Abstract
Background
The evidence of using JAK inhibitors among hospitalized patients with COVID-19 is conflicting. The systematic review and meta-analysis aimed to address the efficacy of Janus Kinase (JAK) Inhibitors in reducing risk of mortality among hospitalized patients with COVID-19.
Methods
Several electronic databases, including PubMed, EuropePMC, and the Cochrane Central Register of Controlled Trials, with relevant keywords “COVID-19″ AND (“JAK inhibitor” OR “Ruxolitinib” OR “Tofacitinib” OR “Fedratinib” OR “Baricitinib”) AND (“Severe” OR “Mortality”), were used to perform a systematic literature search up to December 11, 2020. All studies pertinent to the predetermined eligibility criteria were included in the analysis. Our outcome of interest was all types of mortality, clinical improvement, and clinical deterioration. Dichotomous variables of our outcomes of interest were analyzed using Maentel-Haenszel formula to obtain odds ratios (ORs) and 95% confidence intervals (CI) with random-effects modeling regardless of heterogeneity.
Results
Five studies with a total of 1190 patients and were included in this systematic review and meta-analysis. The use of JAK inhibitors was associated with a reduced risk of mortality (OR 0.51, 95% CI 0.28–0.93, P = 0.02; I2: 7.8%, P = 0.354) and clinical improvement (OR 1.76, 95% CI 1.05–2.95, P = 0.032; I2: 26.4%, P = 0.253). The use of JAK inhibitors was not associated with a reduced risk of clinical deterioration (OR 0.58, 95% CI 0.28–1.19, P = 0.136; I2: 24.1%, P = 0.267).
Conclusion
The use of JAK inhibitors was significantly associated with a reduced risk of mortality, and clinical improvement in hospitalized patients with COVID-19.
Keywords: Janus kinase inhibitors, COVID-19, SARS-CoV-2, Mortality, Clinical outcome
1. Introduction
Although the advent of promising COVID-19 vaccine seems could end this catastrophe by developing antibodies to prevent COVID-19 infection, treatment options for hospitalized patients with COVID-19 are still urgently needed to be addressed,1 especially in high-risk patients who are at greater risk of developing severe or critical COVID-19.2, 3, 4 The only effective treatment for severe COVID-19 by far is dexamethasone,5 while others turn out to be inconclusive and might be inefficacious.6
Hyperinflammatory state induced by severe COVID-19, which is characterized by massive cytokine release and increased of pro-inflammatory markers, is a critical condition that may culminate into acute respiratory distress syndrome (ARDS), fatal thrombosis, and multi-organ failure.7, 8, 9 Drugs that act to inhibit cytokine production are proposed to be beneficial in rescuing immense cytokine derangements in severe COVID-19. Janus Kinase (JAK) inhibitors presents as attractive therapeutic strategy, as this type of medication could deter intracellular signalling pathway in cytokine productions.
The evidence of using JAK inhibitors among hospitalized patients with COVID-19 is conflicting. Ruxolitinib, one of the JAK inhibitors, has previously been reported in a non-randomized trial to be efficacious in reducing mortality among hospitalized patients with severe COVID-19.10 D'Alessio et al. showed significantly higher overall survival among COVID-19 patients receiving ruxolitinib [Hazard ratio (HR) for overall survival 4.65, 95% CI 1.65–12.7; p = 0.0034].10 Remarkably, Kalil et al. reported insignificant mortality reduction using a combination of JAK inhibitors with remdesivir in a multi-center randomized controlled trial (HR 0.65; 95% CI, 0.39–1.09); though, accelerated clinical improvement was documented [Odds ratio (OR) 1.3; 95% CI, 1.0–1.6].11 Therefore, this systematic review was aimed to assess the efficacy of JAK inhibitors in terms of clinical outcome, including mortality, clinical improvement, and clinical deterioration among hospitalized COVID-19 patients.
2. Methods
The protocol of this study is registered in PROSPERO (CRD42021228844).
