Abstract
Background:
Skeletal muscle mass is an important factor for various diseases’ outcomes. As for its indicators, temporal muscle thickness (TMT) and temporal muscle area (TMA) on the head computed tomography are useful, and TMT and TMA were reported as potential prognostic factors for aneurysmal subarachnoid hemorrhage (SAH). We examined the clinical characteristics, including TMT and TMA, of SAH patients aged 75 or younger.
Methods:
We retrospectively investigated 127 SAH patients with all World Federation of Neurosurgical Societies (WFNS) grades and treated by clipping between 2009 and 2019. Clinical outcome was measured with the modified Rankin Scale (mRS) at 6 months, with favorable outcome defined as mRS 0–2. The associations between the clinical variables and the outcomes were analyzed.
Results:
The mean age was 60.6 (32–74) years, and 65% were women. The mean ± standard deviation of WFNS grade was 2.8 ± 1.4. TMT and TMA were larger in the favorable outcome group than the poor one. Multivariate analysis revealed that age, smoking, WFNS grade, and TMT or TMA were associated with favorable outcome. Receiver operating characteristic analysis found that the threshold of TMT was 4.9 mm in female and 6.7 mm in male, and that of TMA was 193 mm2 in female and 333 mm2 in male.
Conclusion:
The odds ratios for TMT and TMA related to clinical outcome were lower than for smoking and WFNS grade; however, on multivariate analysis they remained independent prognostic factors in SAH patients aged 75 or younger treated by clipping. Further studies are needed to confirm these findings.
Keywords: Cerebral aneurysm, Clipping, Prognostic factor, Sarcopenia, Subarachnoid hemorrhage, Temporal muscle thickness and area

INTRODUCTION
Low skeletal muscle mass due to low nutrition or aging (sarcopenia in the broadest sense[5,8,27,31]) is clinically important in the various diseases both in the elderly[6,38,45] as well as young and middle-aged populations.[39,40] In stroke patients, including those with subarachnoid hemorrhage (SAH), total body skeletal muscle mass is important so that they can obtain better functional outcomes after rehabilitation.[28,32] To measure the total body skeletal muscle mass, psoas muscle cross-sectional area at the level of the third lumbar vertebra on the abdominal computed tomography (CT),[18] gait speed, and handgrip strength is usually used.[8] However, it is difficult to measure muscle mass or muscle function of the SAH patients in those ways, because we do not usually perform abdominal CT and the SAH patients often have impaired consciousness and need of rest considering the risk of rerupture.
Therefore, we focused on the temporal muscle thickness (TMT), and temporal muscle area (TMA) on the head CT, because TMT and TMA are substituted as useful measures of the total body skeletal muscle mass.[13,26,34] TMT and TMA are recently featured and reported as clinically useful parameters; they are indicators of nutrition,[13] prognosis of glioblastoma,[11,15] and brain metastasis.[9,10] They are also predictors of sarcopenia in neurological patients[41] or major complications following surgery for nonsyndromic craniosynostosis.[35] Furthermore, they are reported as potential prognostic factors for aneurysmal SAH patients treated by endovascular coiling,[20,21] and those over 75 years of age treated by clipping.[23,24] However, especially in aneurysmal SAH patients aged 75 or younger treated by clipping, the association between TMT and TMA and prognosis has not been investigated.
This study was performed to analyze the clinical characteristics of aneurysmal SAH patients aged 75 or younger who were treated by clipping, with a focus on the temporal muscle. To the best of our knowledge, this is the first study, including the young and middle ages, to examine the relationship between TMT and TMA and outcomes of SAH patients treated by clipping.
MATERIALS AND METHODS
Study population
We retrospectively retrieved data from medical records of all the 127 aneurysmal SAH patients who were admitted between 2009 and 2019 and treated by clipping at our institution. All the patients included in this study had been independent in their activities in daily livings (ADLs) before the onset of SAH. The diagnosis of SAH was based on the clinical history and the presence of SAH on CT. The hospital’s research ethics committee approved this study, and we gained written informed consent for this study from all of the patients, the legally authorized representative of the patients, or next of kin of the deceased patients. All methods were carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki).
General management of SAH was similar in all cases: all patients were first treated with nicardipine and kept normovolemic with normal blood pressure (systolic blood pressure <140 mmHg). Indication for surgery was established according to the Japanese Guidelines for the Management of Stroke 2009[43] and 2015.[44] Both versions describe samely as follows; patients classified as World Federation of Neurological Surgeons (WFNS) grades[42] I-III were considered eligible to undergo aneurysm treatment, whereas those with WFNS Grades IV and V were not regarded as suitable for such treatment, except for young and middle-aged patients or patients with large intra-parenchymal hematoma or hydrocephalus. We mainly performed clipping, but endovascular coiling was considered when it seemed superior to clipping, such as in the posterior circulation aneurysm. Patients with SAH treated by coiling, of unverified etiology and due to trauma, arteriovenous malformation, or dissection were excluded from this study. This direct surgery was performed within 72 h after onset.
