Abstract
Lymphoproliferative disorders are one of the most frequent hematological malignancies affecting the blood and lymphatic system. To better stratify patients, an accurate imaging evaluation is needed. Although computed tomography and positron emission tomography are considered the standard methods, these procedures have several clinical drawbacks, such as biological risk and high costs. Ultrasound (US) is a rapid and user-friendly method to evaluate lymph node (LN) and organ enlargements. US imaging provides more sensitive information about LN structure, vascularization, and metabolism and new techniques have increased its specificity, especially in malignant setting. However, validated and standardized criteria for its use are missing, with only several single-center experiences reported. Therefore, the aim of this paper is to review and briefly illustrate the status of the US knowledge and applications in lymphoproliferative workup, particularly concerning malignant LN pathology.
Keywords: Malignant lymphoproliferative disorders, Ultrasound, Lymphoma, Lymph node, Lymphoproliferative imaging
INTRODUCTION
Lymphoproliferative disorders are heterogeneous diseases characterized by uncontrolled lymphocyte proliferation, accumulating in the blood flow and bone marrow, and determining lymphadenopathies or another organ enlargement. To obtain an accurate diagnosis and staging (which will determine treatment strategy) and ensure a correct follow-up, informative clinical, laboratory, and imaging data are needed.
Although computed tomography (CT) and positron emission tomography (PET) are considered the standard methods for lymphoproliferative disease assessment and follow-up,[1,2] these procedures have several clinical drawbacks, such as biological risk and high costs.
Ultrasound (US) is a rapid, easily available, and user-friendly method to evaluate lymph node (LN) enlargement, as well as LN structure, vascularization, and metabolism, with new techniques increasing its sensitivity and specificity, especially in malignant LN disease. Conventional US diagnostic criteria combine classical bidimensional gray scale (B-mode) and color Doppler imaging (CDI), with more modern US techniques such as elastography and contrast-enhanced US (CEUS) to provide further dynamic information. Its increasing accuracy and user-friendliness have made US one of the most widely employed imaging techniques in clinical practice. In diagnostic workup, US LN characteristics assessments lead clinician in deciding if biopsy or other imaging tools are required to exclude malignancy influencing which node should be biopsied.
However, validated and standardized criteria for its use are missing, with only several single-center experiences reported. Therefore, the aim of this paper is to review and briefly illustrate the status of the US knowledge and the US applications in malignant lymphoproliferative disease workup.
THE ROLE OF US IN DIFFERENT MOMENTS OF LN DISEASE
US applications at diagnosis
In the past decade, many attempts have been made to reach a lymphoproliferative disease diagnosis with less invasive techniques but, despite the considerable advantages offered by radiological methods, the histological assessment is required.[1,2] With the aim of improving diagnostic clinical workup, the US performance was studied.
Open surgical biopsy is considered the standard and some studies have tested the accuracy of US-guided core needle biopsies and have compared them to standard biopsies. In an Italian single-center report, US-guided core needle biopsies using a 21-gauge needle resulted in a high yield rate (diagnostic tissue obtained in 96% of the 55 patients examined and suitable for diagnosis in 87% of them) as well as 100% success rate in reaching a definitive diagnosis, which lead to appropriate therapy. This technique was successfully applied applied to obtain tissue from LN, but also liver, kidney, and spleen, and could therefore be considered for other uses outside of LN biopsies; and on the other hand, that it had similar results in both new diagnoses and cases of relapse or progression, suggesting an use during follow up as well as the initial diagnosis.[3] A following, larger single-center study confirmed these findings, reporting that patients had a good experience with the service, and minimal complications.[4-6]
A first study compared Doppler US-guided core needle biopsies (using a 16-gauge needle) with open surgical biopsies for the initial work-up of suspected lymphoma cases: The US-guided procedure resulted more sensitive in identifying malignancies, as well as having other advantages. More specifically, US-guided biopsies were associated with a shorter waiting time to undergo biopsy, fewer complications resulting from the procedure (e.g., less pain, numbness, or paresthesia; fewer wound infections; lower incidence of lymphorrhea; and smaller scars) and lower economic costs (with a 24-fold lower cost than surgical biopsies). In addition, power Doppler contributed to a more accurate selection of the biopsy site, reducing the risk of removing necrotic and/or reactive LN that would have been inadequate for diagnosis.[5] Finally, one last study compared traditional US with CDI and/or CEUS for core needle biopsy targeting of deep sited lesions. CEUS will be examined more in depth in the following sections, but this analysis showed that CDI and CEUS lead to a better sensitivity, with a higher proportion of LN diagnostic compared to conventional US imaging. The possibility to obtain a diagnostic amount of tissue avoiding large blood vessels, the possibility to have a precise spatial correlation in real time, and the cost-effectiveness according to preliminary analyses further represent advantages. In addition, blood flow, transverse axis of the lesion, and mean PET standardized uptake value represented predictor of successful diagnosis.[7]
