Abstract
Microbial fuel cells (MFCs) have shown immense potential as a one-stop solution for three major sustainability issues confronting the world today—energy security, global warming and wastewater management. MFCs represent a cross-disciplinary platform for research at the confluence of the natural and engineering sciences. The diversity of variables influencing performance of MFCs has garnered research interest across varied scientific disciplines since the beginning of this century. The increasing number of research publications has made it necessary to keep track of work being carried out by research groups across the globe and consolidate significant findings on a regular basis. Review articles are often the nodal points for beginners who are required to undertake an exploratory survey of literature to identify a suitable research problem. This ‘review of reviews’ is a ready-reckoner that directs readers to relevant reviews and research articles reporting significant developments in MFC research in the last two decades. The article also highlights the areas needing research attention which when addressed could take this technology a few more steps closer to practical implementation.
Keywords: Microbial fuel cells, Microbial electrochemistry, Microbial electron transfer, Wastewater treatment, Alternate energy, Bioanode, Biocathode
Introduction
The Earth presently plays host to almost 8 billion human beings (UN DESA Population Division 2019) and the number is expected to go up further and level out by the latter half of the Twenty-first century (Gonzalo et al. 2016). Sustainability of natural resources has been a cause for concern (Buhaug and Urdal 2013) due to ambitious social and economic goals. Dwindling reserves of fossil fuels (Hallenbeck and Ghosh 2009) account for over 80% of the world's primary energy consumption (Mohr et al. 2015). Greenhouse effect, a natural phenomenon that is chiefly responsible for the habitability of earth, appears to be assuming unmanageable proportions. Unregulated release of carbon dioxide and other greenhouse gases resulting from anthropocentric activities have led to increased absorption of infrared radiation from the sun leading to above-normal surface temperatures on earth (IPCC 2014). The need to curb such emissions underlines the search for sustainable, carbon–neutral sources of energy (Arent et al. 2011; Villano et al. 2012). Reinforcing the need to shift to renewable energy, Rittman (2008) specifically outlines the potential of microorganisms as a source of energy.
Urbanization is on the rise in developing nations (Buhaug and Urdal 2013) and the resultant increase in average income has ameliorated food preferences, putting pressure on water resources (de Fraiture and Wichelns 2010). The increased demand for water has impacted water availability (Haddeland et al. 2014) and has promoted reuse of wastewater for applications such as irrigation (Toze 2006) and landscaping. However, in many developing countries, advances in sanitation infrastructure and wastewater treatment have been outpaced by population growth (Qadir et al. 2010). As a result, many of them are on the lookout for reliable and low-cost means for treatment of domestic, agricultural and industrial wastewater to make it reusable (Massoud et al. 2009). An informative and well-illustrated review article by Larsen et al. (2016) discusses the need to adopt innovative strategies for arriving at resource-efficient solutions for issues related to urban water management.
Past and present of MFCs
Electrical effects resulting from microbial disintegration of organic compounds were first described by Potter (1911) over a hundred years ago. In the subsequent decades leading to the next century, there were only a few isolated reports of attempts to extend this fascinating discovery towards practical applications. Schröder (2011) traces the century-long history of microbial electrochemical systems from the time they were first reported, highlights significant milestones, succinctly outlines the reasons for the initial dearth of interest in taking this technology further, and finally describes the relevance and future scope of this discipline following its resurgence at the turn of the century.
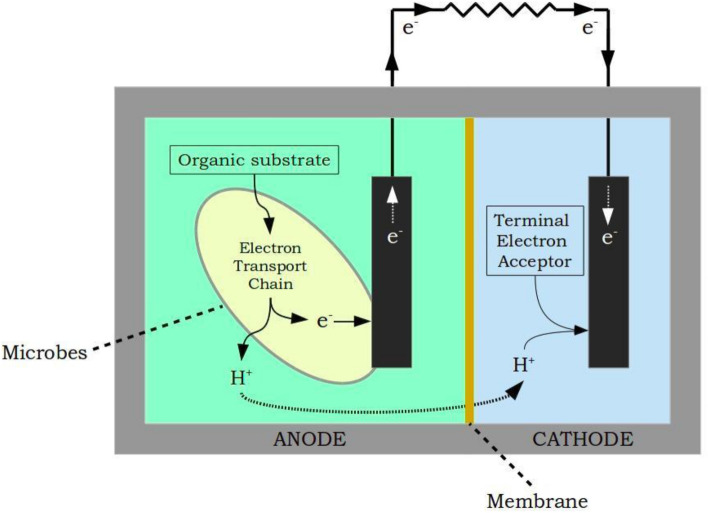
Microbial Fuel Cells (MFCs) have been aptly described by Du et al. (2007) as “bioreactors that convert the energy in the chemical bonds of organic compounds into electrical energy through catalytic activity of microorganisms under anaerobic conditions”. Figure 1 is a graphical representation of a generic two-chambered MFC comprising an anode and a cathode chamber separated by a selectively permeable membrane. The microbes’ need for a compatible electron acceptor to deposit electrons is readily fulfilled by the anode of an MFC in the absence of a more suitable acceptor (Stams et al. 2006). These electrons collected by the anode are channelised across an external load (resistor) to harness usable energy. The final step of the electron transport occurs at the cathode in the presence of a terminal electron acceptor. Thus, a ‘quasi-engineered’ electron transport chain that mimics the bacterial respiratory chain forms the core of an MFC. Basic concepts relating to MFCs are presented in a lucidly written lecture text by Schröder (2018). The technical foundations and principles which form the basis of this technology are presented in comprehensive review articles by Logan et al. (2006) and Santoro et al. (2017). These microbe-catalysed electrochemical devices are viewed as a potential solution for wastewater management and as a source of sustainable and clean energy. To make this solution practically viable, research on microbial electrochemical technologies has primarily focused on four aspects, viz. minimizing electrochemical losses, improving performance efficiency, lowering working costs and scaling up systems for practical applications (Fig. 2).
Fig. 1.
Schematic of a generic two-chambered MFC
Fig. 2.
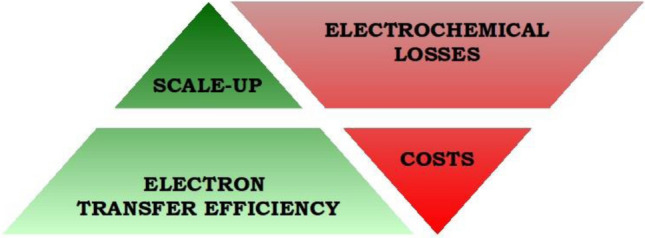
Four major focus areas of MFC research
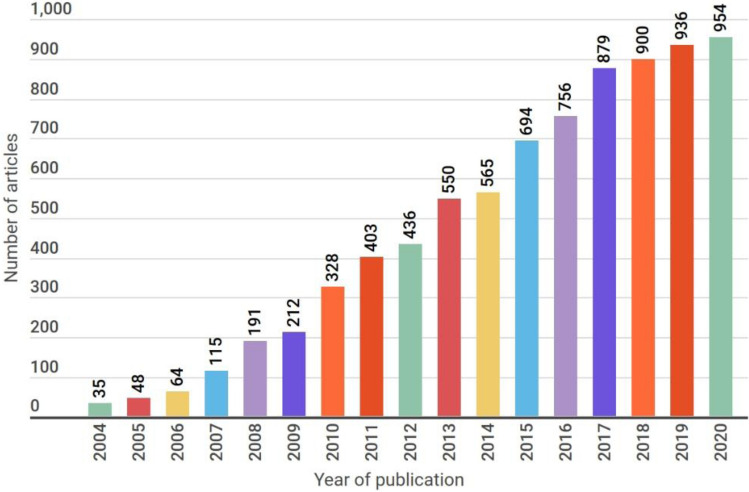
A query submitted for the term ‘microbial fuel cells’ on the Web of Science™ platform of Clarivate Analytics (Fig. 3) showed a gradual increase in the number of research articles on MFCs that were published in the years 2004–2020 in scientific, peer-reviewed journals. It must be noted that this figure serves to only emphasize the growth trend and that the output of a similar query in different search engines would obviously return varying numbers based on the websites and databases that are indexed by the respective algorithms.
Fig. 3.
Year-wise trend of publications based on a search on the Web of Science™ portal for the keyword 'microbial fuel cell' (2004–2020)
Among the different types of articles that are published in scientific journals, review articles represent a starting point for budding researchers and a vade mecum for established scientists. In general, reviews primarily serve to fulfil the following tasks:
-
i.
classifying the ever-growing information in a subject into relevant categories,
-
ii.
providing references to research papers that describe significant advancements, and
-
iii.
highlighting lacunae to be addressed by researchers.
This article has been compiled with the primary objective of aiding beginners to sift through the abundantly available scientific literature on MFCs by directing them to focused reviews and relevant breakthrough research articles highlighting significant advances in the field. The content has been divided into independent sub-sections pertaining to configuration, microbes, materials, performance characterization, scale-up and applications for the sake of convenience. The choice of references cited in this article is based entirely on their content and is not influenced by any intentional bias whatsoever.
