Abstract
Objectives:
Measurement of hepatic vein pressures is the accepted gold standard for the evaluation of portal hypertension. This study was conducted to evaluate the correlation between hepatic vein pressure measurements and histologic findings from transjugular liver biopsies. The hypothesis was that higher hepatic venous pressure gradients would correlate with a histologic diagnosis of cirrhosis.
Material and Methods:
We identified all patients who underwent transjugular liver biopsies at our institution between January 2015 and December 2019. Of these, 178 patients who had undergone hemodynamic evaluations during the biopsy procedure were included in the study. Demographic information and laboratory data were extracted from the patients’ electronic medical records. The hepatic vein pressure gradient (HVPG) was determined by subtracting the free hepatic venous pressure from the wedged hepatic venous pressure (WHVP), and the portosystemic gradient (PSG) was determined by subtracting the right atrial pressure from the WHVP. HVPG and PSG were compared by linear regression analysis and by calculating their receiver operating characteristics (ROC).
Results:
HVPG and PSG measurements were significantly associated with cirrhosis, with area under the ROC curve of 0.79 and 0.78, respectively. At the optimal cutoff of 9 mmHg, sensitivity and specificity for HVPG were 71% and 83% for HVPG and 67 % and 81% for PSG, respectively. No statistical difference was observed between the two measurements.
Conclusion:
A transhepatic venous pressure gradient above a cutoff of 9 mmHg is predictive of histologic cirrhosis, regardless of whether it is expressed as HVPG or PSG, with acceptable to excellent performance characteristics.
Keywords: Transjugular liver biopsy, Hepatic vein pressure gradient, Portosystemic gradient, Cirrhosis, Positive predictive value, Sensitivity, Specificity
INTRODUCTION
The measurement of hepatic venous pressures and the calculation of the hepatic vein pressure gradient (HVPG) represent the gold standard for the evaluation of portal hypertension. The HVPG is defined as the pressure difference between the wedge hepatic vein pressure (WHVP) and the free hepatic vein pressure (FVHP).[1,2] According to hemodynamic parameters, patients with liver disease are categorized as having subclinical or clinically significant portal hypertension (CSPH).[2] The procedure is invasive and requires technical expertise to achieve reliable results.[2] Close attention to detail during the hemodynamic procedure is imperative as the specific technique used may influence the results and subsequently patient’s management and prognosis.[3-6] Despite its invasive nature, hemodynamic evaluation of hepatic venous pressures is considered safe, with minimal reported complications.[2]
CSPH is defined as a HVPG >10 mmHg.[1,2] Patients with CSPH are at high risk of developing clinical complications including variceal bleeding, ascites, and encephalopathy.[1,7,8] Some patients with CSPH remain unidentified because they are asymptomatic while other patients with CSPH who are assumed to be cirrhotic do in fact have non-cirrhotic portal hypertension.[1,9] These patients may require a liver biopsy to confirm the diagnosis of cirrhosis.[2]
Transjugular liver biopsy (TJLB) is an established method to obtain liver tissue with minimal complication risks.[10-13] The technique was initially used for patients at risk of a complication from a percutaneous biopsy, such as patients with thrombocytopenia, ascites, or elevated INR.[10,11] Technological advances have resulted in the development of reliable and safe biopsy systems.[12,14] Along with the development of aggressive protocols to manage complications of portal hypertension,[2] TJLB is now often used in conjunction with hemodynamic evaluation in the work-up of patients with abnormal liver function and suspected portal hypertension.[11,14]
The main purpose of this study was to determine the predictive value of hemodynamic measurements for the diagnosis of cirrhosis. The hypothesis was that hepatic venous pressure gradients higher than 10 mmHg would be associated with the diagnosis of cirrhosis. A secondary goal was to describe the procedural technical features as performed at our center, a tertiary care, community hospital system.[15,16]
MATERIAL AND METHODS
Patient population and clinical data
This retrospective study was approved by the Institutional Review Board (IRB). The medical and angiographic records of patients who underwent a TJLB with hemodynamic evaluation between January 1, 2015, and December 31, 2019, were reviewed. At our center, TJLBs are performed by the interventional radiology service. A search of our institution’s picture archiving and communication system under “SP Transvascular Biopsy” was used to retrieve the information. A total of 179 patients (99 women and 80 men) with a mean age of 60.9 (26–86) years were identified. One patient was excluded from analysis due to incomplete data. Three patients with potentially resectable liver tumors solely underwent hemodynamic measurements. They were included in the analysis since the absence of cirrhosis was established by normal laboratory and imaging data. The final cohort included 178 patients. Demographic data and indications are summarized in Tables 1 and 2.
