Abstract
Aims
The benefit of prophylactic implantable cardioverter-defibrillator (ICD) is not uniform due to differences in the risk of life-threatening ventricular tachycardia (VT)/ventricular fibrillation (VF) and non-arrhythmic mortality. We aimed to develop an ICD benefit prediction score that integrates the competing risks.
Methods and results
The study population comprised all 4531 patients enrolled in the MADIT trials. Best-subsets Fine and Gray regression analysis was used to develop prognostic models for VT (≥200 b.p.m.)/VF vs. non-arrhythmic mortality (defined as death without prior sustained VT/VF). Eight predictors of VT/VF (male, age < 75 years, prior non-sustained VT, heart rate > 75 b.p.m., systolic blood pressure < 140 mmHg, ejection fraction ≤ 25%, myocardial infarction, and atrialarrhythmia) and 7 predictors of non-arrhythmic mortality (age ≥ 75 years, diabetes mellitus, body mass index < 23 kg/m2, ejection fraction ≤ 25%, New York Heart Association ≥II, ICD vs. cardiac resynchronization therapy with defibrillator, and atrial arrhythmia) were identified. The two scores were combined to create three MADIT-ICD benefit groups. In the highest benefit group, the 3-year predicted risk of VT/VF was three-fold higher than the risk of non-arrhythmic mortality (20% vs. 7%, P < 0.001). In the intermediate benefit group, the difference in the corresponding predicted risks was attenuated (15% vs. 9%, P < 0.01). In the lowest benefit group, the 3-year predicted risk of VT/VF was similar to the risk of non-arrhythmic mortality (11% vs. 12%, P = 0.41). A personalized ICD benefit score was developed based on the distribution of the two competing risks scores in the study population (https://is.gd/madit). Internal and external validation confirmed model stability.
Conclusions
We propose the novel MADIT-ICD benefit score that predicts the likelihood of prophylactic ICD benefit through personalized assessment of the risk of VT/VF weighed against the risk of non-arrhythmic mortality.
Keywords: Implantable cardioverter-defibrillator, ICD benefit, Primary prevention, Ventricular tachycardia, Ventricular tachyarrhythmia, Sudden cardiac death, Non-arrhythmic mortality, Risk score
Graphical Abstract
See page 1685 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa1102)
Introduction
Current guidelines provide a Class I recommendation for implantable cardioverter-defibrillator (ICD) implantation in patients with low left ventricular ejection fraction (LVEF). However, not all patients with a low LVEF derive consistent benefit from implantation of a primary prevention ICD,1 , 2 and improved selection of patients at risk of sudden arrhythmic death is warranted, aimed at closing the gap between scientific evidence, avoidable device complications, and the limited resources of healthcare systems.3
Improved selection for primary prevention ICD therapy in patients with a low LVEF can be achieved by weighing the patient-specific risk of life-threatening ventricular tachycardia (VT) and/or ventricular fibrillation (VF) (for which primary device implantation may be life-saving) against the competing risk of non-arrhythmic mortality (for which primary ICD implantation does not provide protection).
In the present study, we aimed to develop a risk prediction score among all ICD patients enrolled in the landmark Multicenter Automatic Defibrillator Implantation (MADIT) Trials that weighs patient-specific risk of VT/VF against the competing risk of non-arrhythmic mortality in order to facilitate the improved selection of patients for primary prevention ICD therapy.
Methods
Study population
The present study cohort comprises all 4503 patients with an ICD who were enrolled in the four MADIT studies conducted from July 1997 to December 2011 (MADIT-II,4 MADIT-CRT,5 MADIT-RIT,6 and MADIT-RISK7). External validation was carried out in patients implanted for primary prevention in the Ranolazine in High-Risk ICD Patients (RAID8) trial conducted between 2011 and 2017.
A brief description of each study is provided in Supplementary material online, Table A.
Endpoints and arrhythmia adjudication
All device therapies delivered in each of the trials were blindly adjudicated by at least two experienced electrophysiologists. Details of device programming and VT/VF definitions are provided in Supplementary material online, Table B.
The primary end point of the present study was the occurrence of life-threatening VT/VF defined as ICD-recorded, treated, or monitored sustained VT ≥ 200 b.p.m. or VF. Non-arrhythmic mortality was defined as death without experiencing sustained VT/VF at any time during follow-up).
We focused on life-threatening VT ≥200 b.p.m. or VF since our prior data from MADIT-RIT suggest that ICD therapy for lower rate ventricular tachyarrhythmias may not be life-saving.6 In a secondary analysis, we also evaluated the consistency of the results for the end point that included lower rates of sustained VTs, defined as of any VT >170 b.p.m. or VF.
Study design
The present study was carried out in three main steps. In the first step, we identified factors associated with increased risk for VT/VF, after accounting for non-arrhythmic mortality as a competing risk, and constructed a VT/VF risk-score based on the relative weight of factors that were predictive of an increased risk for VT/VF. The cohort was subsequently dichotomized based on patient-specific risk for VT/VF (defined as low VT/VF risk, and high VT/VF risk).
In the second step, we used a similar approach to construct the non-arrhythmic mortality risk-score for death without a prior VT/VF as the end point.