MFC design and modeling
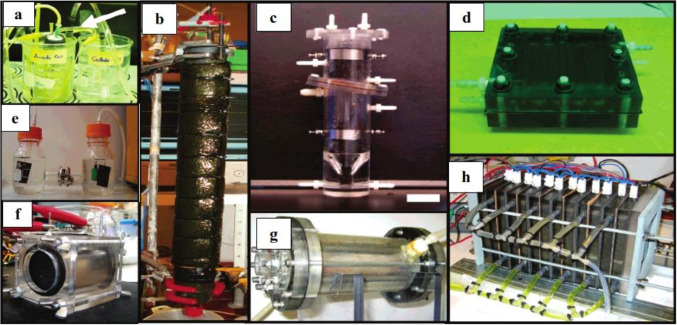
A wide variety of MFC configurations have been designed for specific applications and with the objective of improving performance by minimizing systemic losses. Some of the significant examples include air–cathode single-chamber MFCs (Liu and Logan 2004), flat-plate MFCs (Min and Logan 2004), upflow MFCs (He et al. 2005), tubular MFCs (Rabaey et al. 2005), membrane-electrode assembly MFCs (Pham et al. 2005), stacked MFCs (Aelterman et al. 2006), separator-electrode assembly MFCs (Ahn and Logan 2012). However, the most commonly reported are the two-chambered, 'H-shaped' MFCs which, despite their low current output, have been the most convenient for optimizing performance of new components and characterising operating conditions (Logan et al. 2006). Figure 4 (adapted) presents some of the different experimental designs that have been used in MFC studies and reported in literature.
Fig. 4.
Different designs used in MFC studies: a salt bridge MFC; b, c upflow MFCs; d flat-plate MFC; e h-shaped MFC; f, g single-chamber MFCs; h stacked MFC (The figure has been reprinted (adapted) with permission from Logan BE, Hamelers B, Rozendal R, et al. (2006) Microbial fuel cells: Methodology and technology. Environmental Science & Technology 40:5181–5192. https://doi.org/10.1021/es0605016.
Copyright © 2006 American Chemical Society.)
Discussing essential aspects to be considered while designing MFCs for various practical applications, an article by Logan et al. (2015) highlights the importance of electrode configuration and source of organic substrate in determining performance. Modeling studies, which facilitate detailed analyses of factors affecting the performance of MFCs (Jadhav et al. 2020a), include mathematical modeling (Deb et al. 2020), computer simulations (Xia et al. 2018), neural network modeling (Ma et al. 2019) and electrochemical modeling (Kadivarian and Karamzadeh 2020). Given the diversity of dependent variables that can determine the performance of MFCs (Oliveira et al. 2013; Zhang et al. 2019a), analysing their influence to arrive at a valid conclusion depends to a considerable extent on the number of replicates of an experiment because repeatability is not necessarily assured (Larrosa et al. 2009).
Electroactive microbes
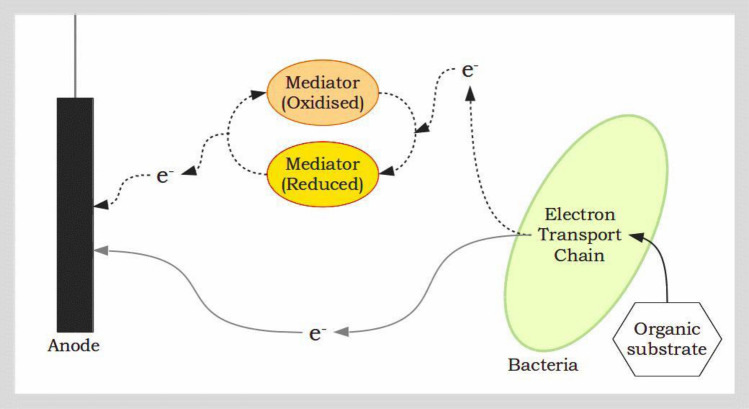
Microbes play a key role in an MFC by catalysing the release of electrons from energy rich bonds of organic substrates under anoxic conditions. Review articles by Pant et al. (2010b) and Pandey et al. (2016) describe different pure substrates and types of wastewater that have been used as a carbon source for microbes in MFCs. The electrons released in this process of oxidation travel through versatile microbial electron transport chains (Fredrickson et al. 2008; Kracke et al. 2015) which comprise serially arranged conductive protein complexes, cytochromes, nanowires and redox proteins (Costa et al. 2018) before being donated to the anode of the MFC. Schröder explains the fundamental mechanisms and energy considerations of anodic electron transfer in a classic review (2007). Electron transfer between microbes and the electrode (Lovley 2012; Kumar et al. 2017) can be either indirect—mediated by naturally produced or artificially added redox shuttles (Martinez and Alvarez 2018)—or by direct extracellular electron transfer (Yang et al. 2012) (Fig. 5). Glasser et al. (2017) provide valuable insights into endogenous extracellular electron shuttles while Lovley (2017) describes the processes associated with direct interspecies electron transfer which enables long-distance transport of electrons in bioelectrochemical systems. Dynamics of electron transfer within microbes (intra), between microbial species (inter), and at the microbe-electrode interface have been detailed in a review article by Zheng et al. (2020).
Fig. 5.
Direct (solid lines) and indirect (dotted lines) electron transfer from bacteria to the anode
Mixed consortia of electrogenic and electrotrophic microbes (Logan 2009; Logan et al. 2019) are known to contribute more effectively to production of current in MFCs as compared to pure cultures of bacteria. This difference could be attributed to synergistic interactions between syntrophic microbial species resulting in effective utilization of available substrates (Kiely et al. 2011) by the formation of electrochemically active biofilms (Borole et al. 2011; Babauta et al. 2012; Reguera 2018; Kiran and Patil 2019). Growth and performance of electroactive biofilms can be enhanced (Li et al. 2018a) by selectively controlling growth conditions (Doyle and Marsili 2015, 2018), using synthetic biology (Glaven 2019) and adopting engineering approaches (Angelaalincy et al. 2018; Chiranjeevi and Patil 2020). Communities of microbial consortia have also been profiled and characterized using ‘omics’ technologies (Rittmann et al. 2008; Lacerda and Reardon 2009; Moran et al. 2013; Franzosa et al. 2015; Kouzuma et al. 2018), flow-cytometric approaches (Koch et al. 2014), computational tools (Haft and Tovchigrechko 2012; Segata et al. 2013) and statistical analysis (Buttigieg and Ramette 2014) to obtain insights from a structural and functional perspective (Zhi et al. 2014).
Electrodes and separators
Efficient electrode materials in MFCs must essentially be biocompatible, electrically conductive, non-corrosive and electrochemically stable. Wei et al. (2011), in their detailed review article, analyse the advantages and disadvantages of different materials used as electrodes in MFCs and discuss the prospects of electrode development. Assessing the performance of electrodes and separators (Hamelers et al. 2010) and use of low-cost materials such as ceramics (Winfield et al. 2016), ligno-cellulosic material (Mehta et al. 2020) and biochar (Chakraborty et al. 2020a) without significantly compromising on efficiency is important for design of efficient MFCs. Breheny et al. (2019) discuss critical aspects for improvement of bioelectrodes in MFCs and Pasternak et al. (2020) present a new dimension for enhancing performance of microbial electrochemical systems using surfactants.
Anodes serve as the substratum for biofilm formation and also function as current collectors in MFCs. Among different materials that have been reported, carbon is most preferred for anodes because of its versatility, non-reactivity, high electrical conductivity and biocompatibility (Logan 2008). While carbon cloth and carbon felt provide more room for colonization of microbes by virtue of being more porous compared to graphite sheets or carbon paper, the innovative introduction of graphite brush anodes (Logan et al. 2007) enabled the incorporation of larger surface area of electrodes for a given volume of the reactor. The high conductivity and surface area provided by nanomaterials resulted in their use in the anode chamber of MFCs (Liu et al. 2020). Gnana kumar et al. (2013) describe the features of anode materials used in MFCs and different processing techniques that can improve efficiency of bacterial adhesion, electron capture and transfer. A comparative account of conventional and modified anodes (Cai et al. 2020) opens up a new window for understanding the characteristics of anode materials and paves the way for development of next generation MFC anodes.
Cathodes provide a common interface for the culmination of the microbial electron transfer process in an MFC resulting in the confluence of electrons, protons and the terminal electron acceptor. On account of their complex role, cathodes have been considered as a critical point to determine the efficiency of MFCs (Rabaey and Keller 2008). Based on the type of electron acceptor used (He et al. 2015), cathodes can be classified as chemical or biological. Oxygen is often preferred as a terminal electron acceptor due to its ubiquity and propensity to get reduced to water. However, poor kinetics of the oxygen reduction reaction led to the use of expensive, precious-metal catalysts such as platinum at the cathode. Studies that focused on reduction of operating costs (Zhang et al. 2009) eventually led the way to development of more economical, alternate cathode materials based on carbon (Peera et al. 2020) and nanocomposites (Dessie et al. 2020) devoid of precious metals for improving efficiency of the oxygen reduction reaction (Yuan et al. 2016). Erable et al. (2012) describe the application of microbes to catalyse the rate-limiting oxygen reduction reaction at the cathode. Biocathodes (He and Angenent 2006), comprising electrotrophic microbes that can directly accept electrons from the electrode (Lovley 2011), can overcome many of the shortcomings encountered using chemical cathodes and are now being actively pursued as a topic of research interest (Song et al. 2019).
