Abstract
Direct Oral Anticoagulants (DOACs) require dose adjustment based on specific patient characteristics, making them prone to incorrect dosing. The current study aimed to evaluate the prevalence of inappropriate DOAC dosing, its predictors, and corresponding outcomes in a single-center cohort of AF patients. We reviewed all patients with AF treated at Mayo Clinic with a DOAC (Apixaban, Rivaroxaban, or Dabigatran) between 2010 and 2017. Outcomes examined were ischemic stroke /transient ischemic attack (TIA)/embolism and bleeding. 8576 patients (mean age 69.5 ± 11.9 years, 35.1 % female, CHA2DS2-VASc 3.0±1.8) received a DOAC (38.6% apixaban, 35.8% rivaroxaban, 25.6% dabigatran). DOAC dosing was inappropriate in 1273 (14.8%) with 1071 (12.4%) receiving an inappropriately low dose, and 202(2.4%) an inappropriately high dose. Patients prescribed inappropriate doses were older (72.4 ± 11.7 vs 69.0 ± 11.8, p<0.0001), more likely to be female (43.1% vs 33.7%, p<0.0001), had a higher CHA2DS2-VASc score (3.4 ± 1.8 vs 2.9 ± 1.8, p<0.0001) and a greater Charlson comorbidity index (3.5 ± 3.3 vs 2.9 ± 3.2, p<0.0001). Over 1.2 ±1.6 years (median 0.5 years) follow up; there was no significant difference in the incidence of stroke/TIA/embolism and bleeding between patients who were inappropriately dosed vs. appropriately dosed. In conclusion, DOAC dosing was not in compliance with current recommendations in 15% of AF patients. Patients at higher risk of stroke/TIA based on older age, female gender, and higher CHA2DS2-VASc score were more likely to be underdosed, but there was no significant difference in outcomes including stroke/TIA/embolism and bleeding.
Keywords: Direct oral anticoagulants, atrial fibrillation, stroke, thromboembolism, bleeding
Introduction
Direct Oral Anticoagulants (DOACs) are now the preferred first-line treatment for stroke risk reduction in patients with atrial fibrillation (AF)1. At present, 4 DOACs (Apixaban, Dabigatran, Rivaroxaban, and Edoxaban) are approved and have been shown in randomized controlled trials to be at least non-inferior 2,3 or superior 4,5 to warfarin in reducing stroke and systemic embolism. Further, DOACs showed a better safety profile with a lower risk of intracerebral hemorrhage but a higher risk of GI bleeding (specifically Dabigatran). Unlike warfarin, DOACs are prescribed in fixed doses but do require dose adjustment based on specific patient characteristics. Alternative dosing regimens are also approved for other indications such as venous thromboembolism, which adds complexity to dosing and may be confusing to the prescribing physician. This complexity, combined with physician preference (whether evidence-based or not), can lead to dosing that deviates from FDA labeling/packaging inserts, thus potentially compromising efficacy and predisposing to adverse effects 6,7. Therefore, our study aimed to describe dosing patterns of DOACs, dosing appropriateness, and the correlation of inappropriate dosing with outcomes in a large single-center cohort of AF patients.
Methods
The Mayo Clinic Institutional Review Board approved the present study. All patients with AF treated with a DOAC (Apixaban, Rivaroxaban, Dabigatran, or Edoxaban) at Mayo Clinic Rochester between December 2010 and December 2017 were identified using the electronic medical record. To identify patients (Figure 1), we screened Mayo Clinic records for the first recorded prescription of a DOAC between 2010 and 2017 and identified those with an atrial fibrillation/flutter diagnosis by International Statistical Classification of Diseases (ICD) 9 and 10 codes (Supplementary Table 1) within 3 months of this prescription. We excluded patients who had a diagnosis or history of deep vein thrombosis or pulmonary embolism. From this group of 10,012 patients, 1419 patients were excluded due to missing data (serum creatinine, weight, or DOAC dosing frequency or strength), which precluded the determination of dose appropriateness. Edoxaban was prescribed to a very small number of patients (n=17) and was excluded due to the inability to draw any definitive conclusions on this group. In the final cohort of 8576 patients, baseline comorbid conditions were identified at the time of index prescription using ICD codes (Supplementary Table 2).
Figure 1.
Study Cohort
The prescription date of a patient’s DOAC was defined as the index date. The appropriateness of the doses of DOAC was determined according to U.S. FDA-approved package inserts, and divided into 3 groups; appropriate dose, inappropriate reduced dose, inappropriate high dose/overdose (Supplementary Table 3). In brief, underdosed patients were prescribed a reduced dose DOAC when they were eligible for a standard dose, and overdosed patients were prescribed a higher dose than recommended. Creatinine clearance was calculated using the Cockcroft–Gault (CG) equation, using the latest creatinine measured within 1 year of the index date and the patient actual body weight.