In the third step, we allocated each individual into a stratum that combined the VT/VF risk score and the non-arrhythmic mortality risk score by cross-tabulation of risks for both outcomes. Based on this analysis, we constructed the MADIT-ICD benefit score that was further subdivided into three groups for the integrated end point of the risk of VT/VF and its inverse correlation with the risk of non-arrhythmic mortality. The three MADIT benefit groups were defined as follows: (i) Highest MADIT benefit (greatest risk of VT/VF and lowest risk of non-arrhythmic mortality), (ii) Intermediate MADIT benefit (increasing risk of VT/VF and declining risk of non-arrhythmic mortality), and (iii) Lowest MADIT benefit (low VT/VF risk and very high risk of non-arrhythmic mortality) group.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Categorical data are summarized as frequencies and percentages. Baseline characteristics are presented for the entire study cohort and by the separate clinical trials comprising the database for descriptive purposes without a formal statistical comparison.
Step 1—VT/VF risk score
We included all potential risk factors for VT/VF that were available for the entire cohort (Supplementary material online, Table C) stratified by ischaemic origin. Multivariate Fine and Gray model, using non-arrhythmic mortality as a competing risk, and VT/VF as the end point, using best subsets selection model (P < 0.05 was determined as sufficient to enter the final model), was used to identify the variables for the final model.
Numeric variables were made binary by the use of cut-off points with the goal of finding a simple, easily implemented scoring method to be derived from them. Thresholds for categorization of numeric variables were pre-specified using clinical well-accepted criteria, with the exception of body mass index (BMI) which was categorized based on the quartile distribution among the cohort.
After selection of binary covariates, each was assigned a numeric value based on the relative value of its regression coefficient in the multivariate regression model. A VT/VF risk score was constructed for by adding the assigned numeric values of the factors identified in each patient, and the study population was dichotomized based on patient-specific score.
Step 2—Non-arrhythmic mortality risk score
A backward stepwise selection Cox regression model for the end point non-arrhythmic mortality was used including all the relevant variables that were included in step one. The same scoring and grouping method as for VT/VF (in step one) was applied for the mortality risk score.
Step 3—Outcome assessment by the MADIT-ICD benefit score
The two dichotomized competing risk scores were subsequently integrated to form the three MADIT-ICD benefit groups: (i) highest benefit group (high VT/VF score and low non-arrhythmic mortality score), (ii) intermediate benefit group (low VT/VF score and low non-arrhythmic score, or high VT/VF score and high non-arrhythmic mortality score), and (iii) lowest benefit group (low VT/VF score and high non-arrhythmic mortality score). Within each group, we used cumulative incidence function (CIF) curves to estimate the probability of first VT/VF, as the event of interest, and the probability of death without VT/VF as a competing risk. A personalized ICD benefit score was created to denote the expected benefit of prophylactic ICD implantation based on the matrix distribution of the competing risks scores in the study population, wherein 100 reflects the highest potential benefit and 0 the lowest potential benefit of the ICD in a primary prevention population.
Number of life-days gained by device implantation was estimated as the area between the survival curves using the restricted mean survival time measure (Supplementary material online, Figure A).9
Internal model validation
The survival prediction models for VT/VF and non-arrhythmic mortality (each tested separately) were validated by measuring discrimination. From the original study cohort, 1000 bootstrapped (by 100) samples were selected randomly with selection probability of 0.26. Discrimination was evaluated using the concordance index (Harrell’s C statistic).
External model validation
The external validation cohort comprised patients implanted with an ICD/cardiac resynchronization therapy with defibrillator (CRT-D) for primary prevention in the RAID trial.8 Importantly, the study did not show a significant effect of ranolazine on the risk of VT/VF and/or death.
All statistical tests were two-sided, a P-value of <0.05 was considered statistically significant. Analyses were carried out with SAS software (version 9.4, SAS institute, NC, USA).
Results
Study population
The baseline clinical characteristics are presented in Table 1. Mean age at enrolment was 64 ± 11 years and 24% were women. Two-thirds had ischaemic cardiomyopathy and mean LVEF was 25 ± 6%. ICDs were implanted in 2700 (60%) patients and 1831 (40%) patients received a CRT-D device. The baseline characteristics of patients who comprised the external validation RAID are presented in Supplementary material online, Table D. In the RAID trial, mean age was 64 ± 10 years, 24% were women, 51% had ischaemic cardiomyopathy, and 53% were implanted with a CRT-D device.
Table 1.
