Abstract
The peptide‐based therapeutic cancer vaccines have attracted enormous attention in recent years as one of the effective treatments of tumour immunotherapy. Most of peptide‐based vaccines are based on epitope peptides stimulating CD8+ T cells or CD4+ T helper cells to target tumour‐associated antigens (TAAs) or tumour‐specific antigens (TSAs). Some adjuvants and nanomaterials have been exploited to optimize the efficiency of immune response of the epitope peptide to improve its clinical application. At present, numerous peptide‐based therapeutic cancer vaccines have been developed and achieved significant clinical benefits. Similarly, the combination of peptide‐based vaccines and other therapies has demonstrated a superior efficacy in improving anti‐cancer activity. We delve deeper into the choices of targets, design and screening of epitope peptides, clinical efficacy and adverse events of peptide‐based vaccines, and strategies combination of peptide‐based therapeutic cancer vaccines and other therapies. The review will provide a detailed overview and basis for future clinical application of peptide‐based therapeutic cancer vaccines.
Keywords: adjuvant and nanomaterial, cancer immunotherapy, combination strategy, epitope peptides, peptide‐based therapeutic cancer vaccine
Despite few clinical successes, peptide‐based therapeutic cancer vaccines have raised hopes in recent years. Therefore, better understanding of immune response, clinical benefits and adverse events, the formulations, and the promising combination strategies with conventional therapies foster development of the next generation of peptide‐based cancer vaccines.
Abbreviations
- AE
adverse event
- ANN
artificial neural network
- CEA
carcinoembryonic antigen
- CpG ODN
cytosine guanine oligodeoxynucleotide
- CR
complete response
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DSPC
distearoyl phosphatidylcholine
- DSPG
distearoyl phosphatidylglycerole
- FDA
Food and Drug Administration
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor
- HLA
human leucocyte antigen
- HLA‐I
HLA class I antigen
- HLA‐II
HLA class II antigen
- IDO
indoleamine 2,3‐dioxygenase
- IEDB
the immune epitope database
- IFA
incomplete Freund adjuvant
- IFN‐γ
interferon‐γ
- IL‐2
interleukin‐2
- ISA
incomplete Seppic adjuvant
- LPS
lipopolysaccharides
- MAGE‐1
melanoma antigen‐1
- MAGE‐A1
melanoma antigen‐A1
- MART‐1
melanoma antigen recognized by T cells 1
- MHC‐I
major histocompatibility complex I
- MHC‐II
major histocompatibility complex II
- MPLA
monophosphoryl lipid A
- MUC
mucin
- OS
overall survival
- PD‐1
programmed death 1
- poly‐ICLC
lysine and carboxymethylcellulose
- ppCT
preprocalcitonin
- PPV
personalized peptide vaccination
- PR3
proteinase‐3
- PSSM
position‐specific scoring matrix
- RAI
reaction at the injection sites
- RFS
disease‐free survival
- RT
radiotherapy
- TA
tumour antigen
- TAA
tumour‐associated antigen
- TAP
transporter associated with antigen processing
- TEIPP
T‐cell epitopes associated with impaired peptide processing
- TIL
tumour infiltrating lymphocyte
- TLR
toll‐like receptor
- TSA
tumour‐specific antigen
- TTK
TTK protein kinase
- VEGFR
vascular endothelial growth factor receptor
1. INTRODUCTION
Immunotherapeutic strategies have dramatically revolutionized cancer treatments, including dendritic cell (DC)‐based cancer vaccines, immune checkpoint inhibitors and chimeric antigen receptor T‐cell immunotherapies (CAR‐T). For example, checkpoint inhibitor‐based immunotherapies that could activate T cells result in an improvement in clinical success, but the tumour targeting was deficient. Despite specific tumour targeting, CAR‐T therapy showed risks of cytokine release syndrome and neurotoxicity, and it could not gain clinical benefits on solid tumours, which caused the limitation of clinical application. Therefore, developing the safe and effective treatments to enhance the specific anti‐tumour activity has become a hot topic in the current field of tumour immunotherapy.
The peptide‐based therapeutic cancer vaccines could offer many advantages with regard to convenient production, cost‐effective manufacture, low carcinogenic potential, insusceptible pathogen contamination and high chemical stability. This type of vaccine contains the distinct 8‐12 aa peptide from tumour antigen (TA) coding sequence. TAs are formed by overexpressing and emerging proteins during the process of tumorigenesis and development. It could be internalized into DCs, where they are degraded into peptides and assemble to human leucocyte antigen (HLA) molecules on DCs surface for T‐cell activation. HLA is the expression product of the human major histocompatibility complex (MHC), which is related to immune response. Regarding the interaction of T cells and DCs, T cells not only recognize specific TA but also recognize the distinct peptide‐HLA complex. The strategy of identifying novel peptides from TA is an attractive method for immunotherapy with clinical benefit and cost‐effectiveness.
Furthermore, the mode of administration is easy and the immune response could be monitored in vitro; thereby, peptide‐based therapeutic cancer vaccines could be a promising approach for cancer therapies. These parameters to develop peptide‐based therapeutic cancer vaccines are critical, such as choices of proper tumour antigens, effective screening and modification methods of epitope peptides, and selections of proper formulations. Furthermore, growing evidence has demonstrated that combination between peptide‐based vaccines and other therapies could offer an ideal view of cancer immunotherapy. In the review, we discussed multiple peptide‐based therapeutic cancer vaccines in various cancer types and their immune response and clinical benefits.
2. TARGET CHOICES OF PEPTIDE‐BASED THERAPEUTIC CANCER VACCINES
The CD8+ T cells are capable of recognizing the peptide‐HLA complex to produce a persistent memory CTL response against target cells expressing the antigen. Therefore, the critical factor is the selection of proper TA for therapeutic cancer vaccines to exert specific cytotoxicity against tumour cells.
TAs can be classified into tumour‐associated antigens (TAA) and tumour‐specific antigens (TSA). Despite TAAs can express in both normal cells and tumour cells, they overexpress in tumour cells but at a low level in normal cells. Therefore, TAAs are attractive targets for developing immunotherapeutic cancer vaccines. Some studies reported that characteristics of proper TAA should be the following: i) differential expression between normal cells and tumour cells; ii) involvement in cell cycle; and iii) association with cell survival. 1 Normally, most of TAAs with low self‐tolerance and strong immunogenicity were used as targets in preclinical studies and clinical trials to evaluate safety and efficacy of peptide‐based therapeutic cancer vaccines. 2 On the other hand, TSA only expressed in tumour cells rather than in normal cells, including mutations of normal proteins, 3 cancer testis antigen, 4 , 5 neoantigens 6 and virus‐related antigens. 7 , 8 Boon et al reported melanoma antigen‐A1 (MAGE‐A1) as the first TSA in humans at 1991. 9 Human leucocyte antigen (HLA) / TSA‐derived peptide complex, could exert higher avidity specific T cells to lead to effective and safe immune response of cancer vaccines against tumour. 10 , 11 TSAs as targets of cancer vaccines demonstrated similar results in both animal models and clinical trials due to the loss of TSA expression in normal tissues, which means non‐immunologically tolerant to TSA and non‐immunity targeting normal tissues. 12 TSAs are attractive for personalized cancer immunotherapy, but it is not cost‐effective. 13 Besides, some studies emerge for the selection of specific epitopes, such as T‐cell epitopes associated with impaired peptide processing (TEIPP), 14 which only express on transporter associated with antigen processing (TAP)‐deficient tumour cell surface. The preprocalcitonin (ppCT) 16–25 antigenic peptide, derived from the calcitonin hormone precursor, as the first human TEIPP Ag, provides a new strategy to counteract immune evasion by antigenic processing machinery defects. 15 Currently, many TAAs and TSAs have been identified as targets for peptide‐based therapeutic cancer vaccines (Figure 1, Table 1), in which most focus on targeting melanoma, 16 lung cancer, 17 breast cancer 18 and leukaemia, 19 , 20 whereas most of them are in phase I and phase II. Recent clinical trials in phase III are only including HER2 (human epidermal growth factor receptor 2)/neu targeting breast cancer 21 ; tyrosinase, gp100 antigen, and MART‐1 (melanoma antigen recognized by T cells 1) antigen targeting melanoma; PR3 (proteinase‐3) targeting leukaemia. 22 TAs, such as Survvin, VEGFR (vascular endothelial growth factor receptor), MUC1 (mucin 1) and TTK (TTK protein kinase), were used most extensively as targets for developing peptide‐based therapeutic cancer vaccines, targeting lung cancer, gastrointestinal cancer and melanoma (Figure 2).
FIGURE 1.
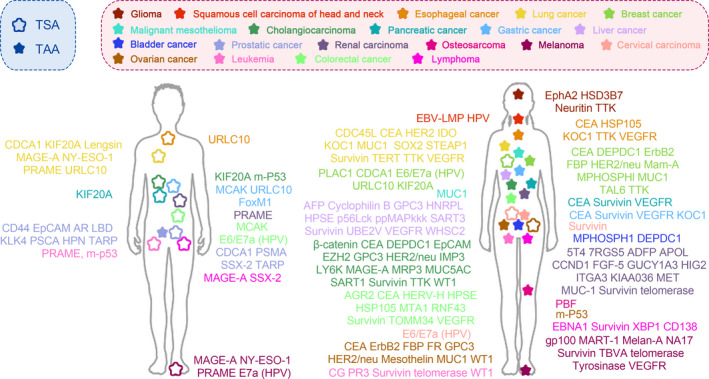
Tumour antigens for peptide‐based therapeutic cancer vaccine in different types of tumours
TABLE 1.