2.1. Study eligibility criteria
The inclusion criteria were as follows: (1) Experimental studies (either non-randomized or randomized studies), (2) in hospitalized adults patients (>18 years old) with confirmed COVID-19 based on reverse transcriptase - polymerase chain reaction (RT-PCR) test, and (3) receiving JAK inhibitors (either ruxolitinib, tofacitinib, fedratinib, or baricitinib), and (4) with our clinical outcomes of interest. Our main outcome of interest was all types of mortality, including in-hospital mortality and 14- or 28-days (30-days) mortality. Additional secondary outcome were clinical improvement and clinical deterioration. Clinical improvement was defined as being discharged or reduction of oxygen requirements after the last treatment dose. Clinical deterioration was defined as intensive care unit (ICU) admission, new use of invasive mechanical ventilation (MV), and or extra-corporeal membrane oxygenation (ECMO). Observational studies and research on pediatric patients (<18 years old) were excluded from this study.
2.2. Search strategy
Several electronic databases, including PubMed, EuropePMC, and the Cochrane Central Register of Controlled Trials, with relevant keywords “COVID-19″ AND (“JAK inhibitor” OR “Ruxolitinib” OR “Tofacitinib” OR “Fedratinib” OR “Baricitinib”) AND (“Severe” OR “Mortality”), were used to perform a systematic literature search from the date of inception up to December 11, 2020. Any duplicate records were removed after obtaining the initial results. By screening the title and abstracts, potential articles were then sorted. Afterwards, the full texts of the remaining articles were assessed for relevance based on eligibility criteria. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was carried out as the core protocol in this study.
2.3. Data collection and risk of bias assessment
Three authors independently performed a systematic search of studies, assessed study inclusion, extracted data, and assessed risk of bias. A pre-built form was used by the authors for data collection, including name of authors, study design and location, total samples, age, intervention, outcome, and key findings of the study. Randomized controlled trials (RCT) were assessed using Cochrane risk-of-bias tool for randomized trials (RoB 2),12 while ROBINS-I tool was used for non-randomized experimental studies.13 Rob 2 is a risk of bias tool which assess bias in RCT based on several domains, including bias due to randomization process, deviations from intended interventions, missing outcome data, measurement of outcome, and reporting bias.12 The ROBINS-I tool also measures risk of bias in multi-dimensional approach which includes bias in confounding factors, selection bias, bias in measurement of intervention, bias in deviations from intervention, missing data, bias in measurement of outcome, and reporting bias.13 Any results disagreement between authors will be determined through discussion.
2.4. Data synthesis and statistical analysis
Meta-analysis of included studies was conducted using STATA 16 (StataCorp LLC, Texas, US). Dichotomous variables of our outcomes of interest were analyzed using Maentel-Haenszel formula to obtain odds ratios (ORs) and 95% confidence intervals (CIs). Random-effects models were used for pooled analysis regardless of heterogeneity. P values were two-tailed, and statistical significance was set at ≤ 0.05. Heterogeneity between studies was analyzed using I2 (I2) statistics with a value of >50% or P-values <0.10 suggesting significant heterogeneity. Small study effects were assessed with Egger test.
3. Results
3.1. Study selection and characteristics of included studies
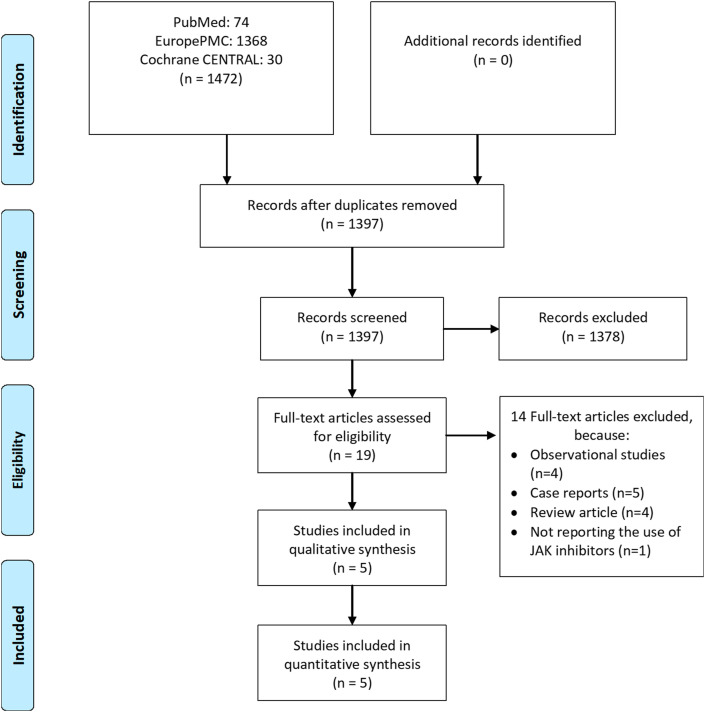
There were 1470 records from our initial searches, which was reduced to 1395 after duplicates removal. We excluded 1376 records after title and abstract screening. After assessing 19 full-texts of the remaining articles for eligibility, 14 articles were excluded from the final analysis. The exclusion were due to observational studies (n = 4), case reports (n = 5), review articles (n = 4), and not reporting the use of JAK inhibitors (n = 1). Thereby, five studies with a total of 1190 patients included in this systematic review and meta-analysis [Fig. 1 ].10 , 11 , 14, 15, 16 Three studies were considered non-randomized experimental studies, while the remaining were RCTs. Ruxolitinib and baricitinib were the only JAK inhibitors used in these trials. The characteristics of the included studies are shown in Table 1 .