Baseline characteristics of the entire cohort
| Study cohort No. (%)/ mean ± SD | MADIT-II | MADIT-CRT | MADIT-RIT | MADIT-Risk | |
|---|---|---|---|---|---|
| Number of patients | 4531 | 742 | 1820 | 1500 | 469 |
| Female | 1088 (24) | 119 (16) | 453 (25) | 436 (29) | 80 (17) |
| Black | 717 (15) | 65 (9) | 143 (8) | 272 (18) | 237 (51) |
| Age (years) | 64 ± 11 | 64 ± 10 | 64 ± 11 | 63 ± 12 | 63 ± 10 |
| Heart rate (b.p.m.) | 70 ± 12 | 72 ± 13 | 68 ± 11 | 72 ± 13 | 69 ± 10 |
| Body mass index (kg/m2) | 29 ± 6 | 28 ± 5 | 29 ± 5 | 29 ± 7 | 29 ± 6 |
| Ejection fraction (%) | 25 ± 6 | 23 ± 5 | 24 ± 5 | 26 ± 6 | 27 ± 5 |
| QRS (ms) | 149 ± 28 | 124 ± 35 | 158 ± 20 | 154 ± 21 | NA |
| Left bundle branch block | 1829 (40) | 129 (19) | 1281 (70) | 388 (80a) | 31 (7) |
| CRT-D | 1831 (40) | NA | 1089 (60) | 758 (51) | NA |
| Ischaemic cardiomyopathy | 3001 (66) | 742 (100) | 999 (55) | 791 (53) | 469 (100) |
| NYHA-I | 673 (15) | 255 (35) | 265 (15) | 35 (2) | 118 (25) |
| NYHA-II | 2783 (62) | 260 (35) | 1555 (85) | 671 (45) | 297 (63) |
| Diabetes | 1443 (32) | 249 (34) | 552 (30) | 485 (33) | 157 (34) |
| Hypertension | 2915 (65) | 393 (53) | 1152 (63) | 1029 (69) | 341 (73) |
| Atrial arrhythmias | 644 (14) | 202 (28) | 209 (12) | 203 (14) | 30 (7) |
| NSVT | 295 (7) | 73 (10) | 128 (7) | 48 (3) | 46 (10) |
| Creatinineb | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 | NA | 1.2 ± 0.4 |
| ACE inhibitor or ARB | 4104 (91) | 655 (88) | 1740 (96) | 1312 (87) | 397 (85) |
| Aldosterone | 1195 (32) | NA | 578 (32) | 544 (36) | 73 (16) |
| Amiodarone | 286 (6) | 50 (7) | 129 (7) | 96 (6) | 11 (2) |
| Aspirin | 3055 (68) | 505 (68) | 1175 (65) | 972 (65) | 403 (87) |
| Beta-blocker | 4025 (89) | 478 (65) | 1702 (94) | 1417 (95) | 427 (93) |
| Digitalis | 1161 (26) | 443 (60) | 468 (26) | 193 (13) | 57 (12) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CRT-D, cardiac resynchronization therapy with defibrillator; NSVT, non-sustained ventricular tachycardia; NYHA, New York Heart Association Class.
Percentage from the total number of MADIT-RIT patients who had EKG data on QRS morphology.
In MADIT-RIT, all patients needed to have creatinine less than 2.5; however, the exact value of creatinine was not further detailed.
VT/VF risk score
Fine and Gray best subset regression identified eight predictors of increased risk for VT/VF (Table 2). These factors were male sex, age <75 years, prior atrial arrhythmia, prior non-sustained ventricular tachycardia (NSVT), LVEF ≤ 25%, systolic blood pressure < 140 mmHg, prior clinical myocardial infarction (MI), and heart rate > 75 b.p.m. at enrolment. Detailed definitions of the variables are found in Supplementary material online, Table C. The study population was dichotomized based on patient-specific VT/VF risk score as described in the Supplementary material online, Table E.
Table 2.
Fine and Gray multivariate model predicting ventricular tachyarrhythmia VT/VF using non-arrhythmic mortality as the competing risk variable, and the corresponding point for the VT/VF risk score
| Variable | Parameter estimate | HR | 95% CI | P-value | Points |
|---|---|---|---|---|---|
| LVEF ≤ 25% | 0.24 | 1.28 | 1.06–1.53 | 0.010 | 1 |
| Atrial arrhythmia | 0.25 | 1.29 | 1.02–1.63 | 0.034 | 1 |
| Heart rate >75 b.p.m. | 0.30 | 1.35 | 1.12–1.63 | 0.002 | 1 |
| SBP < 140 mmHg | 0.40 | 1.49 | 1.15–1.93 | 0.003 | 2 |
| Myocardial infarction | 0.44 | 1.55 | 1.13–2.17 | 0.011 | 2 |
| Age < 75 years | 0.55 | 1.74 | 1.31–2.33 | <0.001 | 2 |
| Male | 0.56 | 1.75 | 1.35–2.27 | <0.001 | 2 |
| Prior NSVT | 0.58 | 1.79 | 1.34–2.37 | <0.001 | 2 |
LVEF, left ventricular ejection fraction; NSVT, non-sustained ventricular tachycardia requiring medication; NSVT, non-sustained ventricular tachycardia.
Non-arrhythmic mortality risk score
Seven factors were identified as predictors of non-arrhythmic mortality: New York Heart Association (NYHA) ≥II, age ≥ 75 years, BMI <23 kg/m2, atrial arrhythmias, LVEF ≤ 25%, and diabetes mellitus were associated with increased risk of non-arrhythmic mortality, whereas treatment with CRT-D vs. ICD-only was associated with reduced risk of non-arrhythmic mortality (Table 3). The study population was dichotomized based on patient-specific non-arrhythmic mortality risk score as described in Supplementary material online, Table F.
Table 3.