The primary outcomes of interest after index DOAC prescription date were the occurrence of (1) ischemic stroke/TIA/embolism, (2) major bleeding, (3) clinically relevant non-major bleeding, and (4) any bleeding which included major, clinically relevant non-major bleeding, and minor bleeding. We also did pre-specified subgroup analysis on patients who experienced intracerebral hemorrhage. To identify these outcomes, we first screened using ICD 9 and 10 codes, followed by manual validation of all outcomes through a thorough review of the electronic medical records (Supplementary Table 4). Major bleeding was defined according to the International Society on Thrombosis and Hemostasis8 as acute or subacute clinically overt bleeding that meets 1 or more of the following criteria: (1) Fatal bleeding and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or intramuscular with compartment syndrome (2) Bleeding causing a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more over a 24 hr period, or (3) leading to transfusion of 2 or more units of whole blood or packed red cells. A clinically relevant non-major bleed is an acute or subacute clinically overt bleed that does not meet the criteria for a major bleed but prompts a clinical response, in that it leads to at least 1 of the following: a hospital admission for bleeding, or a physician-guided medical or surgical treatment for bleeding, or a change in antithrombotic therapy. All acute clinically overt bleeding events that did not meet the criteria for either major bleeding or clinically relevant non-major bleeding were classified as minor bleeding and included in the outcome – any bleed.
Baseline characteristics are reported as absolute numbers and percentages for categorical variables, and as medians (interquartile range) or means (standard deviation) for continuous variables as appropriate. Comparisons between groups were made using the chi‐squared test for categorical variables and the Kruskal-Wallis for continuous variables. Outcomes were reported both as percentages and as event rates per 100 patient-years. A multivariate regression model was used to identify the association between patient characteristics and inappropriate DOAC dosing. For association with the primary outcomes, adjusted Cox models were created to test the association of inappropriate DOAC dose and outcomes. We performed univariate analysis only to explore outcomes by type of inappropriate dose (high Vs low) and intracerebral hemorrhage due to a low number of events that precluded the use of multivariate analysis. All calculations were performed using SAS Software, SAS Institute, Cary, NC.
Results
The baseline characteristics of 8576 patients who received a DOAC for AF are presented in Table 1. The mean age was 69.5 ± 11.9 years, 3008 (35.1%) patients were female, and CHA2DS2-VASc score was 3.0 ± 1.8 (range 0–9). Patients were followed up for a mean of 1.2 ± 1.6 years (median 0.5 years). Cohort characteristics stratified by the type of DOAC are presented in Supplementary Table 5.
Table 1.
Baseline characteristics of the overall cohort and stratified by the appropriateness of DOAC dosing.