Antigens for peptide‐based therapeutic cancer vaccines
| Tumour | Tumour‐specific antigens | Tumour‐associated antigens |
|---|---|---|
| Glioma | EphA2, HSD3B7, Neuritin, TTK | |
| Squamous cell carcinoma of head and neck | EBV‐LMP, HPV | |
| Oesophageal cancer | URLC10 | CEA, HSP105, KOC1,TTK, VEGFR |
| Lung cancer | CDCA1, KIF20A, Lengsin, MAGE‐A, NY‐ESO‐1, PRAME, URLC10 | CDC45L, CEA, HER2, IDO, KOC1, MUC1, SOX2, STEAP1, Survivin, TERT, TTK, VEGFR |
| Breast cancer | PLAC1, CDCA1, E6/E7a (HPV), URLC10, KIF20A, m‐P53 | CEA, DEPDC1, ErbB2, FBP, HER2/neu, Mam‐A, MPHOSPHl, MUC1, TAL6,TTK |
| Malignant pleural mesothelioma | MUC1 | |
| Liver cancer | AFP, Cyclophilin B, GPC3, HNRPL, HPSE, p56Lck, ppMAPkkk, SART3, Survivin, UBE2V, VEGFR, WHSC2 | |
| Cholangiocarcinoma | KIF20A, MAGE‐A, m‐P53 | β‐catenin, CEA, DEPDC1, EpCAM, EZH2, GPC3, HER2/neu, IMP3, LY6K, MRP3, MUC5AC, SART1, Survivin, TTK, WT1 |
| Pancreatic cancer | KIF20A | CEA, Survivin, VEGFR |
| Gastric cancer | MCAK, URLC10, FoxM1 | CEA, Survivin, VEGFR, KOC1 |
| Bladder cancer | MPHOSPH1, DEPDC1 | |
| Prostatic cancer | CDCA1, PSMA, SSX‐2, TARP | CD44, EpCAM, AR LBD, KLK4, PSCA, HPN |
| Renal carcinoma | PRAME | 5T4, 7RGS5, ADFP, APOL, CCND1, FGF‐5, GUCY1A3, HIG2, ITGA3, KIAA036, MET, MUC1, Survivin, telomerase |
| Osteosarcoma | PBF | |
| Melanoma | MAGE‐A, NY‐ESO‐1, PRAME, E7a (HPV) | gp100, MART‐1, Melan‐A, NA17, Survivin, TBVA, telomerase, Tyrosinase, VEGFR |
| Cervical carcinoma | E6/E7a (HPV) | Survivin |
| Ovarian cancer | m‐P53 | CEA, ErbB2, FBP, FR, GPC3, HER2/neu, Mesothelin, MUC1, WT1 |
| Leukaemia | PRAME, m‐p53 | CG, PR3, Survivin, telomerase, WT1 |
| Colorectal cancer | MCAK, E6/E7a (HPV) | AGR2, CEA, HERV‐H, HPSE, HSP105, MTA1, RNF43, Survivin, TOMM34, VEGFR |
| Lymphoma | MAGE‐A, SSX‐2 | EBNA1, Survivin, XBP1, CD138 |
FIGURE 2.

Frequency of tumour antigens using in clinical trials for peptide‐based therapeutic cancer vaccines
3. STRATEGIES FOR SCREENING EPITOPE PEPTIDES
The anti‐tumour effects of DC‐mediated T‐cell activation are through the stimulation of peptides, terms epitopes, instead of the entire antigen molecule. Normally, the epitope for developing peptide‐based therapeutic cancer vaccines is a short amino sequence derived from TA with immunogenicity and HLA allele compatibility. It has been reported many screening strategies for immunodominant epitopes, such as bioinformatic analysis and HLA ligandome. The affinity of HLA‐I allele and epitopes can be measured and predicted by many methods (Table 2), including the method based on structural analysis, the position‐specific scoring matrix (PSSM), artificial neural network (ANN) method and machine learning. 23 Structural analysis identifies neoepitopes by calculating the minimal free energy of epitope‐HLA complex. 24 , 25 PSSM is produced by measuring the interaction between peptides and specific MHC molecule. 26 The correlation of different positions in sequence was considered into ANN analysis to predict affinity between peptides and MHC molecule. Machine learning could predict affinity of peptides and MHC molecule by learning the affinity of known functional regions with peptides. The immune epitope database (IEDB) predicts the optimal amino binding positions of MHC‐I molecule through a large variety of HLA allele algorithms, thereby being broadly applicated for identifying the epitope peptides. 27 Additionally, HLA ligandome approach could identify naturally HLA‐presented peptides existed in tumour cells by mass spectrometry analysis. 28 It could also be used to identify specifically overexpressed protein‐derived peptides, signal peptide‐derived peptides and antigenic mutation‐derived peptides. 29 This approach could combine with computational biology and bioinformatics, such as functional annotation and gene expression analysis, to identify potential TSA (including neoantigens) and TAA. Based on ligandome analysis, we can observe a few peptides of 11 amino acids, 12 amino acids and 13 amino acids, as their length is outside the consensus of the computer programs for motif prediction of class I peptides.
TABLE 2.
In silico analysis for peptide‐based therapeutic cancer vaccines
| Year | Database | Method | Methodology | Website | Characteristics |
|---|---|---|---|---|---|
| 1994 | BIMAS | Experimental verification | A total of 154 peptides were combined together to generate a table containing 180 coefficients (20 amino acids x 9 positions), each of which represents the contribution of one particular amino acid residue at a specified position within the peptide to binding to HLA‐A2. Provides a predicted t1/2 of dissociation | http://www‐bimas.cit.nih.gov / molbio / hla_bind | |
| 1997 | SYFPEITHI | Experimental verification | The algorithm takes into account a number of characteristics of both the HLA haplotype as well as the peptide of interest, and uses these data to provide a binding score | http:// /www.syfpeithi.de / | |
| 2000 | IEDB | Experimental verification | The IEDB combined all published data associated with epitopes and a large scale of experimentally determined peptides | www.immuneepitope.org | IEDB could provide consistent and accurate data with improved interoperability |
| 2002 | RANKPEP | PSSM | The binding potential of any peptide sequence (query) to a given MHC molecule is linked to its similarity to a group of aligned peptides known to bind to that MHC |
rankpep.html |
|
| 2005 | NetCTL | Bioinformatics prediction | Integration the predictions of proteasomal cleavage, TAP transport efficiency and MHC class I affinity | http://www.cbs.dtu.dk/services/NetCTL | |
| 2008 | NetMHC | PSSM | The software integrates affinity measurements of IEDB database and data of eluting ligands in SYFPEITHI database to train 55 MHC allele‐specific artificial neural networks and additional position‐specific scoring matrix (PSSM) of 67 HLA allele | http://www.cbs.dtu.dk/services/NetMHC. | The binding affinity measurements of 8‐, 10‐ and 11‐mer were predicted based on properties of 9‐mer |
| 2009 | PMBEC | PSSM | PMBEC is derived from the binding affinity data of combinatorial peptide mixtures to build up matrix properties of amino sequence | The software could compensate for missing information on specific residues in the training data | |
| 2015 | ANN‐Hydr | Machine learning | Training on a relative hydrophobicity scale |
Abbreviations: ANN, artificial neural network; BIMAS, bioinformatics and molecular analysis section; CTL, cytotoxic T lymphocyte; HLA, human leucocyte antigen; IEDB, the immune epitope database; MHC, major histocompatibility complex; PSSM, position‐specific scoring matrix; TAP, transporter associated with antigen processing.
4. CLINICAL APPLICATION OF PEPTIDE‐BASED THERAPEUTIC CANCER VACCINES
Since Hu et al reported that MAGE‐1 (melanoma antigen‐1)‐derived peptide can be used as peptide‐based therapeutic cancer vaccine in clinical trial, various TA‐derived epitopes have been identified for clinical application of peptide‐based vaccines. 30 Most recently, peptide‐based vaccines are tested in clinical trials for multiple cancers, including melanoma, 31 oesophageal cancer, 32 , 33 lung cancer, 34 , 35 pancreatic cancer, 36 and head and neck squamous cell carcinoma. 37 The study of Mittendorf et al 38 showed that E75 (nelipepimut‐S), a HLA‐A2/A3‐restricted immunogenic peptide‐derived HER2, was safe and appeared to have clinical efficacy. And a phase III has been initiated. Mittendorf et al also reported the phase II trial evaluating GP2 (a HER2‐derived, HLA‐A2+ restricted peptide) + GM‐CSF (granulocyte‐macrophage colony‐stimulating factor) setting to breast cancer patients to prevent recurrence. Results suggested that the vaccine might be effective in patients with HER2‐positive tumours who also received trastuzumab. 39 Phase II trial of a multivalent WT1 peptide vaccine (galinpepimut‐S) in leukaemia 40 and phase I/II trial of MUC1, HER2 and CEA (carcinoembryonic antigen) HLA‐A2+‐restricted peptides 41 also showed that peptide‐based vaccines were feasible, safe and well tolerated. Sipuleucel‐T for prostate cancer was the first peptide‐based therapeutic cancer vaccine approved to go to the market by Food and Drug Administration (FDA).