All SAH patients who underwent aneurysm clipping received fasudil, cilostazol, and statin as appropriate after the operation. Rehabilitation and nutritional support were started as soon as possible after the operation, and prophylaxis and treatment of complications were also ensured. Intra-arterial infusion of fasudil was performed when necessary for the treatment of symptomatic vasospasm. In addition, a ventriculoperitoneal shunt was performed when hydrocephalus was observed.
Clinical variables
We collected data regarding physiological symptoms at admission for patients included in this study, that is, age, sex, WFNS grade, systolic blood pressure, administration of antithrombotic drugs, history of smoking and drinking, hypertension, diabetes mellitus, dyslipidemia, symptomatic vasospasm, ventriculoperitoneal shunt, and postoperative complications (except for symptomatic vasospasm and hydrocephalus). Postoperative complications included infectious diseases, heart failure, rerupture, seizure, and disuse syndrome. We also measured albumin, lymphocyte, triglycerides, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein (LDL) cholesterol, glucose, and hemoglobin A1c levels at admission. Albumin, lymphocyte, and total cholesterol are known factors for controlling nutritional status score to assess the nutritional status of the patients.[16]
We determined the location and size of the aneurysm, Fisher CT scale, TMT, and TMA based on the results of the CT or CT angiography at admission. We used the Aquilion ONE (Canon Medical Systems Corporation, Tochigi, Japan) to take CT and CT angiography images of 0.5 × 0.5 × 1.0 mm voxels. The slice thickness was reconstructed to 5 mm [Figure 1a]. The window width was adjusted to 300 and the window level to 20. Two investigators who were unaware of the patients’ outcomes measured the TMT and TMA on the CT image at admission using SYNAPSE V 4.1.5 imaging software (Fujifilm Medical, Tokyo, Japan) through methods described previously.[20,24] The TMT was measured bilaterally perpendicular to the long axis of the temporal muscle at the slice 5 mm above the orbital roof and was calculated using averages of the left and right from three determinations of each side [Figure 1b]. The TMA was measured manually by tracing the outline of the temporal muscle on the same slice as used for measuring the TMT [Figure 1c]. The averages of the left and right measurements of the TMT and TMA were calculated and used for analysis. [Figure 1d] shows a patient with large TMT and TMA, while [Figure 1e] shows a small TMT and TMA. TMT and TMA were measured using the CT image at admission, so it has nothing to do with intraoperative electrocoagulation, dissection, nor postoperative atrophy. Furthermore, there were no patients with trigeminal nerve disease nor myopathy which might be related to smaller TMT and TMA.
Figure 1:
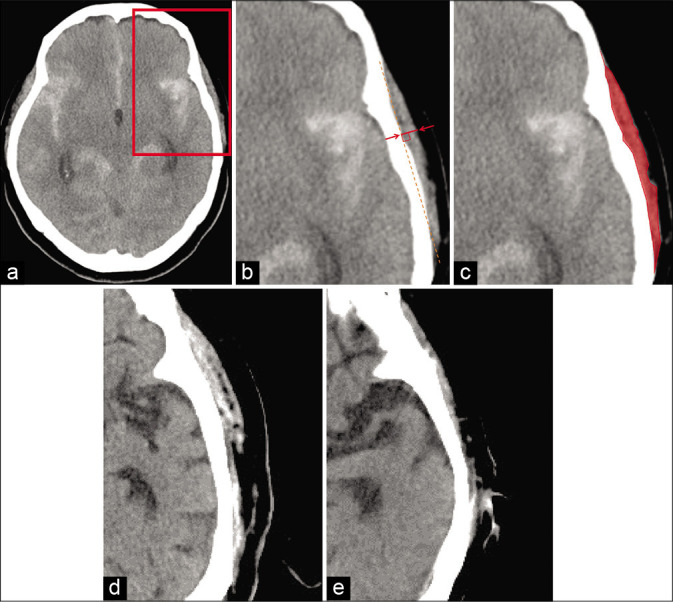
Head CT image measuring temporal muscle thickness (TMT) and area (TMA). The slice is 5 mm above the superior wall of the orbit (a). The rectangular part in (a) is enlarged in (b) and (c). CT image representing TMT. The line between the arrows indicates TMT (b). CT image representing TMA (c). Patient with a large TMT and TMA (d); patient with a small TMT and TMA (e).
To evaluate the outcomes, modified Rankin Scale (mRS) scores at 6 months after the operations of all 127 patients were collected by either personal outpatient interviews, reports from the rehabilitation hospital or home doctor, or interviews over the telephone, once the ethical approval was obtained for the study. We dichotomized mRS scores into favorable (mRS 0–2) or poor (mRS 3–6).