Fig. 1.
Study flow diagram.
Table 1.
Characteristics of included studies.
| No | Authors | Study Design/Location | Total Samples (Intervention vs Control) | Age (Mean or Median) | Intervention (JAK Inhibitors) | Outcome | Result/Key Findings (Intervention vs Control) | Secondary/New Infection (Intervention vs Control) | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| 1 | D'Alessio et al.10 | Non-randomized trial, single center, Italy | 75 (32 vs 43) 100% Severe COVID-19 |
67.5 vs 67.8 | Ruxolitinib 5 mg BID (7 days), then 5 mg OD (10 days) + Methylprednisolone 1 mg/kg IV (3 days), followed by 0.5 mg/kg IV (5 days), then oral prednisone (tapered over 2 weeks) |
1. Clinical recovery without mechanical ventilation 2. Admission to ICU for MV 3. Death 4. Reduction of inflammatory response |
Clinically recovered: 75% (24/32) vs 63% (27/43) ICU (+MV): 16% (5/32) vs 13.9% (6/43) Mortality: 9% (3/32) vs 30% (13/43) Overall Survival: HR 4.65 (95% CI 1.65–12.7; p = 0.0034) Reduction Inflammatory Response: 28/32 (87%) vs 10/43 (23%), p = 0.0001 |
None | Serious |
| 2 | Cantini et al.15 | Non-randomized trial, single center, Italy | 24 (12 vs 12) Moderate COVID-19 100% |
63.5 vs 63 | Baricitinib 4 mg/day (14 days) | 1. Clinical and Laboratory parameters 2. Admission to ICU 3. Discharge at 14 days |
Clinical and Laboratory parameters: Fever, SpO2, PaO2/FiO2, CRP, and MEWS significantly improved (p: 0.000; 0.000; 0.017; 0.023; 0.016, respectively). Admission to ICU: 0% (0/12) vs 33.3% (4/12), p = 0.093 Discharge at 14 days: 58% (7/12) vs 8% (1/12), p = 0.027 |
None | Serious |
| 3 | Giudice et al.16 | Non-randomized trial, Single-Center, Italy | 17 (7 vs 10) 100% Severe COVID-19 |
61 vs 63.5 | Ruxolitinib 10 mg BID (14 days) + Eculizumab 900 mg IV (every 7 day, up to total 3 doses) | Clinical Outcome |
Laboratory parameters: PaO2/FiO2: 370.5 vs 246 (p = 0.0395) ARDS: 14.2% (1/7) vs 40.0% (4/10) Mortality: 14.2% (1/7) vs 10% (1/10) |
None | Serious |
| 4 | Cao et al.14 | RCT, single-blind, Multi-center, China | 41 (20 vs 21) 100% Severe COVID-19 |
