Abstract
Administration of interleukin-2 (IL-2) has led to durable response in patients with advanced renal cancer and melanoma, but is restricted for clinical application due to adverse effects, including the vascular leak syndrome (VLS). VLS is associated with increased circulating levels of the Tie2 antagonist ligand, angiopoietin 2 (Ang2) and decreased Tie2 receptor phosphorylation and downstream signaling in endothelial cells. Given that vascular endothelial protein tyrosine phosphatase (VE-PTP) is a specific membrane phosphatase in endothelial cells that dephosphorylates Tie2, the effects of targeting VE-PTP by a selective inhibitor AKB-9778 (AKB) in terms of VLS and anti-tumor efficacy were examined in this study. We found, by targeting VE-PTP, that the anti-tumor effects induced by IL-2 was augmented (tumor-free 44% (IL-2 alone) vs 87.5% (IL-2+AKB)), associated with enhanced immune cell infiltrate (90% increase for CD8 T cells and NK cells). In addition, the side effects of IL-2 therapy were lessened, as demonstrated by diminished lung weight (less vascular leakage) as well as reduced cytokine levels (Serum HMGB1 from137.04±2.69 to 43.86±3.65 pg/ml, IFN-γ from 590.52 ± 90.52 to 31.37 ± 1.14 pg/ml). We further sought to determine the potential mechanism of the action of AKB-9778. Our findings suggest that AKB-9778 may function through reducing serum Ang2 level and regulating endothelial cell viability. These findings provide insights into the targeting VE-PTP to improve tolerance and efficacy of IL-2 therapy and highlight the clinical potential of AKB-9778 for treating patients with vascular leak syndrome and cancer.
Keywords: IL-2, VLS, VE-PTP, Tie2, HMGB1
INTRODUCTION
Interleukin-2 (IL-2, aldesleukin) was the second exogenously administered cytokine demonstrated to have anti-tumor effects, following interferon alpha. It was approved in 1998 for the treatment of patients with renal cell carcinoma and subsequently those with metastatic melanoma [1,2]. The long-lived efficacy of therapy in some patients clearly established the clinical effectiveness of immunotherapy for cancer. The therapeutic application of IL-2, however, has been limited by dose-dependent and largely reversible toxicity[3,4]. Apart from common side effects including fever, chills, diarrhea and nausea, “capillary/ vascular leak syndrome”(VLS) is a more severe syndrome, resulting in fluid retention, interstitial edema and organ failure[3,5]. Targeting VLS therapeutics might ameliorate the efficacy and tolerance of IL-2 but remain to be further developed[6,7].
Tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2 (Tie2) is a critical component in maintaining endothelial cell viability and vascular integrity[8]. Phospho-Tie2 (pTie2) functions as an active kinase that initiates down-stream AKT-mediated vascular regulation. Activation of Tie2 is modulated by antagonistic effects of the angiopoietins, Ang1 and Ang2. While Ang1 induces Tie2 phosphorylation, Ang2 is a weak partial agonist that competes for Ang1 to limit Tie2 activation[9]. Ten agents targeting Ang/Tie2 axis have been investigated in clinical trials[10], primarily focused on inhibition of angiogenesis. Apart from direct effect on tumor growth and metastasis, inhibition of Ang2 has gained attention as a potential therapeutic strategy based on the pathogenic role of pTie2 blockade by Ang2 in VLS. In sera from patients treated with IL-2, circulating Ang2 levels were strikingly elevated, correlating with VLS development in a specific and time-dependent fashion[11]. Additionally, Ang2 blocks Tie2 phosphorylation induced by Ang1, causing endothelial para-cellular gap formation and increased pulmonary leak in murine models[11]
VE-PTP (vascular endothelial protein tyrosine phosphatase, also known as HPTPβ) is a vascular endothelial cell-specific membrane phosphatase involved in dephosphorylation and consequent inactivation of the Tie2 receptor [12]. Ablation of the VE-PTP gene in mice results in embryonic lethality with defective angiogenesis and establishment of the vasculature[13,14], characterized by induced enlargement of blood vessels within the yolk sac. Specifically, VE-PTP inhibitor, AKB-9778, has been discovered to stabilize breast cancer vasculature and repress metastatic progression with sustained Tie-2 activation[15]. Hence, it becomes of interest whether targeting VE-PTP with inhibitors would be effective to reduce IL-2-induced VLS and/or enhance its anti-tumor activity.
AKB-9778 has been investigated as a novel and potent small molecule inhibitor of the catalytic activity of VE-PTP[16]. We addressed the question if AKB-9778 is a potential adjuvant for IL-2 therapy, with regarding to the efficacy as well as toxicity. Our findings provide evidence for the efficacy of targeting VE-PTP to augment anti-tumor activity as well as diminish the side effects induced by IL-2. Consistently, AKB mitigates the VLS syndrome, possibly through limiting Ang 2 levels, thus increasing pTie2 or directly targeting Tie2. Taken together, these observations serve as a strong basis for further studies to explore the mechanism and efficacy of targeting VE-PTP with AKB-9778 or other agents for treating a broad array of malignant and vascular indications in patients.
