Abstract
After the early advent of the Coronavirus Disease 2019 (COVID-19) pandemic, myriads of FDA-approved drugs have been massively repurposed for COVID-19 treatment based on molecular docking against selected protein targets that play fundamental roles in the replication cycle of the novel coronavirus. Honeybee products are well known of their nutritional values and medicinal effects. Bee products contain bioactive compounds in the form of a collection of phenolic acids, flavonoids, and terpenes of natural origin that display wide spectrum antiviral effects. We revealed by molecular docking the profound binding affinity of 14 selected phenolics and terpenes present in honey and propolis (bees glue) against the main protease (Mpro) and RNA-dependent RNA polymerase (RdRp) enzymes of the novel SARS-CoV-2 virus (the causative agent of COVID-19) using AutoDock Vina software. Of these compounds, p-coumaric acid, ellagic acid, kaempferol, and quercetin have the strongest interaction with the SARS-CoV-2 target enzymes, and it may be considered an effective COVID-19 inhibitor.
Keywords: COVID-19, Honeybee products, Phenolic compounds, Molecular docking, Drug repurposing, Natural products
Introduction
In December 2019, COVID-19 firstly manifested in Wuhan, province of Hubei in China, where frequent number of patients shared similar symptoms of dry cough, fever, and fatigue; then they developed into dyspnea quickly, ending up with acute respiratory distress syndrome (ARDS) in severe cases (Chen et al. 2020; Chan et al. 2020; Zhu et al. 2020; Huang et al. 2020; Zhou et al. 2020).
As of 1st of May 2021, the cumulative number of cases diagnosed with COVID-19 in the world was more than 153 million, whereas more than 3 million cases died (Coronavirus Update (Live): https://www.worldometers.info/coronavirus - Worldometer 2021). As a direct effect of the outbreak, more than 160 countries are fighting to combat the spread of COVID-19 and taking protective measures to save their citizens from the pandemic; at the same time, research institutes, drug corporations, biotechnology institutes, and research groups all over the world are racing to develop effective drugs or potential vaccines for COVID-19 (Sharpe et al. 2020; Thanh Le et al. 2020; Pooladanda et al. 2020; Hachfi and Ben Lasfar 2020; Mullard 2020; Biopharma products in development for COVID-19 2021).
As a fast track to save the time needed for safety and approval studies, researchers started to massively repurpose already FDA-approved drugs for COVID-19 treatment (Kandeel and Al-Nazawi 2020; Harrison 2020). Computational-based techniques like molecular modeling and virtual screening represent magic tools that help to understand the molecular aspects of protein ligand interactions during rational drug design process (Murgueitio et al. 2012). Virtual screening has been encountered in structure-based drug design against emerging and fatal diseases of viral origin (Sirois et al. 2004; Elhefnawi et al. 2012; Raj and Varadwaj 2016; Zhou et al. 2008; Plewczynski et al. 2007).
The key protease (Mpro) and the RNA-dependent RNA polymerase (RdRP), which are responsible for the viral polyprotein proteolytic process as well as viral genome replication and transcription, are two promising drug targets for SARS-CoV-related diseases (Gao et al. 2020) and the main protease (Mpro) responsible for virus maturation in addition to crucial roles in mediating viral replication and transcription (Jin et al. 2020). Based on their crucial role in the life cycle of SARS-CoV-2, these two target sites have been extensively docked to design or distinguish structure-based effective drugs for COVID-19 (Dai et al. 2020).
Bioactive compounds of natural origin are currently screened by molecular docking to in silico test their affinity to molecular targets of COVID-19 taking the advantage that natural products are free from toxic or side effects (Mani et al. 2020; Sayed et al. 2020; Gurung et al. 2020; Khalifa et al. 2020b). Recently, honeybee products have been proposed as a potential compatible antiseptic to help protect against the COVID-19 based on biocidal effect of hydrogen peroxide and other phytochemicals existing in bee products (Al Naggar et al. 2020). These phenolic compounds and terpenes found in honeybee products were documented to possess variable medicinal effects including wound healing, antioxidant, antimicrobial, antiviral, anti-inflammatory, cardioprotective, and neuroprotective activities (Küçük et al. 2007; Mohamed et al. 2009; Al Naggar et al. 2016; Pasupuleti et al. 2017; Jibril et al. 2019; El-Seedi et al. 2020; Al Naggar et al. 2020; Al Naggar et al. 2021).