The peptide‐based therapeutic cancer vaccines in clinical trials often combine multiple targets with multiple epitopes by different screening strategies, unlike in vitro studies that usually focus on a single antigen. Due to the presence of multiple epitopes, T cells that recognize different targets can be activated to minimize tumour immune escape caused by antigen loss. Moreover, the combination of HLA‐I and HLA‐II class epitopes increases the possibility of both CD4+ and CD8+ effector T‐cell activation, which contributes to the persistence and survival of effector cells in vivo. 42 Therefore, these peptide‐based therapeutic cancer vaccines have been reported to be well tolerated and have shown clinical benefits against tumours. In the following paragraphs, we focused on introductions of targets, sequences and research progress of epitope peptides in recent 5 years (Table 3).
TABLE 3.
Clinical trials of peptide‐based therapeutic cancer vaccines in recent five years
| Tumour | Targets | Epitopes | Reference | Phase |
|---|---|---|---|---|
| Solid tumour | GPC3 | FVGEFFTDV | 92 | Ⅰ |
| KOC1, DEPDC1, MPHOSPH1, TTK, URLC10 | KTVNELQNL, EYYELFVNI, IYNEYIYDL, SYRNEIAYL, RYCNLEGPPI | 48 | Ⅰ | |
| WT1 | CYTWNQMNL | 66 | I/II | |
| Melanoma | Tyrosinase, gp100, MART‐1 | YMDGTMSQV, IMDQVPFSV, LAGIGILTV | 93 | Ⅲ |
| Breast cancer | HER2 | IISAVVGIL | 39 | I/II |
| E75(nelipepimut‐S, KIFGSLAFL) | 38 | I/II | ||
| IISAVVGIL, LRMKGVGSPYVSRLLGICL | 94 | II | ||
| Breast cancer, ovarian cancer | MUC1, ErbB2, CEA | SAPDNRPAL, KIFGSLAFL, YLSGADLNL | 41 | I/II |
| Leukaemia | WT1 | YMFPNAPYL, RSDELVRHHNMHQRNMTKL, PGCNKRYFKLSHLQMHSRKHTG, SGQAYMFPNAPYLPSCLES | 40 | I/II |
| KRYFKLSHLQMHSRKH | Ⅰ | |||
| Renal carcinoma | APOL‐1, APOL‐2, KIAA0367, ITGA3, MUC‐1, ADFP, MET, CCND1, RGS5, GUCY1A3 | FLGENISNFL, ALADGVQKV, ALFDGDPHL, SVFAGVVGV, LLYPTEITV, STAPPVHNV, SVASTITGV, YVDPVITSI, LAALPHSCL, LLGATCMFV | 95 | I/II |
| HIG2 | VLNLYLLGV | 96 | Ⅰ | |
| Glioma | ANKRD40, BCA, CDK4, EIF4E, PTP, USP11, et al | 33 HLA‐A*02:01‐binding and 26 HLA‐A*24:02‐binding peptides | 63 | Ⅰ |
| WT1 | CYTWNQMNL, KRYFKLSHLQMHSRKH | 56 | I/II | |
| Survivin | DLAQMFFCFKEL | 97 | Ⅰ | |
| BCAN, CHI3L2, CSPG4, FABP7, IGF2BP3, NLGN4X, NRCAM, PTPRZ1, TNC | ALWAWPSEL, SLWAGVVVL, TMLARLASA, LTFGDVVAV, KIQEILTQV, NLDTLMTYV, GLWHHQTEV, AIIDGVESV, KVFAGIPTV, AMTQLLAGV | |||
| Neuroblastoma | NY‐ESO‐1 | SLLMVVITQV | 49 | |
| Colorectal cancer | RNF43, TOMM34 | NSQPVWLCL, KLRQEVKQNL | 98 | II |
| RNF43, TOMM34, KOC1, VEGFR1/2 | NSQPVWLCL, KLRQEVKQNL, KTVNELQNL, SYGVLLWEI, RFVPDGNRI | 99 | II | |
| Oesophageal cancer | DEPDC1, MPHOSPH1, URLC10, CDCA1, KOC1 | EYYELFVNI, IYNEYIYDL, RYCNLEGPPI, KTVNELQNL, YMMPVNSEV, KLATAQFKI | 43 | |
| Colorectal cancer, oesophageal cancer | HSP105 | NYGIYKQDL, EYVYEFRDKL, RLMNDMTAV, KLMSSNSTDL | 100 | Ⅰ |
| Gastric cancer | FOXM1, DEPDC1, KIF20A, URLC10, VEGFR | IYTWIEDHF, RYCNLEGPPI, EYYELFVNI, KVYLRVRPLL, SYGVLLWEIF | 53, 55 | I/II |
| Gastrointestinal cancer | HSP70, GPC3 | YGAAVQAAI, MVNELFDSL | 91 | Ⅰ |
| Pancreatic cancer | KIF20A, VEGFR1/2 | KVYLRVRPLL, SYGVLLWEI, RFVPDGNRI | 90 | II |
| WT1 | RMFPNAPYL, CYTWNQMNL | 61 | II | |
| Lung cancer | IDO | ALLEIASCL | 47 | Ⅰ |
| Bladder cancer | DEPDC1, MPHOSPH1 | EYYELFVNI, MVNELFDSL / LFDSLFPVI / SLQVTRIFL | 44 | I/II |
| Prostatic cancer | Personalized peptide vaccination (PPV) | LLQAEAPRL / KLKHYGPGWV / KLVERLGAA / DVWSFGILL / DLLSHAFFA / ASLDSDPWV / RLQEWCSVI / NVLHFFNAPL / DYSARWNEI/VYDYNCHVDL/HYTNASDGL/DYLRSVLEDF/RYLTQETNKV/LYCESVHNF/HYRKWIKDTI/DYVREHKDNI/WLEYYNLER/QIRPIFSNR/ILEQSGWWK/VIQNLERGYR/GIHKQKEKSR/GAAPLILSR/APAGRPSASR/KIREEYPDR | 83 | II |
| CDCA1 | VYGIRLEHF | 62 | Ⅰ | |
| Cervical carcinoma | FOXM1, MELK, HJURP, VEGFR1/2 | YLVPIQFPV, SLVLQPSVKV, GLMDLSTTPL, RFVPDGNRI | 57 | Ⅰ |
| Ovarian cancer | FBP | EIWTHSYKV / EIWTFSTKV | 64 | I/II |
4.1. Study design and treatment
Peptide‐based therapeutic cancer vaccines are usually administered in a 7‐ to 15‐day interval with subcutaneous axillary and/or inguinal injection of 1‐3 mg/dose per peptide per person. Patients usually complete a course of at least 2 months to a maximum of 12 months unless patients experience disease progression or unacceptable toxicity. The primary end points are safety, tolerability, immunogenicity and operational feasibility of the peptide‐based vaccines. The secondary end points are evaluations of anti‐tumour effects, overall survivals (OS) and disease‐free survivals (RFS).
4.2. Clinical efficacy and immune response
Analysis on patients treated with peptide‐based vaccines showed that the production of epitope‐specific CTLs could be induced in most patients, and even tumour infiltrating lymphocyte (TIL) activation could be induced in individual patients. 43 The CD8+ T cells in lymph nodes and the infiltration of CD8+ T cells in the tumour microenvironment increased in about 30%‐60% of patients, and the secretion of granzyme B and interferon‐γ (IFN‐γ) also increased. Patients who showed a strong epitope‐specific CTL response had longer OS than those with non‐ or low immune response, demonstrating that peptide‐based vaccines could be effective in patients who showed a peptide‐specific immune response. Compared with the placebo group, patients receiving the peptide‐based vaccine showed a tendency of improved OS and RFS, and their condition was more stable. The peptide‐based vaccine therapy usually shows delayed immune response and tumour growth inhibition, but does not show significant tumour shrinkage. 44 , 45 Additionally, the epitope peptide could induce anti‐tumour response over a long period of time. 46 Kjeldsen et al reported that 13.3% of patients showed anamnestic immune response 6 years after primary immunization. 47 In another case of oesophageal cancer, the patient received 8 vaccinations every 6 months, a total of 38 vaccinations, and finally obtained a complete response (CR) lasting for 5 years. 48 Although peptide‐specific responses also were elicited in high‐risk patients, previous studies showed that patients in the early stage of tumour progression or with a low disease burden could obtain better clinical benefits. 49 , 50 , 51 This is because the immunosuppressive tumour microenvironment was the stronger in high‐risk patients compared with low‐risk patients. There were no significant differences in OS and RFS between the vaccine treatment group and the control group in some clinical trials. For example, Brian IRini et al reported that the peptide‐based vaccine did not improve any clinically relevant indicators in advanced metastatic renal cancer in a phase III study. 52 Possible reasons for lack of clinical benefits include the patient's low immune status, the limited response to the vaccine, and the poor dose and/or the short duration of treatment. The clinical effect of peptide‐based vaccine may be delayed compared with chemotherapy due to the mechanism of immune response, which may lead to a longer observation period to evaluate the clinical benefits. Some studies also recruited patients with advanced disease who were resistant to multiple chemotherapies, and it was difficult for these patients to gain clinical benefits from the peptide‐based vaccines due to the poor state of their immune systems. Therefore, peptide‐based vaccines may be suitable as an adjuvant therapy for cancer patients after surgery. 53
4.3. Adverse events
The peptide‐based vaccines have distinct characteristics of better tolerance and safety compared with conventional anti‐tumour therapies, such as chemotherapy and immune checkpoint inhibitors, and the vaccines generally could not cause serious systemic adverse events (AEs). The most common AEs related to the peptide‐based vaccine are erythema and induration related to the injection site with grade 1 or 2, 53 which are easy to be reversed. Patients with reaction at the injection sites (RAI) generally showed a better prognosis than those without skin reaction, 53 , 54 suggesting that RAI might be a surrogate predictor of CTL response to peptide‐based vaccine. Other grade 1 or 2 AEs include nausea, diarrhoea, myalgia, fatigue, increased aspartate aminotransaminase, and increased blood alkaline phosphatase 55 , 56 , 57 and urinary irritation in bladder cancer. 58 No dose‐related toxicity and treatment‐related death were observed. Some studies reported grade 1‐3 AEs in haematology, such as hypoalbuminemia, thrombocytopenia, leukopenia, neutropenia, anaemia and bone marrow suppression, which were mainly related to the cancer progression. 57 , 59 , 60 , 61 , 62 , 63 However, the causal relationship between anaemia and the peptide‐based vaccine cannot be ruled out in the vaccine targeting VEGFR. 55 Moreover, the most common grade 3 or higher AEs were RAIs (including ulcers and diffuse maculopapular rash) and headache. The peptide‐based vaccine‐related grade 3 AEs included chest pain, dyspnoea and pulmonary embolism, which may be due to the expression of epitope‐related TA in lung tissue, leading to a direct immune response (on‐target and off‐tumour). 64 , 65 During the six‐year follow‐up, IDO (indoleamine 2,3‐dioxygenase)‐specific peptide vaccine showed no grade 3 or 4 AEs, which ensured the long‐term safety of peptide‐based vaccines. 47 Sawada et al found the TA‐specific CD8+ T cells showed exhausted phenotypes in individual patients, which may be due to over‐activation of CD8+ T cells in patients with high tumour mutation burden or over‐frequent vaccinations. 66 In summary, patients could gain clinical benefits from peptide‐based therapeutic cancer vaccines with distinct advantages of safety, good tolerance and effective immunization.