| Patient Demographics | Total Cohort (n=8576) | Appropriate Dose (n=7303) | Inappropriate Dose (n=1273) | P value |
|---|---|---|---|---|
| Age, (years) | 69.5 ± 11.9 | 69.0 ± 11.8 | 72.4 ± 11.7 | <0.001 |
| Women | 3008 (35.1%) | 2459 (33.7%) | 549 (43.1%) | <0.001 |
| White | 8094 (94.4%) | 6911 (94.6%) | 1183 (92.9%) | |
| American Indian/Alaskan Native | 20 (0.2%) | 19 (0.3%) | 1 (0.1%) | |
| Asian | 69 (0.8%) | 54 (0.7%) | 15 (1.2%) | |
| Black | 63 (0.7%) | 52 (0.7%) | 11 (0.9%) | |
| Native Hawaiian/Pacific Islander | 9 (0.1%) | 7 (0.2%) | 2 (0.2%) | |
| Other/Unknown/Not Disclosed | 321 (3.7%) | 260 (3.6%) | 61 (4.8%) | |
| Body Mass Index (kg/m2) | 30.4 ± 6.8 | 30.5 ± 6.8 | 29.5 ± 6.6 | <0.001 |
| Charlson co-morbidity Index, median (range) | 2 (0–24) | 2 (0–23) | 3 (0–24) | <0.001 |
| CHA2DS2-VASc score | 3.0 ± 1.8 | 2.9 ± 1.8 | 3.4 ± 1.8 | <0.001 |
| Creatinine (mg/dl) | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.2 ± 0.6 | <0.001 |
| Creatinine Clearance ( ml/min) | 84.8 ± 38.1 | 86.7 ± 37.4 | 73.5 ± 39.9 | <0.001 |
| Diabetes Mellitus | 2091 (24.4%) | 1725 (23.6%) | 366 (28.8%) | <0.001 |
| Hypertension | 5811 (67.8%) | 4909 (67.2%) | 902 (70.9%) | 0.01 |
| Hyperlipidemia | 4633 (54.0%) | 3916 (53.6%) | 717 (56.3%) | 0.10 |
| Heart Failure | 2801 (32.7%) | 2339 (32.0%) | 462 (36.3%) | 0.003 |
| Myocardial infarction | 1026 (12.0%) | 833 (11.4%) | 193 (15.2%) | <0.001 |
| Peripheral vascular disease | 787 (9.2%) | 651 (8.9%) | 136 (10.7%) | 0.04 |
| Aortic Atherosclerotic Disease | 751 (8.8%) | 626 (8.6%) | 123 (9.7%) | 0.21 |
| Liver Disease | 1003 (11.7%) | 820 (11.2%) | 183 (14.4%) | 0.001 |
| Anemia | 1051 (12.3%) | 832 (11.4%) | 219 (17.2%) | <0.001 |
| Alcoholism | 383 (4.5%) | 312 (4.3%) | 71 (5.6%) | 0.02 |
Mean ± SD unless otherwise specified
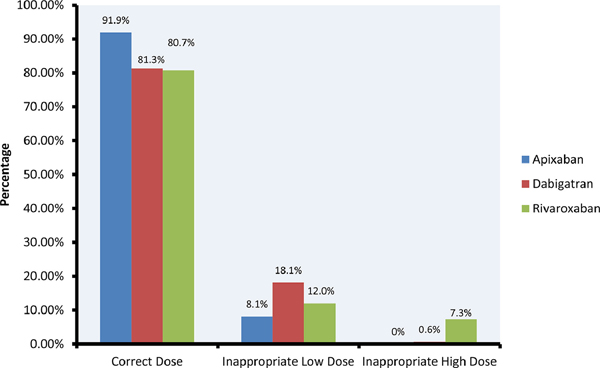
Apixaban (3312, 38.6%) was the most frequently prescribed DOAC, followed by Rivaroxaban (3066, 35.8%) and Dabigatran (2198, 25.6%). DOAC dosing did not conform to the recommended dose in 1273 (14.8%) patients. Of these, 1071 (12.4%) received an inappropriately low dose, and 202 (2.4%%) an inappropriate high dose. Over the study period, there was no clear temporal trend in inappropriate or appropriate prescribing (Supplemental Table 6). The characteristics of patients who received appropriate vs. inappropriate dosing are compared in Table 1. Patients who received an inappropriate dose were older (72.4 ± 11.7 vs 69.0 ± 11.8, p<0.0001), more likely to be female (43.1% vs 33.7%, p<0.0001), have a lower creatinine clearance (73.5 ± 39.9 vs 86.7 ± 37.4 ml/min, p<0.0001), higher CHADSVASC score (3.4 ± 1.8 vs 2.9 ± 1.8, p<0.0001) and a greater Charlson comorbidity index (3.5 ± 3.3 vs 2.9 ± 3.2, p<0.0001). Breakdown of patient characteristics by inappropriate high vs. low dose (Table 2) revealed that patients who received an inappropriately low dose vs high dose were younger (71.1 ± 11.9 vs 79.0 ± 7.6, p<0.0001), less likely to be female (38.7% vs 66.3%, p<0.0001), have a greater creatinine clearance (78.8 ± 40.4 vs 40.2 ± 8.4 ml/min, p<0.0001) and a lower CHADSVASC score (3.3 ± 1.8 vs 3.9 ± 15, p<0.0001).
Table 2.
Baseline characteristics of the overall cohort and stratified by the appropriateness of DOAC dosing.