Table 1:
Patient characteristics.
| Variable | Average | SD | Range |
|---|---|---|---|
| Female, n | 80 | - | 45% |
| Male, n | 99 | - | 55% |
| Age, years | 60.9 | 12.8 | 26–86 |
| TBili | 3.0 | 6.0 | 0.2–38.7 |
| AlkPhos | 176 | 157 | 36–1.200 |
| SGOT | 138 | 320 | 15–2.843 |
| SGPT | 134 | 336 | 7–3.524 |
| PltCt (in 1000 s) | 176 | 84 | 13–400 |
| INR | 1.2 | 0.4 | 0.8–3.7 |
Table 2:
Indications for transjugular liver biopsy and portal pressure measurement.
| Indication | n | % |
|---|---|---|
| Total | 178 | 100.0 |
| Abnormal liver function tests | 28 | 15.7 |
| History of alcohol use | 20 | 11.2 |
| Autoimmune disorder | 18 | 10.1 |
| Hepatitis C | 15 | 8.4 |
| History of cancer | 13 | 7.3 |
| Ascites and abnormal liver function | 11 | 6.1 |
| Pre-surgical evaluation | 10 | 5.6 |
| Heart failure and abnormal liver function | 10 | 5.6 |
| Fatty liver and abnormal liver function | 10 | 5.6 |
| Morbid obesity and abnormal liver function | 8 | 4.4 |
| Previous drug exposure | 6 | 3.7 |
| Hepatitis B | 5 | 2.8 |
| History of cirrhosis | 3 | 1.7 |
| Other indications | 21 | 11.8 |
The following data were retrieved: Procedure indication, results of liver function tests closest to the date of the procedure, recorded results of the hemodynamic evaluation (pressure measurements in mmHg), technique for pressure measurement (wedged catheter or occlusion balloon), type of needle used for biopsy, number of liver samples obtained per procedure, histologic diagnosis as recorded in the pathology report, size of the liver samples as measured by the pathologist, fluoroscopy time, and cumulative radiation dose. The medical records were reviewed for up to 30 days after the procedure to identify procedural complications.
Procedural techniques
Six fellowship-trained interventional radiologists performed the procedures, using previously described methods.[2,11,14] Minor interindividual variations in technique or instrumentation were dictated by operator preference. Transvascular pressures were measured using NAMIC PERCEPTOR Manifold transducers (Navilyst Medical, Marlborough, MA) connected to Philips IntelliVue X2 monitors. The pressure transducer was calibrated to 0 mmHg at the mid-right atrial (RA) level as described.[2] Permanent tracing recording of pressures is available but not routinely charted at our center. Standard measurements included inferior vena cava and RA pressures, free hepatic venous pressure (FHVP), and wedged hepatic venous pressure (WHVP). Pressure gradients were calculated according to previously established literature.[2] The HVPG was calculated by subtracting the FHVP from the WHVP. The portosystemic gradient (PSG) was calculated as the difference between the WHVP and the right atrial pressure (RAP). The use of wedge catheter technique versus balloon occlusion technique was annotated. The hemodynamic findings were classified as: Normal (HVPG = 0–5 mmHg), “subclinical” portal hypertension (HVPG = 6–9 mmHg), and “clinically significant” portal hypertension (HVPG = 10 mmHg or higher).[1,2]
Biopsy samples were sent to the department of pathology following a standard protocol.[14] Pathologists with experience in liver diseases reviewed the samples, and patients were classified as cirrhotic or non-cirrhotic based on standard histologic criteria.