These studies demonstrated that US-guided core needle biopsy could be a tool to obtain an effective, safe, fast, and low-cost routine biopsy for patients with suspected lymphoproliferative disease, avoiding the psychological and physical pain of an unnecessary surgical intervention. The current indications, which reserve this options only to those patients too fragile to undergo a surgical intervention or to document relapse,[1] should be revised to allow for a more widespread use. Further studies are needed to assess which is the best US modality to use, with CDI (and CEUS) possibly being better options than conventional US.
Prognostic role of US
Size and extension of lymphadenopathies are important factors in definition the prognosis and the appropriate treatment in lymphoma, with bulky disease and an advanced stage being recognized as poor prognostic factors.[1] In clinical practice, CT, PET, and clinical examination are considered the standard methods for mapping disease sites, estimating tumor burden at all phases.[1] High-resolution US with power Doppler has also been investigated to stage disease, thanks to its ability to accurately define the morphologic and vascular characteristics of LN and therefore precisely identify malignant ones.
About Hodgkin’s lymphoma, one study compared staging by CT and clinical examination with power Doppler US, with particular attention on their accuracy in identifying bulky disease and its value in predicting the response to treatment. The identification of bulky disease was somewhat discordant between clinical/CT examination and US examination, and power Doppler US was found to be the best predictor of no treatment failure, with only power Doppler US identified bulky disease and advanced stage identified as statistically significant prognostic factors in the multivariate analysis. Therefore, it would appear that a power Doppler examination could give useful prognostic information even beyond those offered by traditional imaging and other commonly evaluated risk factors, which, in turn, would result in a more accurate staging and better treatment decisions for patients.[8]
US application during follow-up
Treatments and outcomes of lymphoproliferative disorders have improved in recent decades, and therefore, newer, more sensitive approaches needed to evaluate response, verify response durability, and identify early disease recurrence. For interim and end of treatment assessment, PET-CT is recommended as a first choice, with CT scans used together with PET to complete the assessment in certain cases, or as the only method in tumors with low or variably glucose uptake.[1,2] Then, the follow-up program depends on the age of patients, on the disease phase (initial therapy or relapse/ progression), on staging at the time of diagnosis, on the type of treatment administered and the response obtained, and on whether they are participating in a clinical trial. In this setting, is usually recommended, due to the high false positive rates of PET imaging, to resort to imaging exams only when there are clinical indications.[1]
Therefore, US is still not included in this setting by official guidelines, and little is known about its usefulness in detecting relapse. However, a randomized study compared the use of US and chest radiography with the standard PETCT imaging during follow-up of patients with Hodgkin lymphoma who were in complete remission after first-line treatment but at high risk for relapse. In this setting, the two follow-up approaches showed comparable sensitivity, but the combination of US and chest radiography showed better specificity and positive predictive value, with fewer false-positive results (which meant fewer unnecessary invasive procedures); in addition, it resulted in a much lower exposure to radiation and economic costs. These results seem to indicate that in high-risk patients, the use of US for most sites and chest radiography for the deeper compartment of the mediastinum can be an appropriate method to detect early relapse and allow for prompt intervention.[9]
NOVEL US IMAGING MODALITIES
Newer US techniques are becoming more widespread in recent years, complimenting conventional B-mode US and CDI to provide more information: For instance, CEUS had better evaluate vascularity, and elastography measures relative tissue stiffness. Both techniques have been applied to lymphoma, and the available studies will be briefly discussed here.