In our study, we performed deep virtual screening via molecular docking to test binding affinity of various selected bioactive compounds such as terpenes and flavonoids of honey and propolis as inhibitors against the COVID-19 essential enzymes: RNA-dependent RNA polymerase and the main protease.
Docking methodology
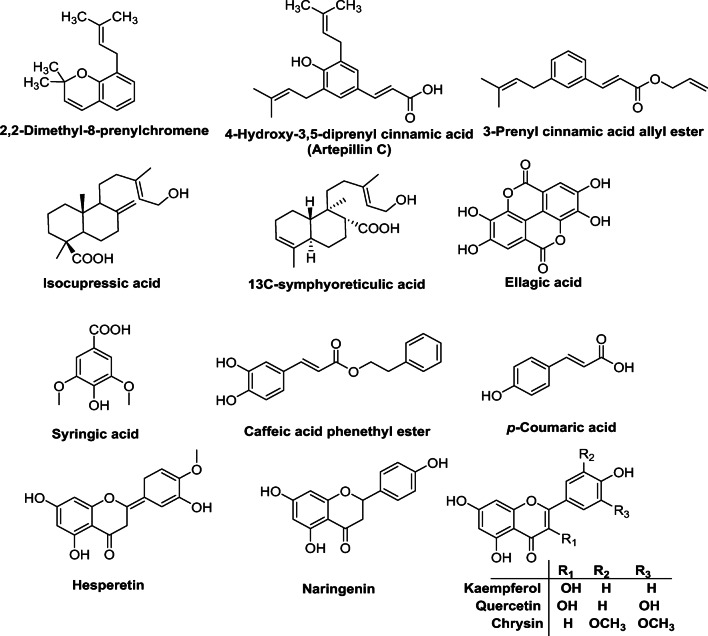
The crystal structures of COVID-19 RNA-dependent RNA polymerase (RdRp) (PDB code: 6M71) (Gao et al. 2020) and the main protease (Mpro) (PDB code: 6LU7) (Jin et al. 2020) were retrieved from Protein Data Bank. This docking study was carried out on 14 compounds (Fig. 1) from honey and propolis into the receptor active site using AutoDock Vina (Trott and Olson 2010). These compounds were selected based on previously reported antiviral activities against related viruses to COVID-19 (Al Naggar et al. 2020; Shahidi and Yeo 2018); in the same context, several studies employing virtual screening of closely related members or categories of the selected phytochemicals were performed against SARS-CoV-2 proteins since these phytochemicals are naturally existing in plants and spices (Sayed et al. 2020; Ibrahim et al. 2020; Umesh et al. 2020).
Fig. 1.
Chemical structure of important bioactive compounds in honeybee products
Ligand structures were drawn into Marvin Sketch V19.12 (Marvin | ChemAxon 2020), and the most energetically favored conformer was exported as (*.pdb) file format. AutoDockTools package (Morris et al. 2009) was used to assign Gasteiger atomic partial charges, and all the rotatable bonds in ligands were set to be flexible. For receptor preparation, all water molecules were removed, the co-crystalized ligand was removed, Gasteiger atomic partial charges were assigned, and all receptors and ligands were converted to the PDBQT format using AutoDockTools package for docking process. In the AutoDock Vina configuration files, the parameter num modes was set to 10 and exhaustiveness to 14. The grid boxes of center (x= 118.23, y= 103.32, and z= 118.37) with size (x=17, y=25, z=17) for the RNA-dependent RNA polymerase and center (x= −10.71, y= 12.41, and z= 68.83) with size (x=16, y=18, z=16) for the main protease were used to define the active site. AutoDock Vina was executed. Pymol (PyMOL Molecular Visualization System 2020) was used for 3D visualization, and the 2D schematic presentation was generated using LigPlot+ V1.4.5 (Laskowski and Swindells 2011).