Multivariate Cox regression model predicting non-arrhythmic mortality and the corresponding point for the non-arrhythmic mortality risk score
| Variable | Parameter estimate | HR | 95% CI | P-value | Points |
|---|---|---|---|---|---|
| CRT-D | −0.27 | 0.76 | 0.60–0.98 | 0.032 | −1 |
| NYHA ≥ II | 0.33 | 1.43 | 1.18–2.24 | 0.003 | 1 |
| Diabetes | 0.37 | 1.47 | 1.27–2.00 | <0.001 | 1 |
| Body mass index <23 kg/m2 | 0.48 | 1.62 | 1.20–2.18 | <0.001 | 2 |
| Atrial arrhythmia | 0.47 | 1.60 | 1.22–2.10 | <0.001 | 2 |
| LVEF ≤ 25% | 0.51 | 1.66 | 1.32–2.09 | <0.001 | 2 |
| Age ≥ 75 years | 0.56 | 1.75 | 1.36–2.25 | <0.001 | 2 |
CRT-D, cardiac resynchronization therapy with defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association Class.
The MADIT-ICD benefit score
The graphical abstract shows: (i) the components of the two risk scores (top-right panel); (ii) the integrated strata of the MADIT-ICD benefit groups with the personalized ICD benefit score (top left panel); (iii) the predicted risk of life-threatening VT/VF by the three benefit groups (bottom left panel); and (iv) the predicted risk of non-arrhythmic mortality by the three benefit groups (bottom right panel).
Observed risk of VT/VF and non-arrhythmic mortality by the MADIT-ICD benefit groups
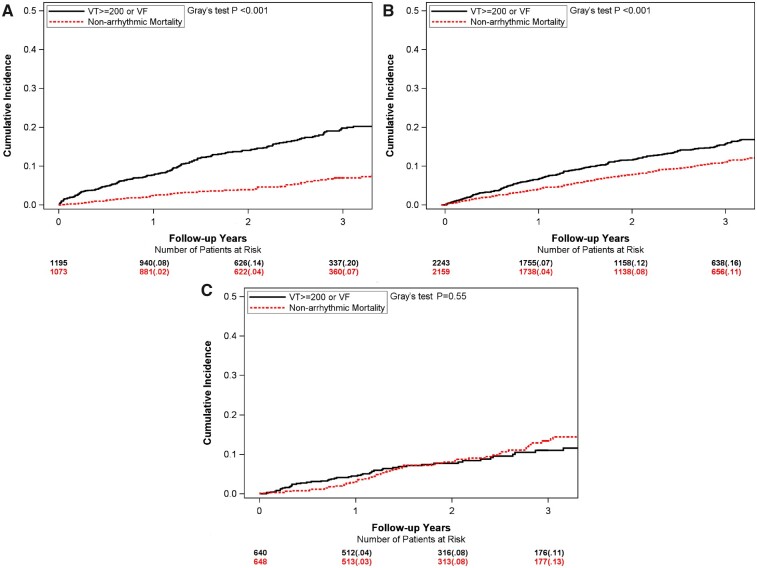
Figure 1A–C illustrates the CIF curves for the observed risk of VT/VF and for non-arrhythmic mortality in each of the three MADIT-ICD benefit groups. In the highest MADIT-ICD benefit group, the 3-year observed risk of VT/VF was >3-fold higher than the corresponding risk of non-arrhythmic mortality (20% vs. 7%, P < 0.001, respectively; Figure 1A). In the intermediate group, the 3-year observed risk of VT/VF was also higher than the corresponding risk of non-arrhythmic mortality, but the difference was attenuated (16% vs. 11%, P < 0.01; Figure 1B). In contrast, in the lowest MADIT-ICD benefit group, the 3-year observed risk of VT/VF was similar to the risk of non-arrhythmic (11% vs. 13%, P = 0.55; Figure 1C).
Figure 1.
Cumulative incidence curve for ventricular tachyarrhythmia (VT ≥ 200 b.p.m., or VF) and for the competing risk of non-arrhythmic mortality by MADIT-ICD benefit groups. (A) Highest; (B) intermediate; and (C) lowest.
At 3 years following ICD implantation, the life-gain was 74 days in the highest group, 31 days in the intermediate group, and 6 days in the lowest group (Supplementary material online, Table G).
Predicted risk of VT/VF and non-arrhythmic mortality by the MADIT-ICD benefit groups
The predicted risk estimate graphs among the three MADIT-ICD benefit score groups for the end points of VT/VF and non-arrhythmic mortality are shown in the graphical abstract (bottom left and right panels, respectively) and the corresponding predicted rates are provided in Table 4. Predicted rates were similar to observed rates (Table 4 and Figure 1A–C, and Supplementary material online, Figure B). The highest MADIT-ICD benefit group showed a 3-year predicted risk of VT/VF that was nearly three-fold higher than the corresponding predicted risk of non-arrhythmic mortality [20% (range 17–23) vs. 7% (range 6–8), respectively; P < 0.001]. The intermediate benefit group showed an attenuated difference between the two corresponding predicted risks [15% (range 13–17) vs. 9% (range 8–10), respectively; P < 0.01], whereas in the lowest MADIT-ICD benefit group, the 3-year predicted risk of VT/VF was similar to the risk of non-arrhythmic mortality [11% (range 10–13) vs. 12% (range 11–14), respectively; P = 0.41].
Table 4.