| Patient Demographics | Appropriate Dose (n=7303) | Inappropriate Low Dose (n=1071) | Inappropriate High Dose (n=202) | P value |
|---|---|---|---|---|
| Age (years) | 69.0 ± 11.8 | 71.1± 11.9 | 79.0 ± 7.6 | <0.001 |
| Women | 2459 (33.7%) | 415 (38.7%) | 134 (66.3%) | <0.001 |
| Body Mass Index(kg/m2) | 30.5 ± 6.8 | 30.3 ± 6.6 | 25.5 ± 5.1 | <0.001 |
| Charlson co-morbidity Index, median (range) | 2 (0–23) | 3 (0–24) | 3 (0–15) | <0.001 |
| CHA2DS2-VASc score | 2.9 ± 1.8 | 3.5 ± 3.3 | 3.6 ± 3.3 | <0.001 |
| Creatinine (mg/dl) | 1.1 ± 0.4 | 1.1 ± 0.5 | 1.4 ± 0.9 | <0.001 |
| Creatinine Clearance (ml/min) |
86.7 ± 37.4 | 79.8 ± 40.4 | 40.2 ± 8.4 | <0.001 |
| Diabetes Mellitus | 1725 (23.6%) | 328 (30.6%) | 38 (18.8%) | <0.001 |
| Hypertension | 4909 (67.2%) | 768 (71.7%) | 134 (66.3%) | 0.01 |
| Hyperlipidemia | 3916 (53.6%) | 615 (57.4%) | 102 (50.5%) | 0.04 |
| Heart Failure | 2339 (32.0%) | 385 (35.9%) | 77 (38.1%) | 0.01 |
| Myocardial infarction | 833 (11.4%) | 165 (15.4%) | 28 (13.9%) | 0.0006 |
| Peripheral vascular disease | 651 (8.9%) | 114 (10.6%) | 22 (10.9%) | 0.12 |
| Aortic Atherosclerotic Disease | 626 (8.6%) | 109 (10.2%) | 14 (6.9%) | 0.15 |
| Liver Disease | 820 (11.2%) | 159 (14.8%) | 24 (11.9%) | 0.003 |
| Anemia | 832 (11.4%) | 188 (17.6%) | 31 (15.3%) | <0.001 |
| Alcoholism | 312 (4.3%) | 63 (5.9%) | 8 (4.0%) | 0.06 |
Mean ± SD unless otherwise specified
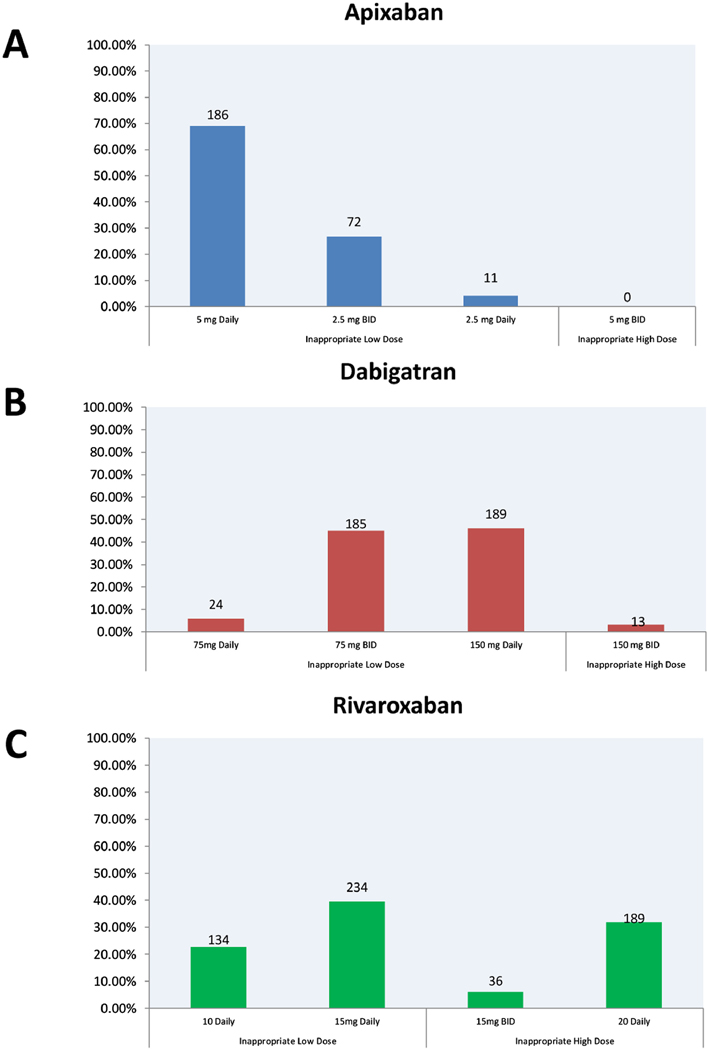
Inappropriate dosed DOACs were more commonly prescribed in patients who received Dabigatran and Rivaroxaban compared to Apixaban (18.6% vs. 19.4% vs. 8.1%, respectively, p<0.0001). Figure 3 shows the distribution of inappropriate dosing across all DOAC groups. The most common inappropriate prescription pattern was underdosing with 5 mg daily of Apixaban, 150 mg daily of Dabigatran, and 15 mg daily of Rivaroxaban. In a multivariate model (Table 3), factors associated with inappropriate dosing were older age [odds ratio (OR) per year, 1.008 (95% CI, 1.00 to 1.016), p=0.037], female sex [OR, 1.27(1.11 to 1.44), p<0.0003], lower creatinine clearance [OR, 1.10(1.08 to 1.14) per 10 ml/min decrease, p<0.0001], and history of diabetes [OR, 1.23(1.07 to 1.43) p=0.005], liver disease[OR, 1.23(1.02 to 1.48), p<0.01], and anaemia[OR, 1.25(1.05 to 1.50), p=0.03].