Statistical analysis
Categorical variables were compared using Chi-square tests. Continuous variables were compared using t-tests. A linear regression analysis was used to compare HVPG and PSG. The sensitivity and specificity of HVPG or PSG in predicting the presence of liver cirrhosis were analyzed by plotting the receiver operating characteristics (ROC) and by calculating the area under the ROC curve (AUROC) with its 95% confidence intervals. Using the ROC data, the Youden index was calculated to determine the optimum cutoff values of HVPG and PSG for the prediction of cirrhosis. The joint influence of multiple predictor variables on the occurrence of liver cirrhosis (as outcome variable) was tested, using multivariable logistic regression analyses. The list of potential predictor variables included demographic characteristics, as well as laboratory parameters, such as pressure gradients, liver function tests, and platelet counts.
RESULTS
Technical and procedural features
A 19-gauge Argon t-lab needle (Argon Medical Devices, Frisco, TX) was used in 168 patients (95.4%), and an 18- or 19-gauge Quick-Core Cook needle (Cook, Bloomington, IN) was used in eight cases. The average number of tissue samples obtained per procedure was 3.6 (range: 2–8). The mean length of the samples was 1.5 cm (0.6–2.3 cm).
The WHVP was obtained using an occlusion balloon in 105/178 (58.9%) patients, the wedged catheter technique was used in the remaining cases. The mean fluoroscopy time was 6.5 (range: 2.4–29.6) min, and the mean radiation dose (air kerma) was 280.02 (1.2–3657) mGy.
Procedural complications were reported in 2 patients (1.1%). One patient required overnight hospital admission for the abdominal pain after biopsy. The patient was treated conservatively, and no bleeding was documented. Another patient had a neck hematoma that did not require treatment.
Group comparisons
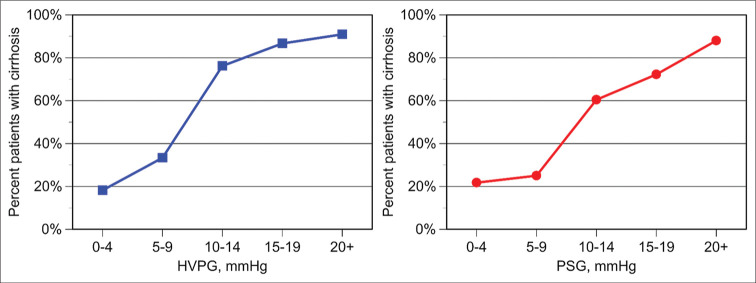
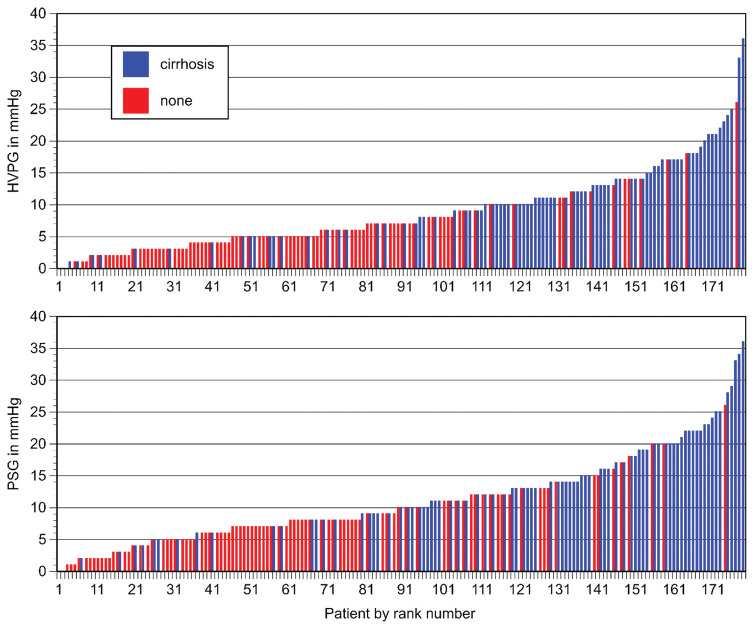