Predicted risk estimate based on the hazard estimates from Fine and Gray model
| At 1 yeara
|
At 2 yearsa
|
At 3 yearsa
|
||||
|---|---|---|---|---|---|---|
| MADIT-ICD benefit group | VT/VF | Non-arrhythmic mortality | VT/VF | Non-arrhythmic mortality | VT/VF | Non-arrhythmic mortality |
| Highest MADIT-ICD benefit group | ||||||
| Predicted mean rate (%) | 9 | 2 | 15 | 4 | 20 | 7 |
| Predicted range (%) | 8–10 | 2–3 | 13–17 | 3–5 | 17–23 | 6–8 |
| Intermediate MADIT-ICD benefit group | ||||||
| Predicted mean rate (%) | 7 | 3 | 11 | 6 | 15 | 9 |
| Predicted range (%) | 6–8 | 2–3 | 10–12 | 5–7 | 13–17 | 8–10 |
| Lowest MADIT-ICD benefit group | ||||||
| Predicted mean rate (%) | 5 | 4 | 8 | 8 | 11 | 12 |
| Predicted range (%) | 4–6 | 3–5 | 7–10 | 6–10 | 10–13 | 11–14 |
VT/VF stands for life-threatening ventricular tachycardia ≥200 b.p.m. or ventricular fibrillation.
Non-arrhythmic mortality is defined as death without having any VT/VF in life.
Time since defibrillator implantation.
Consistent results were obtained when the MADIT-ICD benefit score was assessed for the secondary end point of sustained VT/VF (≥170 b.p.m.) vs. death w/o VT/VF (Supplementary material online, Figure C), demonstrating a significantly higher risk of VT/VF vs. non-arrhythmic mortality in the highest group (28% vs. 7%, respectively; P < 0.001); an attenuated difference in the intermediate group (23% vs. 11%, respectively; P < 0.001), and a lower risk of VT/VF vs. non-arrhythmic mortality in the lowest group (20% vs. 14%, respectively; P = 0.03).
Personalized ICD benefit score
We developed the personalized ICD benefit score based on integrated assessment of the VT/VF and non-arrhythmic mortality scores with a range of 0–100, wherein a score of 100 denotes the highest potential ICD benefit and 0, the lowest potential benefit. Thus, within the highest benefit group, the personalized ICD benefit score is in the range of 76–100. In the intermediate benefit group, the corresponding range is 26–75; and in the lowest benefit group, it is ≤25. The personalized ICD benefit score can be derived from the online calculator (https://is.gd/madit) or by using the conversion Supplementary material online, Table in page 8.
Validation of the models
Internal validation using bootstrapping showed good correlation between the original cohort and the derivate cohort (C index for the non-arrhythmic mortality score 0.68 and for VTVF score 0.71).
External validation confirmed model stability with similar C indices between the original cohort and the RAID population for the non-arrhythmic mortality score (0.67), and for the VT/VF score (0.75). The receiver operating characteristic curves are presented in Supplementary material online, Figure D1–2.
Discussion
In this study, comprising all ICD patients enrolled in the landmark MADIT trials, we provide a simple clinical score that can help identify patients who are more likely to benefit from primary ICD therapy through an assessment of individualized predicted risk for life-threatening VT/VF and the competing risk of non-arrhythmic mortality. The proposed MADIT-ICD benefit score can be applied for improved risk stratification by identifying candidates for primary ICD implantation with a greater potential for survival benefit, in whom the predicted risk of VT/VF is a higher than the competing risk of non-arrhythmic mortality. In contrast, ICD candidates in whom the predicted risk of non-arrhythmic mortality is similar to or higher than the risk of VT/VF have a lower likelihood to derive a survival benefit from primary ICD implantation.
Benefit of ICD in patients with a low LVEF
In a contemporary setting, it is important to incorporate additional parameters, beyond LVEF, to identify patients who will derive significant benefit from primary ICD therapy. This is further supported by recent data on the declining incidence of sudden cardiac death (SCD) in patients with a low LVEF,10 growing pathological heterogeneity,11 the proven benefit of cardiac resynchronization therapy in reducing heart failure mortality, and new pharmacotherapy therapy for heart failure reduction.12 These recent advances pose challenges in selecting patients for ICD implantation for primary prevention and stress the need for contemporary innovative risk stratification strategies for primary device therapy within the low LVEF population.13
Assessment of VT/VF vs. non-arrhythmic mortality risk for improved selection of ICD candidates
Standard statistical methods such as Kaplan–Meier and Cox regression for risk assessment in ICD candidates ignore death as a competing risk. However, the low LVEF population represents a heterogeneous group with varying degrees of risk for VT/VF (associated with arrhythmogenic substrate) vs. non-arrhythmic mortality (associated with the presence of comorbidities and declining cardiac function). Therefore, weighing between VT/VF and non-arrhythmic mortality needs to be individually performed in order to appropriately assess the potential benefit of the ICD in a candidate with a low LVEF.
Previous well-known risk scores, such as the MADIT-II ICD risk stratification score or the Seattle Heart Failure Score, were developed only in cohorts prior to the introduction of CRT, and included mainly ischaemic patients.14 , 15 The Seattle Heart Failure Score was validated recently using a cohort of nearly 100 000 patients.16 This study identified 21 979 of ICD candidates who did not derive any survival benefit from primary prevention ICD therapy when compared to no ICD therapy. However, several important differences should be noted between the Seattle Heart Failure Score validation study and the present proposed MADIT-ICD benefit score, including (i) CRT-D recipients were not included in the Seattle models as compared with 40% patients with a CRT-D device in the MADIT-trials; (ii) the use of uniformly adjudicated life-threatening arrhythmia as a surrogate for potential arrhythmic death vs. death without a prior arrhythmic event during follow-up, whereas in other studies, a definition of sudden and non-sudden death was employed, which has been shown to be accurate in only about 50% of cases;17 , 18 (iii) the incorporation of external validation of the present findings in the contemporary RAID cohort; and (iv) the ability to provide a personalized ICD benefit score that integrates the competing risks of VT/VF and non-arrhythmic mortality into a score that provides the expected benefit of the defibrillator in the primary prevention population.