Statistical analysis
Intraclass correlation coefficients were used to test inter-rater reliabilities of TMT and TMA. The associations between TMT and TMA and other factors were investigated by the Mann–Whitney U-test, Fisher’s exact test, or Spearman’s coefficient correlation. R > 0.3 was defined that there was a significant correlation. The results are described as mean ± standard deviation (SD). Associations and outcomes between the clinical variables were analyzed using the Mann–Whitney U-test and Fisher’s exact test. Binomial logistic regression analysis was performed using the preoperative factors with P < 0.05 extracted through the univariate analysis described above. Age and sex were also used for the multivariate analysis to adjust the difference of TMT and TMA related to age or sex. Variables with small sample numbers under 80 were excluded from the multivariate analysis. We also performed receiver operating characteristic (ROC) analysis by sex and analyzed the thresholds of the TMT and TMA related to the outcome. The minimum distance point to (0,1) determined the thresholds. A two-tailed p < 0.050 was considered statistically significant. We conducted this calculation using SPSS software version 24.0.0. (IBM, New York, USA).
RESULTS
Clinical characteristics
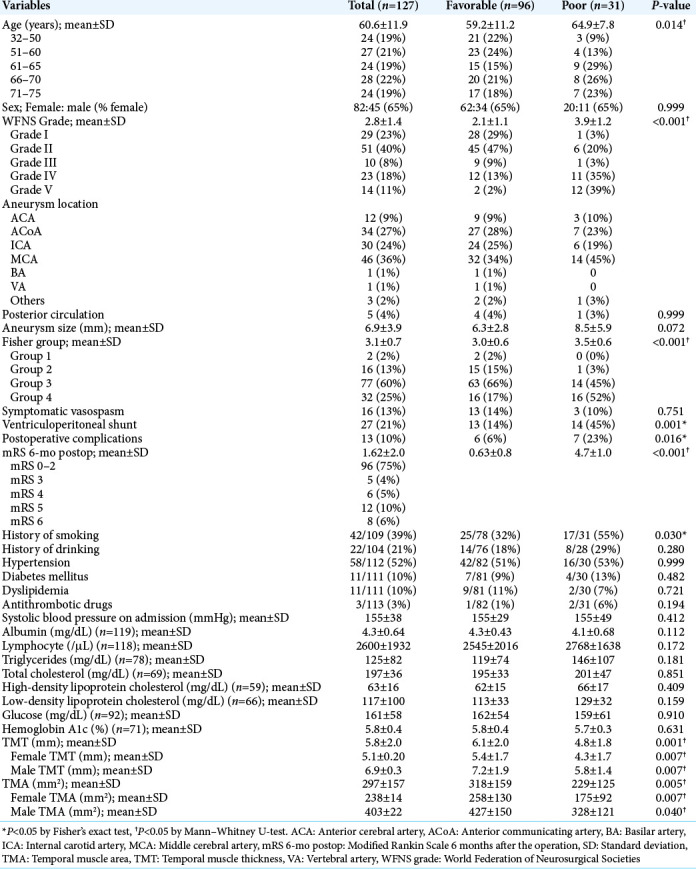
Clinical characteristics of the 127 SAH patients (82 women and 45 men) treated by clipping are summarized in [Table 1]. The mean ± SD WFNS grade was 2.8 ± 1.4. Ninety-six percent of aneurysms were located in the anterior circulation, and the mean ± SD aneurysm size was 6.9 ± 3.9 mm. Fisher Group 3 was 60%, and Group 4 was 25%. Symptomatic vasospasm, ventriculoperitoneal shunt, and other complications were observed in 13%, 21%, and 10%, respectively. Six months after the operation, 96 (75%) patients were independent in ADLs.
Table 1:
Patient characteristics and difference between favorable and poor outcomes.
TMT and TMA and sex, age, laboratory data, or outcome
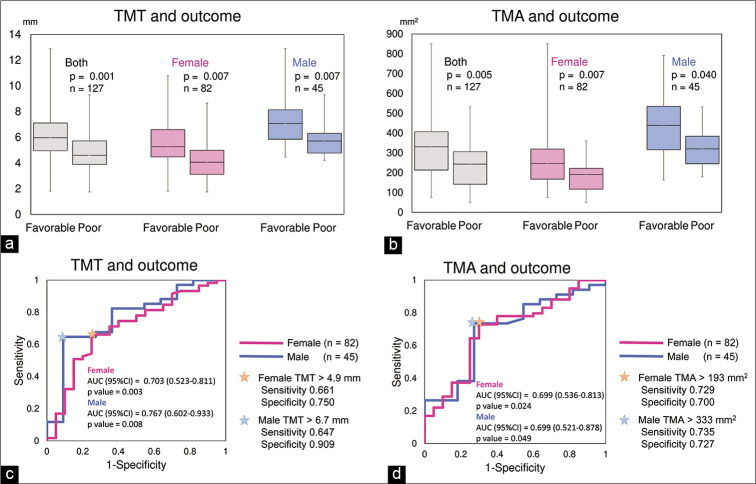
The intraclass correlation coefficients (2, 2) measuring TMT and TMA were 0.803 and 0.724, respectively. TMT and TMA were significantly larger in men than in women [P < 0.001 for both, Table 1], but were not significantly correlated to age (TMT vs. age; r = –0.18, TMA vs. age; r = –0.26). TMT, TMA, and the prognosis were also not correlated to albumin, lymphocytes, or total cholesterol (P > 0.05 for all). TMT was significantly larger in the favorable outcome groups than in the poor outcome groups (P = 0.001 in both sex, P = 0.007 in female, P = 0.007 in male, respectively) [Table 1 and Figure 2a]. TMA was also significantly larger in the favorable outcome groups (P = 0.005 in both sex, P = 0.007 in female, P = 0.040 in male, respectively) [Table 1 and Figure 2b]. WFNS grade, symptomatic vasospasm, or need of shunt for hydrocephalus were not correlated with TMT nor TMA.