A separator in an MFC is a physical barrier that allows charges to pass through but serves as a hurdle to prevent direct electrical contact between the anode and cathode. In the early years, proton exchange membranes such as Nafion® were used in MFCs to selectively allow only protons to the cathode chamber of an MFC (Rahimnejad et al. 2014). Eliminating the use of a proton-specific, separating membrane in MFCs (Jang et al. 2004) was a significant breakthrough for reducing operation costs, but it brought along the twin drawbacks of oxygen diffusion into the anoxic anode chamber and short circuiting of electrons between the anode and cathode, both of which when unregulated have a detrimental impact on performance efficiency. In subsequent years, expensive membranes were substituted with alternatives like Zirfon® (Pant et al. 2010a; Pasupuleti et al. 2016) and low-cost materials having more general transport properties such as ion exchange membranes (Leong et al. 2013), ceramic filtration membranes (Yang et al. 2016a), polymeric membrane separators (Bakonyi et al. 2018), sand/activated carbon separators (Gao et al. 2018), silk fibroin membranes (Pasternak et al. 2019) and polystyrene (Mathuriya and Pant 2019).
Performance characterization
Electrochemical techniques and tools are used to analyze the effect of modifications made to MFCs with the objective of minimizing electrochemical losses and enhancing performance efficiency. Rimboud et al. (2014) present a detailed perspective on the factors to be considered while designing anodes for microbial electrochemical systems. Electroactivity of biofilms has been characterized using techniques such as cyclic voltammetry (Gimkiewicz and Harnisch 2013), electrochemical impedance spectroscopy (ter Heijne et al. 2015), confocal resonance Raman microscopy (Virdis et al. 2016), interdigitated electrode array (Yates et al. 2018) and other methods. Technical aspects such as internal resistance (Zhang and Liu 2010) and anode potential (Aelterman et al. 2008; Wagner et al. 2010; Zhu et al. 2013) have to be understood and commonly encountered issues such as power overshoot (Watson and Logan 2011; Winfield et al. 2011) and voltage reversal (Kim et al. 2020) must be analysed to minimise losses and enhance performance of MFCs. Tutorial articles provide the necessary support to beginners to understand fundamental concepts in electronic circuitry (Sánchez et al. 2020), choice of electrode configurations and operating conditions for electroanalysis (Zhao et al. 2009) and nuances of techniques such as cyclic voltammetry (Harnisch and Freguia 2012; Elgrishi et al. 2018) and electrochemical impedance spectroscopy (He and Mansfeld 2009). Other useful reviews outline performance indicators (Sharma et al. 2014) and terms used to describe performance of microbial electrochemical systems (Wang and He 2020). Challenges encountered due to the diverse configurations of MFCs and different techniques available for characterizing activity of electroactive microbes can be addressed by having a standardized framework (Harnisch and Rabaey 2012) and fundamental guidelines to plan experiments, analyse observations and report results in a more meaningful manner (Logan 2012).
Scaling up
Schröder (2011) reported that the performance of MFCs improved by close to three orders of magnitude—from few μA/cm2 to over 1 mA/cm2—during the first decade of this century. Microscale (Wang et al. 2011; Choi 2015) and microfluidic (Yang et al. 2016b; Parkhey and Sahu 2020) MFCs have shown enhanced performance in terms of power production. Although μL and mL scale laboratory experiments provide cues and clues regarding different mechanisms involved in the functioning of MFCs, systemic understanding obtained from such studies must be transferred and translated (Janicek et al. 2014; Butti et al. 2016) to enable setting up of pilot-scale systems (Logan 2010). Knowledge of the different components and processes involved is critical to make upscaling of MFCs practically feasible (Logan et al. 2015). Significant progress has been achieved over the past decade in developing scaled-up MFC systems for practical applications (Gajda et al. 2018; Abdallah et al. 2019; Jadhav et al. 2020b).
Applications
The primary application of MFCs is wastewater treatment with concomitant production of electricity (Pant et al. 2012). Lefebvre et al. (2011) describe energetics of MFCs with the objective of developing a self-sustaining domestic wastewater treatment process (Oh et al. 2010). Harnessing the potential of MFCs as a power source (Wang et al. 2015) and for production of valuable products by microbial electrosynthesis (Rabaey and Rozendal 2010; Harnisch and Urban 2018) requires an in-depth understanding of factors that limit performance (Sleutels et al. 2012) along with the principles of energy capture and storage (Sun et al. 2016).
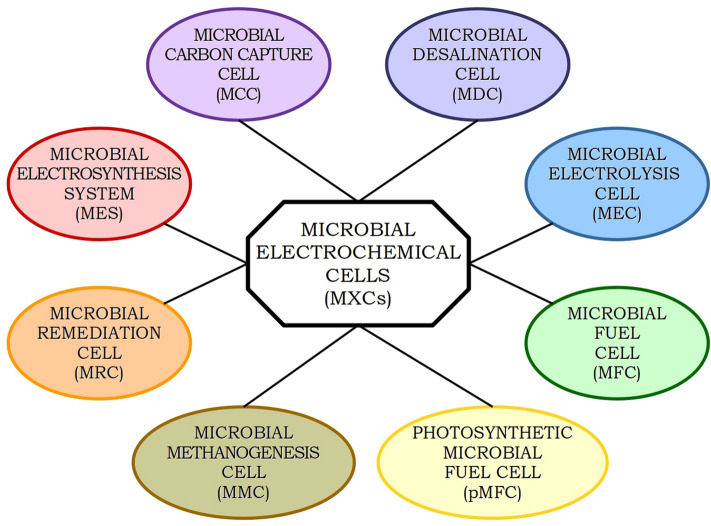
Evolution of microbes has favoured the diversification of MFCs into a number of technologies (Schröder and Harnisch 2017) with varied applications (Schröder et al. 2015), resulting in the more generic term ‘microbial electrochemical cells’ (MXCs) (Fig. 6). Table 1 presents an overview of the multifarious applications of microbial electrochemical technologies and provides references to recently published review articles. Torres (2014) emphasizes on the need to “identify, understand and predict” different phenomena that govern the performance of such systems.
Fig. 6.
Diversification of microbial electrochemical cells
Table 1.
Applications of microbial electrochemical technologies
| Application | Reference | |
|---|---|---|
| 1 | Electrobioremediation of recalcitrant pollutants and xenobiotics | (Sevda et al. 2018; Chakraborty et al. 2020b; Chandrasekhar et al. 2020; Wang et al. 2020b) |
| 2 | In situ remediation of groundwater and soil | (Cecconet et al. 2020; Li et al. 2020) |
| 3 | MFC-coupled hybrid systems for environmental remediation and resource recovery | (Yang et al. 2019; Zhang et al. 2019b; Elmaadawy et al. 2020; Wang et al. 2020a) |
| 4 | Energy recovery from urine and solid wastes | (Gurjar and Behera 2020; Santoro et al. 2020) |
| 5 | Recycling elements using self-sustaining photosynthetic MFCs | (Greenman et al. 2019; Mekuto et al. 2020) |
| 6 | MFC-based biosensors | (Sun et al. 2015; ElMekawy et al. 2018; Jiang et al. 2018; Sevda et al. 2020) |
| 7 | Energy harvesting and storage in combination with electrochemical capacitors and as supercapacitive MFCs | (Caizán-Juanarena et al. 2020; Soavi and Santoro 2020) |
| 8 | Carbon capture and microbial electrosynthesis of value-added products | (Roy et al. 2015; Das et al. 2019; Chu et al. 2020) |
| 9 | Platform technology for novel applications | (Butti et al. 2016; Zou and He 2018; Hoareau et al. 2019) |
Conclusions
The pursuit of alternate sources of energy due to the consequences of an unabated rise in human population has directed attention of researchers towards MFCs which essentially perform a dual-role of wastewater treatment and clean energy production. The steady increase in the number of research articles published on MFCs (Md Khudzari et al. 2018) over the last 15 years is an indicator of the steadfastness and commitment of the research community. Moreover, among the several books written or compiled on MFCs, the following three which encapsulate significant advances pertaining to construction, characterization, applications and diversification of this technology deserve a mention: Microbial fuel cells (Wiley-Interscience) (Logan 2008), Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation (Elsevier) (Venkata Mohan et al. 2018) and Microbial Electrochemical Technologies (Routledge/CRC Press) (Tiquia-Arashiro and Pant 2020).
However, what goes unnoticed is the increasing number of students opting for MFCs and related technologies for their projects at high school and university levels due to the societal relevance of these topics. Considering the fact that data presented in such project reports often trigger more specialized and resource intensive studies, it might be worthwhile exploring the creation of a platform to document and collate promising results from such projects. Moreover, acknowledging the efforts of these young contributors, in a noteworthy manner, in research publications resulting from these leads would encourage more exploratory studies by students.
Planning for a student project or designing a research experiment might seem to be an elementary process because of the seemingly limitless possibilities that exist to observe the effects of tweaking the diverse physico-chemical and biological variables that directly or indirectly influence MFC performance. However, it would preferable to align the scope of such investigations to the aforementioned four major objectives of MFC research—keeping electrochemical losses under check, boosting electron transfer efficiency, bringing down operational costs and upscaling systems for practical applications—so that it results in a significant contribution to the existing body of knowledge.
