Abstract
Purpose:
The objective of this review was to assess the efficacy of non-pharmacological interventions on endometrial cancer (EC) survivors QOL, and their use of patient reported outcome measures (PROMs).
Methods:
We conducted a systematic review of randomized controlled trials (RCTs) of non-pharmacological interventions that assessed the impact of intervention on EC survivors’ general and domain-specific QOL (i.e., physical, psychological, and social well-being) using PROMs.
Results:
Of the 3,178 studies identified, 28 full-text articles were reviewed, and 10 were included in the review. Nine RCTs assessed at least one PROM as a primary outcome and six assessed a PROM as a secondary outcome, but few studies used validated PROMs. Significant improvements in general QOL were found in two studies, domain-specific QOL in three studies, and both general and domain-specific QOL in three studies; however, effect sizes ranged from small to large and no significant effects were found for social well-being and few were found for psychological well-being.
Conclusions:
Few non-pharmacological interventions for EC survivors targeted QOL, even though QOL was assessed as either a primary or secondary outcome of the RCT. Despite this, findings suggest that non-pharmacological interventions for EC survivors hold promise for improving general and domain specific QOL. Use of validated PROMs would greatly enhance outcome reporting and facilitate comparisons across studies. More interventions are also needed that address social and psychological functioning in this population.
Implications for Cancer Survivors:
Our review highlights a need to 1) expand non-pharmacological RCTs for EC survivors, 2) increase the use of validated PROMs measuring QOL, and 3) address psychosocial domains of QOL when developing interventions for this population.
INTRODUCTION
Women with endometrial cancer (EC) are the second largest population of female cancer survivors in the United States [1, 2]. It is estimated that 81% will live 5 years or more after diagnosis [3] thanks to treatment advances and the fact that most EC tumors are well-differentiated and localized [1, 4]. However, EC survivors have poorer quality of life (QOL) relative to the general population [4], with poorer outcomes linked to higher rates of obesity and sedentary lifestyle [5, 6]. They also suffer from obesity-related comorbidities including diabetes, hypertension, and cardiovascular disease [7, 8], which can adversely affect QOL and increase mortality risk [9, 10]. Non-pharmacological interventions (i.e., interventions that do not include pharmaceutical therapies as a component of their treatment) that encourage EC patients to increase physical activity, make healthier food choices and lose weight have sought to improve health outcomes (e.g., survival, lifestyle risk factors) in this population [11], but they have yielded small effects that have not been sustained over time [12]. One possibility is that most of these interventions have focused on improving health outcomes (e.g., improving survival, reducing adverse events and lifestyle risk behaviors) without addressing the variety of physical, functional, and psychosocial sequelae that EC survivors experience that can contribute to poor QOL [13]. For example, many women struggle with depression and anxiety symptoms, concerns about recurrence, and changes in sexual and social functioning after being successfully treated for EC [13–17]. Developing a greater understanding of the effects of non-pharmacological interventions on EC survivors’ general and domain-specific QOL (i.e., physical, psychological, and social well-being) could therefore elucidate new targets and opportunities for enhancing intervention efficacy[18].
QOL can be assessed using a variety of patient reported outcome measures (PROMs), including general and domain-specific questionnaires. General QOL questionnaires encompass generic instruments that can either be used across a wide range of diseases or disease-specific instruments that assess a broad range of symptoms and side effects. Data obtained from PROMs can be leveraged to assess healthcare quality and used to make comparisons across different cancers [17]. The latter is particularly important as relatively little research has been carried out on PROMs and QOL in EC compared to other cancers (e.g., breast cancer) [13]. Given this and increasing recognition of the importance of QOL in the management of EC survivors, researchers have called for the incorporation of PROMs as secondary or co-primary end points in EC randomized controlled trials (RCTs) [13]. As a growing number of RCTs targeting EC survivors are now including PROMs, a rigorous evaluation is needed to identify service gaps, directions for new research, and to provide evidence-based recommendations to health care providers and patients. To address these gaps, we conducted a systematic review of RCTs of non-pharmacological interventions for EC survivors that assessed the impact of intervention on survivors’ general and domain-specific QOL using PROMs.