Results and discussion
Computational docking was implemented to predict the binding mode of 14 compounds representing flavonoids, phenolic acids, and terpenes from honey and propolis (Fig. 1) with two different targets from COVID-19.
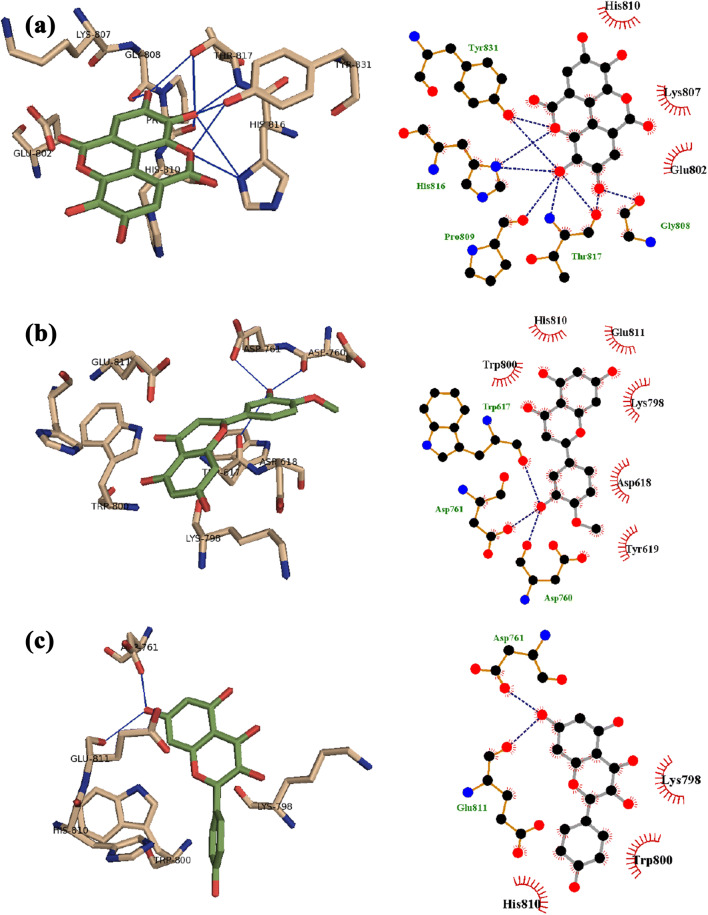
The bioactive compounds, ellagic acid, hesperetin, and kaempferol, are the most promising compounds on COVID-19 RdRp, while artepillin C, ellagic acid, hesperetin, kaempferol, and quercetin were the most active on the main protease (Mpro). The binding scores for each compound into the two targets are shown in Table 1. The binding mode for ellagic acid to COVID-19 RdRb was attributed to H-bond interaction with Gly808, pro809, His816, Thr817, and Tyr 831, while amino acid residues Trp617, Asp760, and Asp761 are positioned at distance of H-bond with hesperetin, and also kaempferol interacts with Glu811 and Asp761 by H-bond. Furthermore, the aromatic ring system of ellagic acid, hesperetin, and kaempferol makes π-ion hydrophobic interaction with Lys798 (Fig. 2). We repurpose the compounds of interesting binding scores as potent inhibitors of viral replication.
Table 1.