MATERIALS AND METHODS
Animals and tumor cell lines
Female C57BL/6 (B6, H-2b) mice, 8- to 10-week-old, were purchased from Taconic. Animals were maintained in a specific pathogen-free facility at the University of Pittsburgh Cancer Institute (Pittsburgh, PA) and used in accordance with institutional and NIH guidelines. MC38 murine colorectal carcinoma and Panc02 adenocarcinoma cells (C57BL/6 syngeneic) were purchased from The American Type Culture Collection. Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) medium supplemented with 5% heat-inactivated FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin.
Liver metastasis model
Liver metastases were obtained by direct portal injection of tumor cells as described previously[17,18]. Briefly, mice were anesthetized with a single intraperitoneal injection of ketamine (50 mg/kg, NLS animal Health) and xylazine (10 mg/kg, NLS animal Health). The portal vein was exposed through a small midline incision. A total of 2X105 luciferase-transfected MC38 tumor cells suspended in 200 mL normal saline were injected. The incision is closed with vicryl suture.
For assessment of AKB-9778 activity to augment anti-tumor IL-2 effect, mice were randomized into 4 groups and received their first bioluminescence imaging (BLI) measurement on the 10th day following tumor inoculation. They then started receiving intraperitoneal injection of rIL-2 (600K IU/mouse, i.p., bid) with or without combination of AKB 9778 (40 mg.kg, subq, bid, administered 30 minutes prior to IL-2) for 5 days. Clinical grade rIL-2 was a kind gift of Prometheus Laboratories, Inc. Untreated control mice (UT) were injected with an equivalent amount of normal saline on the same schedule. Tumor burden was assessed with the IVIS bioluminescence image described later. Blood was collected by direct intracardiac puncture. Lungs were weighed for evaluation of vascular leakage following gentle flushing of the pulmonary artery. Livers and spleens were harvested for isolation of immune cells.
Luciferase transfection of tumor cells and BLI
Stably transduced tumor cells expressing the firefly luciferase gene were generated by lentiviral transfection of the pGL4 Luciferase Reporter Vector (Promega, Madison, WI) and selected with puromycin. Growth characteristics and phenotype of the transfected cells were compared with the parental strain in vitro to verify the absence of any effects secondary to retroviral insertion. Before imaging, mice were anesthetized by isoflurane inhalation followed by intraperitoneal injection of luciferin (300 mg/kg; Caliper Life Sciences, Hopkinton, MA). After waiting 8 minutes to allow proper distribution of luciferin, the mice were imaged with an IVIS 200 system (Xenogen Corporation, Alameda, CA) according to the manufacturer’s instructions. Living Image software (Xenogen) was used to analyze the resultant data. Regions of interest were manually selected and quantification is reported as the average of photon flux within regions of interest. The BLI signal is represented as photons/s/cm2/Sr.
Isolation of nonparenchymal cells and flow cytometry
Mouse livers were minced and digested with 1% collagenase (Sigma, St. Louis, MO) solution at 37°C for 30 minutes. To obtain adequate number of nonparenchymal cells, livers from 3 to 5 animals were pulled together from each treatment group. The nonparenchymal cells were then isolated by centrifugation over a Percoll gradient (Sigma Chemical Co.). Cell surface antigen expression was analyzed by flow cytometry (Becton Dickinson FACScan, BD Biosciences, San Jose, CA) using fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies against mouse CD11b, CD11c, CD4, CD8, F4/80, Gr-1, and NKp46 (all from eBioscience, San Diego, CA). Appropriate isotype and species-matched irrelevant monoclonal antibodies were used as controls.
Serum cytokine determination
Blood was collected from direct intracardiac puncture at individual intervals following tumor inoculation. Serum was used to measure HMGB1 (Shino-test, Japan), IL-6, IL-18, IFN-γ (R&D, Minneapolis, MN), Ang1&2 (USCN, Life Science Inc., China) levels by ELISA, according to the manufacturer’s instruction.
Endothelial cell tube formation on Matrigel
Human dermal microvascular endothelial cells (HDMVEC; VEC Technologies, Rensallaer, NY) were cultured in MCDB-1 complete medial split with DMEM supplemented with 5% fetal bovine serum, penicillin, streptomycin (P/S) and L-glutamine. Cells were plated at a concentration of 25,000cells/well in 24-well plates on pre-hardened growth factor reduced Matrigel (BD Biosciences, San Jose, CA) in the presence of increasing doses of AKB 9778 (0, 0.3, 1, 3,10 μg/ml). After four hours, tube formation on matrigel was digitally captured at 20x using a Nikon inverted microscope and an Olympus DP25 camera.
Statistical analyses
Statistical significance was assessed using the Student t test. A p value less than 0.05 was considered significant. All experiments reported here were repeated at least 2 or 3 times of similar results with representative findings presented.