Figure 2:
Temporal muscle thickness (TMT) was significantly larger in the favorable outcome groups than in the poor outcome groups (P = 0.001 in both sex, P = 0.007 in female, P = 0.007 in male, respectively) (a). Temporal muscle area (TMA) was also significantly larger in the favorable outcome groups (P = 0.005 in both sex, P = 0.007 in female, P = 0.040 in male, respectively) (b). Receiver operating characteristic (ROC) analysis was performed to determine the threshold of the TMT and TMA for outcomes. The threshold of TMT was 4.9 mm in female (sensitivity = 0.661, specificity = 0.750. AUC = 0.703, 95%CI 0.523–0.811, P = 0.003) and 6.7 mm in male (sensitivity = 0.647, specificity = 0.909. AUC = 0.767, 95%CI 0.602–0.933, P = 0.008) (c). The threshold of TMA was 193 mm2 in female (sensitivity = 0.729, specificity = 0.700. AUC = 0.699, 95%CI 0.536–0.813, P = 0.024) and 333 mm2 in male (sensitivity = 0.735, specificity = 0.727. AUC = 0.699, 95%CI 0.521–0.878, P = 0.049) (d).
Relationship between variables and outcome
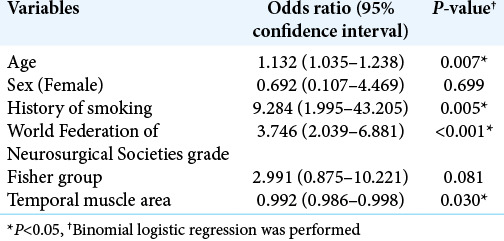
Age, WFNS grade, smoking, Fisher group, LDL cholesterol, ventriculoperitoneal shunt, and postoperative complications were also significantly associated with poor outcomes in the univariate analysis (P = 0.014, P < 0.001, P = 0.030, P < 0.001, P = 0.04, P = 0.001, P = 0.016, respectively) [Table 1]. Using binomial logistic regression, we analyzed age, sex, WFNS grade, Fisher group, history of smoking, and TMT as potential preoperative prognostic factors. LDL cholesterol was excluded since the sample number was small. TMT and TMA have the collinearity (r = 0.83, P < 0.001 by Spearman’s coefficient correlation), so TMT or TMA was, respectively, used for the multivariate analysis. The multivariate analysis revealed that age, history of smoking, WFNS grade, and TMT were independent factors related to outcomes (P = 0.010, P = 0.006, P < 0.001, and P = 0.023, respectively) [Table 2]. It also demonstrated that age, history of smoking, WFNS grade, and TMA were independent factors related to outcomes (P = 0.007, P = 0.005, P < 0.001, and P = 0.030, respectively) [Table 3].
Table 2:
Multivariate analysis of the characteristics and outcomes.
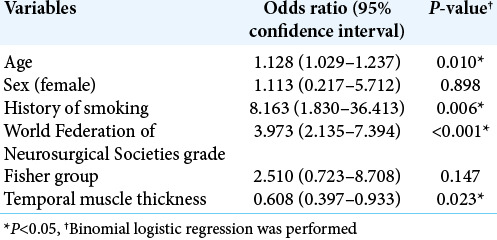
Table 3:
Multivariate analysis of the characteristics and outcomes.
Threshold of TMT and TMA for outcome
ROC analysis revealed that the threshold of TMT was 4.9 mm in female (sensitivity = 0.661, specificity = 0.750. area under the ROC curve [AUC] = 0.703, 95% CI 0.523–0.811, P = 0.003) and 6.7 mm in male (sensitivity = 0.647, specificity = 0.909. AUC = 0.767, 95%CI 0.602–0.933, P = 0.008) [Figure 2c]. It also demonstrated that the threshold of TMA was 193 mm2 in female (sensitivity = 0.729, specificity = 0.700. AUC = 0.699, 95%CI 0.536–0.813, P = 0.024) and 333 mm2 in male (sensitivity = 0.735, specificity = 0.727. AUC = 0.699, 95% CI 0.521–0.878, P = 0.049) [Figure 2d].
