Abstract
Aim
A lack of known guidelines for the provision of extracorporeal cardiopulmonary resuscitation (ECPR) to patients with out‐of‐hospital cardiac arrest (OHCA) has led to variability in practice between hospitals even in the same country. Because variability in ECPR practice has not been completely examined, we aimed to describe the variability in ECPR practice in patients with OHCA from the emergency department (ED) to the intensive care units (ICU).
Methods
An anonymous online questionnaire to examine variability in ECPR practice was completed in January 2020 by 36 medical institutions who participated in the SAVE‐J II study. Institutional demographics, inclusion and exclusion criteria, initial resuscitation management, extracorporeal membrane oxygenation (ECMO) initiation, initial ECMO management, intra‐aortic balloon pumping/endotracheal intubation/management during coronary angiography, and computed tomography criteria were recorded.
Results
We received responses from all 36 institutions. Four institutions (11.1%) had a hybrid emergency room. Cardiovascular surgery was always involved throughout the entire ECMO process in only 14.7% of institutions; 60% of institutions had formal inclusion criteria and 50% had formal exclusion criteria. In two‐thirds of institutions, emergency physicians carried out cannulation. Catheterization room was the leading location of cannulation (48.6%) followed by ED (31.4%). The presence of formal exclusion criteria significantly increased with increasing ECPR volume (P for trend <0.001). Intra‐aortic balloon pumping was routinely initiated in only 25% of institutions. Computed tomography was routinely carried out before coronary angiography in 25% of institutions.
Conclusions
We described the variability in ECPR practice in patients with OHCA from the ED to the ICU.
Keywords: Emergency department, extracorporeal cardiopulmonary resuscitation, out‐of‐hospital cardiac arrest
Four institutions (11.1%) had a hybrid emergency room. Cardiovascular surgery was always involved throughout the entire extracorporeal membrane oxygenation process in only 14.7% of institutions. The presence of formal exclusion criteria significantly increased with increasing extracorporeal cardiopulmonary resuscitation volume.

Introduction
The prevalence of extracorporeal cardiopulmonary resuscitation (ECPR) in patients with out‐of‐hospital cardiac arrest (OHCA) has been rapidly increasing worldwide, and countries in East Asia, such as Japan, Korea, and Taiwan, are leading in the field of ECPR, followed by European countries and the USA. 1
Although ECPR has been provided in many countries, guidelines do not determine the details of ECPR practice. Therefore, ECPR practice varies in each individual hospital even in the same country, and the variability of ECPR practice has been examined only in Korea and the USA. 2 , 3 However, as several studies have reported ECPR since the 1980s in Japan 4 , 5 and Sakamoto et al. undertook a prospective observational study (i.e., the SAVE‐J study) to evaluate the effectiveness of ECPR and conventional cardiopulmonary resuscitation (CPR) for patients with OHCA, 6 the variability of ECPR practice has not been fully described. At present, we are preparing for the next SAVE‐J II study to provide real‐world data on ECPR in Japan and to examine the indications, management, and prediction of neurologic outcomes.
This study aimed to describe the variability of ECPR practice in patients with OHCA from the emergency department (ED) to the intensive care unit (ICU) based on their responses on questionnaires from institutions in the SAVE‐J II study and also to clarify the points for improvement in ECPR practice through the present study.
Methods
The institutional review board approved this survey‐based study (approval number 19‐R155). The local committee waived the approval of review boards in participating institutions because the present questionnaire survey did not contain individual patients’ information.
Study setting
We prepared a questionnaire to examine the variability of ECPR practice from ED to ICU. The basic questionnaire was distributed to 36 medical institutions who participated in the SAVE‐J II study (UMIN‐ID, UMIN000036490), which is a retrospective multicenter observational study to examine the effectiveness of ECPR in Japan. The survey was carried out using an anonymous questionnaire system through the Internet 7 (Appendix S1) and was distributed in January 2020 and collected by February 2020.
Data collection
In the basic questionnaire, the following parameters were recorded: demographics of institutions, inclusion and exclusion criteria, initial resuscitation management, drug administration for persistent ventricular fibrillation/pulseless ventricular tachycardia, chest compression methods during the cannulation process, venoarterial extracorporeal membrane oxygenation (VA‐ECMO) initiation, initial ECMO management, intra‐aortic balloon pumping (IABP)/endotracheal intubation/management during coronary angiography (CAG), and computed tomography (CT) criteria. The questions are shown in Appendix S1.