CEUS has been investigated both as a diagnostic tool and as a way to evaluate treatment response. Intense homogeneous enhancement in CEUS was identified as a characteristic of benign LN, while perfusion defects were identified as characteristics of malignant LN – although these criteria lead to false-positive diagnoses in case of necrosis and false-negative ones in some cases of lymphoma. Nonetheless, when compared to traditional US, the addition of CEUS resulted in an increase in accuracy.[10] Another study focused on the differential diagnosis between head–and-neck lymphoma and cancerous LN, finding that the best sensitivity, specificity, and accuracy were reached when combining the data from CEUS images and CEUS dose parameters (more specifically, the peak intensity and the area under the curve). It confirmed homogeneous enhancement and centrifugal filling as characteristic of lymphomatous LN, while cancer metastases more commonly showed inhomogeneous centripetal enhancement and perfusion defects, and were the only lesions to display a ring enhancement, although this feature was not present in all cases.[11] These results were confirmed by studies focusing on the US characteristics of lymphomatous nodes only. One study compared the results of CEUS and PET-CT in the diagnostic work-up of lymphomas and found that the variations of CEUS dose parameters were correlated with PET-CT results. This study was in accordance with the previous ones in that the perfusion pattern most commonly seen was intense, homogeneous, and centrifugal and with a clear boundary.[12] Another study examined the CEUS characteristics of lymphomatous LN and compared the accuracy of CEUS, PET-CT, and contrast-enhanced CT, finding a comparable accuracy across the three methods. In this case, the patterns observed were characterized either by a rapid, well-distributed hyperactive enhancement (which was the pattern found in the two indolent lymphoma cases examined) or a rapid heterogeneous enhancement.[13]
About treatment response evaluation, a first study compared CEUS parameters before and after the first two cycles of R-CHOP in aggressive B-cell lymphoma. The variations in peak intensity and mean intensity after the two cycles were significantly associated with response and showed a positive correlation with PET-CT data; in addition, both imaging modalities were also found to be good predictors of overall survival and progression-free survival.[14] After this, a single-center report examined the conventional US as well as CEUS parameters of LN before and after the first three chemotherapy cycles. A significant difference was found both in the patients who responded and in those who did not when considering the maximum diameter of the LN (which decreased in the former and increased in the latter) and the peak systolic flow velocity as measured by power Doppler. CEUS imaging showed a rapid homogeneous enhancement, consistent with the previous report, and sometime, intensity curve features changed in a significant manner before and after treatment, to the point that it was the best predictor of the efficacy of treatment.[15]
In conclusion, CEUS shows promise both as a diagnostic tool and as a way to predict response to treatment (and adjust the treatment plan accordingly), but there are still far too little data to reach a proper characterization of CEUS enhancement patterns in lymphomatous LN, which are necessary to make a precise diagnosis.
Elastography is another imaging modality that has received a certain interest in lymphoma diagnosis, staging, and treatment response evaluation. A pilot study investigated its contribution to the differential diagnosis of superficial lymphadenopathies, finding that shear wave velocity was able to discriminate with good accuracy between benign and malignant lesions and, within the latter, between metastatic and lymphomatous involvement (with the velocity significantly higher in malignant compared to benign lesions and in metastatic compared to lymphomatous ones).[16] Then, two studies investigated elastography as a means to predict treatment response. In the first one, heavily pretreated Hodgkin lymphoma patients undergoing brentuximab vedotin treatment were examined before and after treatment: At the baseline scan, no statistically significant difference was found between the strain ratio and LN volume of patients who would later respond or not to treatment, while after treatment, there were differences in both parameters between the two groups.[17] The second study, already cited above, also evaluated the elasticity index before and after chemotherapy, again finding no significant difference between that of patients who responded to treatment and those who did not.[15] Therefore, elastography could be an interesting tool to include in the diagnostic workup of lymphadenopathies, while it may not be useful as a predictor for treatment response; however, there are far too few studies on the matter to reach a definitive conclusion.