MFCs have been prototyped in various shapes and sizes; each new configuration presenting an improvement over the others in some aspect of performance. Alterations to MFC configurations will continue in the quest for models that can be effectively implemented on a large scale. The understanding of variables associated with MFC performance has certainly improved over the years and the inventory of materials that improve the performance of MFCs is also continually expanding. These must go hand-in-hand with efforts to curb costs of scaled-up systems. Agricultural wastes, for instance, are carbon-rich materials that can be carbonized and exploited as low-cost electrode material. However, such substitutions can imply a trade-off with performance efficiency, opening up new avenues for detailed optimization studies using statistical methods such as response surface methodology.
Carrying out mathematical modeling and computer simulations can provide a near-realistic estimate of the optimal configuration, components and operating parameters to be employed under a given set of conditions for specific applications. Designing high-throughput methods for screening performance of components and operating parameters is a challenge that is still relevant and needs attention; especially because of the inter-relationships among the physico-chemical and variables influencing MFCs.
The fact remains, however, that the biological component will always be a complex variable that cannot be precisely modelled; and thus needs more focused attention for unravelling unknown facets of bacterial metabolism and energetics specifically in the context of bioelectrochemical systems. Community dynamics of microbial consortia in electroactive biofilms powering microbial electrochemical systems are still being understood. In silico analyses of genomic and proteomic data in openly available repositories such as the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov), Worldwide Protein Data Bank (www.wwpdb.org), European Bioinformatics Institute (www.ebi.ac.uk) and many others make it possible to gain insights into the mechanistic aspects of bacterial electron transfer systems and processes. Metagenomic approaches for microbial community profiling are gaining relevance as they also account for bacteria which cannot be easily cultured in laboratory conditions. Sophisticated protein modelling and visualization tools available today can uncover hitherto unknown aspects of bacterial respiratory proteins and biofilm-associated proteins (www.biofilms.biosim.pt). Protocols employed for control of biofilms, especially in the food and healthcare sectors where they are known to be a nuisance, could provide useful hints to develop methods for promoting their growth in bioelectrochemical systems.
Tutorial articles on a design of experiments approach to effectively plan experiments and on electrochemical techniques for performance characterization will help in hand-holding students and scientists from diverse backgrounds to set-up the working environment. Limited access to equipment for electrochemical characterization, often not affordable for school and colleges not having established routes to obtain funding, can be a major bottleneck for obtaining reliable results. Efforts to bring down costs of basic instrumentation using micro-controllers (Meloni 2016; Li et al. 2018b) would bolster the quality of results of academic projects relating to MFCs.
As evidenced by literature, what began as a fascinating phenomenon over a century ago has evolved into a fertile avenue for researchers from different disciplines to converge and contribute (Fig. 7). The journey of MFCs seems to be akin to the folk-tale of the six blind men who tried to describe an elephant; each one basing his judgement on a part of the animal that he felt with his hands. It was only when all their views were rationally consolidated that they perceived the bigger picture and came to the conclusion that an elephant is actually much more than just fan-like ears, pillar-like legs, spear-like tusks, a tube-like trunk, a rope-like tail and a wall-like body. Multidisciplinary approaches and transdisciplinary efforts have demolished traditional barriers and bridged the gaps which had prevailed in the earlier years on account of adopting a simplex approach towards harnessing energy from wastewater using microbial catalysts.
Fig. 7.

MFC research can be classified under many subject areas
The plethora of applications conceptualized, demonstrated and envisaged portray microbial electrochemical technologies as a 'magic bullet' for impending sustainability crises. However, global sustainability issues can be successfully addressed by MFCs only if the efforts are collated, structured and directed towards a common objective of practical application of these technologies. Singular efforts in multiple directions would only result in a tug-of-war between research groups of varying skills and capabilities. Rather, a collaborative approach at the regional level could optimally utilize the available pool of expertise for the output of MFCs to reach usable levels in large scale applications at affordable costs. Established groups must take the lead in their respective regions for drawing up a framework and charting a roadmap for other fledgling groups to also contribute in their respective niche areas towards a common objective of societal benefit. The untiring efforts of the International Society for Microbial Electrochemistry and Technology (www.is-met.org) in this direction will certainly go a long way in making this possible. As it has been rightly said: “Coming together is a beginning. Keeping together is progress. Working together is success.”
Acknowledgements
This work is dedicated to Bhagawan Sri Sathya Sai Baba, the founder chancellor of the Sri Sathya Sai Institute of Higher Learning. Continued support of my research supervisors—Prof. Govind Rao, UMBC and Prof. S. Siva Sankara Sai, SSSIHL—is gratefully acknowledged. Insightful suggestions provided by my students—Sahashransu Satyajeet Mahapatra and Mayur Mukhi—are greatly appreciated.
Declarations
Conflict of interest
The author declares no conflict of interest in the publication.
References
- Abdallah M, Feroz S, Alani S, et al. Continuous and scalable applications of microbial fuel cells: a critical review. Rev Environ Sci Biotechnol. 2019;18:543–578. doi: 10.1007/s11157-019-09508-x. [DOI] [Google Scholar]
- Aelterman P, Rabaey K, Pham HT, et al. Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ Sci Technol. 2006;40:3388–3394. doi: 10.1021/es0525511. [DOI] [PubMed] [Google Scholar]
- Aelterman P, Freguia S, Keller J, et al. The anode potential regulates bacterial activity in microbial fuel cells. Appl Microbiol Biotechnol. 2008;78:409–418. doi: 10.1007/s00253-007-1327-8. [DOI] [PubMed] [Google Scholar]
- Ahn Y, Logan BE. A multi-electrode continuous flow microbial fuel cell with separator electrode assembly design. Appl Microbiol Biotechnol. 2012;93:2241–2248. doi: 10.1007/s00253-012-3916-4. [DOI] [PubMed] [Google Scholar]
- Angelaalincy MJ, Navanietha Krishnaraj R, Shakambari G, et al. Biofilm engineering approaches for improving the performance of microbial fuel cells and bioelectrochemical systems. Front Energy Res. 2018;6:1–12. doi: 10.3389/fenrg.2018.00063. [DOI] [Google Scholar]
- Arent DJ, Wise A, Gelman R. The status and prospects of renewable energy for combating global warming. Energy Econ. 2011;33:584–593. doi: 10.1016/j.eneco.2010.11.003. [DOI] [Google Scholar]
- Babauta JT, Renslow R, Lewandowski Z, Beyenal H. Electrochemically active biofilms: facts and fiction. A review. Biofouling. 2012;28:789–812. doi: 10.1080/08927014.2012.710324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakonyi P, Koók L, Kumar G, et al. Architectural engineering of bioelectrochemical systems from the perspective of polymeric membrane separators: a comprehensive update on recent progress and future prospects. J Memb Sci. 2018;564:508–522. doi: 10.1016/j.memsci.2018.07.051. [DOI] [Google Scholar]
- Borole AP, Reguera G, Ringeisen B, et al. Electroactive biofilms—current status and future research needs. Energy Environ Sci. 2011;4:4813. doi: 10.1039/c1ee02511b. [DOI] [Google Scholar]
- Breheny M, Bowman K, Farahmand N, et al. Biocatalytic electrode improvement strategies in microbial fuel cell systems. J Chem Technol Biotechnol. 2019;94:2081–2091. doi: 10.1002/jctb.5916. [DOI] [Google Scholar]
- Buhaug H, Urdal H. An urbanization bomb? Population growth and social disorder in cities. Glob Environ Chang. 2013;23:1–10. doi: 10.1016/j.gloenvcha.2012.10.016. [DOI] [Google Scholar]