The binding scores for each compound into the two target enzymes of SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) and the main protease (Mpro)
| Bioactive compounds | SARS-CoV-2 RNA-dependent RNA polymerase | SARS-CoV-2 main protease (Mpro) |
|---|---|---|
| 2,2-Dimethyl-8-prenylchromene | −5.6 | −6.8 |
| Artepillin C | −5.9 | −7.5 |
| 3-Prenyl cinnamic acid allyl ester | −5.3 | −6.2 |
| Isocupressic acid | −5.8 | −6.4 |
| 13C-symphyoreticulic acid | −5.7 | −6.9 |
| Ellagic acid | −6.4 | −7.5 |
| Syringic acid | −5.5 | −5.6 |
| Caffeic acid phenethyl ester | −5.4 | −7.0 |
| p-Coumaric acid | −5.3 | −5.6 |
| Hesperetin | −6.3 | −7.4 |
| Naringenin | −6.0 | −6.5 |
| Kaempferol | −6.2 | −7.8 |
| Quercetin | −6.1 | −7.4 |
| Chrysin | −6.1 | −7.2 |
Fig. 2.
The docking complex of a ellagic acid, b hesperetin, and c kaempferol (green) with the X-ray structure of 6M71; SARS-CoV-2 RNA-dependent RNA polymerase (left, tint) that showed hydrogen bond (blue) interaction and 2D schematic diagram of the interaction (right)
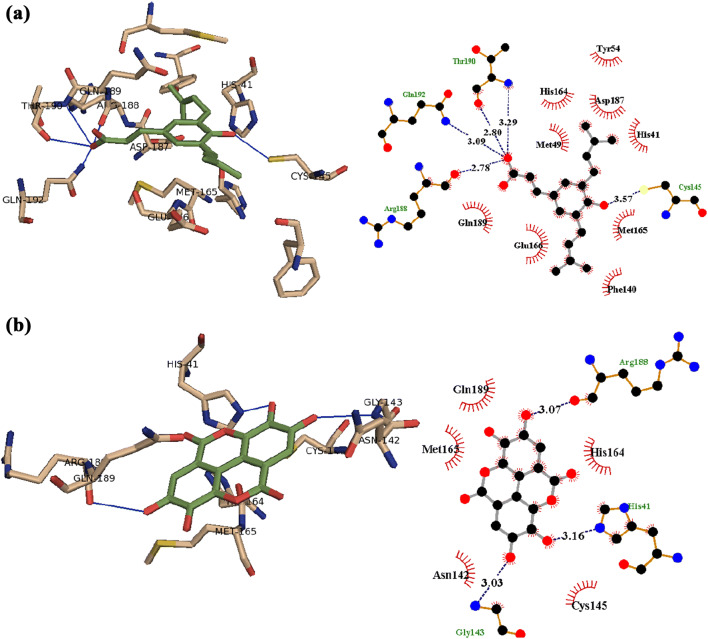
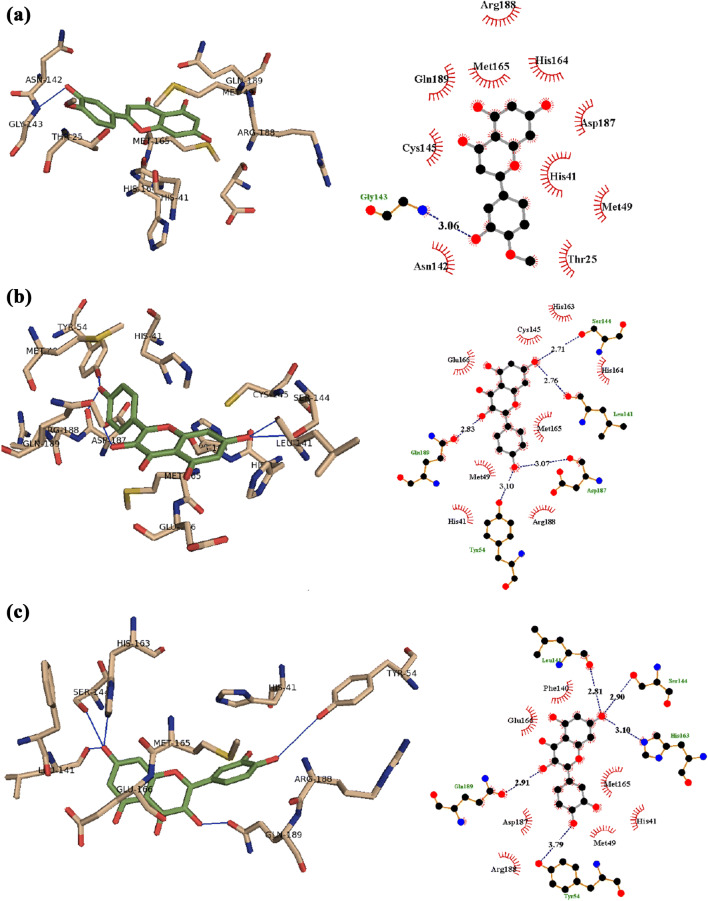
From the docking of all identified compounds into the active site of SARS-CoV-2 main protease (Mpro) in the current study, artepillin C showed H-bond interaction with Cys145, Arg188, Thr190, and Gln192, while amino acid residues His41, Gly143, and Arg188 are positioned at distance of H-bond with ellagic acid (Fig. 3). In addition, hesperetin interacts with Gly143 by H-bond, while amino acid residues Tyr54, Leu141, Ser144, Asp 187, and Gln189 are positioned at distance of H-bond with kaempferol, and also quercetin makes H-bond with Tyr54, Leu141, Ser144, His163, and Gln189. Furthermore, the aromatic ring system of artepillin C, ellagic acid, hesperetin, kaempferol, and quercetin makes π-ion hydrophobic interaction with either Met165 or Glu166 (Fig. 4). Taken together we propose the indicated flavonoids as potential inhibitors of the main protease of COVID-19, thus limiting viral maturation.
Fig. 3.
The docking complex of a artepillin C and b ellagic acid (green) with the X-ray structure of 6LU7; SARS-CoV-2 main protease (Mpro) (left, tint) that showed hydrogen bond (blue) interaction and 2D schematic diagram of the interaction (right)