METHODS
Search Strategy and Selection Criteria
Our review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19]. A medical librarian conducted searches within the MEDLINE Ovid, EMBASE, Scopus, Psyclnfo, PubMed, and Cochrane electronic databases using MeSH headings and equivalent words and phrases. Search terms and the complete search strategy are detailed in Appendix A. Results were verified by reviewing reference lists from the publications retrieved, including relevant systematic reviews and meta-analyses. In a blind review, titles and abstracts of publications were screened by three reviewers -- a health psychologist (HB), a clinical psychology doctoral student (MKR), and a Masters level research coordinator with experience working with EC patients (AB). Articles selected for screening were 1) focused on EC survivors or included EC survivors in their sample, 2) RCTs of non-pharmacological interventions (i.e., behavioral, psychological, or educational interventions), and 3) involved outcome assessment of PROMs. Articles were excluded if they 1) were not in English, 2) did not report data (e.g., study protocols) or full text was not available (e.g. conference abstracts, poster presentations), or 3) did not report the percentage of EC participants in the study sample.
Study Selection, Data Extraction, and Guality Assessment
The three reviewers screened each study independently by title and abstract based on the predefined eligibility criteria. Full texts of eligible studies were then reviewed by AB and MKR for data extraction. Study characteristics including author, year of publication, intervention characteristics, participant characteristics, PROs assessed, statistical analysis method, and outcomes were extracted and inputted into Eppi-Reviewer 4. Disagreements in study eligibility, data extraction, and quality assessment were resolved by consultation with the third author, HB. Enrollment and retention rates are reported in Table 2. Enrollment rates reflect the number of individuals successfully enrolled in the intervention, and were computed by dividing the total number who consented by the total number invited to participate. Attrition rates reflect the number of individual who dropped out of the study either during intervention or at follow-up. When not reported, these rates were computed by dividing the number of participants who failed to complete follow-up assessments by the total number of participants randomized. Finally, two team members (AB and HB) reviewed all data to ensure accuracy before analysis. Studies were assessed for quality using a modified 11-item version of the well-established Physiotherapy Evidence Database (PEDro) coding scheme [20].
Table 2.
Systematic Review of Randomized Control Trial Interventions Involving Endometrial Cancer Survivors (k = 10)
| Sample Demographics | Enrollment and Attrition Rates | Study Design | Study Arms: | Key Findings | |
|---|---|---|---|---|---|
| Brotto, 2012 [21] |
N: 31 Mean age: 54 % EC: 64.5 % |
Enrollment: 5.3% Attrition: 0% |
Theory: CBT Blinding: No Randomization: Yes Delivery/dosage: 3 months/once a month Primary Outcome(s): Psychological Well-Being, Social Well-Being Secondary Outcome(s): Psychological Well-Being |
Intervention: mindfulness-based cognitive behavioral intervention for sexual dysfunction (psychoeducation + mindfulness stress reduction) Control: wait-list control Interventionist: Psychologist Assessment: Baseline, 3 months, 6 months |
General QOL – N/A Physical Well-Being – N/A Psychological Well-Being • Depressive Symptoms - Beck Depression Inventory (BDI), ns • Sex-related Distress - Female Sexual Distress Scale (FSDS) ↓ (d =0.22) Social Well-Being • Relationship Functioning - Sexual Function Questionnaire (SFQ), ns • Sexual Functioning - Female Sexual Function Index (FSFI) ↑ (d =0.53) |
| Donnelly, 2011 [22] |
N: 33 Mean age: 53 % EC: 45.4% |
Enrollment: 8.3% Attrition: 15.2% |
Theory: Trans-Theoretical Model (TTM) Blinding: Yes Randomization: Yes Delivery/dosage: 12 weeks/ 5-7 times per week Primary Outcome(s): Physical Well-Being Secondary Outcome(s): General QOL, Psychological |
Intervention: Physical Activity Behavioral Change Intervention Control: Usual Care (+ weekly symptom screens over phone) Interventionist: Physiotherapist Assessment: Baseline, 12 weeks, 6 months |
General QOL • Functional Assessment of Cancer Therapy-General (FACT-G) Total Score, ns Physical Well-Being • Fatigue - Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) ↓ (d =0.21 )‡ • Fatigue - Functional Assessment in Chronic Illness Therapy —Fatigue (FACIT-F), ns • Sleep dysfunction (Pittsburgh Sleep Quality Index; PSQI), ns Psychological Well-Being • Depressive Symptoms (BDI), ns |
RESULTS
Our search strategy identified 5,137 references and 1 additional article was identified through searching references of systematic reviews. Following removal of 1,960 duplicates, we screened titles and abstracts of 3,178 unique studies. After reviewing titles and abstracts, 28 articles were retrieved in full, of which 10 [8, 21–29] were eligible for this review (see Figure 1).
Figure 1.