- Butti SK, Velvizhi G, Sulonen MLK, et al. Microbial electrochemical technologies with the perspective of harnessing bioenergy: maneuvering towards upscaling. Renew Sustain Energy Rev. 2016;53:462–476. doi: 10.1016/j.rser.2015.08.058. [DOI] [Google Scholar]
- Buttigieg PL, Ramette A. A guide to statistical analysis in microbial ecology: a community-focused, living review of multivariate data analyses. FEMS Microbiol Ecol. 2014;90:543–550. doi: 10.1111/1574-6941.12437. [DOI] [PubMed] [Google Scholar]
- Cai T, Meng L, Chen G, et al. Application of advanced anodes in microbial fuel cells for power generation: a review. Chemosphere. 2020 doi: 10.1016/j.chemosphere.2020.125985. [DOI] [PubMed] [Google Scholar]
- Caizán-Juanarena L, Borsje C, Sleutels T, et al. Combination of bioelectrochemical systems and electrochemical capacitors: principles, analysis and opportunities. Biotechnol Adv. 2020;39:107456. doi: 10.1016/j.biotechadv.2019.107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconet D, Sabba F, Devecseri M, et al. In situ groundwater remediation with bioelectrochemical systems: a critical review and future perspectives. Environ Int. 2020;137:105550. doi: 10.1016/j.envint.2020.105550. [DOI] [PubMed] [Google Scholar]
- Chakraborty I, Sathe SM, Dubey BK, Ghangrekar MM. Waste-derived biochar: applications and future perspective in microbial fuel cells. Bioresour Technol. 2020;312:123587. doi: 10.1016/j.biortech.2020.123587. [DOI] [PubMed] [Google Scholar]
- Chakraborty I, Sathe SM, Khuman CN, Ghangrekar MM. Bioelectrochemically powered remediation of xenobiotic compounds and heavy metal toxicity using microbial fuel cell and microbial electrolysis cell. Mater Sci Energy Technol. 2020;3:104–115. doi: 10.1016/j.mset.2019.09.011. [DOI] [Google Scholar]
- Chandrasekhar K, Kumar G, Venkata Mohan S, et al. Microbial Electro-Remediation (MER) of hazardous waste in aid of sustainable energy generation and resource recovery. Elsevier BV; 2020. [Google Scholar]
- Chiranjeevi P, Patil SA. Strategies for improving the electroactivity and specific metabolic functionality of microorganisms for various microbial electrochemical technologies. Biotechnol Adv. 2020;39:107468. doi: 10.1016/j.biotechadv.2019.107468. [DOI] [PubMed] [Google Scholar]
- Choi S. Microscale microbial fuel cells: advances and challenges. Biosens Bioelectron. 2015;69:8–25. doi: 10.1016/j.bios.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Chu N, Liang Q, Jiang Y, Zeng RJ. Microbial electrochemical platform for the production of renewable fuels and chemicals. Biosens Bioelectron. 2020;150:111922. doi: 10.1016/j.bios.2019.111922. [DOI] [PubMed] [Google Scholar]
- Costa NL, Clarke TA, Philipp LA, et al. Electron transfer process in microbial electrochemical technologies: the role of cell-surface exposed conductive proteins. Bioresour Technol. 2018;255:308–317. doi: 10.1016/j.biortech.2018.01.133. [DOI] [PubMed] [Google Scholar]
- Das S, Das S, Das I, Ghangrekar MM. Application of bioelectrochemical systems for carbon dioxide sequestration and concomitant valuable recovery: a review. Mater Sci Energy Technol. 2019;2:687–696. doi: 10.1016/j.mset.2019.08.003. [DOI] [Google Scholar]
- de Fraiture C, Wichelns D. Satisfying future water demands for agriculture. Agric Water Manag. 2010;97:502–511. doi: 10.1016/j.agwat.2009.08.008. [DOI] [Google Scholar]
- Deb D, Patel R, Balas VE. A review of control-oriented bioelectrochemical mathematical models of microbial fuel cells. Processes. 2020;8:583. doi: 10.3390/pr8050583. [DOI] [Google Scholar]
- Dessie Y, Tadesse S, Eswaramoorthy R. Review on manganese oxide based biocatalyst in microbial fuel cell: nanocomposite approach. Mater Sci Energy Technol. 2020;3:136–149. doi: 10.1016/j.mset.2019.11.001. [DOI] [Google Scholar]
- Doyle LE, Marsili E. Methods for enrichment of novel electrochemically-active microorganisms. Bioresour Technol. 2015;195:273–282. doi: 10.1016/j.biortech.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Doyle LE, Marsili E. Weak electricigens: a new avenue for bioelectrochemical research. Bioresour Technol. 2018;258:354–364. doi: 10.1016/j.biortech.2018.02.073. [DOI] [PubMed] [Google Scholar]
- Du Z, Li H, Gu T. A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv. 2007;25:464–482. doi: 10.1016/j.biotechadv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Elgrishi N, Rountree KJ, McCarthy BD, et al. A practical beginner’s guide to cyclic voltammetry. J Chem Educ. 2018;95:197–206. doi: 10.1021/acs.jchemed.7b00361. [DOI] [Google Scholar]
- Elmaadawy K, Liu B, Hu J, et al. Performance evaluation of microbial fuel cell for landfill leachate treatment: research updates and synergistic effects of hybrid systems. J Environ Sci (China) 2020;96:1–20. doi: 10.1016/j.jes.2020.05.005. [DOI] [PubMed] [Google Scholar]
- ElMekawy A, Hegab HM, Pant D, Saint CP. Bio-analytical applications of microbial fuel cell–based biosensors for onsite water quality monitoring. J Appl Microbiol. 2018;124:302–313. doi: 10.1111/jam.13631. [DOI] [PubMed] [Google Scholar]
- Erable B, Féron D, Bergel A. Microbial catalysis of the oxygen reduction reaction for microbial fuel cells: a review. Chemsuschem. 2012;5:975–987. doi: 10.1002/cssc.201100836. [DOI] [PubMed] [Google Scholar]
- Franzosa EA, Hsu T, Sirota-Madi A, et al. Sequencing and beyond: integrating molecular “omics” for microbial community profiling. Nat Rev Microbiol. 2015;13:360–372. doi: 10.1038/nrmicro3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson JK, Romine MF, Beliaev AS, et al. Towards environmental systems biology of Shewanella. Nat Rev Microbiol. 2008;6:592–603. doi: 10.1038/nrmicro1947. [DOI] [PubMed] [Google Scholar]
- Gajda I, Greenman J, Ieropoulos IA. Recent advancements in real-world microbial fuel cell applications. Curr Opin Electrochem. 2018;11:78–83. doi: 10.1016/j.coelec.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Liu L, Yang F. A novel bio-electrochemical system with sand/activated carbon separator, Al anode and bio-anode integrated micro-electrolysis/electro-flocculation cost effectively treated high load wastewater with energy recovery. Bioresour Technol. 2018;249:24–34. doi: 10.1016/j.biortech.2017.09.134. [DOI] [PubMed] [Google Scholar]
- Gimkiewicz C, Harnisch F. Waste water derived electroactive microbial biofilms: growth, maintenance, and basic characterization. J Vis Exp. 2013 doi: 10.3791/50800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser NR, Saunders SH, Newman DK. The colorful world of extracellular electron shuttles. Annu Rev Microbiol. 2017;71:731–751. doi: 10.1146/annurev-micro-090816-093913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaven SM. Bioelectrochemical systems and synthetic biology: more power, more products. Microb Biotechnol. 2019;12:819–823. doi: 10.1111/1751-7915.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo JA, Alfonseca M, Muñoz F-F. World population: past, present, & future. Singapore: World Scientific; 2016. [Google Scholar]
- Greenman J, Gajda I, Ieropoulos I. Microbial fuel cells (MFC) and microalgae; photo microbial fuel cell (PMFC) as complete recycling machines. Sustain Energy Fuels. 2019;3:2546–2560. doi: 10.1039/c9se00354a. [DOI] [Google Scholar]
- Gurjar R, Behera M. Treatment of organic fraction of municipal solid waste in bioelectrochemical systems: a review. J Hazardous Toxic Radioact Waste. 2020;24:04020018. doi: 10.1061/(asce)hz.2153-5515.0000505. [DOI] [Google Scholar]
- Haddeland I, Heinke J, Biemans H, et al. Global water resources affected by human interventions and climate change. Proc Natl Acad Sci U S A. 2014;111:3251–3256. doi: 10.1073/pnas.1222475110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft DH, Tovchigrechko A. High-speed microbial community profiling. Nat Methods. 2012;9:793–794. doi: 10.1038/nmeth.2080. [DOI] [PubMed] [Google Scholar]
- Hallenbeck PC, Ghosh D. Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol. 2009;27:287–297. doi: 10.1016/j.tibtech.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Hamelers HVM, Ter Heijne A, Sleutels THJ, a, , et al. New applications and performance of bioelectrochemical systems. Appl Microbiol Biotechnol. 2010;85:1673–1685. doi: 10.1007/s00253-009-2357-1. [DOI] [PubMed] [Google Scholar]
- Harnisch F, Freguia S. A basic tutorial on cyclic voltammetry for the investigation of electroactive microbial biofilms. Chem An Asian J. 2012;7:466–475. doi: 10.1002/asia.201100740. [DOI] [PubMed] [Google Scholar]
- Harnisch F, Rabaey K. The diversity of techniques to study electrochemically active biofilms highlights the need for standardization. Chemsuschem. 2012;5:1027–1038. doi: 10.1002/cssc.201100817. [DOI] [PubMed] [Google Scholar]
- Harnisch F, Urban C. Electrobiorefineries: unlocking the synergy of electrochemical and microbial conversions. Angew Chemie Int Ed. 2018;57:10016–10023. doi: 10.1002/anie.201711727. [DOI] [PubMed] [Google Scholar]