Definitions
Hybrid emergency room
Based on the concept of combining “examination” and “treatment” in the same space, the hybrid emergency room (ER) is equipped with a multislice interventional radiology–CT system in the ER. 8 , 9 Emergency physicians (EPs) carry out ECPR.
Study end‐points
The primary end‐point of the present study was to describe the variability of ECPR practice in patients with OHCA from ED to ICU in Japan.
Primary data analysis
Data are summarized as absolute values and percentages. Demographics of institutions, inclusion and exclusion criteria, initial resuscitation management, ECMO initiation, initial ECMO management, IABP/endotracheal intubation/management during CAG, and CT criteria are described. Missing data were not imputed. We used the Cochran–Armitage test for trend of categorical variables. Statistical analyses were undertaken using JMP statistical software (version 12; SAS Institute, Cary, NC, USA). A two‐sided P‐value of <0.05 was considered statistically significant for all analyses.
Results
We received responses from all 36 institutions (Fig. 1). Most participating institutions were distributed in the surrounding areas of big cities such as Tokyo and Osaka.
Fig. 1.
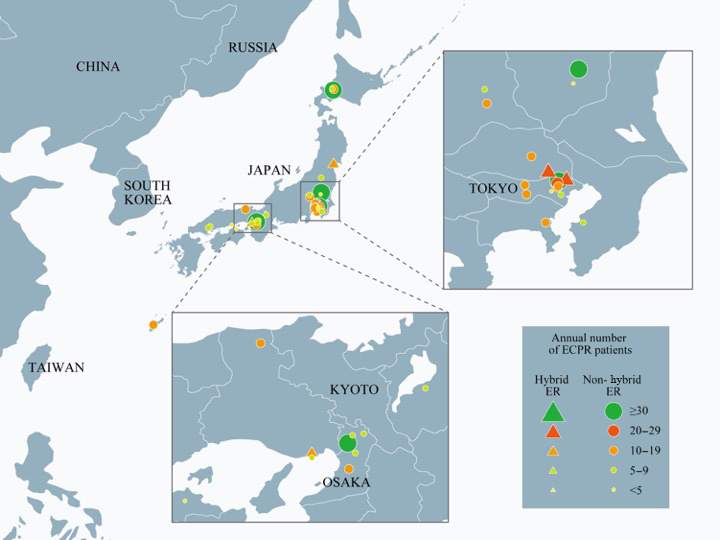
Thirty‐six medical institutions in Japan were included in the SAVE‐J II study. ECPR, extracorporeal cardiopulmonary resuscitation; ER, emergency room.
Demographics of institutions
The details of study institution demographics are described in Table 1. Four institutions (11.1%) who responded had a hybrid ER. The leading historical number of ECPR per year was 10–19 cases, followed by 5–9 cases. Cardiovascular surgery always involved the entire ECMO process in only 14.7% of institutions.
Table 1.
Demographics of institutions that participated in the SAVE‐J II study
| Variable | Level | % | N |
|---|---|---|---|
| Hybrid ER versus nonhybrid ER | Hybrid ER | 11.1 | 4 |
| Non‐hybrid ER | 88.9 | 32 | |
| Historical rate of ECPR per year | <5/year | 11.1 | 4 |
| 5–9/year | 33.3 | 12 | |
| 10–19/year | 36.1 | 13 | |
| 20–29/year | 8.3 | 3 | |
| ≥30/year | 11.1 | 4 | |
| Historical rate of arrival of CPA patients per year in the emergency department | <100/year | 16.7 | 6 |
| 100–199/year | 36.1 | 13 | |
| 200–299/year | 22.2 | 8 | |
| ≥300/year | 25.0 | 9 | |
| Historical rate of VA‐ECMO in patients with circulatory failure after ROSC per year | <5/year | 44.4 | 16 |
| 5–9/year | 38.9 | 14 | |
| 10–19/year | 13.9 | 5 | |
| ≥20/year | 2.8 | 1 | |
| Service involved throughout the entire ECMO process | |||
| Emergency medicine | Yes, always | 91.7 | 33 |
| Yes, as needed | 5.6 | 2 | |
| No | 2.8 | 1 | |
| Cardiology | Yes, always | 52.8 | 19 |
| Yes, as needed | 44.4 | 16 | |
| No | 2.8 | 1 | |
| Cardiovascular surgery (missing obs. = 2) | Yes, always | 14.7 | 5 |
| Yes, as needed | 61.8 | 21 | |
| No | 23.5 | 8 | |
| Radiology (missing obs. = 2) | Yes, always | 3.0 | 1 |
| Yes, as needed | 29.4 | 10 | |
| No | 67.6 | 23 | |
CPA, cardiopulmonary arrest; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; ER, emergency room; obs., observations; ROSC, return of spontaneous circulation; VA‐ECMO, venoarterial extracorporeal membrane oxygenation.