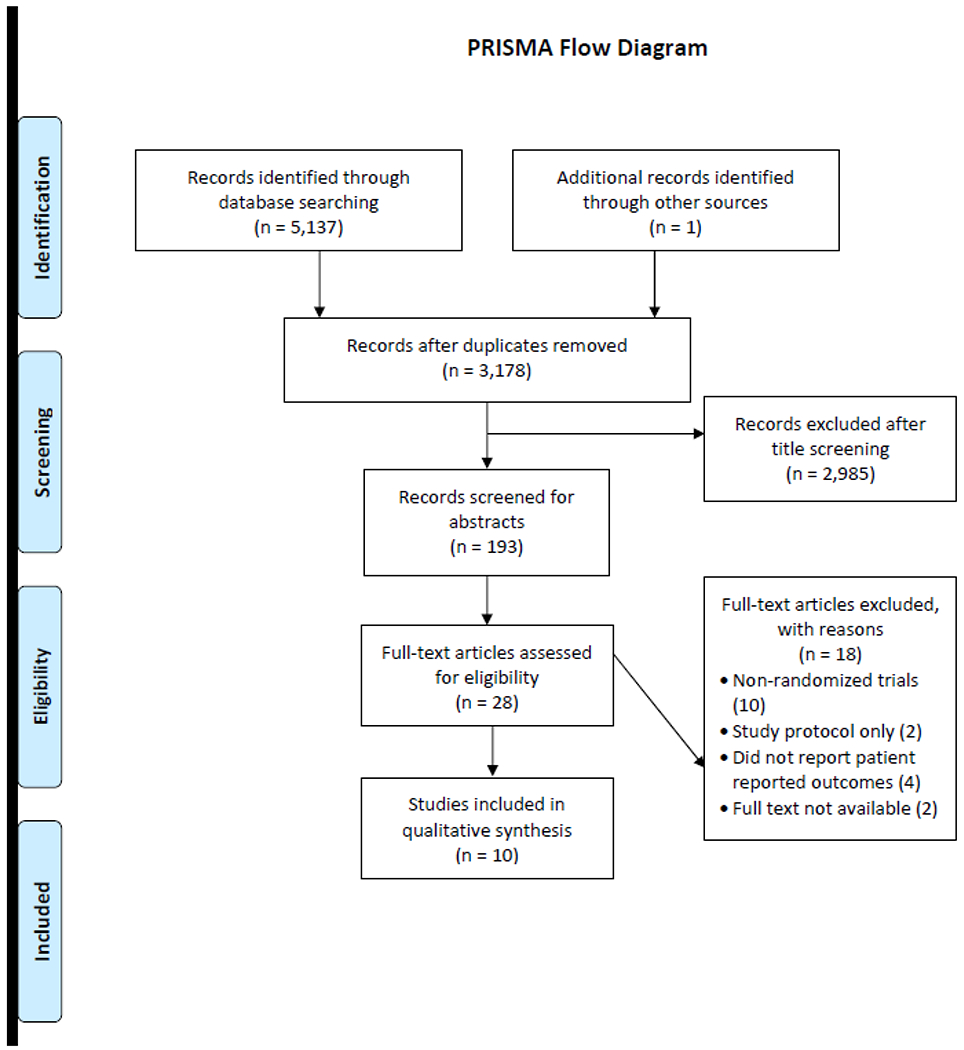
Flow Diagram Depicting the Systematic Review Process
Participant Characteristics
As Table 1 shows, patients were predominately white (82.3%); mean age was 56.7 years (SD = 4.6, range = 31 [21] to 79 years [29]). Across studies, 558 EC survivors were enrolled, but sample size varied considerably, ranging from 28 [28] to 165 [26]. Only one study had a sample size > 100 [26]. Four studies focused exclusively on EC or uterine cancer survivors [8, 24, 27, 28]; the rest involved mixed samples of gynecological cancer survivors [21–23, 25, 26, 29]. The mean enrollment rate was 22.1% (range = 5.3% [21] to 69% [23]), and the mean attrition rate was 14.4% (range = 0% [23] to 39.3% [28]).
Table 1.