- He Z, Angenent LT. Application of bacterial biocathodes in microbial fuel cells. Electroanalysis. 2006;18:2009–2015. doi: 10.1002/elan.200603628. [DOI] [Google Scholar]
- He Z, Mansfeld F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ Sci. 2009;2:215. doi: 10.1039/b814914c. [DOI] [Google Scholar]
- He Z, Minteer SD, Angenent LT. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ Sci Technol. 2005;39:5262–5267. doi: 10.1021/es0502876. [DOI] [PubMed] [Google Scholar]
- He C-S, Mu Z-X, Yang H-Y, et al. Electron acceptors for energy generation in microbial fuel cells fed with wastewaters: a mini-review. Chemosphere. 2015;140:12–17. doi: 10.1016/j.chemosphere.2015.03.059. [DOI] [PubMed] [Google Scholar]
- Hoareau M, Erable B, Bergel A. Microbial electrochemical snorkels (MESs): a budding technology for multiple applications. A mini review. Electrochem commun. 2019;104:106473. doi: 10.1016/j.elecom.2019.05.022. [DOI] [Google Scholar]
- IPCC (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland
- Jadhav DA, Carmona-Martínez AA, Chendake AD, et al. Modeling and optimization strategies towards performance enhancement of microbial fuel cells. Bioresour Technol. 2020 doi: 10.1016/j.biortech.2020.124256. [DOI] [PubMed] [Google Scholar]
- Jadhav DA, Das I, Ghangrekar MM, Pant D. Moving towards practical applications of microbial fuel cells for sanitation and resource recovery. J Water Process Eng. 2020;38:101566. doi: 10.1016/j.jwpe.2020.101566. [DOI] [Google Scholar]
- Jang JK, Pham TH, Chang IS, et al. Construction and operation of a novel mediator- and membrane-less microbial fuel cell. Process Biochem. 2004;39:1007–1012. doi: 10.1016/S0032-9592(03)00203-6. [DOI] [Google Scholar]
- Janicek A, Fan Y, Liu H. Design of microbial fuel cells for practical application: a review and analysis of scale-up studies. Biofuels. 2014;5:79–92. doi: 10.4155/bfs.13.69. [DOI] [Google Scholar]
- Jiang Y, Yang X, Liang P, et al. Microbial fuel cell sensors for water quality early warning systems: fundamentals, signal resolution, optimization and future challenges. Renew Sustain Energy Rev. 2018;81:292–305. doi: 10.1016/j.rser.2017.06.099. [DOI] [Google Scholar]
- Kadivarian M, Karamzadeh M. Electrochemical modeling of microbial fuel cells performance at different operating and structural conditions. Bioprocess Biosyst Eng. 2020;43:393–401. doi: 10.1007/s00449-019-02235-1. [DOI] [PubMed] [Google Scholar]
- Kiely PD, Regan JM, Logan BE. The electric picnic: synergistic requirements for exoelectrogenic microbial communities. Curr Opin Biotechnol. 2011;22:378–385. doi: 10.1016/j.copbio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Kim B, Mohan SV, Fapyane D, Chang IS. Controlling voltage reversal in microbial fuel cells. Trends Biotechnol. 2020;38:667–678. doi: 10.1016/j.tibtech.2019.12.007. [DOI] [PubMed] [Google Scholar]
- Kiran R, Patil SA. Microbial electroactive biofilms. American Chemical Society; 2019. [Google Scholar]
- Koch C, Harnisch F, Schröder U, Müller S. Cytometric fingerprints: evaluation of new tools for analyzing microbial community dynamics. Front Microbiol. 2014;5:273. doi: 10.3389/fmicb.2014.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzuma A, Ishii S, Watanabe K. Metagenomic insights into the ecology and physiology of microbes in bioelectrochemical systems. Bioresour Technol. 2018;255:302–307. doi: 10.1016/j.biortech.2018.01.125. [DOI] [PubMed] [Google Scholar]
- Kracke F, Vassilev I, Krömer JO. Microbial electron transport and energy conservation—the foundation for optimizing bioelectrochemical systems. Front Microbiol. 2015;6:1–18. doi: 10.3389/fmicb.2015.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GG, Sarathi VGS, Nahm KS. Recent advances and challenges in the anode architecture and their modifications for the applications of microbial fuel cells. Biosens Bioelectron. 2013;43:461–475. doi: 10.1016/j.bios.2012.12.048. [DOI] [PubMed] [Google Scholar]
- Kumar A, Hsu LHH, Kavanagh P, et al. The ins and outs of microorganism-electrode electron transfer reactions. Nat Rev Chem. 2017;1:1–13. doi: 10.1108/10662240210438434. [DOI] [Google Scholar]
- Lacerda CMR, Reardon KF. Environmental proteomics: applications of proteome profiling in environmental microbiology and biotechnology. Brief Funct Genomic Proteomic. 2009;8:75–87. doi: 10.1093/bfgp/elp005. [DOI] [PubMed] [Google Scholar]
- Larrosa A, Lozano LJ, Katuri KP, et al. On the repeatability and reproducibility of experimental two-chambered microbial fuel cells. Fuel. 2009;88:1852–1857. doi: 10.1016/j.fuel.2009.04.026. [DOI] [Google Scholar]
- Larsen TA, Hoffmann S, Lüthi C, et al. Emerging solutions to the water challenges of an urbanizing world. Science. 2016;352:928–933. doi: 10.1017/CBO9781107415324.004. [DOI] [PubMed] [Google Scholar]
- Lefebvre O, Uzabiaga A, Chang IS, et al. Microbial fuel cells for energy self-sufficient domestic wastewater treatment-a review and discussion from energetic consideration. Appl Microbiol Biotechnol. 2011;89:259–270. doi: 10.1007/s00253-010-2881-z. [DOI] [PubMed] [Google Scholar]
- Leong JX, Daud WRW, Ghasemi M, et al. Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: a comprehensive review. Renew Sustain Energy Rev. 2013;28:575–587. doi: 10.1016/j.rser.2013.08.052. [DOI] [Google Scholar]
- Li M, Zhou M, Tian X, et al. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol Adv. 2018;36:1316–1327. doi: 10.1016/j.biotechadv.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Li YC, Melenbrink EL, Cordonier GJ, et al. An easily fabricated low-cost potentiostat coupled with user-friendly software for introducing students to electrochemical reactions and electroanalytical techniques. J Chem Educ. 2018;95:1658–1661. doi: 10.1021/acs.jchemed.8b00340. [DOI] [Google Scholar]
- Li T, Li R, Zhou Q. The application and progress of bioelectrochemical systems (BESs) in soil remediation: a review. Green Energy Environ. 2020 doi: 10.1016/j.gee.2020.06.026. [DOI] [Google Scholar]
- Liu H, Logan BE. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol. 2004;38:4040–4046. doi: 10.1021/es0499344. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang X, Zhang Q, Li C. Microbial fuel cells: nanomaterials based on anode and their application. Energy Technol. 2020 doi: 10.1002/ente.202000206. [DOI] [Google Scholar]
- Logan BE. Microbial fuel cells. New Jersey: John Wiley & Sons Inc; 2008. [Google Scholar]
- Logan BE. Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol. 2009;7:375–381. doi: 10.1038/nrmicro2113. [DOI] [PubMed] [Google Scholar]
- Logan BE. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl Microbiol Biotechnol. 2010;85:1665–1671. doi: 10.1007/s00253-009-2378-9. [DOI] [PubMed] [Google Scholar]
- Logan BE. Essential data and techniques for conducting microbial fuel cell and other types of bioelectrochemical system experiments. Chemsuschem. 2012;5:988–994. doi: 10.1002/cssc.201100604. [DOI] [PubMed] [Google Scholar]
- Logan BE, Hamelers B, Rozendal R, et al. Microbial fuel cells: methodology and technology. Environ Sci Technol. 2006;40:5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- Logan BE, Cheng S, Watson V, Estadt G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ Sci Technol. 2007;41:3341–3346. doi: 10.1021/es062644y. [DOI] [PubMed] [Google Scholar]
- Logan BE, Wallack MJ, Kim K, et al. Assessment of microbial fuel cell configurations and power densities. Environ Sci Technol Lett. 2015;2:206–214. doi: 10.1021/acs.estlett.5b00180. [DOI] [Google Scholar]
- Logan BE, Rossi R, Ragab A, Saikaly PE. Electroactive microorganisms in bioelectrochemical systems. Nat Rev Microbiol. 2019;17:307–319. doi: 10.1038/s41579-019-0173-x. [DOI] [PubMed] [Google Scholar]
- Lovley DR. Powering microbes with electricity: direct electron transfer from electrodes to microbes. Environ Microbiol Rep. 2011;3:27–35. doi: 10.1111/j.1758-2229.2010.00211.x. [DOI] [PubMed] [Google Scholar]
- Lovley DR. Electromicrobiology. Annu Rev Microbiol. 2012;66:391–409. doi: 10.1146/annurev-micro-092611-150104. [DOI] [PubMed] [Google Scholar]
- Lovley DR. Happy together: microbial communities that hook up to swap electrons. ISME J. 2017;11:327–336. doi: 10.1038/ismej.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Yin Y, Pang S, et al. A data-driven based framework of model optimization and neural network modeling for microbial fuel cells. IEEE Access. 2019;7:162036–162049. doi: 10.1109/ACCESS.2019.2951943. [DOI] [Google Scholar]