DISCUSSION
The natural history of lymphoproliferative disorders has dramatically improved in recent decades, partly as a result of the increased imaging techniques accuracy. CT and PET examinations are considered the standard imaging modalities, but they have a number of clinical drawbacks. The biological exposition, the low sensitivity of CT in some cases, or the inability to use PET imaging in tumors without avid glucose uptake represent serious limits.[2] Furthermore, the high cost of these procedures is not a secondary problem.
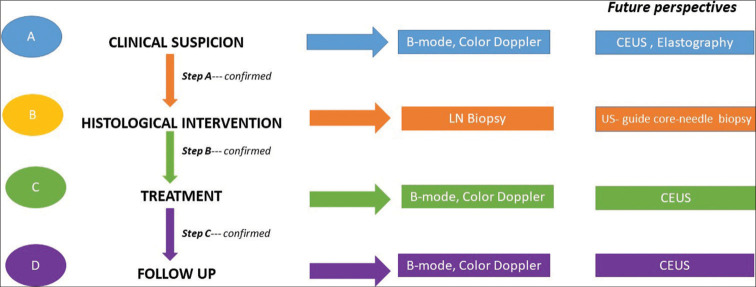
US is a fast, readily available, and easy-to-use method to evaluate LN [Figure 1]. New techniques are increasing its sensitivity and specificity, making US one of the most attractive and usable methods in all phases of LN study. The reports described have validated the applicability of this method at all stages, from initial diagnostic workup to staging, prognostic evaluation, and follow-up [Figure 2]. Despite these advantages, histological examination is still irreplaceable.
Figure 1:
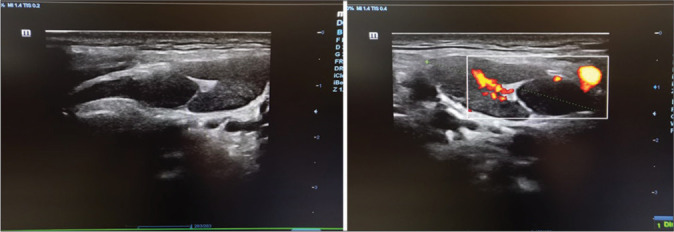
Examples of normal/reactive lymph nodes in B-mode and color Doppler studies.
Figure 2:
Proposed US-guided diagnostic workflow in clinical suspected lymphadenopathies. PET and CT scans are recommended according to the international guidelines. (1,2) Step A – When a pathological lymph node is identified at the clinical examination, a first level US evaluation with B-mode and color Doppler can be obtained. If pathologic suspicion is confirmed, additional information can be acquired from CEUS and elastographic study or patient should performed histological confirmation. The role of elastography is poorly defined in hematological setting. Step B – Having reached the pathological suspicion, histological examination must be performed. Step C – During treatment, disease LN responsiveness can be tracked. The role of CEUS is poorly defined. Step D – During follow-up, US allows monitoring of disease remission. The role of CEUS is not studied. If LN recurrence is suspected, CEUS could be recommended.