Table 3 shows the population stratified by demographic characteristics and laboratory parameters. Patients with and without cirrhosis were similar in gender and age distribution. Serum bilirubin, alkaline phosphatase, and INR levels were also similar. Liver enzymes and platelet counts were significantly lower in cirrhotic as compared to non-cirrhotic patients. HVPG and PSG values were significantly higher in patients with cirrhosis. Table 4 shows the stratification of the patient population into five pressure gradient categories. An association between the transhepatic pressure gradient and the presence of liver cirrhosis was noticed. This association was statistically highly significant for both HVPG and PSG (P < 0.0001). Figure 1 shows the distribution of pressure gradients in individual patients with or without cirrhosis. At gradients of 10 mmHg or higher, the majority of patients were cirrhotic, regardless of whether the gradient was expressed as HVPG or PSG.
Table 3:
Study population stratified by the presence of liver cirrhosis and laboratory parameters.
| Parameter | With Cirrhosis | Without cirrhosis | P-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Total, n | 85 | 100% | 93 | 100% | |
| Female, n | 44 | 52% | 54 | 58% | ns |
| Male, n | 41 | 48% | 39 | 42% | |
| Age, years | 61 | 12 | 61 | 13 | ns |
| TBili | 3.2 | 6.7 | 2.9 | 5.3 | ns |
| AlkPhos | 181 | 149 | 172 | 165 | ns |
| SGOT | 70 | 53 | 201 | 430 | <0.005 |
| SGPT | 42 | 28 | 217 | 448 | <0.001 |
| PltCnt | 153 | 80 | 189 | 84 | <0.005 |
| INR | 1.21 | 0.22 | 1.22 | 0.49 | ns |
| HVPG, mmHg | 11.8 | 6.7 | 6.0 | 4.1 | <0.001 |
| PSG, mmHg | 14.5 | 7.3 | 8.0 | 4.6 | <0.001 |
Table 4:
Pressure gradients in patient with and without cirrhosis.
| Pressure gradient | HVPG, mmHg | PSG, mmHg | ||||
|---|---|---|---|---|---|---|
| Cirrhosis | None | Total | Cirrhosis | None | Total | |
| 0–4 mmHg | 8 | 36 | 44 | 5 | 18 | 23 |
| 5–9 mmHg | 22 | 44 | 66 | 16 | 48 | 64 |
| 10–14 mmHg | 32 | 10 | 42 | 29 | 19 | 48 |
| 15–19 mmHg | 13 | 2 | 15 | 13 | 5 | 18 |
| 20+ mmHg | 10 | 1 | 11 | 22 | 3 | 25 |
| Total | 85 | 93 | 178 | 85 | 93 | 178 |
For HVPG, Chi-square=51.85, df=4, P<0.0001; for PSG, Chi-square=43.15, df=4, P<0.0001. HVPG: Hepatic vein pressure gradient; PSG: Portosystemic gradient.
Figure 1:
Relationship between pressure gradient and the frequency of cirrhosis on biopsy. Left: Hepatic venous pressure gradient (HVPG). Right: Portosystemic gradient (PSG).