5. COMMON PHARMACEUTICAL FORMULATIONS OF PEPTIDE‐BASED THERAPEUTIC CANCER VACCINES
The peptide‐based therapeutic cancer vaccines can improve the prognosis of cancer patients, while a more effective vaccine is needed to improve PFS and OS of patients. One of the strategies is developing a safe and effective immune formulation to enhance TA‐derived peptide‐specific immunity. The epitope peptides with instinct features of low molecular weight, easy to degradation and short half‐life accelerated the development of pharmaceutical formulations of peptide‐based vaccines. The preparation of formulations usually by prolongating epitope persistence, enhancing co‐stimulation signal, increasing local inflammation and triggering non‐specific proliferation of lymphocytes enhanced the efficacy of peptide‐based vaccines. These formulations can be divided into immune stimulation adjuvants and vaccine delivery systems according to the main mechanism of action.
5.1. Immune stimulation adjuvants
Immune stimulation adjuvants could enhance humoral immune and Ⅳ type allergy to induct IFN‐γ secretion, regulate MHC‐II class antigen expression for producing TA‐specific CTLs, such as complete Freund's adjuvant, incomplete Freund's adjuvant (IFAs), toll‐like receptor (TLR) agonists and cytokines. The incomplete Freund's adjuvant, Montanide ISA (incomplete Seppic adjuvant) 51, and the cytokine, GM‐CSF, are widely used in clinical trials.
5.1.1. Montanide ISA 51
Not only could Montanide ISA 51 trigger immune responses, but also enhance the depot effect of vaccines. Due to the non‐absorbable mineral oil composition, it remains at the subcutaneous injection site for weeks to months, helping maintain persistence of epitopes to active T cells. 67 Combining the epitope peptides with Montanide ISA 51 may cause a stronger immune response and kill more tumour cells. Before vaccination, lyophilized powder of epitope peptides was dissolved in the appropriate solvent, such as normal saline or dimethyl sulphoxide diluted with normal saline (Figure 3A). Then, solvent mixed with Montanide ™ ISA 51 VG (Seppic Inc, Paris, France) at ratio of 1:1 until the two liquids generating a thick, creamy, opaque and consistent emulsion (Figure 3B). The patients are usually vaccinated at a 1.0 mL dose level containing 1‐3 mg epitopes. Valmori D et al tested different formulations to improve the CTL immune response. The results showed that IFA injection significantly increased the CTL response. 36 Sher YP et al used Montanide ISA 51 combined with Th epitopes derived from TAL6 antigen and CpG ODN (cytosine guanine oligodeoxynucleotide, TLR9 agonist) to make the immune stimulation adjuvant, showing that the epitope with adjuvant was more effective in inhibiting tumour growth and metastasis than the epitope alone. 68 Although Montanide ISA 51 is generally safe, it often causes local side effects, such as skin irritation and inflammation, even ulcers.
FIGURE 3.
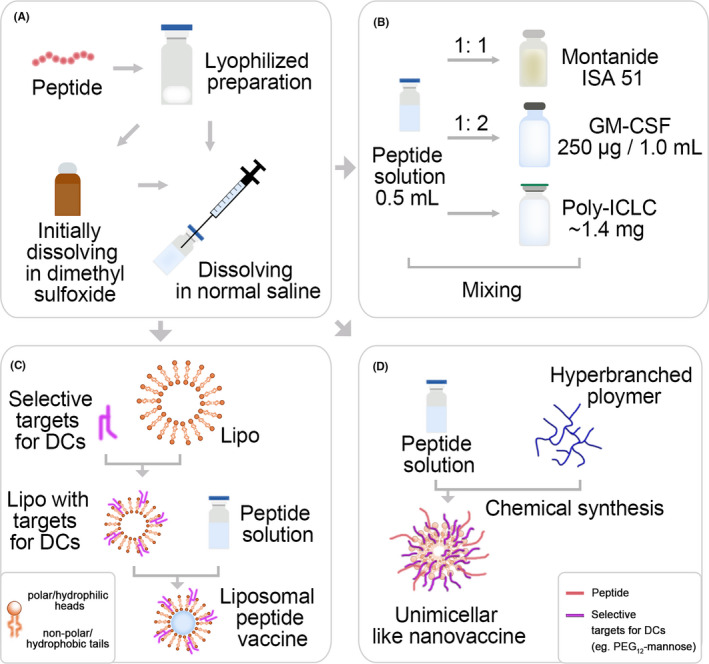
Preparing emulsions, micelles and nanoparticles for epitope peptides. a, Dissolution of lyophilized preparation of epitope peptides. b, Epitope peptides mixing with immune adjuvants. c, Design diagram of liposomal peptide vaccine. d, Peptide amphiphile micelles for vaccine delivery
5.1.2. GM‐CSF
GM‐CSF is usually utilized as an adjuvant due to it can enhance effective priming of T‐cell responses by attracting and stimulating DCs in skin loaded with tumour‐associated epitope peptides upon vaccination. It may also have antiangiogenic activity and has been successfully applied in late‐stage clinical trials. The lyophilized powder of peptides was dissolved in 0.5 mL suitable solvent and mixed with 250 μg / 1.0 mL GM‐CSF, and the total volume was 1.5 mL (Figure 3B). 38 Previous studies of peptide‐GM‐CSF clinical trials demonstrated that side effects could be attributed to the toxicity of GM‐CSF rather than the immune activity of peptides, 39 , 69 and the most serious side effect was only RAI.
5.1.3. TLR agonist
TLR agonists are effective adjuvants that could enhance epitopes‐induced CTL memory activation. 70 TLR3 agonist poly‐ICLC (lysine and carboxymethylcellulose) is widely used to stimulate tumour‐specific T‐cell response to prevent T cell from exhausting and to improve immunotherapy outcomes. 71 The vaccines with combination of epitope solutions and 1.4 mg poly‐ICLC could effectively induce epitope‐specific CTL activity (Figure 3B). Melssen et al 72 reported poly‐ICLC can be used as an effective vaccine adjuvant to induce CD8+ T‐cell immune response with targeting action and acceptable safety. TLR4 agonists as vaccine adjuvants have also been used in clinical trials, but the classic TLR4 agonist LPS (lipopolysaccharides) has been considered to be toxic. Besides, CD8+ T‐cell immune response induced by poly‐ICLC may be marginally more responsive than LPS.
5.2. Vaccine design and delivery system
Optimized delivery systems have been developed to design rational vaccines, which usually consist of comparable size, such as liposomes, microemulsions, immune‐stimulating complexes, and other nanometre or microparticle systems. The delivery system being especially suitable for the development of vaccines could improve clinical benefits of vaccines.
In recent years, more and more attention has been paid to the design of peptide‐based nanoparticle vaccines for tumour immunotherapy (Figure 3C). The optimized liposome‐based vaccines could co‐deliver peptides and adjuvants to promote their delivery to lymphoid organs and to draining lymph nodes (dLNs), which shows the acceptable clinical potential of liposome as delivery system. 73 The bioconjugation strategy links the target to the particle to improve the peptides/adjuvant co‐delivery to the DCs in lymph nodes for immune response enhancement. Additionally, liposomes can encapsulate multiple epitopes to target different TAs, which can better meet the needs of clinical application. Rueda F et al 74 used liposomes to encapsulate B epitopes, T‐cell epitopes, Th epitopes and TLR ligands to improve the immunity of the vaccine. Arab A et al 75 developed effective vaccine delivery/auxiliary systems by connecting the epitope E75, which was derived from the highly expressed antigen HER2 in breast cancer patients, with the liposome containing distearoyl phosphatidylcholine (DSPC) and distearoyl phosphatidylglycerole (DSPG). Martine A et al 68 also developed liposome‐based co‐delivery system containing melanoma‐associated antigen‐derived peptide GP100280‐288 and TLR4 ligand monophosphoryl lipid A (MPLA), which could be phagocytized by subcutaneous DCs and significantly enhanced the epitope‐specific T‐cell response. These results indicated that strategy of nanocarriers based on liposome is effective to induce anti‐tumour immune response.