The use of US-guided core needle biopsies has received a certain interest, as it represents an opportunity to avoid surgical biopsies. A number of drawbacks have been described making not all patients clinical eligible. Core needle biopsies are not recommended by guidelines because it is feared that they might not provide enough adequate tissue,[1] but in most cases, reported sufficient tissue was collected for architectural, immunohistochemical, and molecular evaluation.[4,5] However, something which was stressed by all groups is that the expertise of the team is crucial in obtaining good results.[4-6] Furthermore, it seems that power Doppler and CEUS techniques can further improve the accuracy of the procedure, thanks to their ability to examine intranodal vessels.[5,7]
Indeed, the ability of power Doppler, and even more so of CEUS, to identify blood vessels has proved to be extremely promising in a variety of different settings. Indeed, blood flow is considered a marker of viable tissue and makes it possible to distinguish it from inflammation, necrosis, or fibrosis, which may all be found in the setting of lymphomatous disease. In addition, neoangiogenesis is recognized as a critical component of malignancies, since it enables their growth and invasion, and, therefore, can provide useful diagnostic, prognostic, and predictive indications.[18] In the first place, the study of LN vasculature is a reliable method for the differential diagnosis of lymphadenopathies, to distinguish between benign and malignant lesions and, among the latter, between tumor metastases and lymphomas.[10-13] Perfusion is distinctly different between benign and malignant lesions. Specific characteristics setting characterizes metastases and lymphoma. Metastases reach the edge of the node through peripheral lymph vessels and spread toward the center, creating a centripetal perfusion, and their growth is more rapid, resulting in immature vessels, which do not ensure sufficient oxygenation, which, in turn, leads to necrosis and perfusion defects. On the other hand, lymphomas spread from the center of the node to the periphery and develop hyperplastic vessels, leading to the characteristic homogeneous centrifugal enhancement with no perfusion defects.[11,19] Then, during the diagnostic workup, vasculature studies can provide indications on which LN to sample[5,7] and can be useful in staging and predicting outcome.[8] It has been found that angiogenesis is more prominent in more aggressive lymphoma subtypes and seems to correlate with more aggressive disease and poorer outcome.[18] Although these findings are still provisional, they would explain the correlation between CEUS and PET-CT findings[12,13] and their prognostic and predictive value: A higher vessel density, as identified by CEUS, would identify cells with a more intense metabolism and therefore a more rapid growth, which are characteristics of more aggressive lymphomas.[12] Finally, ad interim analyses during chemotherapy can provide further information on prognosis and response to treatment.[14,15] Blood flow to LN which are responding to treatment decreases, leading to a less intense signal; while in lymphomas which are resistant neoangiogenesis continues undisturbed, leading to a more intense signal.[15]
CONCLUSION
Ultrasound is a user-friendly method to evaluate malignant LN disease. Its increasing accuracy have made US one of the most widely employed imaging techniques in clinical practice.
However, there is still much to learn about the role of US techniques in lymphoproliferative diseases and some technical considerations are required. Indeed, US does not provide information about the mediastinum and there is a lack of information about its usefulness during follow-up, and about newer US techniques, such as elastography. In addition, newer techniques require more specific software/ equipment and expertise.
Therefore, further studies are needed to better define how US can be integrated into the diagnostic workup and long-term management of these disorders: In these settings, it could complement standard imaging modalities, offering an accurate, rapid, and cost-effective alternative that does not expose patients to radiation.
Footnotes
How to cite this article: Tavarozzi R, Manzato E, Lombardi A. Lymph Node Ultrasound in Lymphoproliferative Disorders: Where Are We Now? J Clin Imaging Sci 2021;11:22.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-hodgkin lymphoma: The lugano classification. J Clin Oncol. 2014;32:3059–67. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32:3048–58. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zinzani PL, Colecchia A, Festi D, Magagnoli M, Larocca A, Ascani S, et al. Ultrasound-guided core-needle biopsy is effective in the initial diagnosis of lymphoma patients. Haematologica. 1998;83:989–92. [PubMed] [Google Scholar]