Fig. 4.
The docking complex of a hesperetin, b kaempferol, and c quercetin (green) with the X-ray structure of 6LU7; SARS-CoV-2 main protease (Mpro) (left, tint) that showed hydrogen bond (blue) interaction and 2D schematic diagram of the interaction (right)
In line with our finding, promising candidates identified in our study like p-coumaric acid, ellagic acid, kaempferol, and quercetin were previously found to have potential antiviral activity against the common cold human rhinovirus which is RNA virus like SARS-CoV-2; surprisingly the mentioned bioactive compounds were suggested in the same study to block or reduce the viral entry into the cells to protect the cells from the virus cytopathic effects and subside virus replication (Kwon et al. 2019), supporting our virtual screening. Moreover, quercetin and its derivatives were previously confirmed to inhibit the SARS-CoV proteases of other coronaviruses including SARS-CoV proteases (3CLpro and PLpro) which share 97% homology to COVID-19 main protease (Bafna et al. 2020) as well as the Middle Eastern respiratory syndrome coronavirus (MERS-CoV) 3CLpro protease (Nguyen et al. 2012). Quercetin was also able to inhibit both enzymes in vitro in micromolar doses (Park et al. 2017); in general SARS-CoV and MERS-CoV share 82.45 and 69.58 percentage identity of their genome to SARS-CoV-2 (Kaur et al. 2020). The existence of this mixture of phytomedicines in bee products create a broad spectrum anti-COVID-19 cocktail that targets more than one crucial enzyme of the virus. Aside from the two enzymes we docked in our sample, other studies investigated the binding affinity of bee phytochemicals, especially propolis from various geographical origins, against other COVID-19 targets through virtual screening (Khalifa et al. 2020a; Ibrahim et al. 2020; Khalifa et al. 2020b; Khayrani et al. 2021; Güler et al. 2020). Taken all together, from our study and other studies, we spot the light on the protective and preventive role of honeybee products against COVID-19.