Inclusion and exclusion criteria
Approximately 60% of institutions had a formal inclusion criterion, and no limitation on age was provided in 40% of institutions (Table 2). Half of the institutions had a formal exclusion criterion. No institutions responded that obesity, cachexia, or initial rhythm pulseless electrical activity were the exclusion criteria of ECPR in the present study, and only 10% of institutions responded that ECPR was not carried out in patients with unwitnessed or no bystander CPR.
Table 2.
Inclusion and exclusion criteria of extracorporeal cardiopulmonary resuscitation reported by 36 Japanese medical institutions
| Variable | Level | % | n |
|---|---|---|---|
| Formal inclusion criteria | Yes | 61.1 | 22 |
| Age (years) (included number = 10) | <80 | 10.0 | 1 |
| <75 | 50.0 | 5 | |
| No limitation of age | 40.0 | 4 | |
| Time from witnessed to hospital arrival (min) (included number = 10) | <30 | 10.0 | 1 |
| <45 | 30.0 | 3 | |
| <60 | 20.0 | 2 | |
| No limitation of time | 40.0 | 4 | |
| Formal exclusion criteria | Yes | 50.0 | 18 |
| Exclusion criteria (any) (included number = 18) | Age | 55.6 | 10 |
| Obesity | 0.0 | 0 | |
| Cachexia | 0.0 | 0 | |
| Impaired ADL | 66.7 | 12 | |
| Apparent dialysis access | 22.2 | 4 | |
| Concomitant major trauma | 5.6 | 1 | |
| Suspected drug overdose | 0.0 | 0 | |
| Unwitnessed | 11.1 | 2 | |
| No bystander CPR | 11.1 | 2 | |
| Initial rhythm PEA | 0.0 | 0 | |
| Initial rhythm Asystole | 22.2 | 4 | |
| Time from collapse to hospital arrival | 27.8 | 5 | |
| Terminal state of cancer | 77.8 | 14 | |
| DNAR | 88.9 | 16 | |
| Terminal state of pre‐existing disease | 72.2 | 13 | |
| Children | 5.6 | 1 |
ADL, activities of daily living; CPR, cardiopulmonary resuscitation; DNAR, do not attempt resuscitation; PEA, pulseless electrical activity.
Initial resuscitation management
Approximately 90% of institutions initiated blood drawing during CPR, and C‐reactive protein and fibrinogen degradation products/d‐dimer were evaluated in nearly all institutions (Table 3).
Table 3.
Initial resuscitation management reported by 36 Japanese medical institutions
| Variables | Level | % | n |
|---|---|---|---|
| Timing of blood drawing | During CPR (from arrival to VA‐ECMO initiation) | 86.1 | 31 |
| After VA‐ECMO initiation | 13.9 | 5 | |
| Blood drawing item (any) | CBC | 100 | 36 |
| Chemistry | 100 | 36 | |
| Blood gas | 100 | 36 | |
| CRP | 97.2 | 35 | |
| FDP/d‐dimer | 94.4 | 34 | |
| AT‐III | 38.9 | 14 | |
| Defibrillation for persistent VF/pulseless VT | Following ACLS algorithm | 33.3 | 12 |
| If DC was applied three times in the prehospital setting, no initiation of further DC | 13.9 | 5 | |
| Limited use of DC | 11.9 | 4 | |
| No further initiation of DC | 13.9 | 5 | |
| Decision by physicians in charge | 27.8 | 10 | |
| Drug administration (epinephrine, amiodarone) for persistent VF/pulseless VT | Following ACLS algorithm | 72.2 | 26 |
| Limited use | 8.3 | 3 | |
| No use | 5.6 | 2 | |
| Use epinephrine only | 0.0 | 0 | |
| Use amiodarone only | 8.3 | 3 | |
| Others | 5.6 | 2 | |
| Chest compression methods during the cannulation process | Mechanical | 33.3 | 12 |
| Hand | 44.4 | 16 | |
| Both (mechanical and hand) | 22.2 | 8 |
ACLS, advanced cardiovascular life support; AT‐III, antithrombin III; CBC, complete blood count; CPR, cardiopulmonary resuscitation; CRP, C‐reactive protein; DC, direct current; FDP, fibrin/fibrinogen degradation products; VA‐ECMO, venoarterial extracorporeal membrane oxygenation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Extracorporeal membrane oxygenation initiation
Vascular access was obtained percutaneously using ultrasound (47.2%), followed by percutaneously without ultrasound (44.4%). No institution reported cut‐down vascular access (Table 4). In two‐thirds of institutions, EPs carried out the cannulation. The catheterization room for CAG (48.6%) was the leading location of cannulation, followed by the ED (31.4%).