DISCUSSION
Our findings show that TMT and TMA would be ones of the prognostic factors of SAH patients aged 75 or younger treated by clipping, and our study found that the threshold for the outcome of TMT was 4.9 mm in female and 6.7 mm in male, and that of TMA was 193 mm2 in female and 333 mm2 in male.
Skeletal muscle mass and neurosurgical diseases
In addition to our report, several reports on the association between skeletal muscle mass and neurosurgical diseases are reported. Higher skeletal muscle mass reduces the risk of intracranial arterial stenosis and may protect against ischemic stroke.[19,29] Sarcopenia, loss of skeletal muscle mass in the elderly, also relates to the medium-term outcomes of carotid artery stenting,[17] mortality after percutaneous vertebral augmentation,[1] and traumatic brain injury.[46]
These are some speculations of why low skeletal muscle mass is related to the poor prognosis of SAH. Patients with neurosurgical diseases or after surgical treatment are usually bedridden for some time due to impaired consciousness, sedation, or surgical invasion. The skeletal muscle would be further catabolized during such acute treatment and hospitalization, so those with low skeletal muscle mass may have difficulty performing rehabilitation. In addition, low skeletal muscle mass and sarcopenia are related to malnutrition.[25] It is reported that TMT and TMA related to lymphocytes in SAH patients over 75 years.[24] Total lymphocyte count is one of the indicators of nutrition,[16] and this indicated the association between low TMT and TMA and malnutrition in patients over 75 years old. However, results in this study did not reproduce these trends. Hence, another explanation as to why SAH patients of 75 years or younger with low skeletal muscle mass have poor outcomes regardless of malnutrition should exist.
As a potential explanation, the skeletal muscle is an endocrine organ and secretes myokines. One of the myokines, interleukin-6 (IL), is associated with inflammation, and elevated plasma concentrations of IL-6 are seen in patients with lower muscle mass or sarcopenia.[3,36] The higher early serum IL-6 levels after SAH are associated with poor outcomes.[14] Furthermore, IL-10, another myokine, is an anti-inflammatory cytokine[36] that facilitates the resolution of inflammatory cascades which, if prolonged, cause secondary brain damage,[12] and sarcopenia was correlated with a high IL-6/IL-10 ratio in the population over 60 years.[36] Therefore, low skeletal muscle mass can be associated with poor prognosis in young and middle-aged patients through elevated inflammatory cytokines secreted from the skeletal muscle. Further investigation using patients’ blood samples on the association between myokines and patients’ characteristics is needed.
Usefulness of TMT and TMA as indicators of skeletal muscle mass in neurosurgical diseases
Many studies on the association between skeletal muscle mass and outcomes used psoas muscle cross-sectional area at the level of the third lumbar vertebra,[18] systemic muscle mass, gait speed, and handgrip strength as indicators of sarcopenia.[7] Mainly, the systemic muscle mass and its function are important for diagnosis and indicators of sarcopenia,[5] and muscle strength was at the forefront as it was recognized that strength was better than mass in predicting adverse outcomes.[4,8,22,37] However, we were unable to perform muscle function tests due to the patients’ impaired consciousness and rerupture concerns. Patients with SAH naturally undergo head CT, so we can easily and safely obtain information about the temporal muscle from CT. Therefore, in the present study, we focused on the temporal muscle, and this method of measurement can be applied to other neurosurgical diseases. Moreover, combined with the previous report on older patients[24] and on the patients treated by endovascular coiling,[20] our study suggests that the temporal muscle mass (TMT and TMA) was related to the prognosis of patients of all ages with SAH.
Previously, thresholds of TMT in the magnetic resonance imaging (MRI) for the outcomes in various diseases were investigated. TMT in MRI over the median was associated with favorable outcomes in brain metastasis.[10] (The medians were not described, but mean TMT was 5.0 (range 2.0–8.9) mm in female, 6.2 (1.7–10.8) in male, respectively.) TMT in MRI over 7.2 mm was a threshold for the prognosis of glioblastoma patients.[11] The cutoff points of TMT in MRI to diagnose sarcopenia of 6.3 mm for male patients and 5.2 mm for female patients, respectively.[41] Our study found that the threshold for the outcome of TMT in CT was 4.9 mm in female and 6.7 mm in male, respectively. It is not easy to compare the threshold values of TMT to those in the previous reports because the modality was different in which TMT was measured. However, investigating the threshold is meaningful for the clinical practice, and further prospective studies are desired.
Clinical application
Awareness of the relationship between loss of muscle mass and outcome may lead to new therapeutic targets for patients with unruptured aneurysms before developing SAH. Low skeletal muscle mass is related to malnutrition and aging,[25] so nutritional intervention[33] and exercise training[2,30] are supplemental therapies for patients with cancer and rheumatic diseases. Therefore, these therapies could be applied to patients with unruptured cerebral aneurysms to the extent that the aneurysm would not rupture due to vigorous exercise.[47]
Limitations
First, the sample size was small. Second, previous studies reported that sex and age affect temporal muscle mass,[10,34] and our study showed that sex was related to TMT and TMA. We performed multivariate analysis to adjust for confounders, such as age and sex, to solve this problem. Third, we did not measure actual skeletal muscle volume nor muscle function. Therefore, it is uncertain that TMT and TMA were really surrogate markers of skeletal muscle mass in this study. Furthermore, it is unknown that TMT and TMA were determined congenitally and whether they can be enlarged by training like chewing. Fourth, it is unknown whether TMT or TMA is better. TMT is fast and has better reproducibility to be measured in our methods. TMA has a statistically stronger association with the SAH outcomes compared to TMT in the previous reports,[20,24] but not in this study.