- Martinez CM, Alvarez LH. Application of redox mediators in bioelectrochemical systems. Biotechnol Adv. 2018;36:1412–1423. doi: 10.1016/j.biotechadv.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Massoud MA, Tarhini A, Nasr JA. Decentralized approaches to wastewater treatment and management: applicability in developing countries. J Environ Manage. 2009;90:652–659. doi: 10.1016/j.jenvman.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Mathuriya AS, Pant D. Assessment of expanded polystyrene as a separator in microbial fuel cell. Environ Technol (United Kingdom) 2019;40:2052–2061. doi: 10.1080/09593330.2018.1435740. [DOI] [PubMed] [Google Scholar]
- Md Khudzari J, Kurian J, Tartakovsky B, Raghavan GSV. Bibliometric analysis of global research trends on microbial fuel cells using Scopus database. Biochem Eng J. 2018;136:51–60. doi: 10.1016/j.bej.2018.05.002. [DOI] [Google Scholar]
- Mehta S, Jha S, Liang H. Lignocellulose materials for supercapacitor and battery electrodes: a review. Renew Sustain Energy Rev. 2020;134:110345. doi: 10.1016/j.rser.2020.110345. [DOI] [Google Scholar]
- Mekuto L, Olowolafe AVA, Pandit S, et al. Microalgae as a biocathode and feedstock in anode chamber for a self-sustainable microbial fuel cell technology: a review. S Afr J Chem Eng. 2020;31:7–16. doi: 10.1016/j.sajce.2019.10.002. [DOI] [Google Scholar]
- Meloni GN. Building a microcontroller based potentiostat: a inexpensive and versatile platform for teaching electrochemistry and instrumentation. J Chem Educ. 2016;93:1320–1322. doi: 10.1021/acs.jchemed.5b00961. [DOI] [Google Scholar]
- Min B, Logan BE. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ Sci Technol. 2004;38:5809–5814. doi: 10.1021/es0491026. [DOI] [PubMed] [Google Scholar]
- Mohr SH, Wang J, Ellem G, et al. Projection of world fossil fuels by country. Fuel. 2015;141:120–135. doi: 10.1016/j.fuel.2014.10.030. [DOI] [Google Scholar]
- Moran MA, Satinsky B, Gifford SM, et al. Sizing up metatranscriptomics. ISME J. 2013;7:237–243. doi: 10.1038/ismej.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ST, Kim JR, Premier GC, et al. Sustainable wastewater treatment: how might microbial fuel cells contribute. Biotechnol Adv. 2010;28:871–881. doi: 10.1016/j.biotechadv.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Oliveira VB, Simões M, Melo LF, Pinto AMFR. Overview on the developments of microbial fuel cells. Biochem Eng J. 2013;73:53–64. doi: 10.1016/j.bej.2013.01.012. [DOI] [Google Scholar]
- Pandey P, Shinde VN, Deopurkar RL, et al. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy. 2016;168:706–723. doi: 10.1016/j.apenergy.2016.01.056. [DOI] [Google Scholar]
- Pant D, Van Bogaert G, De Smet M, et al. Use of novel permeable membrane and air cathodes in acetate microbial fuel cells. Electrochim Acta. 2010;55:7710–7716. doi: 10.1016/j.electacta.2009.11.086. [DOI] [Google Scholar]
- Pant D, Van Bogaert G, Diels L, Vanbroekhoven K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol. 2010;101:1533–1543. doi: 10.1016/j.biortech.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Pant D, Singh A, Van Bogaert G, et al. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012;2:1248–1263. doi: 10.1039/c1ra00839k. [DOI] [Google Scholar]
- Parkhey P, Sahu R. Microfluidic microbial fuel cells: recent advancements and future prospects. Int J Hydrogen Energy. 2020 doi: 10.1016/j.ijhydene.2020.07.019. [DOI] [Google Scholar]
- Pasternak G, Yang Y, Santos BB, et al. Regenerated silk fibroin membranes as separators for transparent microbial fuel cells. Bioelectrochemistry. 2019;126:146–155. doi: 10.1016/j.bioelechem.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Pasternak G, Askitosari TD, Rosenbaum MA. Biosurfactants and synthetic surfactants in bioelectrochemical systems: a mini-review. Front Microbiol. 2020;11:1–9. doi: 10.3389/fmicb.2020.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupuleti SB, Srikanth S, Dominguez-Benetton X, et al. Dual gas diffusion cathode design for microbial fuel cell (MFC): optimizing the suitable mode of operation in terms of bioelectrochemical and bioelectro-kinetic evaluation. J Chem Technol Biotechnol. 2016;91:624–639. doi: 10.1002/jctb.4613. [DOI] [Google Scholar]
- Peera SG, Maiyalagan T, Liu C, et al. A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells. Int J Hydrogen Energy. 2020 doi: 10.1016/j.ijhydene.2020.07.252. [DOI] [Google Scholar]
- Pham TH, Jang JK, Moon HS, et al. Improved performance of microbial fuel cell using membrane-electrode assembly. J Microbiol Biotechnol. 2005;15:438–441. [Google Scholar]
- Potter MC. Electrical effects accompanying the decomposition of organic compounds. Proc R Soc B Biol Sci. 1911;84:260–276. doi: 10.1098/rspb.1911.0073. [DOI] [Google Scholar]
- Qadir M, Wichelns D, Raschid-Sally L, et al. The challenges of wastewater irrigation in developing countries. Agric Water Manag. 2010;97:561–568. doi: 10.1016/j.agwat.2008.11.004. [DOI] [Google Scholar]
- Rabaey K, Keller J. Microbial fuel cell cathodes: from bottleneck to prime opportunity? Water Sci Technol. 2008;57:655–659. doi: 10.2166/wst.2008.103. [DOI] [PubMed] [Google Scholar]
- Rabaey K, Rozendal RA. Microbial electrosynthesis—revisiting the electrical route for microbial production. Nat Rev Microbiol. 2010;8:706–716. doi: 10.1038/nrmicro2422. [DOI] [PubMed] [Google Scholar]
- Rabaey K, Clauwaert P, Aelterman P, Verstraete W. Tubular microbial fuel cells for efficient electricity generation. Environ Sci Technol. 2005;39:8077–8082. doi: 10.1021/es050986i. [DOI] [PubMed] [Google Scholar]
- Rahimnejad M, Bakeri G, Ghasemi M, Zirepour A. A review on the role of proton exchange membrane on the performance of microbial fuel cell. Polym Adv Technol. 2014;25:1426–1432. doi: 10.1002/pat.3383. [DOI] [Google Scholar]
- Reguera G. Microbial nanowires and electroactive biofilms. FEMS Microbiol Ecol. 2018;94:1–13. doi: 10.1093/femsec/fiy086. [DOI] [PubMed] [Google Scholar]
- Rimboud M, Pocaznoi D, Erable B, Bergel A. Electroanalysis of microbial anodes for bioelectrochemical systems: Basics, progress and perspectives. Phys Chem Chem Phys. 2014;16:16349–16366. doi: 10.1039/c4cp01698j. [DOI] [PubMed] [Google Scholar]
- Rittmann BE. Opportunities for renewable bioenergy using microorganisms. Biotechnol Bioeng. 2008;100:203–212. doi: 10.1002/bit.21875. [DOI] [PubMed] [Google Scholar]
- Rittmann BE, Krajmalnik-Brown R, Halden RU. Pre-genomic, genomic and post-genomic study of microbial communities involved in bioenergy. Nat Rev Microbiol. 2008;6:604–612. doi: 10.1038/nrmicro1939. [DOI] [PubMed] [Google Scholar]
- Roy S, Schievano A, Pant D. Electro-stimulated microbial factory for value added product synthesis. Bioresour Technol. 2015;213:129–139. doi: 10.1016/j.biortech.2016.03.052. [DOI] [PubMed] [Google Scholar]
- Sánchez C, Dessì P, Duffy M, Lens PNL. Microbial electrochemical technologies: electronic circuitry and characterization tools. Biosens Bioelectron. 2020;150:111884. doi: 10.1016/j.bios.2019.111884. [DOI] [PubMed] [Google Scholar]
- Santoro C, Arbizzani C, Erable B, Ieropoulos I. Microbial fuel cells: From fundamentals to applications. a review. J Power Sources. 2017;356:225–244. doi: 10.1016/j.jpowsour.2017.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C, Garcia MJS, Walter XA, et al. Urine in bioelectrochemical systems: an overall review. ChemElectroChem. 2020;7:1312–1331. doi: 10.1002/celc.201901995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys. 2007;9:2619–2629. doi: 10.1039/b703627m. [DOI] [PubMed] [Google Scholar]
- Schröder U. Discover the possibilities: microbial bioelectrochemical systems and the revival of a 100-year–old discovery. J Solid State Electrochem. 2011;15:1481–1486. doi: 10.1007/s10008-011-1395-7. [DOI] [Google Scholar]
- Schröder U. A basic introduction into microbial fuel cells and microbial electrocatalysis. ChemTexts. 2018;4:19. doi: 10.1007/s40828-018-0072-1. [DOI] [Google Scholar]
- Schröder U, Harnisch F. Life electric—nature as a blueprint for the development of microbial electrochemical technologies. Joule. 2017;1:244–252. doi: 10.1016/j.joule.2017.07.010. [DOI] [Google Scholar]
- Schröder U, Harnisch F, Angenent LT. Microbial electrochemistry and technology: terminology and classification. Energy Environ Sci. 2015;8:513–519. doi: 10.1039/C4EE03359K. [DOI] [Google Scholar]
- Segata N, Boernigen D, Tickle TL, et al. Computational meta’omics for microbial community studies. Mol Syst Biol. 2013;9:666. doi: 10.1038/msb.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevda S, Sreekishnan TR, Pous N, et al. Bioelectroremediation of perchlorate and nitrate contaminated water: a review. Bioresour Technol. 2018;255:331–339. doi: 10.1016/j.biortech.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Sevda S, Garlapati VK, Naha S, et al. Biosensing capabilities of bioelectrochemical systems towards sustainable water streams: technological implications and future prospects. J Biosci Bioeng. 2020;129:647–656. doi: 10.1016/j.jbiosc.2020.01.003. [DOI] [PubMed] [Google Scholar]