63 vs 64 | Ruxolitinib 5 mg BID | 1. Time to clinical improvement 2. CT Scans Improvement on Day 14 3. Time to Lymphocyte recovery 4. Invasive MV 5. Mortality 6. Viral Clearance Time |
Time to clinical improvement: 12 vs 15 days (p = 0.147) Clinical improvement Day 14: 60.0% (12/20) vs 42.9% (9/21) Clinical Deterioration: 0% vs 19.0% (4/21), p = 0.107 CT Scan Improvement Day 14: 90.0% (18/20) vs 61.9% (13/21) p = 0.0495 Time to Lymphocyte Recovery: 5 vs 8 days (p = 0.033) Invasive MV: 0% vs 14.3% (3/21), p = 0.232 Mortality: 0% (0/20) vs 14.3% (3/21) p = 0.232) Viral Clearance Time: 13 vs 12 days (p = 0.649) |
0% (0/20) vs (9.5%) 2/21 | Some Concerns |
| 5 | Kalil et al.11 | RCT, double blind, Multi-center (USA- Singapore-South Korea- Mexico, Japan, Spain, UK, Denmark) | 1033 (515 vs 518) 31.7% Severe COVID-19 |
55.0 vs 55.8 | Baricitinib 4 mg/day (14 days) 2 mg/day if eGFR <60 |
1. Time to recovery 2. Clinical Status at Day 15 3. Number of Days Supplemental O2 4. NIV or High Flow 5. Invasive MV or ECMO 6. Mortality 7. Adverse Events |
Median time to recovery and Clinical Recovery: 7 vs 8 days, 84.1% (433/515) vs 78.4% (406/518), RR for recovery, 1.16; 95% CI 1.01–1.32; p = 0.03 Improvement in clinical status at day 15: OR 1.3; 95% CI, 1.0–1.6). Supplemental Oxygen use: 10 vs 12 days (MD -2.0 95% CI -5.2 to 1.2) NIV or High Flow: 4 vs 5 days (MD -1.0, 95% CI -2.9 to 0.9) Invasive MV or ECMO: (1) Duration of use 20 vs 25 days (MD -5.0, 95% CI -12.9 to 2.9) (2) New use 9.9% (46/461) vs 15.2% (70/461), RR 0.64, 95% CI 0.44 to 0.93) 28-day mortality: 4.7% (24/515) vs 7.1% (37/518) HR 0.65; 95% CI, 0.39–1.09 Serious Adverse Events: 16.0% vs 21.0% (MD -5.0%; 95% CI, −9.8 to −0.3; p = 0.03) |
5.9% (30/515) vs 11.2% (57/519), (MD -5.3%; 95% CI, −8.7 to −1.9; p = 0.003) | Low |
JAK: Janus Kinase; RCT: Randomized Controlled Trials; ICU: Intensive Care Unit; MV: Mechanical Ventilation; NIV: Non-Invasive Ventilation; ECMO: Extra-Corporeal Membrane Oxygenation; SpO2: Oxygen Saturation; PaO2: Partial pressure of oxygen; FiO2: Fraction of oxygen; CRP: C-Reactive Protein; S: Modified Early Warning Score; HR: Hazard Ratio; OR: Odds Ratio; MD: Mean Difference; CI: Confidence Interval.