Furthermore, accurate and standard methods of TMT and TMA measurements were not established. Ranganathan[34] and Rinkinen[35] used image analysis and engineering software to measure temporal muscle. Furtner[9-11,41] did not use image analysis and engineering software, but used MRI and measured TMT perpendicular to the long axis of the temporal muscle at the level of the orbital roof. Hsieh[15] measured the TM width using an average of the four slices of head CT images. Here, we used CT to measure TMT and TMA.[20,24] Measurement using CT images is easy because we routinely perform head CT, which does not require special software for analysis. However, TMT was manually measured perpendicular to the long axis of the temporal muscle, and TMA was calculated by tracing the outline of the temporal muscle, so lack of accuracy and reproducibility is possible. Therefore, we calculated these values using averages of the left and right from three determinations of each side by two investigators, and the intraclass correlation coefficients were sufficient.
CONCLUSION
TMT and TMA were independent prognostic factors for mRS 6 months after aneurysm clipping in aneurysmal SAH patients 75 years of age or younger of all WFNS grades. Our study found that the threshold for the outcome of TMT was 4.9 mm in female and 6.7 mm in male and that of TMA was 193 mm2 in female and 333 mm2 in male. Further studies are needed to confirm these findings.
Footnotes
How to cite this article: Katsuki M, Kakizawa Y, Nishikawa A, Yamamoto Y, Uchiyama T. Temporal muscle thickness and area are an independent prognostic factors in patients aged 75 or younger with aneurysmal subarachnoid hemorrhage treated by clipping. Surg Neurol Int 2021;12:151.
Contributor Information
Masahito Katsuki, Email: ktk1122nigt@gmail.com.
Yukinari Kakizawa, Email: ykakizawajp@yahoo.co.jp.
Akihiro Nishikawa, Email: aki.west@gmail.com.
Yasunaga Yamamoto, Email: yamamotoyasunaga@gmail.com.
Toshiya Uchiyama, Email: u_tosh@gmail.com.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bayram S, Akgül T, Adıyaman AE, Karalar Ş Dölen D, Aydoseli A. Effect of sarcopenia on mortality after percutaneous vertebral augmentation treatment for osteoporotic vertebral compression fractures in elderly patients: A retrospective cohort study. World Neurosurg. 2020;138:e354–60. doi: 10.1016/j.wneu.2020.02.121. [DOI] [PubMed] [Google Scholar]
- 2.Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol. 2015;11:86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 3.Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. 2017;22:25. doi: 10.1186/s40001-017-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binay Safer V, Safer U. Comment on. “Clinical characteristics of aneurysmal subarachnoid hemorrhage (SAH) in the elderly over 75: Would temporal muscle be a potential prognostic factor as an indicator of sarcopenia? ” Clin Neurol Neurosurg. 2020;188:105600. doi: 10.1016/j.clineuro.2019.105600. [DOI] [PubMed] [Google Scholar]
- 5.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Costa TM, Costa FM, Moreira CA, Rabelo LM, Boguszewski CL, Borba VZC. Sarcopenia in COPD: Relationship with COPD severity and prognosis. J Bras Pneumol. 2015;41:415–21. doi: 10.1590/S1806-37132015000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furtner J, Berghoff AS, Albtoush OM, Woitek R, Asenbaum U, Prayer D, et al. Survival prediction using temporal muscle thickness measurements on cranial magnetic resonance images in patients with newly diagnosed brain metastases. Eur Radiol. 2017;27:3167–73. doi: 10.1007/s00330-016-4707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furtner J, Berghoff AS, Schöpf V, Reumann R, Pascher B, Woitek R, et al. Temporal muscle thickness is an independent prognostic marker in melanoma patients with newly diagnosed brain metastases. J Neurooncol. 2018;140:173–8. doi: 10.1007/s11060-018-2948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furtner J, Genbrugge E, Gorlia T, Bendszus M, Nowosielski M, Golfinopoulos V, et al. Temporal muscle thickness is an independent prognostic marker in patients with progressive glioblastoma: Translational imaging analysis of the EORTC 26101 trial. Neuro Oncol. 2019;21:1587–94. doi: 10.1093/neuonc/noz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia JM, Stillings SA, Leclerc JL, Phillips H, Edwards NJ, Robicsek SA, et al. Role of interleukin-10 in acute brain injuries. Front Neurol. 2017;8:244. doi: 10.3389/fneur.2017.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa Y, Yoshida M, Sato A, Fujimoto Y, Minematsu T, Sugama J, et al. Temporal muscle thickness as a new indicator of nutritional status in older individuals. Geriatr Gerontol Int. 2019;19:135–40. doi: 10.1111/ggi.13570. [DOI] [PubMed] [Google Scholar]