Table 4.
Extracorporeal membrane oxygenation (ECMO) initiation reported by 36 Japanese medical institutions
| Variables | Level | % | N |
|---|---|---|---|
| Vascular access obtained by | Percutaneous access with ultrasound | 47.2 | 17 |
| Percutaneous access without ultrasound | 44.4 | 16 | |
| Cut‐down | 0 | 0 | |
| Modified (percutaneous + cut‐down) | 5.6 | 2 | |
| Others | 2.8 | 1 | |
| Role of cannulator (check all that apply) | Emergency medicine | 66.7 | 24 |
| Cardiology | 63.9 | 23 | |
| Vascular surgery | 0 | 0 | |
| Locations where cannulation has been performed at the institution (missing obs. = 1) | Emergency department | 31.4 | 11 |
| Hybrid ER | 8.6 | 3 | |
| Catheter room | 48.6 | 17 | |
| Operating room | 2.9 | 1 | |
| Others | 8.6 | 3 | |
| Cannula selection: typical arterial cannula size range (Fr) | <15 | 5.6 | 2 |
| 15–17 | 72.2 | 26 | |
| 19–21 | 16.7 | 6 | |
| 23–25 | 5.6 | 2 | |
| Cannula selection: typical venous cannula size range (Fr) | <15 | 5.6 | 2 |
| 15–17 | 5.6 | 2 | |
| 19–21 | 69.4 | 25 | |
| 23–25 | 19.4 | 7 | |
| Priming of ECMO (check all that apply) | Clinical engineering technologist | 94.4 | 34 |
| Emergency medicine | 11.1 | 4 | |
| Cardiology | 5.6 | 2 | |
| Timing of priming of ECMO | Before patient arrival | 11.1 | 4 |
| Within 5 min after arrival | 69.4 | 25 | |
| 5–10 min after arrival | 16.7 | 6 | |
| After 10 min | 2.8 | 1 |
ER, emergency room; obs., observations.
With regard to the typical cannula size range, 19–21 Fr was used in approximately 70% of institutions for venous ECMO and 15–17 Fr was used in >70% of institutions for arterial ECMO.
Initial ECMO management
Only one‐quarter of institutions routinely placed distal limb perfusion catheters (Table S1). Seventy percent of EPs actually carried out the placement of distal limb perfusion catheters.
Intra‐aortic balloon pumping/endotracheal intubation/management during CAG
Intra‐aortic balloon pumping is routinely initiated in only 25% of institutions. In approximately 10% of institutions, IABP was placed by EPs, who are responsible for airway management during CAG (Table S2).
Computed tomography
Computed tomography was routinely undertaken before CAG in 25% of institutions, and nine institutions routinely carried out CT after CAG (Table S3). Head and whole‐body non‐contrast CTs were mainly carried out with consideration of contrast CT as needed in both situations (before and after CAG).
Trend of presence of formal inclusion and exclusion criteria for ECPR volume
The presence of formal exclusion criteria significantly increased with increasing ECPR volume (P for trend <0.001; Fig. 2A), but the presence of formal inclusion criteria did not (P for trend = 0.646; Fig. 2B).
Fig. 2.