Similarly, unimicellar nanostructures based on amphiphilic dendrimers, hyperbranched polymers and cross‐linked block copolymer micelles are another acceptable strategy, which could not depolymerize when diluted. Additionally, Rui Zhang et al 76 reported that the antimicrobial peptide with low toxic cholesterol modification, DP7‐C, showed a dual role as carrier and immune adjuvant. DP7‐C with hydrophilic DP7 and hydrophobic cholesterol could self‐assemble into amphiphilic micellar structure in aqueous solution, improving the efficacy of DC‐based vaccines (Figure 3D). The toxicity of peptide‐based vaccines may be related to the membrane instability caused by the hydrophobicity of peptides, which can be reduced by fusing the peptides with the polymer into the micellar structure. 72 In general, the micelle‐based could elicit the significant immune response to inhibit tumour growth.
Short peptide‐based supramolecular hydrogel with three dimensional networks of nanofibres, nanotubes and nanoparticles 77 was a novel and promising immunostimulant, which could improve the biostability and bioactivity of peptides. The hydrogel formulation could protect the peptide against enzyme digestion and nanofibres in gels facilitated the uptake of peptides by DCs, thereby increasing the accumulation of peptides in lymph nodes to activate immune response. Yang et al 78 describe a supramolecular hydrogel of a self‐assembling D‐tetra‐peptide capable of evoking both humoral and cellular immune responses. The D‐tetra‐peptide (Nap‐GFFY) could form hydrogels by a heating‐cooling process or simply by an autoclave in phosphate‐buffered saline (PBS, pH 7.4), and allow the incorporation of different peptides by mixing through vortex or shaking. Moreover, the Nap‐GDFDFDYTKPR hydrogel discovered on this basis combined tuftsin (TKPR) and Nap‐GDFDFDY, which showed an excellent anti‐tumour efficacy by stimulating a powerful CD8+ T‐cell immune response, enhancing the phagocytic activity of macrophages and promoting the maturation of DCs. 79 Due to the very simple preparation process, the good biocompatibility and strong vaccine adjuvant potency, short peptide‐based supramolecular hydrogel suggested a great potential in vaccine development.
6. COMBINATION OF PEPTIDE‐BASED THERAPEUTIC CANCER VACCINES AND OTHER THERAPIES
Although many studies have demonstrated the effectiveness of peptide‐based therapeutic cancer vaccines, no vaccine has shown significant OS benefits in randomized phase III clinical trials. However, combination of therapies aimed at controlling immune tolerance might improve outcomes, such as chemotherapy, radiotherapy (RT), biological agents and immune checkpoint inhibitors (Table 4). In addition to TA‐derived peptide vaccination, the personalized peptide vaccination (PPV), a novel immunotherapeutic approach based on a specific pool of peptides, is usually used on the combination strategy with other therapies in clinical trials. The peptide pool of PPV includes all information on the HLA‐A type, and the peptide candidate library includes mutated peptides and highly expressed peptides. Considering the heterogeneous antigen expressions of different patients before vaccination, four specific epitopes aiming to the individual patient were selected from the candidate peptides into combination application strategy of peptide‐based therapeutic cancer vaccines.
TABLE 4.
Therapeutic value of combined application of peptide‐based vaccines and other therapies in recent five years
| Combined classification | Combined drug | Tumour | Targets | Epitopes | Combined effect | Reference | Phase |
|---|---|---|---|---|---|---|---|
| Chemotherapy | Cyclophosphamide | Solid tumour | RNF43 | ALWPWLLMAT / ALWPWLLMAT | Decreased ratio of Tregs, increased tumour‐specific immune responses and clinical efficacy | 81 | Ⅰ |
| KOC1, DEPDC1, MPHOSPH1, TTK, URLC10 | KTVNELQNL, EYYELFVNI, IYNEYIYDL, SYRNEIAYL, RYCNLEGPPI | Decreased ratio of Tregs | 48 | Ⅰ | |||
| Cholangiocarcinoma | CypB, NRPL, p56Lck, ppMAPkkk, SART3, UBE2V, WHSC2 | KLKHYGPGWV; ALVEFEDVL; NVLHFFNAPL; KLVERLGAA; DVWSFGILL; DLLSHAFFA; LLQAEAPRL; RLAEYQAYI; RLQEWCSVI; LIADFLSGL; ASLDSDPWV; ILGELREKV | T‐cell response enhancement, significant PFS and OS elongation | 82 | II | ||
| Docetaxel | Lung Cancer | PPV | 12 peptides for HLA‐A2, 14 peptides for HLA‐A24, 9 peptides for HLA‐A3, and 4 peptides for HLA‐A26 | No survival improvement | 101 | II | |
| Gemcitabine | Pancreatic cancer | WT1 | RMFPNAPYL / CYTWNQMNL | PFS and OS elongation | 61 | II | |
| KIF20A, VEGFR1/2 | KVYLRVRPLL, SYGVLLWEI, RFVPDGNRI | Good tolerance, clinical benefits | 54, 102 | II | |||
| 5‐Fluorouracil, cyclophosphamide, levofolinic acid, oxaliplatin | Colorectal cancer | TS | YMIAHITGLFLDSLGFSTTLGDAHIYL | Lymphocytes response enhancement, PFS and OS elongation | 103 | Ⅰ | |
| Dexamethasone | Prostate cancer | PPV: SART3, Cyclophilin B, p56lck, ppMAPkkk, WHSC2, UBE2V, HNRPL, SART2, MRP3, PAP, PSA, EGF‐R, IEX‐1, β‐tublin5 | LLQAEAPRL / KLKHYGPGWV / KLVERLGAA / DVWSFGILL / DLLSHAFFA / ASLDSDPWV / RLQEWCSVI / NVLHFFNAPL / DYSARWNEI / VYDYNCHVDL / HYTNASDGL / DYLRSVLEDF / RYLTQETNKV / LYCESVHNF / HYRKWIKDTI / DYVREHKDNI / WLEYYNLER / QIRPIFSNR / ILEQSGWWK / VIQNLERGYR / GIHKQKEKSR / GAAPLILSR / APAGRPSASR / KIREEYPDR | Significant OS elongation | 98 | II | |
| Platinum‐containing chemotherapy plus best supportive care | Bladder cancer | PPV | 31 candidate peptides for patients with positive HLA‐A2, ‐A3, ‐A11, ‐A24, ‐A26, ‐A31 or ‐A33 alleles | OS elongation | 84 | II | |
| Radiotherapy | Radiotherapy | Liver cancer | PPV |
P1: CORE‐18, MUC‐12, KRAS‐A02‐G13D1, PSCA‐76 P2: PI3KCA‐A02‐H1047L‐1, CORE‐35, WTP53‐149, AFP‐137 P3: EGFR‐800, KRAS‐A11‐G13D, CYPB‐84, CTNNB1‐A11‐S45F P4: KRAS11‐12C, EGFR‐54,AFP‐403, Survivin28‐80 P5: AFP‐357, VEGFR2‐169, KRAS‐A11‐12C, MRP3‐1293 P6:KRAS‐A11‐12D, CTNNB1‐A11‐41A, CTNNB1‐A11‐S45F, KRAS‐A11‐12R P7:SART3‐109, CORE‐18, PSCA‐7, hTERT‐540 P8:AFP‐357, KRAS‐A11‐12D, VEGFR2‐169, PSCA‐776 P9:CTNNB1‐A11‐S45F, CTNNB11‐41A, CTNNB11‐45P, EGFR‐54 |
Regression of tumour, decrease of AFP level | 85 | Ⅰ |
| Targeted therapy | Trastuzumab | Breast cancer | HER2 | E75(nelipepimut‐S, KIFGSLAFL) | No added cardiac toxicity | 88 | II |
| Biological agents | Bacillus Calmette‐Guérin | Bladder cancer | DEPDC1, MPHOSPH1 | EYYELFVNI, IYNEYIYDL | Good tolerance | 58 | II |
6.1. The effect of combined chemotherapy and peptide‐based vaccine
Causes of low immune responses may be associated with high Treg number. Since cyclophosphamide could selectively deplete Tregs 80 and regulate dendritic cell homoeostasis, the combination of low‐dose cyclophosphamide and peptide‐based therapeutic cancer vaccines may provide clinical benefits. 81 , 82 However, the peptide‐based vaccines combined with low‐dose IL‐2 (interleukin‐2) may exert negative effects on anti‐cancer therapies due IL‐2 may increase Tregs. 48 In addition, compared with Treg inhibitor gemcitabine alone, more than half of patients treated with peptide‐based vaccine combined with gemcitabine showed long‐lasting epitope‐specific T‐cell immune responses, reduced tumour burden, and long‐term stable disease. 61 However, the peptide‐based vaccine in combination with gemcitabine was not effective in patients with advanced metastatic disease, which was consistent with the opinion that the optimal condition for obtaining long‐term clinical benefits was in the early stage of tumour or with a low disease burden described above. Besides, for prostate cancer patients treated with peptide‐based vaccine and low‐dose dexamethasone, OS was significantly prolonged compared with dexamethasone alone due to induction of the specific anti‐tumour immunity. 83 In addition, OS also appeared to be improved when combined with peptide‐based vaccines and platinum drugs. 84
6.2. The effect of combined radiotherapy and peptide‐based vaccine
The radiation may not reach all tumour focuses due to metastases or the large size of the tumour during radiotherapy. The combination of radiotherapy and peptide‐based vaccines can effectively prevent tumours. 85 Release of danger‐associated molecular patterns by RT‐induced cell death, resulting in the facilitation of tumour antigen uptake by DCs and cross‐presentation on MHC class I, is the molecular mechanism by which the combination strategy modifies the tumour microenvironment and enhances anti‐tumour immune response. The other advantage is that the combination strategy is expected to reduce the dosage of chemotherapy drugs to avoid the side effects of chemotherapy, which has great potential clinical application values.
