently Simple Naturally Occurring Proteins. Protein Sci. 2014, 23 (1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Vallée-Bélisle A; Plaxco KW Structure-Switching Biosensors: Inspired by Nature. Curr. Opin. Struct. Biol 2010, 20 (4), 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Vallee-Belisle A; Ricci F; Plaxco KW Thermodynamic Basis for the Optimization of Binding-Induced Biomolecular Switches and Structure-Switching Biosensors. Proc. Natl. Acad. Sci. U. S. A 2009, 106 (33), 13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Zou L; Sun W Human Urine Proteome: A Powerful Source for Clinical Research. Advances in experimental medicine and biology 2015, 845, 31–42, DOI: 10.1007/978-94-017-9523-4_4. [DOI] [PubMed] [Google Scholar]
- (36).Arroyo-Currás N; Scida K; Ploense KL; Kippin TE; Plaxco KW High Surface Area Electrodes Generated via Electrochemical Roughening Improve the Signaling of Electrochemical Aptamer-Based Biosensors. Anal. Chem 2017, 89 (22), 12185–12191. [DOI] [PubMed] [Google Scholar]
- (37).Lai RY; Seferos DS; Heeger AJ; Bazan GC; Plaxco KW Comparison of the Signaling and Stability of Electrochemical DNA Sensors Fabricated from 6- or 11-Carbon Self-Assembled Monolayers†. Langmuir 2006, 22 (25), 10796–10800. [DOI] [PubMed] [Google Scholar]
- (38).Shaver A; Curtis SD; Arroyo-Currás N Alkanethiol Monolayer End Groups Affect the Long-Term Operational Stability and Signaling of Electrochemical, Aptamer-Based Sensors in Biological Fluids. ACS Appl. Mater. Interfaces 2020, 12 (9), 11214–11223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.