Conclusions
Molecular docking of honeybee products’ set of bioactive compounds against unique COVID-19 targets, including Mpro and RdRb enzymes, has distinguished promising compounds of natural origin with deep binding to the respective COVID-19 targets. P-coumaric acid, ellagic acid, kaempferol, and quercetin are the most promising compounds on COVID-19 active sites (RdRb and Mpro). These bioactive compounds were also found to have potential antiviral activity against the common cold human rhinovirus which is RNA virus like SARS-CoV-2. In summary and based on our theoretical studies supported by previous in vitro confirmatory studies, we recommend further in vivo investigations to assess the predicted affinity of the selected compounds against the novel coronavirus target enzymes.
Acknowledgements
YA and GY are grateful to Alexander von Humboldt (AvH) Foundation for post-doc fellowships.
Author contribution
Conceptualization: Yahya Al Naggar and Galal Yahya. Methodology: Moataz A. Shaldam. Writing original draft and preparation: Moataz A. Shaldam, Galal Yahya, Nashwa Mohamed, and Yahya Al Naggar. Writing, review, and editing: Galal Yahya, Mohamed Abdel-Daim, and Yahya Al Naggar.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
No human or animal specimens were used in this work.
Consent for publication
Not applicable
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al Naggar, Yahya, Sun J, Robertson A, Giesy JP, Wiseman S (2016) Chemical characterization and antioxidant properties of Canadian propolis. In J Apic Res 55(4):305–314.10.1080/00218839.2016.1233700
- Naggar A, Yahya, Giesy JP, Abdel-Daim MM, Ansari J, Mohammad, Al-Kahtani SN, Yahya G. Fighting against the second wave of COVID-19: can honeybee products help protect against the pandemic? In Saudi J Biol Sci. 2020;28:1519–1527. doi: 10.1016/j.sjbs.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Naggar Y, Yahya G, Al-Kahtani S, Stangaciu S, El-Seedi H (2021) Back to Ancient Remedy: Could Inhalation of Aerosolised-Honey and Propolis Tincture Protect Against the COVID-19 Pandemic? J Apither 8(2):1–5
- Bafna K, Krug RM, Montelione GT (2020) Structural similarity of SARS-CoV2 Mpro and HCV NS3/4A proteases suggests new approaches for identifying existing drugs useful as COVID-19 therapeutics. In ChemRxiv. 10.26434/chemrxiv.12153615
- Biopharma products in development for COVID-19 (2021). Available online at https://www.bioworld.com/COVID19products, updated on 1/14/2021, checked on 1/14/2021.
- Chan JF-W, Yuan S, Kok K-H, Kai-Wang TK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. In Lancet (London, England) 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J'a, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. In Lancet (London, England) 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus Update (Live): 93,163,080 Cases and 1,993,578 Deaths from COVID-19 virus pandemic - Worldometer (2021). Available online at https://www.worldometers.info/coronavirus/, updated on 1/14/2021, checked on 1/14/2021.
- Dai W, Zhang B, Jiang X-M, Su H, Li J, Zhao Y, Xie X, Jin Z, Peng J, Liu F, Li C, Li Y, Bai F, Wang H, Cheng X, Cen X, Hu S, Yang X, Wang J, Liu X, Xiao G, Jiang H, Rao Z, Zhang L-K, Xu Y, Yang H, Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368(6497):1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhefnawi M, ElGamacy M, Fares M. Multiple virtual screening approaches for finding new hepatitis C virus RNA-dependent RNA polymerase inhibitors: structure-based screens and molecular dynamics for the pursue of new poly pharmacological inhibitors. In BMC Bioinformatics. 2012;13(17):S5. doi: 10.1186/1471-2105-13-S17-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Seedi HR, Khalifa SAM, El-Wahed AA, Gao R, Guo Z, Tahir HE, et al. Honeybee products: an updated review of neurological actions. In Trends Food Sci Technol. 2020;101:17–27. doi: 10.1016/j.tifs.2020.04.026. [DOI] [Google Scholar]