MADIT-ICD benefit score
The MADIT-ICD benefit score may be easily calculated using the free available website (https://is.gd/madit) or manually (outlined in the Supplementary material online, Pages 8–10) and can be used for patient–physician shared decision-making on the need for primary device therapy. Based on our study, the highest MADIT-ICD benefit group comprises patients with the highest 3-year predicted risk for VT/VF events and with the lowest 3-year predicted risk for non-arrhythmic mortality (74 days life-gain at 3 years). Thus, patients in this category should be encouraged to receive an ICD/CRT-D device for the primary prevention of SCD. The corresponding difference between the risks of VT/VF vs. non-arrhythmic mortality is attenuated in the intermediate benefit group (31 days life-gain at 3 years). Thus, patients in this group should be also be considered for primary prevention ICD/CRT-D, with a focus on concomitant management of associated comorbidities to also reduce the risk of non-arrhythmic mortality. In the lowest benefit group, our findings show that the risk of experiencing VT/VF (11% at 3 years) is still sufficiently high to warrant ICD implantation. However, the fact that the risk of non-arrhythmic mortality (without a prior VT/VF event) is at least equal to the arrhythmic risk suggests that an individualized approach to primary device implantation should be considered in this population, with more focus on the treatment of comorbidities to maximize the benefit of the ICD in patients who have the highest likelihood to die from non-arrhythmic causes. These data also highlight the need for early consideration of ICD therapy, prior to the development of more advanced risk factors associated with non-arrhythmic mortality.
When the components of the non-Arrhythmic Mortality Score are assessed individually, we see that age plays a significant role. Older patients may have less arrhythmic risk due to the competing risk of non-arrhythmic mortality.19 Similarly, diabetes mellitus was identified to confer a high-risk for non-arrhythmic mortality, due to associated cardiovascular and non-cardiovascular comorbidities in this population.20 Other markers of more advanced cardiac disease and comorbidities comprising the non-arrhythmic mortality score include increased NYHA class,21 low LVEF,22 and a low BMI,23 which may be a marker of cardiac cachexia, whereas treatment with CRT-D is associated with a reduction in the risk of non-arrhythmic mortality, consistent with prior data.24
Components of the VT/VF risk score include established factors such as prior NSVT25; a faster baseline heart rate (independently of beta-blocker therapy), which may be a marker of increased sympathetic activity27; and a lower systolic blood pressure, which is shown to be a marker of sudden death risk in patients with heart failure.26 Male sex and prior MI are also included in the VT/VF risk score, similar to prior reports that showed a higher ventricular arrhythmic risk among these groups.27 Of note, prior MI was identified as the most powerful predictor of VT/VF among collinear variables that included ischaemic cardiomyopathy and aspirin treatment. It should be noted that four variables (age, gender, atrial arrhythmia,28 and LVEF) were identified to be associated with both VT/VF and non-arrhythmic mortality, but were assigned different points in each respective score due to a differential effect of those variables on the two outcomes (graphical abstract: top right panel tables). Medications were considered as candidates in the score development, yet due to collinearity with more powerful baseline clinical predictors, these variables did not enter the final models.
Our study has several limitations. The impact of very recent heart failure drugs (including angiotensin receptor neprilysin inhibitor and Sodium glucose co-transporter 2 inhibitors) on the VT/VF risk in ICD candidates remains unknown. However, current European Society of Cardiology (ESC)/US guidelines do not require treatment with newer heart failure medications as a pre-requisite for primary ICD implantation.29 , 30 According to the guidelines, optimal medical therapy includes beta-blockers, angiotensin-converting enzyme/angiotensin receptor blockers and aldosterone antagonists, all utilized at a very high frequency in the MADIT and RAID trials compared to contemporary real-world data.31 , 32 Additionally, the external validation RAID cohort supports the validity of our data in a contemporary setting.
CRT use was not incorporated into MADIT-II which may lead to an overestimation of the mortality impact of CRT vs. ICD. Yet, separate analysis excluding MADIT-II patients depicted similar findings.
Finally, despite the many advantages of using data from multi-centre randomized controlled trials, patients from these trials may do better than real-world patients. Thus, our results may not be applicable to elderly (age > 80 years) or those with advance renal dysfunction. In addition, the retrospective nature and the length of follow-up of the current analysis might have an impact on the result. Therefore, our findings should be further validated in contemporary prospective ICD registries to ensure generalizability to real-world primary prevention candidates.
Conclusion
Based on the combined MADIT population, we propose to apply the MADIT-ICD benefit score (https://is.gd/madit) that defines three ICD benefit groups and provides patient-specific estimates of the expected benefit of the ICD in a primary prevention population based on simple clinical variables. The proposed MADIT-ICD benefit score can be used for shared decision-making by utilizing personalized integrated assessment of the competing risk of VT/VF vs. non-arrhythmic mortality in ICD candidates.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The data that support the findings of this study are owned by the University of Rochester and Boston Scientific. Data are available upon reasonable request from the authors (AY and IG) with the permission of Boston Scientific and the University of Rochester.