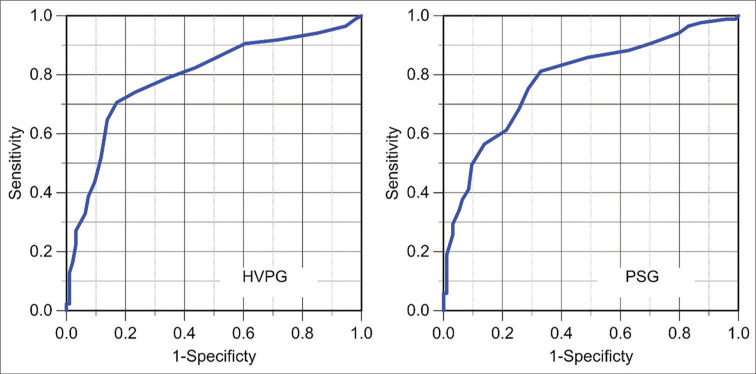
Figure 2 shows the correlation between pressure gradients and histologic findings and Figure 3 shows the ROC curves for HVPG and PSG. Both curves displayed a similar behavior. The AUROC with 95% confidence intervals was 0.791 (0.724–0.858) for HVPG and 0.78 (0.718–0.853) for PSG. The Youden index suggests that a gradient of 9 mmHg represents the best cutoff point to determine the presence of cirrhosis. The likelihood of having a biopsy positive for cirrhosis was unlikely with a gradient lower than 9 mmHg. Using 9 mmHg as the cutoff pressure gradient, the sensitivity and specificity for the diagnosis of cirrhosis for HVPG were 71% and 83%. The sensitivity and specificity for the PSG were 67% and 81%. The difference was not significant. Figure 4 shows the linear regression analysis between HVPG and PSG. The two gradients were closely correlated (R = 0.90, P < 0.0001).
Figure 2:
Relationship between pressure gradient and the presence of cirrhosis on biopsy in individual patients. Top: Hepatic venous pressure gradient (HVPG). Bottom: Portosystemic gradient (PSG).
Figure 3:
Receiver operating characteristics of hepatic venous pressure gradient (HVPG, left) and portosystemic gradient (PSG, right) in predicting the presence of cirrhosis on liver biopsy.
Figure 4:
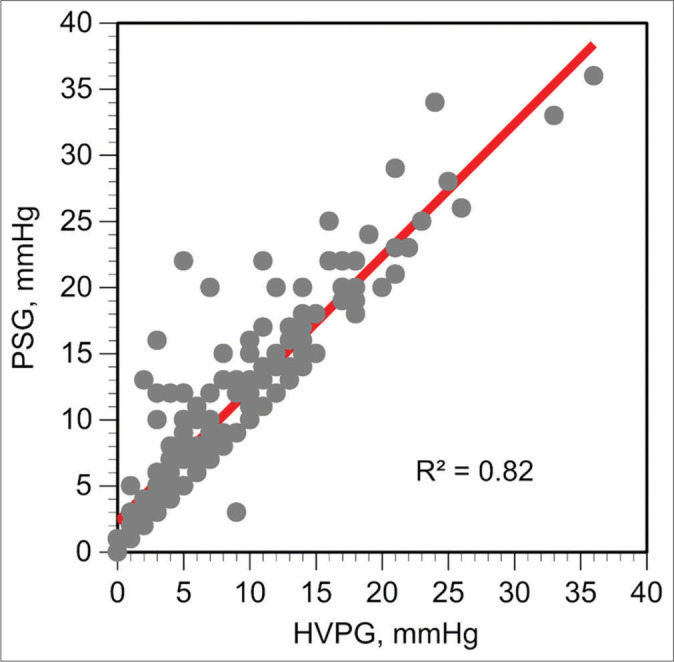
Linear regression analysis between hepatic venous pressure gradient and portosystemic gradient.