Participant and Design Characteristics of Studies in the Systematic Review (k = 10)
| Mean(SD) | Number (%) of Studies | |
|---|---|---|
| Cancer Type | ||
| Endometrial/Uterine Only | 4 (40.0) | |
| Endometrial and Other | 6 (60.0) | |
| Sample Size | 55.8 (38.8) | |
| Participant Age | 56.7 (4.6) | |
| Percent White/Caucasian* | 82.3% | |
| Enrollment Rate | 22.1% (18.7) | |
| Attrition Rate | 14.4% (11.6) | |
| Type of Control Group | ||
| Usual Care | 8 (80.0) | |
| Wait-list Control | 2 (20.0) | |
Design Characteristics
As Table 2 shows, seven of the 10 RCTs sought to improve lifestyle risk behaviors (e.g., reducing overweight/obesity, increasing physical activity, and improving dietary intake) [8, 22, 24–28]; three targeted different domains of QOL (i.e., physical, social, and psychological well-being) [21,23, 29]. Intervention duration ranged from 8 [23, 25] to 24 weeks [27]. The average number of sessions was 12 (range = 3 [21] to 24 [28]), and session length varied from 20-120 minutes. Five interventions were delivered in person [21, 23, 25, 26, 28], one was delivered by telephone/text [24], and four incorporated both modalities [8, 22, 27, 29].
When reviewing behavioral health interventions, theoretical frameworks used for intervention development were noted to determine if there were trends in phenomenological agreement and effectiveness across interventions. Three studies [23, 24, 29] had no explicit or implied conceptual framework. Social Cognitive Theory, which posits individual behavioral change is achieved through skill building in self-efficacy and positive reinforcement of goals [30] grounded four interventions [8, 25, 27, 28]. Cognitive behavioral and/or behavioral change theories, which posit that behavioral change is influenced by readiness, intention and commitment to change [31] were utilized in two interventions [22, 23]. Finally, Self-Determination Theory [32], which focuses on individual needs for autonomy (intrinsic motivation), competence, and relatedness grounded one intervention [26]. Therapeutic techniques that were employed included Cognitive Behavior Therapy (CBT), health education, individual and group counseling, pelvic floor muscle training, and Japanese massage therapy.
PROMs Used
Nine of the ten RCTs included at least one PROM as a primary outcome measure [8, 21–23, 25–29], six included at least one PROM as a secondary outcome measure [21–24, 26, 27]. As Table 2 shows, choice of PROMs varied widely. The most commonly used PROM was the FACT-G [33] (4 studies) [8, 22, 26, 28]. Other PROMs used included the EORTC QLQ-30 [34] (N=2) [23, 25]. Beck Depression Inventory [35] (BDI; N=2) [21, 22] and Hospital Anxiety and Depression Scale [36] (HADS; N=2) [23, 27]. Five studies assessed changes in PROMs at one follow-up time point [23, 24, 26, 28, 29], three assessed changes at two follow-ups [21, 22, 25], and two assessed changes at three follow-ups [8, 27].
Intervention Effects on QOL
Theoretical frameworks proved to have a significant impact on study design and intervention effectiveness. Studies that utilized cognitive behavioral approaches and interpersonal counseling in their intervention design were more likely to yield significant effects across all three QOL domains. Session length and frequency of intervention were not predictors of efficacy across QOL domains, however adherence to traditional treatment lengths for behavioral interventions seemed to have contributed to their effectiveness within each study.
As Table 2 shows, eight studies assessed general QOL, but domain-specific measures of QOL were not consistently included in assessment batteries. For example, only three studies included a domain-specific measure of physical well-being (i.e., fatigue, urinary incontinence) [8, 22, 29]. Several studies identified physical activity or physical health as a primary outcomes, however these outcomes were assessed using anthropometric measures (e.g. waist circumference, six minute walk test, body mass index) as opposed to PROMs [27, 28]. Eight studies assessed psychological well-being [8, 21–24, 26, 27, 29], but the constructs measured varied widely and included depression, anxiety, self-efficacy, mood, distress, body image, and perceived impact of cancer. Only two studies assessed social well-being [21,24]; and neither reported any significant changes over time.
Two studies reported no improvements in either general or domain-specific QOL [8, 29]. Of the remaining eight studies, two reported improvements in general QOL [25, 28], three reported improvements in domain-specific QOL [21,22, 27], and three reported improvements in both general and domain-specific QOL [23, 24, 26]. Although we could not calculate effect sizes based on the available data reported in two studies [23, 24], effect sizes for at least one QOL outcome were of small magnitude in four studies [21,22, 25, 26], medium magnitude in one study [21], and large magnitude in two studies [27, 28] (see Table 2)[37].