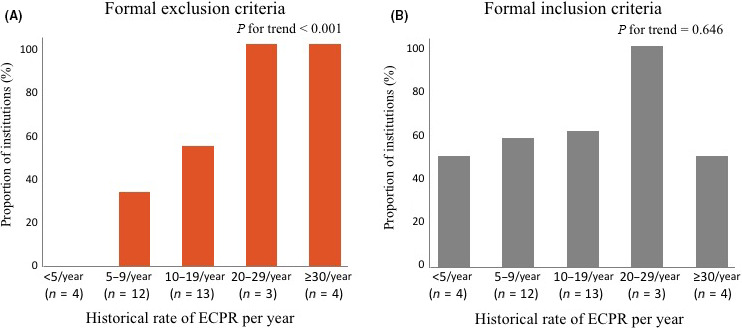
Trend of presence of (A) inclusion and (B) exclusion criteria for extracorporeal cardiopulmonary resuscitation (ECPR) volume in 36 medical institutions in Japan.
Discussion
The details of VA‐ECMO, such as inclusion and exclusion criteria, initial resuscitation management, ECMO initiation, initial ECMO management, IABP/endotracheal intubation/management during CAG, and CT criteria differed in each participating institution.
Demographics of institutions
In the present study, approximately 10% of institutions had the so‐called hybrid ER. Several studies on ECPR in hybrid ER have been recently reported. 8 , 10 This system has the possibility of seamless ECPR from ECMO induction to CAG, followed by percutaneous coronary intervention without any patient transfer. 10 Regarding the historical rate of ECPR, more than one‐third of institutions carried out ECPR on 10–19 patients/year, which was higher than that of the former study with 40% of institutions having 0–1 patients/year in the USA in 2016. 3 Recently, excellent data regarding the effectiveness of ECPR for OHCA patients has been reported, 11 and ECPR will continue to be provided in institutions around the world.
Inclusion and exclusion criteria
Less than half of institutions do not limit the age in the inclusion criteria of ECPR. This is closely related to the national health insurance system in Japan. 12 Because the Japanese government mainly pays the medical bills, physicians in charge do not pay attention to the unpaid medical bills in the ECPR initiation. A systematic review provides age cut‐offs, usually <75 years, 13 and Otani et al. also recently addressed the inclusion criteria of those aged ≤75 years. 14 Goto et al. examined elderly patients with OHCA who were treated with ECPR and concluded that ECPR might not be beneficial for patients aged ≥70 years who had OHCA. 15 Thus, the limitation of age <75 years in the inclusion criteria of ECPR could be warranted. Regarding exclusion criteria, only 50% of institutes have a formal exclusion criterion, which is a lower figure than that in the USA or Korea. 2 The establishment of inclusion and exclusion criteria is fundamentally required to provide a universally acceptable algorithm in ECPR. To date, it is difficult to create specific inclusion and exclusion criteria due to low certainty of evidence.
Initial resuscitation management
Blood drawing, including not only complete blood count and blood gas but also C‐reactive protein and fibrinogen degradation products/d‐dimer, was undertaken during CPR in almost all institutions in the present study. This mainly reflects the characteristics of clinical practice in Japan. 16 , 17 Because Otani et al. reported that the d‐dimer level could predict major bleeding in ECPR on admission, 18 evaluating these markers could have some significance in predicting the outcomes.
Extracorporeal membrane oxygenation initiation
Venous cut‐down was not used to obtain vascular access in this study. More than one‐third of institutions use this procedure for vascular access in the USA. 3 We speculate that because the number of obese patients is relatively low in Japan compared with other developed countries, 19 vascular access can be obtained percutaneously. Regarding the role of the cannulator, EPs carried out cannulation in two‐thirds of institutions, but the proportion is only 10% in the USA. 3 This is mainly due to the unavailability of cardiothoracic surgeons throughout the entire ECMO process in Japan. Approximately 50% of institutions in the present survey carried out cannulation in the catheterization room, whereas cannulation was undertaken mainly in the ED and operating room in the USA. 3 With regard to the typical cannula size range, 19–21 Fr was used in venous ECMO and 15–17 Fr was used in arterial ECMO in approximately 70% of institutions in the present study. However, 23–25 Fr is used in venous and 19–21 Fr is used in arterial ECMO in approximately 50% of institutions in the USA. 3 Safe cannulation using larger catheters could be preferable for ECPR in Japan.