In a multivariate regression analysis, the HVPG was a significant predictor of cirrhosis, with an R2 = 0.19. Lower SGOT and SGPT levels acted as additional and independent statistically significant variables in predicting the presence of cirrhosis [Table 3]. A similar result was also observed using HAG instead of HVG as the primary predictor variable (data not shown). The inclusion of SGOT and SGPT levels improved the model with an R2 = 0.38 [Table 3]. The addition of any other of the variables did not improve the model.
DISCUSSION
The results of the present study showed significant correlation between the magnitude of the pressure gradient and the presence of cirrhosis. The AUROCs of both the HVPG and PSG and their 95% confidence limits were 0.791 (0.724– 0.858) and 0.785 (0.718–0.853), respectively. The calculation of the Youden index identified a pressure gradient of 9 mmHg as the cutoff with the optimum performance characteristics. Its application resulted in a sensitivity of 71% and specificity of 83% for the HVG, and a sensitivity of 67% and a specificity of 81% for the PSG. Using the traditional academic point system, the performance of both measurements was in the acceptable (0.70–0.80) to excellent (0.80–0.90) range.[17,18]
The results of the present study agree with previous reported data showing a significant association of HVPG with Stage IV fibrosis or cirrhosis on histologic examination.[9,19,20] In this regard, our findings confirm the validity of the method and demonstrate its applicability in a community-based interventional radiology practice. Vincent et al. found a sensitivity of 71.3% and a specificity of 79.6% for the diagnosis of advanced fibrosis in a group of 340 patients using a cutoff point of 6 mmHg.[19] Sourianarayanane et al. identified higher levels of fibrosis at lower HVPG (6 mmHg), specifically looking at patients with non-alcoholic steatohepatitis.[20] Rodrigues et al. reported a specificity of 92% for the diagnosis of cirrhosis using a HVPG cutoff of 12 mmHg.[9]
The significant association of lower SGOT and SGPT values with the presence of cirrhosis is likely related to our patient selection. In our clinical practice, transjugular liver biopsies were frequently performed to determine the disease etiology in patients with acute hepatitis – this group of patients typically presents with marked liver enzyme elevations and no advanced fibrosis on biopsy. A second subgroup includes patients with suspected or likely cirrhosis – characterized by less inflammation but advanced fibrosis.
In spite of their sensitivity and specificity, hemodynamic measurements alone should not be used to differentiate between cirrhotic and non-cirrhotic portal hypertension. Along these lines, we observed that 15/178 (8.4%) of patients had histologic cirrhosis but pressure gradients below the cutoff of 9 mmHg, likely due to the presence of spontaneous portosystemic collaterals. Hemodynamic measurements lend themselves to be combined with hepatic tissue sampling. Because of the random nature of tissue sampling, liver biopsies occasionally understage or mis-stage the degree of fibrosis. Based on the results of our analysis, we would suggest that pressure measurements could serve as a helpful adjunct in cases with a questionable outcome of liver biopsy.
Comparison of HVPG and PSG revealed no significant difference between the two reference standards with regard to the prediction of cirrhosis. This was reflected by the corresponding AUROCs and by the statistically significant correlation between the two measurements in our patient population. There has been a longstanding debate in the field with regard to the optimum reference measurement,[3] with most authors favoring the HVPG as the accepted standard.[2,5] Other authors have argued for the superiority of the RAP. Rossle et al. found that FVHP could be inaccurate secondary to abnormal vein anatomy caused by cirrhosis.[3] According to these authors, measurement of pressures in the hepatic veins could result in a falsely elevated reference pressure leading to underestimation of the pressure gradient.[3] Since we routinely measure both reference pressures, we were able to perform a direct comparison. While our study was not designed to specifically address this question, our data suggest that both calculations are equivalent in the clinical practice setting. We conclude that both approaches are valid.
Our results showed high technical success rates for TJLB (99.4%) and hemodynamic measurements (99.4%). Furthermore, 98.2% of the biopsies were diagnostic, indicative of optimal tissue samples. The average number of biopsy passes per procedure was 3.6 and the mean total sample length was 15 mm. These results compare favorably with previously published guidelines and case series.[10,12,14,21] Importantly, we documented only two minor complications none of which required major intervention.