Quality of Studies
A review of findings using the modified PEDro scale [20] revealed that all 10 studies specified eligibility criteria and randomly allocated participants to groups. In most cases, random allocation was concealed with the exception of three studies [21,26, 28]. Subjects and study administrators were not blinded to group allocation after randomization in any studies, but two studies reported blinding assessors to group allocation at follow-up assessment [21, 22]. Only five studies obtained measures of at least one key outcome from more than 85% of the original participants [21–23, 27, 29]. All studies reported results of between-group comparisons for at least one key outcome, but only two studies reported both point measures and measures of variability for at least one key outcome [22, 23]. Of the eight studies that failed to report both measures, two studies reported effect sizes [21,28], five studies reported standard deviation and/or confidence intervals as measures of variability [8, 24–27], and one study reported neither [29].
DISCUSSION
This systematic review was conducted to understand the effects of non-pharmacological interventions on EC survivors’ general and domain-specific QOL. To date, three systematic reviews have been conducted to examine QOL in EC survivors [12, 17, 38]. Our findings augment and extend findings from these reviews by examining a broader array of non-pharmacological interventions that have been tested in RCTs and distinguishing between general and domain-specific aspects of QOL. Specifically, we found that three interventions demonstrated significant improvements in both general and domain-specific QOL, two demonstrated improvements for general QOL only, and three demonstrated improvements for domain-specific QOL only. However, effect sizes ranged from small to large, validated PROMs were infrequently used, and a wide variety of QOL domains were examined, making cross-study comparisons difficult.
This review uncovered several limitations of the non-pharmacological intervention literature in EC that represent directions for future research. First, several studies were identified but were excluded because they either involved pharmacological interventions [39], were single-arm trials or quasi-experimental studies [40–43], or did not include at least one PROM as an outcome measure [44]. The fact that all the studies meeting our inclusion criteria were conducted in the past decade reflects this burgeoning research area and need for more RCTs that utilize validated PROMs so conclusions can be drawn regarding the efficacy of interventions on general and domain-specific aspects of QOL. Second, only four RCTs focused exclusively on EC survivors [8, 24, 27, 28], and only two of these demonstrated significant effects on QOL [24, 28]. More studies are thus needed that either address the unique health and QOL needs of EC survivors or that report outcomes separately by cancer type. Third, most studies had small sample sizes and thus were underpowered to examine changes in the multiple QOL outcomes that were assessed and enrollment rates varied widely from 5.3% [21] to 69% [23]. In the larger context of enrollment in clinical trials, only 5–30% of adult cancer patients participate [45, 46]. Documented barriers such as distance from the trial center, fear of randomization, and perceived burden of participation were all likely factors as most of the RCTs we reviewed were delivered in person, did not involve participant blinding, and involved numerous sessions delivered over several weeks. Incorporating strategies to reduce burden such as decreasing the number of sessions or assessments, and conducting sessions by phone, the Internet, or in patients’ homes may help reduce burden and bolster enrollment. Likewise, enlisting physicians or clinic staff to introduce the study may also be helpful. Fourth, most studies included white participants, ignoring the possibility that race and culture may influence survivor QOL. Study demographics also likely reflect who has access and ability to attend intervention programs and may have excluded patients who lived far away from their care center, had transportation problems, or had physical limitations that made travel difficult. Emerging communication technologies (e.g. internet, mobile health technologies, and social media) may help to improve equal access and facilitate dissemination. However, more research is needed to determine survivors’ intervention preferences, whether factors such as advanced disease status, age, or comfort with technology affect receptivity and uptake, and whether such interventions are feasible and cost-effective. Finally, in our quality assessment of each intervention, we uncovered inconsistencies in study methodology and outcome reporting that impacted comparability of results. Although quality scores on the PEDro scale were generally high, most studies did not report both point measures and variability measures for key outcomes (criterion 11 on the PEDro scale). Some studies also did not include information on refusal or attrition rates, suggesting that reporting standards could improve.
None of the interventions in this review explicitly targeted social functioning and only one targeted psychological functioning. This could explain why no significant effects on social well-being, depression, anxiety, or general distress were found. Another possibility is that the lack of significant results in individual studies may be attributed to some participants having very little distress or relationship problems to begin with, or that some studies did not use stringent screening criteria prior to enrolling participants. More research is needed to determine whether there are profiles of at-risk survivors who may benefit from intervention and whether it may be necessary to screen for psychological distress or poorer physical well-being from the outset. Finally, research has shown that cancer patient distress often alleviates over time as patients adjust to the “new normal” of their lives after treatment [47, 48]. This natural trajectory may have washed out any positive intervention effects.