Initial ECMO management
With regard to the placement of distal limb perfusion catheters, routine catheter placement was carried out in only 25% of institutions, whereas >90% was routinely initiated in the USA. 3 This is mainly due to patient size, degree of obesity, 19 and catheter size. 20 Limb ischemia is less likely due to smaller catheter use without distal limb perfusion flows compared with Europe or North America. 20 , 21 Approximately 90% of distal limb perfusion catheters are used by cardiothoracic surgeons in the USA, whereas >70% are used by Eps, including acute care surgeons trained in cardiovascular surgery, in Japan. 3
Intra‐aortic balloon pumping/endotracheal intubation/management during CAG
Intra‐aortic balloon pumping is recognized as the bundle treatment option used during ECPR; 13 , 22 however, to our knowledge, ours is the first questionnaire to determine the details of IABP. Intra‐aortic balloon pumping was routinely initiated in only 25% of institutions, initiated as needed in 60%, and in 15% for cardiac origin only. This could reflect the variance of ECPR strategies in each participating hospital.
Computed tomography
Japan has the highest number of CT scanners worldwide; 23 thus, CTs were commonly used in the present survey and might be useful for diagnosis and prognostic prediction in ECPR. 24
Current issues and future challenges in ECPR in Japan
Regarding the low rates of inclusion and exclusion criteria, only high‐volume centers tend to have the latter. Considering the cost of ECPR, universally acceptable exclusion criteria need to be accepted, even at low‐volume centers. Establishing inclusion and exclusion criteria is a first step toward organizing a universal algorithm. To this end, the results of the upcoming SAVE‐J II study, with >2,000 ECPR cases, will therefore provide a robust conclusion in terms of the inclusion and exclusion criteria.
Approximately 10% of institutions had a hybrid ER, and the number of hybrid ERs will continue to increase. The advantages of ECPR initially carried out in a hybrid ER are considered to be earlier initiation of ECMO, safer procedures (cannulation under the X‐ray system), and shorter time required for the completion of percutaneous coronary intervention. These advantages could contribute to the development of better outcomes in Japan. Moreover, considering these advantages, the inclusion/exclusion criteria and management strategies in those facilities could be optimized.
Although aggressive blood drawing and routinely obtained CT before admittance to the ICU are among Japan’s ECPR strengths, more data are required to make the argument more effective in ECPR. As the present study showed that EPs carried out the cannulation in 66.7% of the surveyed institutions, the findings should be applicable to the USA and other countries. As mentioned above, the results of the SAVE‐J II study will also determine the association between cannulation by EPs in the ED and complications or good outcomes.
Limitations
This study had several limitations. First, because this is a questionnaire survey, more detailed clinical practice on ECPR was not obtained. For example, three‐choice questions answerable with “Yes, always”, “Yes, as needed”, and “No” did not obtain detailed information. Second, although 36 institutions were included in the present study, a previous report suggested that 69 institutes were capable of ECPR in Japan. 25 Therefore, not all Japanese ECPR facilities took part in the present study. However, almost all high‐volume ECPR centers are included in the present SAVE‐J II study, and responses were received from all 36 participating facilities. Finally, ICU management was not evaluated in the current study; therefore, an advanced questionnaire containing questions arising from ICU management of ECPR is being designed for future studies.
Conclusions
The variability of ECPR practice in patients with OHCA from ED to ICU has been described. We have some advantages with regard to hospital facilities, such as the availability of hybrid ER and CT; therefore, clinical research using the data of those Japanese facilities needs to be better coordinated. Extracorporeal cardiopulmonary resuscitation practice varied; therefore, establishing the inclusion/exclusion criteria will be required to provide the most appropriate and standardized management strategies in ECPR practice.
Disclosure
Approval of the research protocol: Approval number 19‐R155.
Informed consent: N/A.
Registry and registration no. of the study/trial: Not applicable.
Animal studies: N/A.
Conflict of interest: None.
Supporting information
Appendix S1. Details of the questionnaire regarding extracorporeal cardiopulmonary resuscitation practice in patients with out‐of‐hospital cardiac arrest in Japan.
Table S1. Initial extracorporeal membrane oxygenation (ECMO) management. CT, computed tomography; ICU, intensive care unit.