The mean fluoroscopy time was 6.5 min with a range between 2.4 and 29.6 min. The mean cumulative fluoroscopy dose delivered during the procedure was 280.2 mgy. TJLB is not classified as a lengthy procedure in regard to total fluoroscopy time,[22] but the findings in the present study reflect that this procedure may be technically difficult, resulting in prolonged fluoroscopy times and higher radiation doses. The main technical difficulty of this procedure is catheterization of a hepatic vein suitable for pressure measurements and execution of the biopsy. The dose to the operator was not calculated in our study but the use of additional protective gear has proven to be useful.[15]
Potential limitations of our study relate its retrospective design and the inevitable interoperator variability in some technical aspects of pressure measurements and biopsies. For example, the use of an occlusion balloon has been recommended as the most accurate modality to determine the WHVP.[5,6,23] This approach was used in 59% of patients. Most procedures were performed with the Argon needle, reflecting physician consensus in favor of one needle system.[14] Optimization of these technical factors would require standardized, prospective studies.
CONCLUSION
Our retrospective study in a cohort of 178 patients demonstrates that the presence of a transhepatic venous pressure gradient above a cutoff of 9 mmHg is predictive of histological cirrhosis. Transjugular hemodynamic measurements and biopsies were safe and resulted in high-quality samples.
Footnotes
How to cite this article: Ferral H, Fimmel CJ, Sonnenberg A, Alonzo MJ, Aquisto TM. Transjugular liver biopsy with hemodynamic evaluation: Correlation between hepatic venous pressure gradient and histologic diagnosis of cirrhosis. J Clin Imaging Sci 2021;11:25.
Declaration of patient consent
The informed consent requirement was waived by the Institutional Review Board since the study was retrospective only, and since all patient information was deidentified.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Magaz M, Baiges A, Hernandez-Gea V. Precision medicine in variceal bleeding: Are we there yet? J Hepatol. 2020;72:774–84. doi: 10.1016/j.jhep.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7:141–55. doi: 10.1586/egh.12.83. [DOI] [PubMed] [Google Scholar]
- 3.Rossle M, Blanke P, Fritz B, Schultheiss M, Bettinger D. Free hepatic vein pressure is not useful to calculate the portal pressure gradient in cirrhosis: A morphologic and hemodynamic study. J Vasc Interv Radiol. 2016;27:1130–7. doi: 10.1016/j.jvir.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 4.La Mura V, Abraldes JG, Berzigotti A, Erice E, Flores-Arroyo A, Garcia-Pagan JC, et al. Right atrial pressure is not adequate to calculate portal pressure gradient in cirrhosis: A clinical-hemodynamic correlation study. Hepatology. 2010;51:2108–16. doi: 10.1002/hep.23612. [DOI] [PubMed] [Google Scholar]
- 5.Bosch J, Garcia-Pagan JC. Calculating hepatic venous pressure gradient: Feel free to stay free. J Vasc Interv Radiol. 2016;27:1138–9. doi: 10.1016/j.jvir.2016.03.048. [DOI] [PubMed] [Google Scholar]
- 6.Smith TP, Kim CY, Smith AD, Janas G, Miller MJ, Sopko DR, et al. Hepatic venous pressure measurements: Comparison of end-hole and balloon catheter methods. J Vasc Interv Radiol. 2012;23:219–26.e6. doi: 10.1016/j.jvir.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 7.La Mura V, Garcia-Guix M, Berzigotti A, Abraldes JG, Garcia-Pagan JC, Villanueva C, et al. A prognostic strategy based on stage of cirrhosis and HVPG to improve risk stratification after variceal bleeding. Hepatology. 2020;72:1353–65. doi: 10.1002/hep.31125. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, et al. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis. Hepatol Int. 2018;12(Suppl 1):34–43. doi: 10.1007/s12072-017-9808-z. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues SG, Montani M, Guixe-Muntet S, de Gottardi A, Berzigotti A, Bosch J. Patients with signs of advanced liver disease and clinically significant portal hypertension do not necessarily have cirrhosis. Clin Gastroenterol Hepatol. 2019;17:2101–9.e1. doi: 10.1016/j.cgh.2018.12.038. [DOI] [PubMed] [Google Scholar]