- Gao Y, Yan L, Huang Y, Liu F, Zhao Y, Cao L, et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. In Science (New York, NY) 2020;368(6492):779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güler, Halil Ibrahim; Tatar, Gizem; Yildiz, Oktay; Belduz, Ali Osman; Kolayli, Sevgi (2020) Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by Molecular Docking Study. [DOI] [PMC free article] [PubMed]
- Gurung AB, Ali MA, Lee J, Farah MA, Al-Anazi KM. Unravelling lead antiviral phytochemicals for the inhibition of SARS-CoV-2 Mpro enzyme through in silico approach. In Life sciences. 2020;255:117831. doi: 10.1016/j.lfs.2020.117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachfi W, Ben Lasfar N. COVID-19: Main therapeutic options. La Tunisie medicale. 2020;98(4):299–303. [PubMed] [Google Scholar]
- Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020;38(4):379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. In The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MAA, Abdelrahman AHM, Hussien TA, Badr EAA, Mohamed TA, El-Seedi HR, et al. In silico drug discovery of major metabolites from spices as SARS-CoV-2 main protease inhibitors. In Comput Biol Med. 2020;126:104046. doi: 10.1016/j.compbiomed.2020.104046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jibril FI, Hilmi ABM, Manivannan L (2019) Isolation and characterization of polyphenols in natural honey for the treatment of human diseases. In Bull Natl Res Cent 43(1). 10.1186/s42269-019-0044-7
- Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. In Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Kandeel M, Al-Nazawi M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. In Life sciences. 2020;251:117627. doi: 10.1016/j.lfs.2020.117627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur N, Singh R, Dar Z, Bijarnia RK, Dhingra N, Kaur T. Genetic comparison among various coronavirus strains for the identification of potential vaccine targets of SARS-CoV2. Infection Genetics and Evolution: Infect Genet Evol. 2020;104490:104490. doi: 10.1016/j.meegid.2020.104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa SAM, Mohamed BS, Elashal MH, Du M, Guo Z, Zhao C et al (2020a) Comprehensive overview on multiple strategies fighting COVID-19. In Int J Environ Res Public Health 17(16) [DOI] [PMC free article] [PubMed]
- Khalifa SAM, Yosri N, El-Mallah MF, Ghonaim R, Guo Z, Musharraf SG et al (2020b) Screening for natural and derived bio-active compounds in preclinical and clinical studies: one of the frontlines of fighting the coronaviruses pandemic. In Phytomedicine:153311. 10.1016/j.phymed.2020.153311 [DOI] [PMC free article] [PubMed]
- Khayrani AC, Irdiani R, Aditama R, Pratami DK, Lischer K, Ansari MJ, et al. Evaluating the potency of Sulawesi propolis compounds as ACE-2 inhibitors through molecular docking for COVID-19 drug discovery preliminary study. In J King Saud Univ Sci. 2021;33(2):101297. doi: 10.1016/j.jksus.2020.101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küçük M, Kolaylı S, Karaoğlu Ş, Ulusoy E, Baltacı C, Candan F. Biological activities and chemical composition of three honeys of different types from Anatolia. In Food Chem. 2007;100(2):526–534. doi: 10.1016/j.foodchem.2005.10.010. [DOI] [Google Scholar]
- Kwon MJ, Shin HM, Perumalsamy H, Wang X, Ahn Y-J (2019) Antiviral effects and possible mechanisms of action of constituents from Brazilian propolis and related compounds. In J Apic Res:1–13. 10.1080/00218839.2019.1695715
- Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. In J Chem Inf Model. 2011;51(10):2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Mani JS, Johnson JB, Steel JC, Broszczak DA, Neilsen PM, Walsh KB, Naiker M. Natural product-derived phytochemicals as potential agents against coronaviruses: a review. In Virus Res. 2020;284:197989. doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin | ChemAxon. version 19.12, by ChemAxon (2020) Available online at https://chemaxon.com/products/marvin, updated on 6/11/2020, checked on 6/11/2020.