6.3. The effect of combined other antineoplastic agents and peptide‐based vaccine
The combination of anti‐HER2 antibody trastuzumab with the HER2‐targeting peptide‐based vaccine in preclinical studies led to the proliferation of peptide‐specific CTLs due to trastuzumab‐induced improvement of cross‐presentation of HER2 epitope‐pulsed DCs. 86 , 87 Clifton et al proved that the combination of HER2‐targeting peptide vaccine nelipepimut‐S and trastuzumab is well tolerated. Cardiac dysfunction of class III or IV was observed in the phase III trial of trastuzumab, and the combination of trastuzumab and HER2‐derived peptide vaccine did not increase the cardiotoxicity. 88
Upregulation of immune checkpoint molecule expression on CD8+ T cells, such as PD‐1 (programmed death 1), TIM‐3 (T‐cell immunoglobulin mucin 3) and TIGIT (T‐cell immunoreceptor with Ig and ITIM domains), could inhibit immunopotentiation of the peptide‐based vaccine. The peptide‐based vaccine could also promote the infiltration of CD45RO+ activation/memory T cells into the tumours, which in turn facilitate the increase of PD‐1+ TILs. 89 These suggested that combination strategy of immune checkpoint inhibitors and peptide‐based vaccines may be beneficial for tumour patients. 90 , 91 Indeed, the emergent of preclinical and clinical data demonstrated that the anti‐tumour activity of immune checkpoint inhibitors can be enhanced by peptide vaccination.
7. CONCLUSION AND PERSPECTIVE
The peptide‐based therapeutic cancer vaccines could be well equipped with easy manufacturing, excellent safety profiles and low cost compared with lentivirus‐transduced DC vaccine. The FDA‐approved HLA‐restricted epitope also demonstrated that the strategy based on immune response could revolutionize cancer treatments. Unfortunately, many studies about peptide‐based vaccines have failed in clinical trials due to the immunoevasion of tumour cells and the loss of tumour antigen. Some ‘CTL epitopes’ with low immunogenicity cannot be effectively cross‐presented by DCs in vivo to favour cross‐priming of CTLs. Therefore, it is important to further identify and optimize epitopes with immunogenicity for clinical application.
Despite the peptide‐based cancer vaccines with specific cytotoxicity against tumour cells, there are major challenges of inducing continuous and high immune response level. The results of the early clinical trials thus far conducted suggested that the peptide‐specific immunity gradually decreased over time. The FDA guidelines point that multi‐target vaccines targeting different tumour antigens could generate multiple TA‐specific immune responses, which are expected to overcome resistance of peptide‐based vaccines to effectively inhibit tumour immunoevasion. Therefore, the novel strategy emerging on the identification of epitopes derived from TAs associated with tumour progression can contribute to the development of multi‐target vaccines and improve the efficacy of peptide‐based vaccines. We hope that multi‐peptides therapeutic cancer vaccines could offer a powerful potential in future clinical application at the era of successful immunotherapy.
CONFLICTS OF INTEREST
The author declares that he/she has no competing interests.
ACKNOWLEDGEMENTS
We would like to acknowledge the Key Laboratory of Precision Diagnosis and Treatment of Gastrointestinal Tumors, Ministry of Education (China Medical University, Shenyang, China) for providing the space and equipment for the compilation of the data.
This work was supported by National Natural Science Foundation of China (NSFC)—Liaoning Joint Fund Key Program [No.U1608281], Shenyang S&T Projects [19‐109‐4‐09, 17‐123‐9‐00].
WL wrote the manuscript. HT modified the review. WL, LL and XW collected and summarized the data. ZY and JL conceptualized the review. All authors read and approved the final manuscript.
Liu W, Tang H, Li L, Wang X, Yu Z, Li J. Peptide‐based therapeutic cancer vaccine: Current trends in clinical application. Cell Prolif. 2021;54:e13025. 10.1111/cpr.13025
Contributor Information
Zhaojin Yu, Email: 20101041@cmu.edu.cn, Email: ljp_63@163.com.
Jianping Li, Email: 20101041@cmu.edu.cn, Email: ljp_63@163.com.
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article.
REFERENCES
- 1. Darzynkiewicz Z. Will cancer immunotherapy fail? Scientist. 2006;20(2):14. [Google Scholar]
- 2. Gallou C, Rougeot A, Graff‐Dubois S, Kosmatopoulos K, Menez‐Jamet J. A general strategy to optimize immunogenicity of HLA‐B*0702 restricted cryptic peptides from tumor associated antigens: Design of universal neo‐antigen like tumor vaccines for HLA‐B*0702 positive patients. Oncotarget. 2016;7(37):59417‐59428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen F, Zou Z, Du J, et al. Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J Clin Invest. 2019;129(5):2056‐2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia‐Soto AE, Schreiber T, Strbo N, et al. Cancer‐testis antigen expression is shared between epithelial ovarian cancer tumors. Gynecol Oncol. 2017;145(3):413‐419. [DOI] [PubMed] [Google Scholar]
- 5. Parvizpour S, Razmara J, Pourseif MM, Omidi Y. In silico design of a triple‐negative breast cancer vaccine by targeting cancer testis antigens. Bioimpacts. 2019;9(1):45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vitiello A, Zanetti M. Neoantigen prediction and the need for validation. Nat Biotechnol. 2017;35(9):815‐817. [DOI] [PubMed] [Google Scholar]
- 7. Jabbar B, Rafique S, Salo‐Ahen OMH, et al. Antigenic peptide prediction from E6 and E7 oncoproteins of HPV types 16 and 18 for therapeutic vaccine design using immunoinformatics and MD simulation analysis. Front Immunol. 2018;9:3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krishna S, Ulrich P, Wilson E, et al. Human papilloma virus specific immunogenicity and dysfunction of CD8(+) T cells in head and neck cancer. Cancer Res. 2018;78(21):6159‐6170. [DOI] [PubMed] [Google Scholar]
- 9. van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643‐1647. [DOI] [PubMed] [Google Scholar]
- 10. Robbins PF, Lu Y‐C, El‐Gamil M, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor‐reactive T cells. Nat Med. 2013;19(6):747‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linnemann C, van Buuren MM, Bies L, et al. High‐throughput epitope discovery reveals frequent recognition of neo‐antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21(1):81‐85. [DOI] [PubMed] [Google Scholar]
- 12. Herlyn D, Birebent B. Advances in cancer vaccine development. Ann Med. 1999;31(1):66‐78. [DOI] [PubMed] [Google Scholar]
- 13. Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015;125(9):3413‐3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seidel UJ, Oliveira CC, Lampen MH, Hall T. A novel category of antigens enabling CTL immunity to tumor escape variants: Cinderella antigens. Cancer Immunol Immunother. 2012;61(1):119‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jalali SA, Sankian M, Tavakkol‐Afshari J, Jaafari MR. Induction of tumor‐specific immunity by multi‐epitope rat HER2/neu‐derived peptides encapsulated in LPD Nanoparticles. Nanomedicine. 2012;8(5):692‐701. [DOI] [PubMed] [Google Scholar]
- 16. Ruiter DJ, Mattijssen V, Broecker EB, Ferrone S. MHC antigens in human melanomas. Semin Cancer Biol. 1991;2(1):35‐45. [PubMed] [Google Scholar]
- 17. Mami‐Chouaib F, Echchakir H, Dorothee G, Vergnon I, Chouaib S. Antitumor cytotoxic T‐lymphocyte response in human lung carcinoma: Identification of a tumor‐associated antigen. Immunol Rev. 2002;188:114‐121. [DOI] [PubMed] [Google Scholar]
- 18. de Paula Peres L, da Luz FAC, dos Anjos Pultz B, et al. Peptide vaccines in breast cancer: The immunological basis for clinical response. Biotechnol Adv. 2015;33(8):1868‐1877. [DOI] [PubMed] [Google Scholar]
- 19. Matsushita M, Yamazaki R, Ikeda H, Kawakami Y. Preferentially expressed antigen of melanoma (PRAME) in the development of diagnostic and therapeutic methods for hematological malignancies. Leuk Lymphoma. 2003;44(3):439‐444. [DOI] [PubMed] [Google Scholar]
- 20. Sugiyama H. Cancer immunotherapy targeting WT1 protein. Int J Hematol. 2002;76(2):127‐132. [DOI] [PubMed] [Google Scholar]
- 21. Thundimadathil J. Cancer treatment using peptides: current therapies and future prospects. J Amino Acids. 2012;2012:967347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rezvani K. PR1 vaccination in myeloid malignancies. Expert Rev Vaccines. 2008;7(7):867‐875. [DOI] [PubMed] [Google Scholar]
- 23. Liu G, Li D, Li Z, et al. PSSMHCpan: A novel PSSM‐based software for predicting class I peptide‐HLA binding affinity. Gigascience. 2017;6(5):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu Z, Tang H, Zhang T, et al. TEX19 promotes ovarian carcinoma progression and is a potential target for epitope vaccine immunotherapy. Life Sci. 2020;241:117171. [DOI] [PubMed] [Google Scholar]
- 25. Akiyama Y, Komiyama M, Nakamura Y, et al. Identification of novel MAGE‐A6‐ and MAGE‐A12‐derived HLA‐A24‐restricted cytotoxic T lymphocyte epitopes using an in silico peptide‐docking assay. Cancer Immunol Immunother. 2012;61(12):2311‐2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA‐A2 binding peptides based on independent binding of individual peptide side‐chains. J Immunol. 1994;152(1):163‐175. [PubMed] [Google Scholar]