Despite a growing literature on the functional challenges of EC survivorship [4], few studies have sought to address issues such as sexual dysfunction, incontinence, and dyspareunia in this population. Research in other cancers has shown that these functional challenges adversely affect psychological, relationship, and social functioning [49], and studies have shown that EC survivors are interested in participating in interventions that will positively impact their mental health and sexual/relationship functioning alongside their physical health [4, 50, 51]. In reviewing the excluded studies, we found that a number of studies in this area were either single-arm trials or quasi-experimental studies, so more RCTs are needed that address these aspects of survivor QOL.
In conclusion, although findings of this review suggest that non-pharmacological interventions for EC survivors may hold promise for improving general and domain specific QOL, more studies with methodologically rigorous designs are needed in order to draw definitive conclusions. Even though all the studies in this review included QOL as either a primary or secondary outcome, most focused on improving health outcomes (e.g., clinical outcomes and lifestyle behaviors) and only three sought to directly improve QOL. Thus, effect sizes may be improved by directly targeting QOL and being more discerning about when to target general or domain-specific QOL in future RCTs. Likewise, use of validated PROMs would greatly enhance outcome reporting and facilitate comparisons across studies. Reporting standards also need to be improved. For example, we were unable to examine whether age, cancer type (e.g. uterine, endometrial), stage at diagnosis, or length of remission impacted QOL, as this data was not always reported. Finally, our findings suggest that a research gap exists with regard to interventions that target psychological and social functioning and that more interventions are needed that address these survivor concerns alongside their physical health.
Supplementary Material
Appendix A. Search strategy
| 1. exp Genital Neoplasms, Female/ | 218,385 |
| 2. (genital* and (neoplas* or “neo-plas*” or cancer* or tumor* or oncolog* or carcinoma* or adenocarcinom* or “adeno-carcinom*”) and (female* or woman* or women* or girl*)).ti,ab,kw. | 7,598 tumour* or |
| 3. (gynecol* or gynaecol* or endometr* or “endo-metr*”) and “neo-plas*” or cancer* or tumor* or tumour* or oncolog* or carcinoma* or adenocarcinom* or “adeno-carcinom*”)).ti,ab,kw. | 66,497 (neoplas* or |
| 4. 1 or 2 or 3 | 242,741 |
| 5. Survivors/ or Cancer Survivors/ | 24,586 |
| 6. Survivorship/ | 224 |
| 7. ((survivorship* or “survivor-ship*”) and (plan* or agenda* or treatment* or summar*)).ti,ab,kw. | 5,413 regimen* or |
| 8. 5 or 6 or 7 | 28,554 |
| 9. 4 and 8 | 856 |
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest:
Ashley Buchanan: None to report. McKenzie Roddy: None to report. Hoda Badr: None to report.
References
- [1].Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- [2].Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA: a cancer journal for clinicians. 2019;69:363–85. [DOI] [PubMed] [Google Scholar]
- [3].SEER. SEER Cancer Stat Facts: Endometrial Cancer. Bethesda, MD; 2017. [Google Scholar]
- [4].Rowlands IJ, Lee C, Beesley VL, Janda M, Nagle CM, Webb PM. Women’s perceptions of their lifestyle and quality of life several years after a diagnosis of endometrial cancer. Cancer nursing. 2015;38:E21–E8. [DOI] [PubMed] [Google Scholar]
- [5].Smits A, Lopes A, Das N, Bekkers R, Galaal K. The impact of BMI on quality of life in obese endometrial cancer survivors: Does size matter? Gynecologic Oncology. 2014;132:137–41. [DOI] [PubMed] [Google Scholar]
- [6].Basen-Engquist K, Scruggs S, Jhingran A, Bodurka DC, Lu K, Ramondetta L, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. American journal of obstetrics and gynecology. 2009;200:288. e1–. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New England Journal of Medicine. 2003;348:1625–38. [DOI] [PubMed] [Google Scholar]
- [8].von Gruenigen VE, Gibbons HE, Kavanagh MB, Janata JW, Lerner E, Courneya KS. A randomized trial of a lifestyle intervention in obese endometrial cancer survivors: quality of life outcomes and mediators of behavior change. Health and quality of life outcomes. 2009;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wild S, Bryden J, Lee R, Bishop J, Finlayson A, Byrne C, et al. Cancer, cardiovascular disease and diabetes mortality among women with a history of endometrial cancer. British journal of cancer. 2007;96:1747–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecologic Oncology. 2012;126:176–9. [DOI] [PubMed] [Google Scholar]