3.2. JAK inhibitors, mortality, and clinical improvement
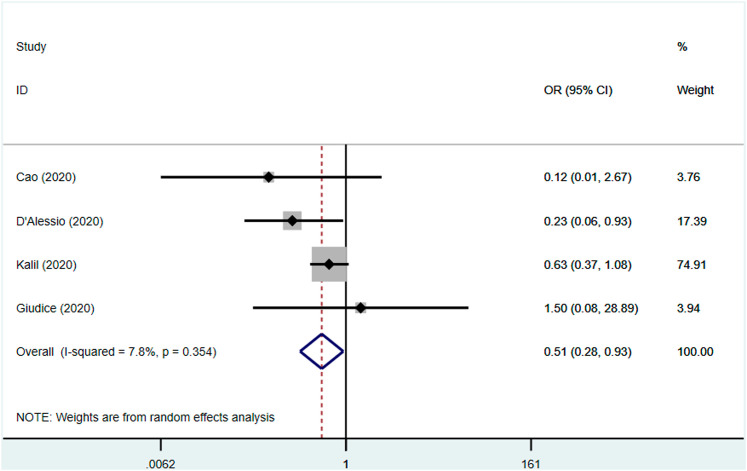
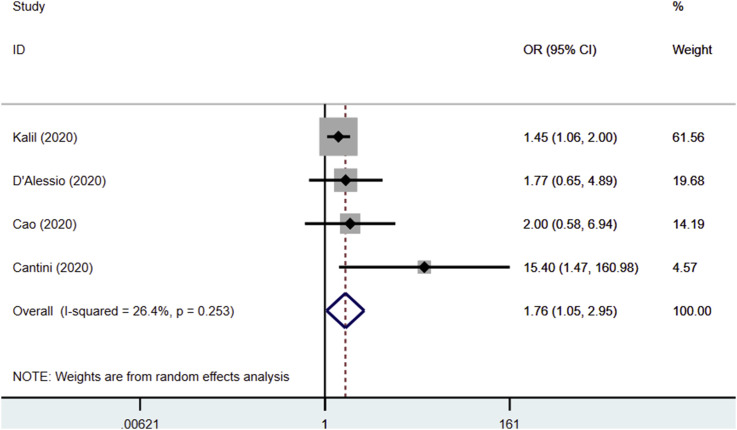
The use of JAK inhibitors was associated with a reduced risk of mortality (OR 0.51, 95% CI 0.28–0.93, P = 0.02; I2: 7.8%, P = 0.354) [Fig. 2 ] and clinical improvement (OR 1.76, 95% CI 1.05–2.95, P = 0.032; I2: 26.4%, P = 0.253) [Fig. 3 ]. Significantly reduced serum C-reactive protein levels after JAK inhibitors administration were reported in two studies.10 , 15 Moreover, significantly faster time to lymphocyte recovery and higher percentage of CT Scan improvement after treatment were seen in the other study.14
Fig. 2.
Forest plot showing overall effect estimates of Janus Kinase inhibitors and risk of mortality. OR: Odds Ratio; CI: Confidence Interval.
Fig. 3.
Forest plot showing overall effect estimates of Janus Kinase inhibitors and risk of clinical improvement. OR: Odds Ratio; CI: Confidence Interval.
3.3. JAK inhibitors and clinical deterioration
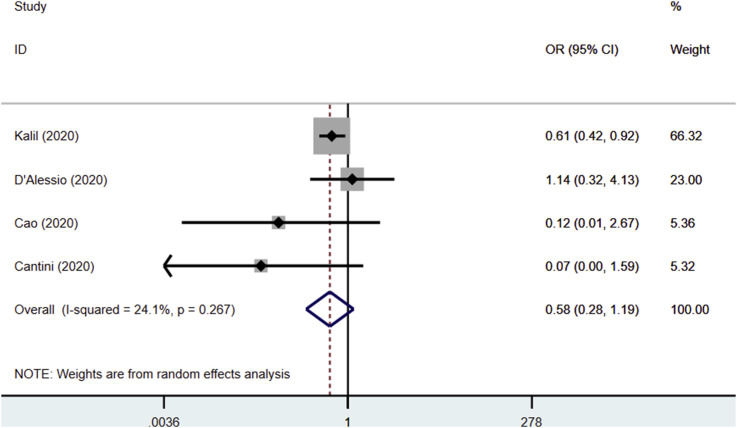
The use of JAK inhibitors was not associated with a reduced risk of clinical deterioration (OR 0.58, 95% CI 0.28–1.19, P = 0.136; I2: 24.1%, P = 0.267) [Fig. 4 ].
Fig. 4.
Forest plot showing overall effect estimates of Janus Kinase inhibitors and risk of clinical deterioration. OR: Odds Ratio; CI: Confidence Interval.
3.4. JAK inhibitors and secondary infection
There were no incidence of new infection or secondary infection among patients receiving JAK inhibitors in three studies.10 , 14 , 15 Remarkably, Kalil et al. reported higher incidence of new infection in patients not receiving JAK Inhibitors (5.9% vs 11.2%, Mean Diff −5.3%; 95% CI -8.7 to −1.9; P = 0.003),11 which was similarly reported by Cao et al. (9.5% vs 0%).14
3.5. Risk of bias
Two of the non-randomized trials were assessed with serious overall risk of bias, which mainly owing to the domain of bias due to deviation from intended interventions. One of the RCT was considered low risk of bias, while some concern in the risk of bias was considered in the other study due to non-blinded measurements of outcome by the treating physician. The risks of bias of the included studies were depicted in Tables 2 and 3 . The small study effects, as shown by the Egger test, were not significant for mortality, clinical improvement, and clinical deterioration (P = 0.812, P = 0.580, and P = 0.645), respectively.