- 14.Höllig A, Remmel D, Stoffel-Wagner B, Schubert GA, Coburn M, Clusmann H. Association of early inflammatory parameters after subarachnoid hemorrhage with functional outcome: A prospective cohort study. Clin Neurol Neurosurg. 2015;138:177–83. doi: 10.1016/j.clineuro.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh K, Hwang M, Estevez-Inoa G, Saraf A, Spina CS, Smith D, et al. Temporalis muscle width as a measure of sarcopenia independently predicts overall survival in patients with newly diagnosed glioblastoma. Int J Radiat Oncol. 2018;102:e225. [Google Scholar]
- 16.Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- 17.Ishihara H, Oka F, Goto H, Nishimoto T, Okazaki K, Sadahiro H, et al. Impact of frailty on medium-term outcome in asymptomatic patients after carotid artery stenting. World Neurosurg. 2019;127:e396–9. doi: 10.1016/j.wneu.2019.03.135. [DOI] [PubMed] [Google Scholar]
- 18.Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Color Dis. 2015;17:20–6. doi: 10.1111/codi.12805. [DOI] [PubMed] [Google Scholar]
- 19.Jung HJ, Jung H, Lee T, Kim J, Park J, Kim H, et al. Decreased muscle mass in Korean subjects with intracranial arterial stenosis: The Kangbuk Samsung Health Study. Atherosclerosis. 2017;256:89–93. doi: 10.1016/j.atherosclerosis.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Katsuki M, Suzuki Y, Kunitoki K, Sato Y, Sasaki K, Mashiyama S, et al. Temporal muscle as an indicator of sarcopenia is independently associated with Hunt and Kosnik grade on admission and the modified Rankin scale score at 6 months of patients with subarachnoid hemorrhage treated by endovascular coiling. World Neurosurg. 2020;137:e526–34. doi: 10.1016/j.wneu.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Katsuki M, Suzuki Y, Kunitoki K, Sato Y, Sasaki K, Mashiyama S, et al. Temporal muscle thickness and area with various characteristics data of the patients with aneurysmal subarachnoid hemorrhage who underwent endovascular coiling. Data Brief. 2020;31:105715. doi: 10.1016/j.dib.2020.105715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsuki M, Suzuki Y, Sato Y, Sasaki K, Shingai Y, Mashiyama S, et al. World Neurosurg. 2020. In reply to the letter to the editor regarding “temporal muscle as an indicator of sarcopenia is independently associated with Hunt and Kosnik grade on admission and the modified rankin scale at 6 months of patients with subarachnoid hemorrhage treated by endovascular coiling; pp. 140–433. [DOI] [PubMed] [Google Scholar]
- 23.Katsuki M, Yamamoto Y, Uchiyama T, Nishikawa A, Wada N, Kakizawa Y. Temporal muscle thickness and area with various characteristics data of the elderly patients over 75 with aneurysmal subarachnoid haemorrhage whose World Federation of Neurosurgical Societies grade were I to III. Data Brief. 2020;28:104832. doi: 10.1016/j.dib.2019.104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsuki M, Yamamoto Y, Uchiyama T, Wada N, Kakizawa Y. Clinical characteristics of aneurysmal subarachnoid hemorrhage in the elderly over 75; would temporal muscle be a potential prognostic factor as an indicator of sarcopenia? Clin Neurol Neurosurg. 2019;186:105535. doi: 10.1016/j.clineuro.2019.105535. [DOI] [PubMed] [Google Scholar]
- 25.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–59. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leitner J, Pelster S, Schöpf V, Berghoff AS, Woitek R, Asenbaum U, et al. High correlation of temporal muscle thickness with lumbar skeletal muscle cross-sectional area in patients with brain metastases. PLoS One. 2018;13:e0207849. doi: 10.1371/journal.pone.0207849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malmstrom TK, Morley JE. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14:531–2. doi: 10.1016/j.jamda.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita T, Nishioka S, Taguchi S, Yamanouchi A, Nakashima R, Wakabayashi H. Sarcopenic obesity and activities of daily living in stroke rehabilitation patients: A cross-sectional study. Healthcare (Basel) 2020;8:255. doi: 10.3390/healthcare8030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minn YK, Suk SH. Higher skeletal muscle mass may protect against ischemic stroke in community-dwelling adults without stroke and dementia: The PRESENT project. BMC Geriatr. 