Figure 3.
Patterns of inappropriate dosing of individual DOAC.
Table 3.
Multivariable model of predictors of inappropriate DOAC dosing
| Variable | Odds ratio | 95% Confidence Limits | P value | |
|---|---|---|---|---|
| Age, per year increase | 1.008 | 1.000 | 1.016 | 0.037 |
| Women | 1.268 | 1.114 | 1.443 | 0.0003 |
| Body Mass Index(kg/m2) | 1.007 | 0.995 | 1.019 | 0.27 |
| Creatinine Clearance, per 10 ml/min decrease | 1.10 | 1.08 | 1.14 | <0.0001 |
| Diabetes Mellitus | 1.232 | 1.065 | 1.427 | 0.005 |
| Hypertension | 0.913 | 0.788 | 1.059 | 0.23 |
| Heart Failure | 1.055 | 0.922 | 1.207 | 0.44 |
| Myocardial Infarction | 1.186 | 0.988 | 1.425 | 0.07 |
| Peripheral Vascular Disease | 0.907 | 0.734 | 1.121 | 0.37 |
| Liver Disease | 1.23 | 1.02 | 1.48 | 0.01 |
| Anaemia | 1.25 | 1.05 | 1.50 | 0.03 |
Over a mean follow up of 1.2 ± 1.6 years (median 0.5 years), there was a trend towards more adverse events in those patients who were inappropriately dosed than those receiving appropriate doses, but this did not reach statistical significance (Table 4).
Table 4.
Outcomes stratified by the appropriateness of DOAC dose.
| Outcome | Appropriate Dose (N=7303) | Inappropriate Dose (N=1273) | Total (N=8576) | Association Effect | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Events | Events/100 PY* (95% CI) | No. of Events | Events/100 PY(95% CI) | No. of Events | Events/100 PY(95% CI) | Hazard ratio (95% CI) | P value | |
| Stroke/TIA/Embolism ^ | 81 (1.1%) | 0.92 (0.72–1.11) | 19 (1.5%) | 1.17 (0.72–1.79) | 100 (1.2%) | .94 (0.78–1.14) | 1.03 (.97–1.09) | 0.36 |
| Major Bleed¶ | 121 (1.7%) | 1.35 (1.12–1.60) | 28 (2.2%) | 1.72 (1.17–2.45) | 149 (1.8%) | 1.4 (1.19–1.64) | 1.03 (.97–1.09) | 0.34 |
| Clinically relevant Non-Major Bleed† | 152 (2.1%) | 1.69 (1.44–1.98) | 32 (2.5%) | 1.97 (1.37–2.74) | 184 (2.1%) | 1.73 (1.5–2.0) | 1.03 (0.97–1.09) | 0.40 |
| Any Bleed‡ | 531 (7.2%) | 5.91 (5.43–6.43) | 110 (8.6%) | 6.7 (5.58–8.11) | 641 (7.4%) | 6.04 (5.57–6.52) | 1.04 (.98–1.10) | 0.23 |
PY- patient year
model was adjusted for age, sex, appropriate dose, creatinine clearance, diabetes, hypertension, heart failure, hyperlipidemia, myocardial infarction, peripheral vascular disease, aortic atherosclerotic disease, and liver disease.
model was adjusted for age, sex, appropriate dose, hypertension, creatinine clearance, diabetes, heart failure, hyperlipidemia, myocardial infarction, peripheral vascular disease, aortic atherosclerotic disease, liver disease, and alcoholism.
model was adjusted for age, sex, appropriate dose, hypertension, creatinine clearance, diabetes, heart failure, hyperlipidemia, myocardial infarction, peripheral vascular disease, aortic atherosclerotic disease, and liver disease.
model was adjusted for age, sex, appropriate dose, hypertension, creatinine clearance, diabetes, heart failure, hyperlipidemia, myocardial infarction, peripheral vascular disease, aortic atherosclerotic disease, liver disease, and alcoholism.
The incidence of the stroke/TIA/embolism [1.17 events per 100 patient-years (95% CI; 0.72–1.79) vs. 0.92 (95% CI 0.72–1.11); p=0.36] was higher in those receiving inappropriate doses but not statistically significant. Additional analysis noted no difference in outcomes if the dose was either inappropriately low or high (Table 5).
Table 5 -.