- [11].von Gruenigen V, Frasure H, Kavanagh MB, Janata J, Waggoner S, Rose P, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecologic Oncology. 2012;125:699–704. [DOI] [PubMed] [Google Scholar]
- [12].Kitson S, Ryan N, MacKintosh ML, Edmondson R, Duffy JM, Crosbie EJ. Interventions for weight reduction in obesity to improve survival in women with endometrial cancer. Cochrane Database of Systematic Reviews. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Joly F, McAlpine J, Nout R, Avall-Lundqvist E, Shash E, Friedlander M, et al. Quality of life and patient-reported outcomes in endometrial cancer clinical trials: a call for action! Int J Gynecol Cancer. 2014;24:1693–9. [DOI] [PubMed] [Google Scholar]
- [14].Bradley S, Rose S, Lutgendorf S, Costanzo E, Anderson B. Quality of life and mental health in cervical and endometrial cancer survivors. Gynecologic Oncology. 2006;100:479–86. [DOI] [PubMed] [Google Scholar]
- [15].Aziz NM. Cancer survivorship research: State of knowledge, challenges and opportunities. Acta Oncologica. 2007;46:417–32. [DOI] [PubMed] [Google Scholar]
- [16].Zandbergen N, de Rooij BH, Vos MC, Pijnenborg JM, Boll D, Kruitwagen RF, et al. Changes in health-related quality of life among gynecologic cancer survivors during the two years after initial treatment: a longitudinal analysis. Acta Oncologica. 2019;58:790–800. [DOI] [PubMed] [Google Scholar]
- [17].Shisler R, Sinnott JA, Wang V, Hebert C, Salani R, Felix AS. Life after endometrial cancer: A systematic review of patient-reported outcomes. Gynecologic Oncology. 2018;148:403–13. [DOI] [PubMed] [Google Scholar]
- [18].Ferrell B, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Quality of life Research. 1995;4:523–31. [DOI] [PubMed] [Google Scholar]
- [19].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [20].Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical therapy. 2003;83:713–21. [PubMed] [Google Scholar]
- [21].Brotto LA, Erskine Y, Carey M, Ehlen T, Finlayson S, Heywood M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecologic Oncology. 2012;125:320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Donnelly CM, Blaney JM, Lowe-Strong A, Rankin JP, Campbell A, McCrum-Gardner E, et al. A randomised controlled trial testing the feasibility and efficacy of a physical activity behavioural change intervention in managing fatigue with gynaecological cancer survivors. Gynecologic Oncology. 2011;122:618–24. [DOI] [PubMed] [Google Scholar]
- [23].Donoyama N, Satoh T, Hamano T, Ohkoshi N, Onuki M. Effects of Anma therapy (Japanese massage) on health-related quality of life in gynecologic cancer survivors: A randomized controlled trial. PLoS ONE. 2018;13:e0196638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Haggerty AF, Hagemann A, Barnett M, Thornquist M, Neuhouser ML, Horowitz N, et al. A Randomized, Controlled, Multicenter Study of Technology-Based Weight Loss Interventions among Endometrial Cancer Survivors. Obesity (Silver Spring). 2017;25 Suppl 2:S102–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koutoukidis DA, Beeken RJ, Manchanda R, Burnell M, Ziauddeen N, Michalopoulou M, et al. Diet, physical activity, and health-related outcomes of endometrial cancer survivors in a behavioral lifestyle program: the Diet and Exercise in Uterine Cancer Survivors (DEUS) parallel randomized controlled pilot trial. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2019;29:531–40. [DOI] [PubMed] [Google Scholar]
- [26].Olesen ML, Duun-Henriksen AK, Hansson H, Ottesen B, Andersen KK, Zoffmann V. A person-centered intervention targeting the psychosocial needs of gynecological cancer survivors: a randomized clinical trial. J. 2016;10:832–41. [DOI] [PubMed] [Google Scholar]
- [27].McCarroll ML, Armbruster S, Frasure HE, Gothard MD, Gil KM, Kavanagh MB, et al. Self-efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial. Gynecologic Oncology. 2014;132:397–402. [DOI] [PubMed] [Google Scholar]
- [28].Rossi A, Garber CE, Ortiz M, Shankar V, Goldberg GL, Nevadunsky NS. Feasibility of a physical activity intervention for obese, socioculturally diverse endometrial cancer survivors. Gynecologic Oncology. 2016;142:304–10. [DOI] [PubMed] [Google Scholar]
- [29].Rutledge TL, Rogers R, Lee SJ, Muller CY. A pilot randomized control trial to evaluate pelvic floor muscle training for urinary incontinence among gynecologic cancer survivors. Gynecologic Oncology. 2014;132:154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bandura A Health promotion from the perspective of social cognitive theory. Psychology and health. 1998;13:623–49. [Google Scholar]