Table S2. Intra‐aortic balloon pumping (IABP)/endotracheal intubation/management during coronary angiography (CAG). CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; PAC, pulmonary artery catheter.
Table S3. Computed tomography. CAG, coronary angiography.
Acknowledgments
We thank all the members of the SAVE‐J II study group who participated in this study: Toru Takiguchi, MD; Kazuhiro Watanabe, MD; Takayuki Ogura, MD; Tomoya Okazaki, MD; Shinichi Ijuin, MD; Ryosuke Zushi, MD; Hideki Arimoto, MD; Hiroaki Takada, MD; Shinichirou Shiraishi, MD; Yuko Egawa, MD; Jun Kanda, MD; Michitaka Nasu, MD; Makoto Kobayashi, MD; Masaaki Sakuraya, MD; Hiromichi Naito, MD; Shunichiro Nakao, MD; Norio Otani, MD; Ichiro Takeuchi, MD; Naofumi Bunya, MD; Takafumi Shimizu, MD; Hirotaka Sawano, MD; Wataru Takayama, MD; Shigeki Kushimoto, MD; Tomohisa Shoko, MD; Makoto Aoki, MD; Takayuki Otani, MD; Yoshinori Matsuoka, MD; Koichiro Homma, MD; Kunihiko Maekawa, MD; Yoshio Tahara, MD; Reo Fukuda, MD; Migaku Kikuchi, MD; Takuo Nakagami, MD; Yoshihiro Hagiwara, MD; Nobuya Kitamura, MD; Kazuhiro Sugiyama MD.
T. Hifumi and A. Inoue contributed equally to this work.
Funding information
SAVE‐J II study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant‐in‐Aid for Scientific Research [C]) Grant Number JP19K09419.
References
- 1. Inoue A, Hifumi T, Sakamoto T, Kuroda Y. Extracorporeal cardiopulmonary resuscitation for out‐of‐hospital cardiac arrest in adult patients. J. Am. Heart Assoc. 2020; 9: e015291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coppler PJ, Abella BS, Callaway CW et al. Variability of extracorporeal cardiopulmonary resuscitation utilization for refractory adult out‐of‐hospital cardiac arrest: an international survey study. Clin. Exp. Emerg. Med. 2018; 5: 100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tonna JE, Johnson NJ, Greenwood J et al. Practice characteristics of Emergency Department extracorporeal cardiopulmonary resuscitation (eCPR) programs in the United States: the current state of the art of Emergency Department extracorporeal membrane oxygenation (ED ECMO). Resuscitation. 2016; 107: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ito MKM, Ujike Y, Imaizumi H, Yoshida M, Sakano S, Asai Y. Clinical application of cardiopulmonary bypass (CPB) to the resuscitation of DOA patients. JJAAM. 1990; 1: 25–33. [Google Scholar]
- 5. Nagao K, Hayashi N, Kanmatsuse K et al. Cardiopulmonary cerebral resuscitation using emergency cardiopulmonary bypass, coronary reperfusion therapy and mild hypothermia in patients with cardiac arrest outside the hospital. J. Am. Coll. Cardiol. 2000; 36: 776–83. [DOI] [PubMed] [Google Scholar]
- 6. Sakamoto T, Morimura N, Nagao K et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out‐of‐hospital cardiac arrest: a prospective observational study. Resuscitation. 2014; 85: 762–8. [DOI] [PubMed] [Google Scholar]
- 7. Macromill . Questant. [cited 1 Jan 2021]. Available from: https://questant.jp/enquete/edit/adhoc/606495.
- 8. Miyazaki K, Hikone M, Kuwahara Y, Ishida T, Sugiyama K, Hamabe Y. Extracorporeal CPR for massive pulmonary embolism in a “hybrid 2136 emergency department”. Am. J. Emerg. Med. 2019; 37: 2132–5. [DOI] [PubMed] [Google Scholar]
- 9. Kinoshita T, Yamakawa K, Matsuda H et al. The survival benefit of a novel trauma workflow that includes immediate whole‐body computed tomography, surgery, and interventional radiology, all in one trauma resuscitation room. Ann. Surg. 2019; 269: 370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayashida K, Kinoshita T, Yamakawa K, Miyara SJ, Becker LB, Fujimi S. Potential impacts of a novel integrated extracorporeal‐CPR workflow using an interventional radiology and immediate whole‐body computed tomography system in the emergency department. BMC Cardiovasc. Dis. 2020; 20: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yannopoulos D, Bartos J, Raveendran G et al. Advanced reperfusion strategies for patients with out‐of‐hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open‐label, randomised controlled trial. Lancet 2020; 396: 1807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ministry of Health LaW . Overview of Medical Service Regime in Japan. [cited 1 Jan 2021]. Available from: https://www.mhlw.go.jp/bunya/iryouhoken/iryouhoken01/dl/01_eng.pdf.