Univariate Outcomes stratified by low and high inappropriate dosing classification
| Outcome | Appropriate Dose (N=7303) | Inappropriate Low Dose (N=1071) | Inappropriate High Dose (N=202) | P value |
|---|---|---|---|---|
| Stroke/ Transient Ischemic Attack /Embolism | 81 (1.1%) | 16 (1.5%) | 3 (1.5%) | 0.50 |
| Major Bleed | 121 (1.7%) | 21 (2.0%) | 7 (3.5%) | 0.13 |
| Clinically relevant Non-Major Bleed | 152 (2.1%) | 22 (2.1%) | 10 (5.0%) | 0.02 |
| Any Bleed | 531 (7.2%) | 84 (7.8%) | 26 (12.9%) | 0.01 |
| Intracerebral Hemorrhage | 112 (1.5%) | 17 (1.6%) | 6 (2.9%) | 0.26 |
In regards to bleeding, similar observations were noted. Major bleeding [1.72 events per 100 patient-years (95% CI; 1.17–2.45) vs. 1.35 (95% CI 1.12–1.60); p=0.34], clinical relevant non major bleeding [1.97 events per 100 patient-years (95% CI; 1.37–2.74) vs. 1.69 (95% CI 1.44–1.98); p=0.40] and any bleeding [6.7 events per 100 patient-years (95% CI; 5.58–8.11) vs. 5.91 (95% CI 5.43–6.43); p=0.23] was higher in those receiving inappropriate doses but did not statistically significant.
When analyzed by low vs high inappropriate dosing using univariate analysis, major bleeding events were similar between the groups (2.0% vs 3.5%, p=0.13) (Table 5). There was an increased incidence of clinically relevant non-major bleeding (2.1% vs 5.0%, p=0.02) and any bleeding (7.8% vs 12.9%, p=0.01) in those who received inappropriate high doses. There was no difference noted in the incidence of intracerebral hemorrhage.
Discussions
In this single-center experience with prescription of DOACs in patients with AF, anticoagulant dosing was inconsistent with the FDA-approved labeling/package inserts in almost 15% of patients. The majority of patients received a dose that was lower than the recommended dose. Older patients, females, and those with lower creatinine clearance, diabetes, anemia, and liver disease were more likely to be prescribed an inappropriate dose of DOAC. Although there was a trend towards adverse events in those receiving inappropriate dosing, there was no statistically significant association between inappropriate dosing and the incidence of stroke/TIA/embolism and bleeding complications.
This study offers valuable insights into dose under-prescription of DOACs, and our findings parallel those of Steinberg, who reported inappropriately low and high dose of DOAC prescriptions in 9.4% and 3.4% of patients respectively in 5,738 patients enrolled in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation AF II registry7. With a similar follow up time to our study, they noted no significant difference in the occurrence of stroke/TIA and significant bleeding events amongst patients treated with inappropriate and appropriate DOAC dosing. Yao reported that of 14,865 with nonvalvular AF patients who were prescribed a DOAC (identified using a large administrative claims database), 13.3% of patients with no indication for dose reduction received a reduced dose without an associated increase in the overall rate of stroke or bleeding events6. Over a similar follow up time to our study, they report that 43% of patients with an indication for dose reduction received a standard dose, and these patients were more prone to bleeding events. This study was, however, limited by the absence of data on patient weight, which is one of the factors necessary to determine the appropriateness of Apixaban dosing and creatinine clearance using the recommended Cockroft-Gault method. In our study, univariate analysis showed a higher incidence of clinically relevant non-major bleed and any bleed, but not major bleed in those receiving a higher dose of DOAC than recommended. The small number of events, however, precluded multivariable analysis. Our findings also parallel the recent publication of the Global Anticoagulant Registry in the Field-Atrial fibrillation (GARFIELD-AF) registry 9. In this publication, the authors highlight that almost 25% of patients received incorrect dosing and that nonrecommended dosing was associated with a higher risk of all cause mortality; however, the risks of stroke, systematic embolism and major bleeding were not significantly different irrespective of the level of dosing. Additionally our study confirms those of other smaller studies worldwide 10–13, which show that 10–15% of patients prescribed a DOAC do not receive the recommended dose; however, outcome data is limited in these smaller cohorts. A recent analysis of ARISTOTLE trial data14 examined the effects of apixaban dose adjustment on clinical and pharmacological outcomes. In this study, the authors noted that appropriate adjustment of Apixaban to the lower dose (2.5 mg twice daily) resulted in lower apixaban concentrations but similar reductions in coagulation activity compared with Apixaban 5 mg twice daily. Additionally, patients prescribed 2.5 mg twice daily vs. 5mg twice daily had no significant difference in stroke, mortality, or bleeding.