- Mohamed M, Sirajudeen K, Swamy M, Yaacob NS, Sulaiman SA. Studies on the antioxidant properties of Tualang honey of Malaysia. In Afr J Tradit Complement Altern Med. 2009;7(1):59–63. doi: 10.4314/ajtcam.v7i1.57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. In J Comput Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A. Flooded by the torrent: the COVID-19 drug pipeline. In Lancet (London, England) 2020;395(10232):1245–1246. doi: 10.1016/S0140-6736(20)30894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgueitio MS, Bermudez M, Mortier J, Wolber G. In silico virtual screening approaches for anti-viral drug discovery. In Drug Discov Today Technol. 2012;9(3):e219–e225. doi: 10.1016/j.ddtec.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TTH, Woo H-J, Kang H-K, van Nguyen D, Kim Y-M, Kim D-W, et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol Lett. 2012;34(5):831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-Y, Yuk HJ, Ryu HW, Lim SH, Kim KS, Park KH, Ryu YB, Lee WS. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. In J Enzyme Inhib Med Chem. 2017;32(1):504–515. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupuleti VR, Sammugam L, Ramesh N, Gan SH. Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. In Oxid Med Cell Longev. 2017;2017:1259510–1259521. doi: 10.1155/2017/1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewczynski D, Hoffmann M, von Grotthuss M, Ginalski K, Rychewski L. In silico prediction of SARS protease inhibitors by virtual high throughput screening. Chem Biol Drug Des. 2007;69(4):269–279. doi: 10.1111/j.1747-0285.2007.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooladanda V, Thatikonda S, Godugu C (2020) The current understanding and potential therapeutic options to combat COVID-19. In Life sciences 254:117765. 10.1016/j.lfs.2020.117765 [DOI] [PMC free article] [PubMed]
- PyMOL Molecular Visualization System. Version 2.0, by Schrödinger (2020). Available online at https://pymol.org/2/, updated on 6/11/2020, checked on 6/11/2020.
- Raj U, Varadwaj PK. Flavonoids as multi-target inhibitors for proteins associated with Ebola virus: in silico discovery using virtual screening and molecular docking studies. In Interdisciplinary Sciences, Computational Life Sciences: INTERDISCIP SCI . 2016;8(2):132–141. doi: 10.1007/s12539-015-0109-8. [DOI] [PubMed] [Google Scholar]
- Sayed AM, Khattab AR, AboulMagd AM, Hassan HM, Rateb ME, Zaid H, Abdelmohsen UR. Nature as a treasure trove of potential anti-SARS-CoV drug leads: a structural/mechanistic rationale. In RSC Adv. 2020;10(34):19790–19802. doi: 10.1039/D0RA04199H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F, Yeo JD (2018) Bioactivities of phenolics by focusing on suppression of chronic diseases: a review. In Int J Mol Sci 19(6). 10.3390/ijms19061573 [DOI] [PMC free article] [PubMed]
- Sharpe HR, Gilbride C, Allen E, Belij-Rammerstorfer S, Bissett C, Ewer K, Lambe T. The early landscape of COVID-19 vaccine development in the UK and rest of the world. In Immunology. 2020;160:223–232. doi: 10.1111/imm.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois S, Wei D-Q, Du Q, Chou K-C. Virtual screening for SARS-CoV protease based on KZ7088 pharmacophore points. In J Chem Inform Comput Sci. 2004;44(3):1111–1122. doi: 10.1021/ci034270n. [DOI] [PubMed] [Google Scholar]
- Thanh Le T, Andreadakis Z, Kumar A, Román G, Raúl, Tollefsen S, Saville M, Mayhew S. The COVID-19 vaccine development landscape. In Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. In J Comput Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesh, Kundu D, Selvaraj C, Singh SK, Dubey VK (2020) Identification of new anti-nCoV drug chemical compounds from Indian spices exploiting SARS-CoV-2 main protease as target. In J Biomol Struct Dyn:1–9. 10.1080/07391102.2020.1763202 [DOI] [PMC free article] [PubMed]
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Khaliq M, Suk J-E, Patkar C, Li L, Kuhn RJ, Post CB. Antiviral compounds discovered by virtual screening of small-molecule libraries against dengue virus E protein. In ACS Chem Biology. 2008;3(12):765–775. doi: 10.1021/cb800176t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.