- 13. Ortega‐Deballon I, Hornby L, Shemie SD, Bhanji F, Guadagno E. Extracorporeal resuscitation for refractory out‐of‐hospital cardiac arrest in adults: a systematic review of international practices and outcomes. Resuscitation 2016; 101: 12–20. [DOI] [PubMed] [Google Scholar]
- 14. Otani T, Sawano H, Hayashi Y. Optimal extracorporeal cardiopulmonary resuscitation inclusion criteria for favorable neurological outcomes: a single‐center retrospective analysis. Acute Med. Surg. 2020; 7: e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goto T, Morita S, Kitamura T et al. Impact of extracorporeal cardiopulmonary resuscitation on outcomes of elderly patients who had out‐of‐hospital cardiac arrests: a single‐centre retrospective analysis. BMJ Open 2018; 8: e019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ono Y, Hayakawa M, Maekawa K, et al. Fibrin/fibrinogen degradation products (FDP) at hospital admission predict neurological outcomes in out‐of‐hospital cardiac arrest patients. Resuscitation 2017; 111: 62–7. [DOI] [PubMed] [Google Scholar]
- 17. Shiba DHT, Tsuchiya M, Hattori K, et al. Pneumonia and extracorporeal cardiopulmonary resuscitation followed by targeted temperature management in patients with out‐of‐hospital cardiac arrest ‐ retrospective cohort study. Circ. Rep. 2019; 1: 575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Otani T, Sawano H, Natsukawa T et al. D‐dimer predicts bleeding complication in out‐of‐hospital cardiac arrest resuscitated with ECMO. Am. J. Emerg. Med. 2018; 36: 1003–8. [DOI] [PubMed] [Google Scholar]
- 19. Collaboration NCDRF . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet. 2016; 387: 1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim J, Cho YH, Sung K et al. Impact of cannula size on clinical outcomes in peripheral venoarterial extracorporeal membrane oxygenation. ASAIO J. 2019; 65: 573–9. [DOI] [PubMed] [Google Scholar]
- 21. Bonicolini E, Martucci G, Simons J et al. Limb ischemia in peripheral veno‐arterial extracorporeal membrane oxygenation: a narrative review of incidence, prevention, monitoring, and treatment. Crit. Care 2019; 23: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shin JS, Lee SW, Han GS, Jo WM, Choi SH, Hong YS. Successful extracorporeal life support in cardiac arrest with recurrent ventricular fibrillation unresponsive to standard cardiopulmonary resuscitation. Resuscitation 2007; 73: 309–13. [DOI] [PubMed] [Google Scholar]
- 23. Sasaki T, Izawa M, Okada Y. Current trends in health insurance systems: OECD countries vs. Japan. Neurol. Med. Chir. (Tokyo) 2015; 55: 267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sugiyama K, Takahashi M, Miyazaki K, Ishida T, Kobayashi M, Hamabe Y. Left ventricular wall findings in non‐electrocardiography‐gated contrast‐enhanced computed tomography after extracorporeal cardiopulmonary resuscitation. Crit. Care 2019; 23: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kitamura T, Iwami T, Atsumi T et al. The profile of Japanese Association for Acute Medicine ‐ out‐of‐hospital cardiac arrest registry in 2014‐2015. Acute Med. Surg. 2018; 5: 249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Details of the questionnaire regarding extracorporeal cardiopulmonary resuscitation practice in patients with out‐of‐hospital cardiac arrest in Japan.
Table S1. Initial extracorporeal membrane oxygenation (ECMO) management. CT, computed tomography; ICU, intensive care unit.
Table S2. Intra‐aortic balloon pumping (IABP)/endotracheal intubation/management during coronary angiography (CAG). CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; PAC, pulmonary artery catheter.
Table S3. Computed tomography. CAG, coronary angiography.