Funding
All MADIT studies were supported by a research grant from Boston Scientific to the University of Rochester, with funds distributed to the coordination and data centre, enrolling centres, core laboratories, committees, and boards under subcontracts from the University of Rochester. RAID trial was supported by grants from the National Heart, Lung, and Blood Institute (Grant No. UO1 HL096607; UO1 HL096610).
Conflict of interest: V.K. has received research grants from Boston Scientific, Biotronik, and ZOLL Inc., Spire Inc., and has consultant agreements with ZOLL Inc., and Biotronik. W.Z.—research grants from Boston Scientific. J.D.—research grants from Boston Scientific. M.E.—research grants from Boston Scientific, consulting Fees, Boston Scientific, Abbott, and Medtronic. D.C.—research grants from Boston Scientific. K.S.—Chief Medical Officer for Rhythm Management at Boston Scientific. I.G.—research grants from Boston Scientific, Medtronic, Zoll. All other authors declared no conflict of interest.
Supplementary Material
Contributor Information
Arwa Younis, Division of Cardiology, Department of Medicine, Clinical Cardiovascular Research Center, University of Rochester Medical Center, 265 Crittenden Blvd CU 420653, NY 14642, USA.
Jeffrey J Goldberger, Division of Cardiology, Miller School of Medicine, University of Miami, 1321 NW 14th St #510, Miami, FL 33125, USA.
Valentina Kutyifa, Division of Cardiology, Department of Medicine, Clinical Cardiovascular Research Center, University of Rochester Medical Center, 265 Crittenden Blvd CU 420653, NY 14642, USA.
Wojciech Zareba, Division of Cardiology, Department of Medicine, Clinical Cardiovascular Research Center, University of Rochester Medical Center, 265 Crittenden Blvd CU 420653, NY 14642, USA.
Bronislava Polonsky, Division of Cardiology, Department of Medicine, Clinical Cardiovascular Research Center, University of Rochester Medical Center, 265 Crittenden Blvd CU 420653, NY 14642, USA.
Helmut Klein, Division of Cardiology, Department of Medicine, Clinical Cardiovascular Research Center, University of Rochester Medical Center, 265 Crittenden Blvd CU 420653, NY 14642, USA.
Mehmet K Aktas, Division of Cardiology, Department of Medicine, Clinical Cardiovascular Research Center, University of Rochester Medical Center, 265 Crittenden Blvd CU 420653, NY 14642, USA.
David Huang, Division of Cardiology, Department of Medicine, Clinical Cardiovascular Research Center, University of Rochester Medical Center, 265 Crittenden Blvd CU 420653, NY 14642, USA.
James Daubert, Division of Cardiology, Duke Medicine Circle Clinic 2F/2G, Durham, NC 27710, USA.
Mark Estes, Division of Cardiology, UPMC Heart and Vascular Institute 1350 Locust Street, Suite 100 Pittsburgh, PA 15219, USA.
David Cannom, Division of Cardiology, Good Samaritan Hospital, 1245 Wilshire Blvd, Ste 703, Los Angeles, CA 90017, USA.
Scott McNitt, Division of Cardiology, Department of Medicine, Clinical Cardiovascular Research Center, University of Rochester Medical Center, 265 Crittenden Blvd CU 420653, NY 14642, USA.
Kenneth Stein, Cardiac Rhythm Management, Boston Scientific Corp., 4100 Hamline Ave N, St Paul, MN 55101, USA.
Ilan Goldenberg, Division of Cardiology, Department of Medicine, Clinical Cardiovascular Research Center, University of Rochester Medical Center, 265 Crittenden Blvd CU 420653, NY 14642, USA.
References
- 1. Bigger JT Jr. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med 1997;337:1569–1575. [DOI] [PubMed] [Google Scholar]
- 2. Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ, Investigators D. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med 2004;351:2481–2488. [DOI] [PubMed] [Google Scholar]
- 3. Colquitt JL, Mendes D, Clegg AJ, Harris P, Cooper K, Picot J, Bryant J. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure: systematic review and economic evaluation. Health Technol Assess 2014;18:1–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial III. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 5. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W; MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 6. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA 3rd, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W; MADIT-RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–2283. [DOI] [PubMed] [Google Scholar]
- 7. Zareba W. Risk Stratification in MADIT II Type Patients. http://grantome.com/grant/NIH/R01-HL077478-04. 2008
- 8. Zareba W, Daubert JP, Beck CA, Huang DT, Alexis JD, Brown MW, Pyykkonen K, McNitt S, Oakes D, Feng C, Aktas MK, Ayala-Parades F, Baranchuk A, Dubuc M, Haigney M, Mazur A, McPherson CA, Mitchell LB, Natale A, Piccini JP, Raitt M, Rashtian MY, Schuger C, Winters S, Worley SJ, Ziv O, Moss AJ; RAID Trial Investigators. Ranolazine in high-risk patients with implanted cardioverter-defibrillators: the RAID trial. J Am Coll Cardiol 2018;72:636–645. [DOI] [PubMed] [Google Scholar]
- 9. Royston P, Parmar MKB. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 2013;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ni H, Coady S, Rosamond W, Folsom AR, Chambless L, Russell SD, Sorlie PD. Trends from 1987 to 2004 in sudden death due to coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2009;157:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lund LH. The inescapable heterogeneity of heart failure. J Card Fail 2017;23:351–352. [DOI] [PubMed] [Google Scholar]