- [31].Marshall SJ, Biddle SJ. The transtheoretical model of behavior change: a meta-analysis of applications to physical activity and exercise. Annals of behavioral medicine. 2001;23:229–46. [DOI] [PubMed] [Google Scholar]
- [32].Patrick H, Williams GC. Self-determination theory: its application to health behavior and complementarity with motivational interviewing. International Journal of behavioral nutrition and physical Activity. 2012;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. [DOI] [PubMed] [Google Scholar]
- [34].Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNCI: Journal of the National Cancer Institute. 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- [35].Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Psychological measurements in psychopharmacology: Karger Publishers; 1974. p. 151–69. [DOI] [PubMed] [Google Scholar]
- [36].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica scandinavica. 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- [37].Cohen J A power primer. Psychological bulletin. 1992;112:155. [DOI] [PubMed] [Google Scholar]
- [38].Smits A, Lopes A, Das N, Bekkers R, Massuger L, Galaal K. The effect of lifestyle interventions on the quality of life of gynaecological cancer survivors: A systematic review and meta-analysis. Gynecologic Oncology. 2015;139:546–52. [DOI] [PubMed] [Google Scholar]
- [39].De Boer SM, Nout RA, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, et al. Long-term impact of endometrial cancer diagnosis and treatment on health-related quality of life and cancer survivorship: results from the randomized PORTEC-2 trial. International Journal of Radiation Oncology* Biology* Physics. 2015;93:797–809. [DOI] [PubMed] [Google Scholar]
- [40].Robertson MC, Lyons EJ, Song J, Cox-Martin M, Li Y, Green CE, et al. Change in physical activity and quality of life in endometrial cancer survivors receiving a physical activity intervention. Health and quality of life outcomes. 2019;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Basen-Engquist K, Carmack C, Brown J, Jhingran A, Baum G, Song J, et al. Response to an exercise intervention after endometrial cancer: differences between obese and non-obese survivors. Gynecologic oncology. 2014;133:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lucas AR, Focht BC, Cohn DE, Buckworth J, Klatt MD. A Mindfulness-based lifestyle intervention for obese, inactive endometrial cancer survivors: a feasibility study. Integrative cancer therapies. 2017;16:263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].McCarroll ML, Armbruster S, Pohle-Krauza RJ, Lyzen AM, Min S, Nash DW, et al. Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application. Gynecologic Oncology. 2015;137:508–15. [DOI] [PubMed] [Google Scholar]
- [44].Maxwell-Smith C, Hince D, Cohen PA, Bulsara MK, Boyle T, Platell C, et al. A randomized controlled trial of WATAAP to promote physical activity in colorectal and endometrial cancer survivors. Psycho-Oncology. 2019. [DOI] [PubMed] [Google Scholar]
- [45].Comis RL, Miller JD, Aldigé CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. Journal of Clinical Oncology. 2003;21:830–5. [DOI] [PubMed] [Google Scholar]
- [46].Lara PN Jr, Higdon R, Lim N, Kwan K, Tanaka M, Lau DHM, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. Journal of Clinical Oncology. 2001;19:1728–33. [DOI] [PubMed] [Google Scholar]
- [47].Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer. 2006;15:306–20. [DOI] [PubMed] [Google Scholar]
- [48].Towsley GL, Beck SL, Watkins JF. “Learning to live with it”: coping with the transition to cancer survivorship in older adults. Journal of Aging Studies. 2007;21:93–106. [Google Scholar]
- [49].Zebrack BJ. Cancer survivor identity and quality of life. Cancer practice. 2000;8:238–42. [DOI] [PubMed] [Google Scholar]
- [50].Koutoukidis D, Beeken R, Lopes S, Knobf M, Lanceley A. Attitudes, challenges and needs about diet and physical activity in endometrial cancer survivors: a qualitative study. European journal of cancer care. 2017;26:e12531. [DOI] [PubMed] [Google Scholar]
- [51].Donnelly C, Lowe-Strong A, Rankin J, Campbell A, Blaney J, Gracey J. A focus group study exploring gynecological cancer survivors’ experiences and perceptions of participating in a RCT testing the efficacy of a home-based physical activity intervention. Supportive Care in Cancer. 2013;21:1697–708. [DOI] [PubMed] [Google Scholar]
- [52].von Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecologic oncology. 2008;109:19–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