- Sharma M, Bajracharya S, Gildemyn S, et al. A critical revisit of the key parameters used to describe microbial electrochemical systems. Electrochim Acta. 2014;140:191–208. doi: 10.1016/j.electacta.2014.02.111. [DOI] [Google Scholar]
- Sleutels THJA, Ter Heijne A, Buisman CJN, Hamelers HVM. Bioelectrochemical systems: an outlook for practical applications. Chemsuschem. 2012;5:1012–1019. doi: 10.1002/cssc.201100732. [DOI] [PubMed] [Google Scholar]
- Soavi F, Santoro C. Supercapacitive operational mode in microbial fuel cell. Curr Opin Electrochem. 2020;22:1–8. doi: 10.1016/j.coelec.2020.03.009. [DOI] [Google Scholar]
- Song H-L, Zhu Y, Li J. Electron transfer mechanisms, characteristics and applications of biological cathode microbial fuel cells—a mini review. Arab J Chem. 2019;12:2236–2243. doi: 10.1016/j.arabjc.2015.01.008. [DOI] [Google Scholar]
- Stams AJM, de Bok FAM, Plugge CM, et al. Exocellular electron transfer in anaerobic microbial communities. Environ Microbiol. 2006;8:371–382. doi: 10.1111/j.1462-2920.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- Sun JZ, Kingori GP, Si RW, et al. Microbial fuel cell-based biosensors for environmental monitoring: a review. Water Sci Technol. 2015;71:801–809. doi: 10.2166/wst.2015.035. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhai L-F, Li W-W, Yu H-Q. Harvest and utilization of chemical energy in wastes by microbial fuel cells. Chem Soc Rev. 2016;45:2847–2870. doi: 10.1039/C5CS00903K. [DOI] [PubMed] [Google Scholar]
- ter Heijne A, Schaetzle O, Gimenez S, et al. Analysis of bio-anode performance through electrochemical impedance spectroscopy. Bioelectrochemistry. 2015;106:64–72. doi: 10.1016/j.bioelechem.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Tiquia-Arashiro SM, Pant D. Microbial electrochemical technologies. CRC Press; 2020. [Google Scholar]
- Torres CI. On the importance of identifying, characterizing, and predicting fundamental phenomena towards microbial electrochemistry applications. Curr Opin Biotechnol. 2014;27:107–114. doi: 10.1016/j.copbio.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Toze S. Reuse of effluent water—benefits and risks. Agric Water Manag. 2006;80:147–159. doi: 10.1016/j.agwat.2005.07.010. [DOI] [Google Scholar]
- UN DESA Population Division (2019) World Population Prospects 2019: Ten Key Findings
- Venkata Mohan S, Pandey A, Varjani S. Microbial electrochemical technology: sustainable platform for fuels, chemicals and remediation. Elsevier; 2018. [Google Scholar]
- Villano M, Aulenta F, Majone M. Perspectives of biofuels production from renewable resources with bioelectrochemical systems. Asia-Pacific J Chem Eng. 2012;7:S263–S274. doi: 10.1002/apj.1643. [DOI] [Google Scholar]
- Virdis B, Millo D, Donose BC, et al. Analysis of electron transfer dynamics in mixed community electroactive microbial biofilms. RSC Adv. 2016;6:3650–3660. doi: 10.1039/c5ra15676a. [DOI] [Google Scholar]
- Wagner RC, Call DF, Logan BE. Optimal set anode potentials vary in bioelectrochemical systems. Environ Sci Technol. 2010;44:6036–6041. doi: 10.1021/es101013e. [DOI] [PubMed] [Google Scholar]
- Wang Z, He Z. Demystifying terms for understanding bioelectrochemical systems towards sustainable wastewater treatment. Curr Opin Electrochem. 2020;19:14–19. doi: 10.1016/j.coelec.2019.09.001. [DOI] [Google Scholar]
- Wang H-Y, Bernarda A, Huang C-Y, et al. Micro-sized microbial fuel cell: a mini-review. Bioresour Technol. 2011;102:235–243. doi: 10.1016/j.biortech.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Wang H, Park J, Ren ZJ. Practical energy harvesting for microbial fuel cells: a review. Environ Sci Technol. 2015;49:3267–3277. doi: 10.1021/es5047765. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang Y, Li M, et al. Operation mechanism of constructed wetland-microbial fuel cells for wastewater treatment and electricity generation: a review. Bioresour Technol. 2020;314:123808. doi: 10.1016/j.biortech.2020.123808. [DOI] [PubMed] [Google Scholar]
- Wang X, Aulenta F, Puig S, et al. Microbial electrochemistry for bioremediation. Environ Sci Ecotechnol. 2020;1:100013. doi: 10.1016/j.ese.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson VJ, Logan BE. Analysis of polarization methods for elimination of power overshoot in microbial fuel cells. Electrochem commun. 2011;13:54–56. doi: 10.1016/j.elecom.2010.11.011. [DOI] [Google Scholar]
- Wei J, Liang P, Huang X. Recent progress in electrodes for microbial fuel cells. Bioresour Technol. 2011;102:9335–9344. doi: 10.1016/j.biortech.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Winfield J, Ieropoulos I, Greenman J, Dennis J. The overshoot phenomenon as a function of internal resistance in microbial fuel cells. Bioelectrochemistry. 2011;81:22–27. doi: 10.1016/j.bioelechem.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Winfield J, Gajda I, Greenman J, Ieropoulos I. A review into the use of ceramics in microbial fuel cells. Bioresour Technol. 2016;215:296–303. doi: 10.1016/j.biortech.2016.03.135. [DOI] [PubMed] [Google Scholar]
- Xia C, Zhang D, Pedrycz W, et al. Models for microbial fuel cells: a critical review. J Power Sources. 2018;373:119–131. doi: 10.1016/j.jpowsour.2017.11.001. [DOI] [Google Scholar]
- Yang Y, Xu M, Guo J, Sun G. Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem. 2012;47:1707–1714. doi: 10.1016/j.procbio.2012.07.032. [DOI] [Google Scholar]
- Yang E, Chae KJ, Kim IS. Assessment of different ceramic filtration membranes as a separator in microbial fuel cells. Desalin Water Treat. 2016;57:28077–28085. doi: 10.1080/19443994.2016.1183523. [DOI] [Google Scholar]
- Yang Y, Ye D, Li J, et al. Microfluidic microbial fuel cells: From membrane to membrane free. J Power Sources. 2016;324:113–125. doi: 10.1016/j.jpowsour.2016.05.078. [DOI] [Google Scholar]
- Yang E, Chae KJ, Choi MJ, et al. Critical review of bioelectrochemical systems integrated with membrane-based technologies for desalination, energy self-sufficiency, and high-efficiency water and wastewater treatment. Desalination. 2019;452:40–67. doi: 10.1016/j.desal.2018.11.007. [DOI] [Google Scholar]
- Yates M, Strycharz-Glaven S, Golden J, et al. Characterizing electron transport through living biofilms. J Vis Exp. 2018;2018:1–8. doi: 10.3791/54671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Hou Y, Abu-Reesh IM, et al. Oxygen reduction reaction catalysts used in microbial fuel cells for energy-efficient wastewater treatment: a review. Mater Horizons. 2016;3:382–401. doi: 10.1039/c6mh00093b. [DOI] [Google Scholar]
- Zhang PY, Liu ZL. Experimental study of the microbial fuel cell internal resistance. J Power Sources. 2010;195:8013–8018. doi: 10.1016/j.jpowsour.2010.06.062. [DOI] [Google Scholar]
- Zhang F, Cheng S, Pant D, et al. Power generation using an activated carbon and metal mesh cathode in a microbial fuel cell. Electrochem commun. 2009;11:2177–2179. doi: 10.1016/j.elecom.2009.09.024. [DOI] [Google Scholar]
- Zhang X, Li X, Zhao X, Li Y. Factors affecting the efficiency of a bioelectrochemical system: a review. RSC Adv. 2019;9:19748–19761. doi: 10.1039/c9ra03605a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu M, Zhou M, et al. Microbial fuel cell hybrid systems for wastewater treatment and bioenergy production: Synergistic effects, mechanisms and challenges. Renew Sustain Energy Rev. 2019;103:13–29. doi: 10.1016/j.rser.2018.12.027. [DOI] [Google Scholar]
- Zhao F, Slade RCT, Varcoe JR. Techniques for the study and development of microbial fuel cells: an electrochemical perspective. Chem Soc Rev. 2009;38:1926–1939. doi: 10.1039/b819866g. [DOI] [PubMed] [Google Scholar]
- Zheng T, Li J, Ji Y, et al. Progress and prospects of bioelectrochemical systems: electron transfer and its applications in the microbial metabolism. Front Bioeng Biotechnol. 2020;8:1–10. doi: 10.3389/fbioe.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi W, Ge Z, He Z, Zhang H. Methods for understanding microbial community structures and functions in microbial fuel cells: a review. Bioresour Technol. 2014;171:461–468. doi: 10.1016/j.biortech.2014.08.096. [DOI] [PubMed] [Google Scholar]
- Zhu X, Tokash JC, Hong Y, Logan BE. Controlling the occurrence of power overshoot by adapting microbial fuel cells to high anode potentials. Bioelectrochemistry. 2013;90:30–35. doi: 10.1016/j.bioelechem.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Zou S, He Z. Efficiently “pumping out” value-added resources from wastewater by bioelectrochemical systems: a review from energy perspectives. Water Res. 2018;131:62–73. doi: 10.1016/j.watres.2017.12.026. [DOI] [PubMed] [Google Scholar]