- 12. Kitai T, Tang WW. Recent advances in treatment of heart failure. F1000Res 2015;4:1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp-Pedersen C, Pehrson S; DANISH Investigators. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 14. Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 2008;51:288–296. [DOI] [PubMed] [Google Scholar]
- 15. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006;113:1424–1433. [DOI] [PubMed] [Google Scholar]
- 16. Bilchick KC, Wang Y, Cheng A, Curtis JP, Dharmarajan K, Stukenborg GJ, Shadman R, Anand I, Lund LH, Dahlström U, Sartipy U, Maggioni A, Swedberg K, O’Conner C, Levy WC. Seattle heart failure and proportional risk models predict benefit from implantable cardioverter-defibrillators. 2017;69:2606–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tseng ZH, Olgin JE, Vittinghoff E, Ursell PC, Kim AS, Sporer K, Yeh C, Colburn B, Clark NM, Khan R, Hart AP, Moffatt E. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation 2018;137:2689–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chernomordik F, Jons C, Klein HU, Kutyifa V, Nof E, Zareba W, Daubert JP, Greenberg H, Glikson M, Goldenberg I, Beinart R. Death with an implantable cardioverter-defibrillator: a MADIT-II substudy. Europace 2019;21:1843–1850. [DOI] [PubMed] [Google Scholar]
- 19. Pellegrini CN, Lee K, Olgin JE, Turakhia MP, Tseng ZH, Lee R, Badhwar N, Lee B, Varosy PD. Impact of advanced age on survival in patients with implantable cardioverter defibrillators. Europace 2008;10:1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zabel M, Willems R, Lubinski A, Bauer A, Brugada J, Conen D, Flevari P, Hasenfuß G, Svetlosak M, Huikuri HV, Malik M, Pavlović N, Schmidt G, Sritharan R, Schlögl S, Szavits-Nossan J, Traykov V, Tuinenburg AE, Willich SN, Harden M, Friede T, Svendsen JH, Sticherling C, Merkely B. Clinical effectiveness of primary prevention implantable cardioverter-defibrillators: results of the EU-CERT-ICD controlled multicentre cohort study. Eur Heart J 2020;41:3437–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J 2006;151:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gula LJ, Klein GJ, Hellkamp AS, Massel D, Krahn AD, Skanes AC, Yee R, Anderson J, Johnson GW, Poole JE, Mark DB, Lee KL, Bardy GH. Ejection fraction assessment and survival: an analysis of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 2008;156:1196–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LAM, Skali H, Pfeffer MA, Yusuf S, Swedberg K, Michelson EL, Granger CB, McMurray JJV, Solomon SD. Body mass index and prognosis in patients with chronic heart failure. Circulation 2007;116:627–636. [DOI] [PubMed] [Google Scholar]
- 24. Wells G, Parkash R, Healey JS, Talajic M, Arnold JM, Sullivan S, Peterson J, Yetisir E, Theoret-Patrick P, Luce M, Tang ASL. Cardiac resynchronization therapy: a meta-analysis of randomized controlled trials. CMAJ 2011;183:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Sousa MR, Morillo CA, Rabelo FT, Nogueira Filho AM, Ribeiro AL. Non-sustained ventricular tachycardia as a predictor of sudden cardiac death in patients with left ventricular dysfunction: a meta-analysis. Eur J Heart Fail 2008;10:1007–1014. [DOI] [PubMed] [Google Scholar]
- 26. Goldenberg I, Moss AJ, McNitt S, Zareba W, Hall WJ, Andrews ML. Inverse relationship of blood pressure levels to sudden cardiac mortality and benefit of the implantable cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 2007;49:1427–1433. [DOI] [PubMed] [Google Scholar]
- 27. Ehdaie A, Cingolani E, Shehata M, Wang X, Curtis AB, Chugh SS. Sex differences in cardiac arrhythmias: clinical and research implications. Circ Arrhythm Electrophysiol 2018;11:e005680. [DOI] [PubMed] [Google Scholar]
- 28. Olesen MS, Yuan L, Liang B, Holst AG, Nielsen N, Nielsen JB, Hedley PL, Christiansen M, Olesen SP, Haunso S, Schmitt N, Jespersen T, Svendsen JH. High prevalence of long QT syndrome-associated SCN5A variants in patients with early-onset lone atrial fibrillation. Circ Cardiovasc Genet 2012;5:450–459. [DOI] [PubMed] [Google Scholar]
- 29. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ. 2015 European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co-chairs. Eur Heart J 2015;36:2793–2799. [DOI] [PubMed] [Google Scholar]
- 30. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 31. Ruwald AC, Gislason GH, Vinther M, Johansen JB, Nielsen JC, Petersen HH, Torp-Pedersen C, Riahi S, Jøns C. The use of guideline recommended beta-blocker therapy in primary prevention implantable cardioverter defibrillator patients: insight from Danish nationwide registers. Europace 2018;20:301–307. [DOI] [PubMed] [Google Scholar]
- 32. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol 2016;67:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are owned by the University of Rochester and Boston Scientific. Data are available upon reasonable request from the authors (AY and IG) with the permission of Boston Scientific and the University of Rochester.