Table 2.
Risk of bias of randomized controlled trials.
| Study | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall Bias |
|---|---|---|---|---|---|---|
| Cao et al. | Low | Low | Low | Some concerns | Low | Some concerns |
| Kalil et al. | Low | Low | Low | Low | Low | Low |
Low risk of bias.
Some concerns.
High risk of bias.
Table 3.
Risk of bias of non-randomized trials.
| Study | Confounding | Selection | Measurement of intervention | Deviations from Intended Interventions | Missing Data | Measurement of outcomes | Reported results | Overall |
|---|---|---|---|---|---|---|---|---|
| D'Alessio et al. | Moderate | Moderate | Low | Serious | Low | Moderate | Moderate | Serious |
| Guidice et al. | Serious | Moderate | Low | Serious | Low | Moderate | Moderate | Serious |
| Cantini et al. | Moderate | Moderate | Low | Serious | Low | Moderate | Moderate | Serious |
Low Risk of Bias.
Moderate Risk of Bias.
Serious Risk of Bias.
4. Discussion
This current meta-analysis of experimental studies showed that the use of JAK inhibitors was significantly associated with a reduced risk of mortality, and clinical improvement in hospitalized patients with COVID-19. Overall, the safety consideration regarding secondary infection among patients receiving JAK inhibitors was not a concern in all studies. Our findings was supported by previous meta-analysis which reported a significantly lower odds of mortality among patients receiving JAK inhibitors (OR 0.12; 95% CI, 0.03–0.39; p = 0.0005).17 The core difference between this study and the previous meta-analysis by Walz et al. was the inclusion of non-experimental studies. We only included experimental studies (non-observational) in order to provide stronger evidence. Despite the difference in study inclusion criteria, the findings of both studies supported the evidence in using JAK inhibitors among hospitalized patients with COVID-19, especially in terms of clinical improvement and reduction of mortality.
Interestingly, the use of JAK inhibitors was not significantly associated with a reduced risk of clinical deterioration which we defined as the ICU admission, new use of invasive MV, and or ECMO, in this study; though, the trend was toward a reduced risk. Hypothetically, this was due to the zero events in the specific outcome of intervention group in two studies. Further inclusion of RCT with higher samples and number of events and/or outcome might enlighten this issue.
The JAKs are families of receptor-associated tyrosine kinase comprising of JAK1, JAK2, JAK3, and tyrosine kinase-2 (TYK2).18 They are all associated with a particular dependent cytokine such as IFN-a, IFN-b, IFN-g, IL-2, IL-4, IL-6, and IL-7 to activate signal transducer and activators of transcription (STATs).18 , 19 Their pivotal role as signalling pathways in cellular survival, proliferation, differentiation, and immigration, provides an ideal therapeutic target for numerous immune, inflammatory, and hematopoietic diseases.20 As of today, JAK inhibitors have been approved for wide range of diseases, including rheumatoid arthritis, psoriasis, atopic dermatitis, ulcerative colitis, myelofibrosis, polycythemia vera, and essential thrombocytosis.21
Several reports have suggested cytokine release syndrome or hypercytokinemia is associated with COVID-19 disease severity through the hyperinflammatory state.8 , 22, 23, 24 A recent study from China had shown IL-6 as the predictor of fatality in COVID-19 patients, convincing that viral-induced hyper inflammation as the leading cause of mortality.25 , 26 Numerous cytokines in COVID-19 patient use one discrete intracellular signalling pathway mediated by JAKs to confer multiple biological functions such as immune regulation, lymphocyte growth and differentiation, and oxidative stress.27 Therefore, JAK inhibitors are hypothetically capable in reducing the severity of hyperinflammatory state in COVID-19.