2017;17:45. doi: 10.1186/s12877-017-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni HJ, Pudasaini B, Yuan XT, Li HF, Shi L, Yuan P. Exercise training for patients pre-and postsurgically treated for non-small cell lung cancer: A systematic review and meta-analysis. Integr Cancer Ther. 2017;16:63–73. doi: 10.1177/1534735416645180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiguchi S, Hino K, Moriya K, Shiraki M, Hiramatsu A, Nishikawa H. Assessment criteria for sarcopenia in liver disease (first edition): Report from the working group for creation of sarcopenia assessment criteria in the Japan Society of Hepatology. Kanzo. 2016;57:353–68. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 32.Nozoe M, Kanai M, Kubo H, Kobayashi M, Yamamoto M, Shimada S, et al. Quadriceps muscle thickness changes in patients with aneurysmal subarachnoid hemorrhage during the acute phase. Top Stroke Rehabil. 2018;25:209–13. doi: 10.1080/10749357.2017.1413762. [DOI] [PubMed] [Google Scholar]
- 33.Paccagnella A, Morassutti I, Rosti G. Nutritional intervention for improving treatment tolerance in cancer patients. Curr Opin Oncol. 2011;23:322–30. doi: 10.1097/CCO.0b013e3283479c66. [DOI] [PubMed] [Google Scholar]
- 34.Ranganathan K, Terjimanian M, Lisiecki J, Rinkinen J, Mukkamala A, Brownley C, et al. Temporalis muscle morphomics: The psoas of the craniofacial skeleton. J Surg Res. 2014;186:246–52. doi: 10.1016/j.jss.2013.07.059. [DOI] [PubMed] [Google Scholar]
- 35.Rinkinen J, Zhang P, Wang L, Enchakalody B, Terjimanian M, Holcomb S, et al. Novel temporalis muscle and fat pad morphomic analyses aids preoperative risk evaluation and outcome assessment in nonsyndromic craniosynostosis. J Craniofac Surg. 2013;24:250–5. doi: 10.1097/SCS.0b013e31827006f5. [DOI] [PubMed] [Google Scholar]
- 36.Rong YD, Bian AL, Hu HY, Ma Y, Zhou XZ. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018;18:308. doi: 10.1186/s12877-018-1007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safer U, Tasci I, Binay Safer V. Letter to the editor regarding “effect of sarcopenia on mortality after percutaneous vertebral augmentation treatment for osteoporotic vertebral compression fractures in elderly patients: A retrospective cohort study”. World Neurosurg. 2020;139:710. doi: 10.1016/j.wneu.2020.04.207. [DOI] [PubMed] [Google Scholar]
- 38.Shibahashi K, Sugiyama K, Kashiura M, Hamabe Y. Decreasing skeletal muscle as a risk factor for mortality in elderly patients with sepsis: A retrospective cohort study. J Intensive Care. 2017;5:8. doi: 10.1186/s40560-016-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Someya Y, Tamura Y, Suzuki R, Kaga H, Kadowaki S, Sugimoto D, et al. Characteristics of glucose metabolism in underweight Japanese women. J Endocr Soc. 2018;2:279–89. doi: 10.1210/js.2017-00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sousa AS, Guerra RS, Fonseca I, Pichel F, Ferreira S, Amaral TF. Financial impact of sarcopenia on hospitalization costs. Eur J Clin Nutr. 2016;70:1046–51. doi: 10.1038/ejcn.2016.73. [DOI] [PubMed] [Google Scholar]
- 41.Steindl A, Leitner J, Schwarz M, Nenning KH, Asenbaum U, Mayer S, et al. Sarcopenia in neurological patients: Standard values for temporal muscle thickness and muscle strength evaluation. J Clin Med. 2020;9:1272. doi: 10.3390/jcm9051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teasdale GM, Drake CG, Hunt W, Kassell N, Sano K, Perat B, et al. A universal subarachnoid hemorrhage scale: Report of a committee of the world federation of Neurosurgical societies. J Neurol Neurosurg Psychiatry. 1988;51:1457. doi: 10.1136/jnnp.51.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The Japan Stroke Society. Tokyo: Kyowa Kikaku; 2009. Japanese Guidelines for the Management of Stroke 2009. [Google Scholar]
- 44.The Japan Stroke Society. Tokyo: Kyowa Kikaku; 2015. Japanese Guidelines for the Management of Stroke 2015. [Google Scholar]
- 45.Tsuchida K, Fujihara Y, Hiroki J, Hakamata T, Sakai R, Nishida K, et al. Significance of sarcopenia evaluation in acute decompensated heart failure. Skeletal muscle mass index versus fat-free mass index. Int Hear J. 2018;59:143–8. doi: 10.1536/ihj.17-057. [DOI] [PubMed] [Google Scholar]
- 46.Uhlich R, Hu P. Sarcopenia diagnosed using masseter muscle area predictive of early mortality following severe traumatic brain injury. Neural Regen Res. 2018;13:2089–90. doi: 10.4103/1673-5374.241451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlak MH, Rinkel GJ, Greebe P, Van Der Bom JG, Algra A. Trigger factors and their attributable risk for rupture of intracranial aneurysms: A case-crossover study. Stroke. 2011;42:1878–82. doi: 10.1161/STROKEAHA.110.606558. [DOI] [PubMed] [Google Scholar]