- 27. Vita R, Overton JA, Greenbaum JA, et al. The immune epitope database (IEDB) 3.0. Nucleic Acids Res. 2015;43(D1):D405‐D412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hunt D, Henderson R, Shabanowitz J, et al. Characterization of peptides bound to the class I MHC molecule HLA‐A2.1 by mass spectrometry. Science. 1992;255(5049):1261‐1263. [DOI] [PubMed] [Google Scholar]
- 29. Barnea E, Beer I, Patoka R, et al. Analysis of endogenous peptides bound by soluble MHC class I molecules: a novel approach for identifying tumor‐specific antigens. Eur J Immunol. 2002;32(1):213‐222. [DOI] [PubMed] [Google Scholar]
- 30. Hu X, Chakraborty NG, Sporn JR, Kurtzman SH, Ergin MT, Mukherji B. Enhancement of cytolytic T lymphocyte precursor frequency in melanoma patients following immunization with the MAGE‐1 peptide loaded antigen presenting cell‐based vaccine. Cancer Res. 1996;56(11):2479‐2483. [PubMed] [Google Scholar]
- 31. Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin‐2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iinuma H, Fukushima R, Inaba T, et al. Phase I clinical study of multiple epitope peptide vaccine combined with chemoradiation therapy in esophageal cancer patients. J Transl Med. 2014;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kono K, Iinuma H, Akutsu Y, et al. Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer‐testis antigens. J Transl Med. 2012;10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki H, Fukuhara M, Yamaura T, et al. Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti‐angiogenic peptides for patients with non‐small cell lung cancer. J Transl Med. 2013;11:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kotsakis A, Papadimitraki E, Vetsika EK, et al. A phase II trial evaluating the clinical and immunologic response of HLA‐A2(+) non‐small cell lung cancer patients vaccinated with an hTERT cryptic peptide. Lung Cancer. 2014;86(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 36. Okuyama R, Aruga A, Hatori T, Takeda K, Yamamoto M. Immunological responses to a multi‐peptide vaccine targeting cancer‐testis antigens and VEGFRs in advanced pancreatic cancer patients. Oncoimmunology. 2013;2(11):e27010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoshitake Y, Fukuma D, Yuno A, et al. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res. 2015;21(2):312‐321. [DOI] [PubMed] [Google Scholar]
- 38. Mittendorf EA, Clifton GT, Holmes JP, et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut‐S) vaccine with booster inoculations to prevent disease recurrence in high‐risk breast cancer patients. Ann Oncol. 2014;25(9):1735‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mittendorf EA, Ardavanis A, Litton JK, et al. Primary analysis of a prospective, randomized, single‐blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget. 2016;7(40):66192‐66201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maslak PG, Dao T, Bernal Y, et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut‐S) in acute myeloid leukemia. Blood Adv. 2018;2(3):224‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Antonilli M, Rahimi H, Visconti V, et al. Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial. Int J Oncol. 2016;48(4):1369‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gerdemann U, Katari U, Christin AS, et al. Cytotoxic T lymphocytes simultaneously targeting multiple tumor‐associated antigens to treat EBV negative lymphoma. Mol Ther. 2011;19(12):2258‐2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daiko H, Marafioti T, Fujiwara T, et al. Exploratory open‐label clinical study to determine the S‐588410 cancer peptide vaccine‐induced tumor‐infiltrating lymphocytes and changes in the tumor microenvironment in esophageal cancer patients. Cancer Immunol Immunother. 2020;69(11):2247‐2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Obara W, Eto M, Mimata H, et al. A phase I/II study of cancer peptide vaccine S‐288310 in patients with advanced urothelial carcinoma of the bladder. Ann Oncol. 2017;28(4):798‐803. [DOI] [PubMed] [Google Scholar]
- 45. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oji Y, Hashimoto N, Tsuboi A, et al. Association of WT1 IgG antibody against WT1 peptide with prolonged survival in glioblastoma multiforme patients vaccinated with WT1 peptide. Int J Cancer. 2016;139(6):1391‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kjeldsen JW, Iversen TZ, Engell‐Noerregaard L, Mellemgaard A, Andersen MH, Svane IM. Durable clinical responses and long‐term follow‐up of stage III‐IV Non‐Small‐Cell Lung Cancer (NSCLC) patients treated with IDO peptide vaccine in a phase I study ‐ A brief research report. Front Immunol. 2018;9:2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murahashi M, Hijikata Y, Yamada K, et al. Phase I clinical trial of a five‐peptide cancer vaccine combined with cyclophosphamide in advanced solid tumors. Clin Immunol. 2016;166–167:48‐58. [DOI] [PubMed] [Google Scholar]
- 49. Camisaschi C, Renne SL, Beretta V, et al. Immune landscape and in vivo immunogenicity of NY‐ESO‐1 tumor antigen in advanced neuroblastoma patients. BMC Cancer. 2018;18(1):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kushner BH, Cheung IY, Modak S, Kramer K, Ragupathi G, Cheung NK. Phase I trial of a bivalent gangliosides vaccine in combination with beta‐glucan for high‐risk neuroblastoma in second or later remission. Clin Cancer Res. 2014;20(5):1375‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Krishnadas DK, Shusterman S, Bai F, et al. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE‐A1, MAGE‐A3 and NY‐ESO‐1 for children with relapsed or therapy‐refractory neuroblastoma and sarcoma. Cancer Immunol Immunother. 2015;64(10):1251‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rini BI, Stenzl A, Zdrojowy R, et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first‐line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open‐label, randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(11):1599‐1611. [DOI] [PubMed] [Google Scholar]
- 53. Fujiwara Y, Okada K, Omori T, et al. Multiple therapeutic peptide vaccines for patients with advanced gastric cancer. Int J Oncol. 2017;50(5):1655‐1662. [DOI] [PubMed] [Google Scholar]
- 54. Suzuki N, Hazama S, Iguchi H, et al. Phase II clinical trial of peptide cocktail therapy for patients with advanced pancreatic cancer: VENUS‐PC study. Cancer Sci. 2017;108(1):73‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sundar R, Rha SY, Yamaue H, et al. A phase I/Ib study of OTSGC‐A24 combined peptide vaccine in advanced gastric cancer. BMC Cancer. 2018;18(1):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tsuboi A, Hashimoto N, Fujiki F, et al. A phase I clinical study of a cocktail vaccine of Wilms' tumor 1 (WT1) HLA class I and II peptides for recurrent malignant glioma. Cancer Immunol Immunother. 2019;68(2):331‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hasegawa K, Ikeda Y, Kunugi Y, et al. Phase I study of multiple epitope peptide vaccination in patients with recurrent or persistent cervical cancer. J Immunother. 2018;41(4):201‐207. [DOI] [PubMed] [Google Scholar]
- 58. Obara W, Hara I, Kato Y, et al. Immunotherapy with cancer peptides in combination with intravesical bacillus Calmette‐Guerin for patients with non‐muscle invasive bladder cancer. Cancer Immunol Immun. 2018;67(9):1371‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kobayashi Y, Sakura T, Miyawaki S, Toga K, Sogo S, Heike Y. A new peptide vaccine OCV‐501: in vitro pharmacology and phase 1 study in patients with acute myeloid leukemia. Cancer Immunol Immunother. 2017;66(7):851‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rampling R, Peoples S, Mulholland PJ, et al. A cancer research UK first time in human phase I trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin Cancer Res. 2016;22(19):4776‐4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nishida S, Ishikawa T, Egawa S, et al. Combination gemcitabine and WT1 peptide vaccination improves progression‐free survival in advanced pancreatic ductal adenocarcinoma: a phase ii randomized study. Cancer Immunol Res. 2018;6(3):320‐331. [DOI] [PubMed] [Google Scholar]
- 62. Obara W, Sato F, Takeda K, et al. Phase I clinical trial of cell division associated 1 (CDCA1) peptide vaccination for castration resistant prostate cancer. Cancer Sci. 2017;108(7):1452‐1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hilf N, Kuttruff‐Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240‐245. [DOI] [PubMed] [Google Scholar]
- 64. Brown TA, Byrd K, Vreeland TJ, et al. Final analysis of a phase I/IIa trial of the folate‐binding protein‐derived E39 peptide vaccine to prevent recurrence in ovarian and endometrial cancer patients. Cancer Med. 2019;8(10):4678‐4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Narita Y, Arakawa Y, Yamasaki F, et al. A randomized, double‐blind, phase III trial of personalized peptide vaccination for recurrent glioblastoma. Neuro Oncol. 2019;21(3):348‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sawada A, Inoue M, Kondo O, et al. Feasibility of cancer immunotherapy with WT1 peptide vaccination for solid and hematological malignancies in children. Pediatr Blood Cancer. 2016;63(2):234‐241. [DOI] [PubMed] [Google Scholar]