This current study identifies key factors associated with inappropriate dosing of DOACs, including older age, female gender, renal dysfunction, and diabetes mellitus. Significantly, many of these factors predispose to a higher risk of stroke and hence the impact of DOAC under-dosing may be more significant and relevant in this population. Our data add to the growing and concerning body of literature published by Steinberg et al 7, Yao et al 6 and other studies from Europe15 and Canada16. Identifying subgroups at higher risk for inappropriate dosing can lead to targeted interventions to improve adherence to dosing recommendations17. Our study did not explore the reason for inappropriate dosing, but several candidate explanations emerge. Although the fixed-dose regimen of DOACs is an important advancement over the international normalized ratio guided dosing of warfarin, certain complexities remain. The dose of DOAC needs to be adjusted based on indication (AF vs. venous thromboembolism) and patient-specific factors, including age, weight, and renal function, leading to confusion amongst prescribers. Second, there may be a general concern regarding bleeding complications amongst physicians and patients despite available evidence to the contrary 18. A meta-analysis pooling the results of all 4 pivotal clinical trials of DOACs showed that DOACs, when compared to warfarin, were associated with a nonsignificant reduction in major bleeding with significant reductions in hemorrhagic stroke and intracranial hemorrhages which should ease concerns regarding bleeding19. Devereaux 20, in an evaluation of 63 physicians and 61 patients, found that that there is a bias towards greater concern for bleeding amongst physicians. Their study highlights that patients placed more value on the avoidance of stroke and less value on the avoidance of bleeding than physicians. Finally, for the first several years of DOAC use, a reversal agent was not available and this may have led to the concern that a significant bleeding event may prove catastrophic 21–23 and hence under-dosing.
Inappropriate DOAC dosing has the potential for significant clinical implications. While data in this regard has not been consistent, there has been a signal towards potential adverse events. In our study, the incidence of stroke/TIA and bleeding was slightly higher in those with inappropriate dosing, but this was not statistically significant. Steinberg7 found no adverse events from under or overdosing, while Yao6 reported that amongst apixaban-treated patients, those who received an inappropriate reduced dose had a significantly higher risk of stroke compared to appropriate dosing. In our study, we report a trend towards worse outcomes but not statistically significant. This may be secondary to lower event rate and short duration of follow-up that may have limited the power to detect any significant differences in outcomes. The general lack of significant difference in outcomes across these observational studies should also lead to a comparison of anticoagulant activity in specific subgroups to identify if factors other than renal function should also guide dose adjustment. Overall, more extensive prospective studies on the impact of DOAC dosing on outcomes in contemporary practice are needed.
The current study has limitations that are inherent to its retrospective observational design. Although outcomes were identified using ICD codes followed by validation using medical records, there could be under-reporting of events if patients were treated at other institutions. The reason for inappropriate dosing and patient compliance could not be determined. The impact of co-prescription of aspirin and other non-steroidal anti-inflammatory agents on DOAC dosing could not be determined due to the unreliability of data on over the counter medication use. Further, we do not have any insight into who prescribed the DOAC (i.e., a primary care physician or cardiologist) and consequently are unable to comment on these prescribing patterns. Lastly, follow-up was relatively short [but similar to previously published studies6,7], limiting conclusions regarding adverse events.
In conclusion, amongst AF patients prescribed a DOAC for stroke prevention at a single center, 15% did not receive a dose that complied with current FDA labeling. Most of these patients were under-dosed, and patients at higher risk of stroke / TIA based on older age, female gender, and higher CHA2DS2-VASc score were more likely to be underdosed.
Supplementary Material
Figure 2.
DOAC Dosing By Drug
Acknowledgments
Funding: This project was supported by CTSA Grant Number UL1TR002377 from the National Center for Advancing Translational Science (NCATS).
Conflict of interest: Dr Madhavan has received research funding from Bristol-Myers Squibb and Pfizer.
Dr Gersh reports personal fees from Janssen Pharmaceuticals and Bristol-Myers Squibb.