- 10.Sue MJ, Lee EW, Saab S, McWilliams JP, Durazo F, ElKabany M, et al. Transjugular liver biopsy: Safe even in patients with severe coagulopathies and multiple biopsies. Clin Transl Gastroenterol. 2019;10:e00063. doi: 10.14309/ctg.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrens G, Ferral H. Transjugular liver biopsy. Semin Intervent Radiol. 2012;29:111–7. doi: 10.1055/s-0032-1312572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorriz E, Reyes R, Lobrano MB, Pulido-Duque JM, San Roman JL, Lonjedo E, et al. Transjugular liver biopsy: A review of 77 biopsies using a spring-propelled cutting needle (biopsy gun) Cardiovasc Intervent Radiol. 1996;19:442–5. doi: 10.1007/BF02577636. [DOI] [PubMed] [Google Scholar]
- 13.Dohan A, Guerrache Y, Dautry R, Boudiaf M, Ledref O, Sirol M, et al. Major complications due to transjugular liver biopsy: Incidence, management and outcome. Diagn Interv Imaging. 2015;96:571–7. doi: 10.1016/j.diii.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Behrens G, Ferral H, Giusto D, Patel J, van Thiel DH. Transjugular liver biopsy: Comparison of sample adequacy with the use of two automated needle systems. J Vasc Interv Radiol. 2011;22:341–5. doi: 10.1016/j.jvir.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Kohlbrenner R, Lehrman ED, Taylor AG, Kohi MP, Fidelman N, Kumar V, et al. Operator dose reduction during transjugular liver biopsy using a radiation-attenuating drape: A prospective, randomized study. J Vasc Interv Radiol. 2018;29:1248–53. doi: 10.1016/j.jvir.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Miller DL, Vano E, Bartal G, Balter S, Dixon R, Padovani R, et al. Occupational radiation protection in interventional radiology: A joint guideline of the cardiovascular and interventional radiology society of Europe and the society of interventional radiology. J Vasc Interv Radiol. 2010;21:607–15. doi: 10.1016/j.jvir.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–6. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 18.Mandrekar JN. Simple statistical measures for diagnostic accuracy assessment. J Thorac Oncol. 2010;5:763–4. doi: 10.1097/JTO.0b013e3181dab122. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JK, Stark C, Shields JT, Bhave AD, Morris CS. Hepatic venous pressure gradient correlates with advanced hepatic fibrosis: A retrospective review. Abdom Radiol (NY) 2017;42:2609–14. doi: 10.1007/s00261-017-1171-y. [DOI] [PubMed] [Google Scholar]
- 20.Sourianarayanane A, Talluri J, Humar A, McCullough AJ. Stage of fibrosis and portal pressure correlation in nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2017;29:516–23. doi: 10.1097/MEG.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 21.Khalifa A, Rockey DC. The utility of liver biopsy in 2020. Curr Opin Gastroenterol. 2020;36:184–91. doi: 10.1097/MOG.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller DL, Balter S, Cole PE, Lu HT, Schueler BA, Geisinger M, et al. Radiation doses in interventional radiology procedures: The RAD-IR study: Part I: Overall measures of dose. J Vasc Interv Radiol. 2003;14:711–27. doi: 10.1097/01.RVI.0000079980.80153.4B. [DOI] [PubMed] [Google Scholar]
- 23.de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI consensus workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]