- 67. Legat A, Maby‐El Hajjami H, Baumgaertner P, et al. Vaccination with LAG‐3Ig (IMP321) and peptides induces specific CD4 and CD8 T‐cell responses in metastatic melanoma patients‐report of a phase I/IIa clinical trial. Clin Cancer Res. 2016;22(6):1330‐1340. [DOI] [PubMed] [Google Scholar]
- 68. Boks MA, Bruijns SCM, Ambrosini M, et al. In situ delivery of tumor antigen‐ and adjuvant‐loaded liposomes boosts antigen‐specific T‐cell responses by human dermal dendritic cells. J Invest Dermatol. 2015;135(11):2697‐2704. [DOI] [PubMed] [Google Scholar]
- 69. Mittendorf EA, Ardavanis A, Symanowski J, et al. Primary analysis of a prospective, randomized, single‐blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann Oncol. 2016;27(7):1241‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Smyth K, Garcia K, Sun Z, Tuo W, Xiao Z. TLR agonists are highly effective at eliciting functional memory CTLs of effector memory phenotype in peptide immunization. Int Immunopharmacol. 2013;15(1):67‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kano Y, Iguchi T, Matsui H, et al. Combined adjuvants of poly(I:C) plus LAG‐3‐Ig improve antitumor effects of tumor‐specific T cells, preventing their exhaustion. Cancer Sci. 2016;107(4):398‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Melssen MM, Petroni GR, Chianese‐Bullock KA, et al. A multipeptide vaccine plus toll‐like receptor agonists LPS or polylCLC in combination with incomplete Freund's adjuvant in melanoma patients. J Immunother Cancer. 2019;7:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kozako T, Arima N, Yoshimitsu M, Honda SI, Soeda S. Liposomes and nanotechnology in drug development: focus on oncotargets. Int J Nanomedicine. 2012;7:4943‐4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rueda F, Eich C, Cordobilla B, et al. Effect of TLR ligands co‐encapsulated with multiepitopic antigen in nanoliposomes targeted to human DCs via Fc receptor for cancer vaccines. Immunobiology. 2017;222(11):989‐997. [DOI] [PubMed] [Google Scholar]
- 75. Arab A, Behravan J, Razazan A, et al. A nano‐liposome vaccine carrying E75, a HER‐2/neu‐derived peptide, exhibits significant antitumour activity in mice. J Drug Target. 2018;26(4):365‐372. [DOI] [PubMed] [Google Scholar]
- 76. Zhang R, Tang L, Tian Y, et al. Cholesterol‐modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials. 2020;241:119852. [DOI] [PubMed] [Google Scholar]
- 77. Wang Z, Liang C, Shang Y, He S, Wang L, Yang Z. Narrowing the diversification of supramolecular assemblies by preorganization. Chem Commun (Camb). 2018;54(22):2751‐2754. [DOI] [PubMed] [Google Scholar]
- 78. Luo ZC, Wu QJ, Yang CB, et al. A powerful CD8(+) T‐cell stimulating D‐Tetra‐peptide hydrogel as a very promising vaccine adjuvant. Adv Mater. 2017;29(5):1601776. [DOI] [PubMed] [Google Scholar]
- 79. Li X, Wang Y, Wang S, et al. A strong CD8(+) T cell‐stimulating supramolecular hydrogel. Nanoscale. 2020;12(3):2111‐2117. [DOI] [PubMed] [Google Scholar]
- 80. Le DT, Jaffee EM. Regulatory T‐cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72(14):3439‐3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hijikata Y, Okazaki T, Tanaka Y, et al. A phase I clinical trial of RNF43 peptide‐related immune cell therapy combined with low‐dose cyclophosphamide in patients with advanced solid tumors. PLoS One. 2018;13(1):e0187878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shirahama T, Muroya D, Matsueda S, et al. A randomized phase II trial of personalized peptide vaccine with low dose cyclophosphamide in biliary tract cancer. Cancer Sci. 2017;108(5):838‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yoshimura K, Minami T, Nozawa M, et al. A phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low‐dose dexamethasone versus dexamethasone alone in chemotherapy‐naive castration‐resistant prostate cancer. Eur Urol. 2016;70(1):35‐41. [DOI] [PubMed] [Google Scholar]
- 84. Noguchi M, Matsumoto K, Uemura H, et al. An open‐label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum‐based chemotherapy. Clin Cancer Res. 2016;22(1):54‐60. [DOI] [PubMed] [Google Scholar]
- 85. Shen J, Wang L‐F, Zou Z‐Y, et al. Phase I clinical study of personalized peptide vaccination combined with radiotherapy for advanced hepatocellular carcinoma. World J Gastroenterol. 2017;23(29):5395‐5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. Ann Surg Oncol. 2006;13(8):1085‐1098. [DOI] [PubMed] [Google Scholar]
- 87. Gall VA, Philips AV, Qiao NA, et al. Trastuzumab increases HER2 uptake and cross‐presentation by dendritic cells. Cancer Res. 2017;77(19):5374‐5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Clifton GT, Peace KM, Holmes JP, et al. Initial safety analysis of a randomized phase II trial of nelipepimut‐S+GM‐CSF and trastuzumab compared to trastuzumab alone to prevent recurrence in breast cancer patients with HER2 low‐expressing tumors. Clin Immunol. 2019;201:48‐54. [DOI] [PubMed] [Google Scholar]
- 89. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558‐562. [DOI] [PubMed] [Google Scholar]
- 90. Shindo Y, Hazama S, Suzuki N, et al. Predictive biomarkers for the efficacy of peptide vaccine treatment: based on the results of a phase II study on advanced pancreatic cancer. J Exp Clin Cancer Res. 2017;36(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nakajima M, Hazama S, Tamada K, et al. A phase I study of multi‐HLA‐binding peptides derived from heat shock protein 70/glypican‐3 and a novel combination adjuvant of hLAG‐3Ig and Poly‐ICLC for patients with metastatic gastrointestinal cancers: YNP01 trial. Cancer Immunol Immunother. 2020;69(8):1651‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tsuchiya N, Hosono A, Yoshikawa T, et al. Phase I study of glypican‐3‐derived peptide vaccine therapy for patients with refractory pediatric solid tumors. Oncoimmunology. 2017;7(1):e1377872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lawson DH, Lee S, Zhao F, et al. Randomized, placebo‐controlled, phase III trial of yeast‐derived Granulocyte‐Macrophage Colony‐Stimulating Factor (GM‐CSF) versus peptide vaccination versus GM‐CSF plus peptide vaccination versus placebo in patients with no evidence of disease after complete surgical resection of locally advanced and/or stage iv melanoma: a trial of the Eastern cooperative oncology group‐American college of radiology imaging network cancer research group (E4697). J Clin Oncol. 2015;33(34):4066‐4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jackson DO, Trappey FA, Clifton GT, et al. Effects of HLA status and HER2 status on outcomes in breast cancer patients at risk for recurrence ‐ Implications for vaccine trial design. Clin Immunol. 2018;195:28‐35. [DOI] [PubMed] [Google Scholar]
- 95. Rausch S, Gouttefangeas C, Hennenlotter J, et al. Results of a phase 1/2 study in metastatic renal cell carcinoma patients treated with a patient‐specific adjuvant multi‐peptide vaccine after resection of metastases. Eur Urol Focus. 2019;5(4):604‐607. [DOI] [PubMed] [Google Scholar]
- 96. Obara W, Karashima T, Takeda K, et al. Effective induction of cytotoxic T cells recognizing an epitope peptide derived from hypoxia‐inducible protein 2 (HIG2) in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2017;66(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ciesielski MJ, Ahluwalia MS, Munich SA, et al. Antitumor cytotoxic T‐cell response induced by a survivin peptide mimic. Cancer Immunol Immunother. 2010;59(8):1211‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kawamura J, Sugiura F, Sukegawa Y, et al. Cytotoxic T lymphocyte response to peptide vaccination predicts survival in stage III colorectal cancer. Cancer Sci. 2018;109(5):1545‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kanekiyo S, Hazama S, Takenouchi H, et al. IgG response to MHC class I epitope peptides is a quantitative predictive biomarker in the early course of treatment of colorectal cancer using therapeutic peptides. Oncol Rep. 2018;39(5):2385‐2392. [DOI] [PubMed] [Google Scholar]
- 100. Shimizu Y, Yoshikawa T, Kojima T, et al. Heat shock protein 105 peptide vaccine could induce antitumor immune reactions in a phase I clinical trial. Cancer Sci. 2019;110(10):3049‐3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Takayama K, Sugawara S, Saijo Y, et al. Randomized phase II study of docetaxel plus personalized peptide vaccination versus docetaxel plus placebo for patients with previously treated advanced wild type EGFR non‐small‐cell lung cancer. J Immunol Res. 2016;2016:1745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Miyazawa M, Katsuda M, Maguchi H, et al. Phase II clinical trial using novel peptide cocktail vaccine as a postoperative adjuvant treatment for surgically resected pancreatic cancer patients. Int J Cancer. 2017;140(4):973‐982. [DOI] [PubMed] [Google Scholar]
- 103. Correale P, Botta C, Martino EC, et al. Phase Ib study of poly‐epitope peptide vaccination to thymidylate synthase (TSPP) and GOLFIG chemo‐immunotherapy for treatment of metastatic colorectal cancer patients. Oncoimmunology. 2016;5(4):e1101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