Dr. Asirvatham reports other from Boston Scientific, other from Medtronic, other from St. Jude Medical, other from Zoll Medical, other from Biotronik, outside the submitted work;
Others: None to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–132. [DOI] [PubMed] [Google Scholar]
- 2.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J. Edoxaban versus warfarin in patients with atrial fibrillation. New Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J. Dabigatran versus warfarin in patients with atrial fibrillation. New Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 5.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A. Apixaban versus warfarin in patients with atrial fibrillation. New Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 6.Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non–vitamin K antagonist Oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol 2017;69:2779–2790. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, Kowey PR, Mahaffey KW, Naccarelli G, Reiffel J. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol 2016;68:2597–2604. [DOI] [PubMed] [Google Scholar]
- 8.Schulman S, Kearon C, Scientific SoCoAot, Thrombosis SCotISo, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemostas 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 9.Camm AJ, Cools F, Virdone S, Bassand J-P, Fitzmaurice DA, Arthur Fox KA, Goldhaber SZ, Goto S, Haas S, Mantovani LG, Kayani G, Grierson Turpie AG, Antoon Verheugt FW, Kakkar AK. Mortality in Patients With Atrial Fibrillation Receiving Nonrecommended Doses of Direct Oral Anticoagulants. J Am Coll Cardiol 2020;76:1425–1436. [DOI] [PubMed] [Google Scholar]
- 10.Howard M, Lipshutz A, Roess B, Hawes E, Deyo Z, Burkhart JI, Moll S, Shilliday BB. Identification of risk factors for inappropriate and suboptimal initiation of direct oral anticoagulants. J Thromb Thrombolysis 2017;43:149–156. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz Ortiz M, Muñiz J, Raña Míguez P, Roldán I, Marín F, Asunción Esteve-Pastor M, Cequier A, Martínez-Sellés M, Bertomeu V, Anguita M. Inappropriate doses of direct oral anticoagulants in real-world clinical practice: prevalence and associated factors. A subanalysis of the FANTASIIA Registry. Ep Europace 2017;20:1577–1583. [DOI] [PubMed] [Google Scholar]
- 12.Pharithi RB, Ranganathan D, O’Brien J, Egom EE, Burke C, Ryan D, McAuliffe C, Vaughan M, Coughlan T, Morrissey E. Is the prescription right? A review of non-vitamin K antagonist anticoagulant (NOAC) prescriptions in patients with non-valvular atrial fibrillation. Safe prescribing in atrial fibrillation and evaluation of non-vitamin K oral anticoagulants in stroke prevention (SAFE-NOACS) group. Irish Journal of Medical Science (1971-) 2019;188:101–108. [DOI] [PubMed] [Google Scholar]
- 13.Diaz H, Bagheri H, Palmaro A, Rousseau V, Bourrel R, Montastruc J-L, Birebent J. Patterns of direct oral anticoagulant drug prescription in France in 2010–2013: a study in the Midi-Pyrénées area. Eur J Clin Pharmacol 2018;74:945–951. [DOI] [PubMed] [Google Scholar]
- 14.Zeitouni M, Giczewska A, Lopes RD, Wojdyla DM, Christersson C, Siegbahn A, De Caterina R, Steg PG, Granger CB, Wallentin L, Alexander JH. Clinical and Pharmacological Effects of Apixaban Dose Adjustment in the ARISTOTLE Trial. J Am Coll Cardiol 2020;75:1145–1155. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez LAG, Martín-Pérez M, Vora P, Roberts L, Balabanova Y, Brobert G, Fatoba S, Suzart-Woischnik K, Schaefer B, Ruigomez A. Appropriateness of initial dose of non-vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation in the UK. BMJ open 2019;9:e031341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAlister FA, Garrison S, Kosowan L, Ezekowitz JA, Singer A. Use of direct oral anticoagulants in Canadian primary care practice 2010–2015: a cohort study from the Canadian Primary Care Sentinel Surveillance Network. J Am Heart Assoc 2018;7:e007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pokorney SD, Gersh BJ, Ahmad A, Al-Khatib SM, Blank M, Coylewright M, DiBattiste P, Healey JS, Hedrich O, Hylek EM, Kline-Rogers E, Peterson ED, Mendys P, Mirro MJ, Naccarelli G, Patel P, Ruff CT, Rutman H, Stockbridge N, Temple R, Granger CB. Stroke prevention in atrial fibrillation: Closing the gap. Am Heart J 2019;210:29–38. [DOI] [PubMed] [Google Scholar]
- 18.Huang D. The fear of bleeding of new oral anticoagulants (NOAC) is not justified by recent NOAC atrial fibrillation outcome trials. Heart, Lung and Circulation 2017;26:S302. [Google Scholar]
- 19.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 20.Devereaux PJ, Anderson DR, Gardner MJ, Putnam W, Flowerdew GJ, Brownell BF, Nagpal S, Cox JL. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001;323:1218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrade JG, Krahn AD, Skanes AC, Purdham D, Ciaccia A, Connors S. Values and preferences of physicians and patients with nonvalvular atrial fibrillation who receive oral anticoagulation therapy for stroke prevention. Can J Cardiol 2016;32:747–753. [DOI] [PubMed] [Google Scholar]
- 22.Devereaux P, Fahey T, Anderson DR, Gardner MJ, Putnam W, Flowerdew GJ, Brownell BF, Nagpal S, Cox JL. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational studyCommentary: Varied preferences reflect the reality of clinical practice. BMJ 2001;323:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barra ME, Fanikos J, Connors JM, Sylvester KW, Piazza G, Goldhaber SZ. Evaluation of dose-reduced direct oral anticoagulant therapy. Am J Med 2016;129:1198–1204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.