Among patients receiving ruxolitinib, the levels of seven cytokines—IL-6, nerve growth factor b, IL-12(p40), migration inhibitory factor, MIP-1a, MIP-1b, and VEGF—were significantly decreased in comparison to the control group.14 Between these cytokines, IL-6 is the main proinflammatory cytokine which mediated organ dysfunction and tissue damage.28 Targeting multiple pro-inflammatory pathways, rather than a single cytokine (e.g. IL-6), is theoretically attractive, as recent RCTs have shown unsatisfactory results of IL-6 inhibitor among hospitalized patients with COVID-19.29 , 30 IL-12(p40), MIP-1a, and MIP-1b are important chemokines for recruiting activated monocytes/macrophages and other inflammatory cells to the site of infection.31, 32, 33 VEGF plays a role in recruiting monocytes/macrophages and increasing capillary permeability syndrome that characterizes some types of viral pneumonia.34 The inhibition of multiple pro-inflammatory cytokines in JAK inhibitors provides the rationalization of superiority in using this class of drug among hospitalized patients with COVID-19.
Baricitinib, another JAK inhibitors, can interfere with the cellular entry of SARS-CoV-2 through the inhibitory effects on the known endocytosis regulators such as AP2-associated protein kinase 1 and cyclin G-associated kinase.35 Several studies have shown the safety and efficacy of baricitinib therapy in COVID-19 patients, with the improved clinical condition and minimal infections or hematologic adverse events.15
Hypothetically, anti-inflammatory drugs are beneficial in patients with COVID-19 patients only when given at the right time. An early administration could intensify viral replication, while a late treatment could worsen the immunological fatigue caused by the prolonged hypercytokinemia.10 Siddiqi and Mehra have proposed three stages of COVID-19 disease progression.36 The third stage is defined as severe extrapulmonary systemic hyperinflammation syndrome, characterized by ARDS, systemic inflammatory response syndrome (SIRS), and impending multi-organ failure. In this phase, anti-inflammatory drugs such as JAK inhibitors may be justified before multiorgan dysfunction occurs.36 Although the most effective timing of administration of these drugs are yet to be reported, prompt initiation within 1–2 weeks after disease onset is a sensible approach.37
Despite concerns about immunosuppression, secondary infections, and thrombosis with use of JAK inhibitors, all the included studies did not report any major issues regarding these adverse effects. On the contrary, lower incidence of secondary infection compared to non-intervention arm was reported in two studies. The latter effect may be associated with shorter recovery time and faster clinical improvement, reducing the risk of secondary infection.11
5. Limitations
This study has several limitations. There were only baricitinib and ruxolitinib in the included studies, therefore whether all JAK inhibitors provide the same benefits need further studies. There were only two RCT included in the analysis and only one RCT provided relatively large sample size. Consequently, more RCTs are still needed to further confirm these findings.
6. Conclusion
The use of JAK inhibitors was significantly associated with a reduced risk of mortality, and clinical improvement in hospitalized patients with COVID-19.
Data availability
The data used to support the findings of this study are included in the article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author contribution
Indra Wijaya: Conceptualization, Methodology, Validation, Resources, Writing – Original Draft, Writing - Review & Editing, Project administration. Rizky Andhika: Conceptualization, Methodology, Writing – Original Draft, Writing – Review & Editing. Ian Huang: Methodology, Investigation, Data Curation, Formal Analysis, Validation, Resources, Writing – Original Draft, Writing – Review and Editing. Aga Purwiga: Software, Validation, Data Curation, Formal Analysis, Visualization. Kevin Yonatan Budiman: Writing – Original Draft, Writing – Review & Editing. Muhammad Hasan Bashari: Writing – Review and Editing, Supervision. Lelani Reniarti: Writing – Review and Editing, Supervision. Rully Marsis Amirullah Roesli: Writing – Review and Editing, Supervision.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgement
None.