- 4.Cohen OC, Brodermann MH, Dervin A, Raja N, Marafioti T, Otero S, et al. Lymph node core biopsies reliably permit diagnosis of lymphoproliferative diseases. Real-world experience from 554 sequential core biopsies from a single centre. Eur J Haematol. 2020;106:267–72. doi: 10.1111/ejh.13545. [DOI] [PubMed] [Google Scholar]
- 5.Pugliese N, di Perna M, Cozzolino I, Ciancia G, Pettinato G, Zeppa P, et al. Randomized comparison of power Doppler ultrasonography-guided core-needle biopsy with open surgical biopsy for the characterization of lymphadenopathies in patients with suspected lymphoma. Ann Hematol. 2017;96:627–37. doi: 10.1007/s00277-017-2926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon M, Yim C, Baek HJ, Lee JS, Seo JH, Kim JP, et al. Ultrasonography-guided core needle biopsy of cervical lymph nodes for diagnosing head and neck lymphoma compared with open surgical biopsy: Exploration for factors that shape diagnostic yield. Am J Otolaryngol. 2018;39:679–84. doi: 10.1016/j.amjoto.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Han J, Wang Y, Mo Y, Li J, Xiang J, et al. Core needle biopsy targeting the viable area of deep-sited dominant lesion verified by color doppler and/or contrast-enhanced ultrasound contribute to the actionable diagnosis of the patients suspicious of lymphoma. Front Oncol. 2020;10:500153. doi: 10.3389/fonc.2020.500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picardi M, Ciancia R, de Renzo A, Montante B, Ciancia G, Zeppa P, et al. Estimation of bulky lymph nodes by power Doppler ultrasound scanning in patients with hodgkin's lymphoma: A prospective study. Haematologica. 2006;91:960–3. [PubMed] [Google Scholar]
- 9.Picardi M, Pugliese N, Cirillo M, Zeppa P, Cozzolino I, Ciancia G, et al. Advanced-stage hodgkin lymphoma: US/ chest radiography for detection of relapse in patients in first complete remission-a randomized trial of routine surveillance imaging procedures. Radiology. 2014;272:262–74. doi: 10.1148/radiol.14132154. [DOI] [PubMed] [Google Scholar]
- 10.Rubaltelli L, Khadivi Y, Tregnaghi A, Stramare R, Ferro F, Borsato S, et al. Evaluation of lymph node perfusion using continuous mode harmonic ultrasonography with a second-generation contrast agent. J Ultrasound Med. 2004;23:829–36. doi: 10.7863/jum.2004.23.6.829. [DOI] [PubMed] [Google Scholar]
- 11.Nie J, Ling W, Yang Q, Jin H, Ou X, Ma X. The value of CEUS in distinguishing cancerous lymph nodes from the primary lymphoma of the head and neck. Front Oncol. 2020;10:473. doi: 10.3389/fonc.2020.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niu X, Jiang W, Zhang X, Ding Z, Xue H, Wang Z, et al. Comparison of contrast-enhanced ultrasound and positron emission tomography/computed tomography (PET/CT) in lymphoma. Med Sci Monit. 2018;24:5558–65. doi: 10.12659/MSM.908849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma X, Ling W, Xia F, Zhang Y, Zhu C, He J. Application of contrast-enhanced ultrasound (CEUS) in lymphomatous lymph nodes: A comparison between PET/CT and contrast-enhanced CT. Contrast Media Mol Imaging. 2019;2019:5709698. doi: 10.1155/2019/5709698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei X, Li Y, Zhang S, Xin XJ, Zhu L, Gao M. The role of contrast-enhanced ultrasound (CEUS) in the early assessment of microvascularization in patients with aggressive B-cell lymphoma treated by rituximab-CHOP: A preliminary study. Clin Hemorheol Microcirc. 2014;58:363–76. doi: 10.3233/CH-131773. [DOI] [PubMed] [Google Scholar]
- 15.Xin L, Yan Z, Zhang X, Zang Y, Ding Z, Xue H, et al. Parameters for contrast-enhanced ultrasound (CEUS) of enlarged superficial lymph nodes for the evaluation of therapeutic response in lymphoma: A preliminary study. Med Sci Monit. 2017;23:5430–8. doi: 10.12659/MSM.907293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Lin X, Chen X, Zheng B. Noninvasive evaluation of benign and malignant superficial lymph nodes by virtual touch tissue quantification: A pilot study. J Ultrasound Med. 2016;35:571–5. doi: 10.7863/ultra.15.05053. [DOI] [PubMed] [Google Scholar]
- 17.Squillaci E, Antonicoli M, Manenti G, Bolacchi F. Real-time ultrasound elastography for assessment of response to brentuximab vedotin treatment in relapsed and refractory hodgkin lymphoma. Eur Rev Med Pharmacol Sci. 2016;20:1628–35. [PubMed] [Google Scholar]
- 18.Ribatti D, Nico B, Ranieri G, Specchia G, Vacca A. The role of angiogenesis in human non-hodgkin lymphomas. Neoplasia. 2013;15:231–8. doi: 10.1593/neo.121962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovangiorio F, Galluzzo M, Andreoli C, de Cicco ML, David V. Color Doppler sonography in the evaluation of superficial lymphomatous lymph nodes. J Ultrasound Med. 2002;21:403–8. doi: 10.7863/jum.2002.21.4.403. [DOI] [PubMed] [Google Scholar]