References
- 1.Permana H., Huang I., Purwiga A., et al. In-hospital use of statins is associated with a reduced risk of mortality in coronavirus-2019 (COVID-19): systematic review and meta-analysis. Pharmacol Rep. 2021;20 doi: 10.1007/s43440-021-00233-3. Published online February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andhika R., Huang I., Wijaya I. Severity of COVID ‐19 in end‐stage kidney disease patients on chronic dialysis. Ther Apher Dial. 2020;27 doi: 10.1111/1744-9987.13597. Published online October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pranata R., Supriyadi R., Huang I., et al. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2020;14 doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pranata R., Lim M.A., Yonas E., et al. Thrombocytopenia as a prognostic marker in COVID-19 patients: diagnostic test accuracy meta-analysis. Epidemiol Infect. 2021 doi: 10.1017/S0950268821000236. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. Published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Solidarity Trial Consortium. Pan H., Peto R., et al. Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N Engl J Med. 2020;2 doi: 10.1056/NEJMoa2023184. Published online December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1–14. doi: 10.1177/.1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonas E., Alwi I., Pranata R., et al. Elevated interleukin levels are associated with higher severity and mortality in COVID 19 – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev. 2020;14(6):2219–2230. doi: 10.1016/j.dsx.2020.11.011. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijaya I., Andhika R., Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with COVID-19: a systematic review. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620960797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Alessio A., Del Poggio P., Bracchi F., et al. Low-dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia. Leukemia. 2020 doi: 10.1038/s41375-020-01087-z. Published online November 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalil A.C., Patterson T.F., Mehta A.K., et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med. 2020;11 doi: 10.1056/NEJMoa2031994. Published online December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 doi: 10.1136/bmj.l4898. Published online August 28. [DOI] [PubMed] [Google Scholar]
- 13.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 doi: 10.1136/bmj.i4919. Published online October 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y., Wei J., Zou L., et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146(1):137–146.e3. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giudice V., Pagliano P., Vatrella A., et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walz L., Cohen A.J., Rebaza A.P., et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. Res Sq. Published online. 2020:1–24. doi: 10.21203/rs.3.rs-64782/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo W., Li Y.X., Jiang L.J., Chen Q., Wang T., Ye D.W. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol Sci. 2020 doi: 10.1016/j.tips.2020.06.007. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roskoski R. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol Res. 2016;111:784–803. doi: 10.1016/j.phrs.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 20.Gao Q., Liang X., Shaikh A.S., Zang J., Xu W., Zhang Y. JAK/STAT signal transduction: promising attractive targets for immune, inflammatory and hematopoietic diseases. Curr Drug Targets. 2018;19(5):487–500. doi: 10.2174/1389450117666161207163054. [DOI] [PubMed] [Google Scholar]
- 21.Damsky W., Peterson D., Ramseier J., et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147(3):814–826. doi: 10.1016/j.jaci.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldmann M., Maini R.N., Woody J.N., et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020 doi: 10.1016/S0140-6736(20)30858-8. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an Analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian W., Jiang W., Yao J., et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. Published online. 2020 doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang S., Tanaka T., Narazaki M., Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019 doi: 10.1016/j.immuni.2019.03.026. Published online. [DOI] [PubMed] [Google Scholar]
- 28.Crayne C.B., Albeituni S., Nichols K.E., Cron R.Q. The immunology of macrophage activation syndrome. Front Immunol. 2019 doi: 10.3389/fimmu.2019.00119. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvarani C., Dolci G., Massari M., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia. JAMA Intern Med. 2021;181(1):24. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone J.H., Frigault M.J., Serling-Boyd N.J., et al. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz O., Hammerschmidt S.I., Moschovakis G.L., Förster R. Chemokines and chemokine receptors in lymphoid tissue dynamics. Annu Rev Immunol. 2016 doi: 10.1146/annurev-immunol-041015-055649. Published online. [DOI] [PubMed] [Google Scholar]
- 32.Cooper A.M., Khader S.A. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007 doi: 10.1016/j.it.2006.11.002. Published online. [DOI] [PubMed] [Google Scholar]
- 33.Menten P., Wuyts A., Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002 doi: 10.1016/S1359-6101(02)00045-X. Published online. [DOI] [PubMed] [Google Scholar]
- 34.Olsson A.K., Dimberg A., Kreuger J., Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006 doi: 10.1038/nrm1911. Published online. [DOI] [PubMed] [Google Scholar]
- 35.Richardson P., Griffin I., Tucker C., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020 doi: 10.1016/S0140-6736(20)30304-4. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.012. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W., Zhao Y., Zhang F., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol. 2020;214(March) doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included in the article.