RESULTS
AKB-9778, in combination with HDIL-2, promoted profound antitumor effects and protected animals from VLS in a liver metastasis model.
In previous experiments, we established a murine liver metastasis tumor model and verified that recombinant interleukin 2 (rIL-2) limited tumor growth in a dose-dependent manner[17]. Administration of 600,000 IU per mouse rIL-2 twice a day can suppress tumor growth, yet is followed by relapse most frequently within months. High dose rIL-2 (HDIL-2) would cause severe systemic toxicity, which extensively limited the amount of the administered dose. In order to investigate the potential of VE-PTP as a therapeutic target for vascular integrity, we sought to determine the effects of administration of the VE-PTP inhibitor AKB-9778 (Aerpio Therapeutics; Cincinnati Ohio) either alone or in combination with IL-2, in a hepatic metastatic tumor model.
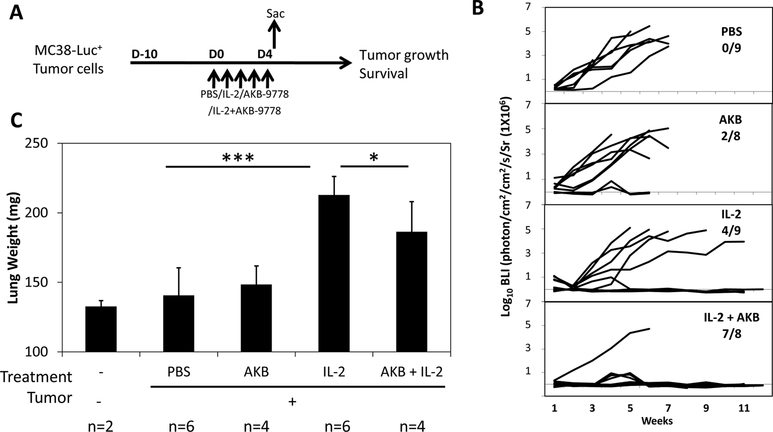
Mice received 2X105 luciferase-labeled murine MC38 colorectal cancer cells via portal vein injection. Ten days after infusion/implantation (Day 0), they were randomly divided into 4 groups that received vehicle control (PBS), AKB-9778 alone 40mg/kg, twice a day for 5 days, rIL-2 600,000 IU per mouse (HDIL- 2), twice a day for 5 day, with or without combination of AKB-9778 (Figure 1A). AKB-9778 was administered 30 min prior to IL-2 according to the protocol others use (Cao M. and Yi T., unpublished data). Tumor growth was measured by bioluminescence imaging (BLI) (Figure 1B–C) once a week and survival of mice was assessed (Supplementary Fig. S1). Treatment with AKB-9778 alone had a negligible effect on tumor growth, whereas HDIL-2 partially inhibited tumor growth. In contrast, a dramatic anti-tumor effect was seen when AKB 9778 was co-administered with HDIL-2 (Figure 1B–C). Although BLI revealed visible tumors during week 4 and 5 in several mice, only 1 of 8 mice developed tumor whereas the others were completely eradicated (87.5% of animals).
Figure 1. AKB-9778 combination with HDIL-2 markedly enhances antitumor effects and protects animals against vascular leak syndrome.
C57BL/6 mice received 2X105 per mouse luciferase expressing MC38 tumor cell via portal vein injection on day −10 (A). Ten days later than tumor implantation, after an initial BLI measurement, mice were randomly divided into four groups and treated with control vehicle (PBS, n=9), AKB 9778 alone (40mg/kg, twice a day for 5 days, n=9), rIL-2 (600,000 IU per mouse, twice a day for 5 day, n=9) or the combination of rIL-2 and AKT 9778 (n=8). Tumor growth was measured by BLI weekly and presented as the intensity of the luciferase signal. B, BLI signals represent tumor development in individual animals) before (day 0) and following treatment. C, On day 4 following IL-2 injection, two hours following the last dose of IL-2 injection, two mice from each group were sacrificed and net lung were weighted. Results shown are the summary of three independent experiments. *p<0.05, ***p<0.001
Clinically, during the course of HDIL-2 therapy, one of the major adverse effects is the vascular leak syndrome (VLS), resulting in fluid retention associated with pulmonary edema[5]. To determine the effect of AKB-9778 on IL-2-induced vascular leakage, murine net lung weight as an indicator of edema was measured two hours following the last dose of IL-2 on Day 4 (Figure 1C). Administration of HDIL-2 dramatically increased the lung weight (p<0.001), which was significantly reduced with AKB 9778 (p<0.05, Figure 1C), demonstrating that the VE-PTP inhibitor, AKB 9778, protected mice against IL-2-induced VLS. Thus, AKB-9778 not only increased its efficacy of IL-2 treatment, but also helped relieve the side effects, slightly prolonging the resultant survival (Supplementary Fig. S1), approaching statistical significance (p = 0.07).
AKB-9778 modulated inflammatory cytokine storm induced by HDIL-2 administration
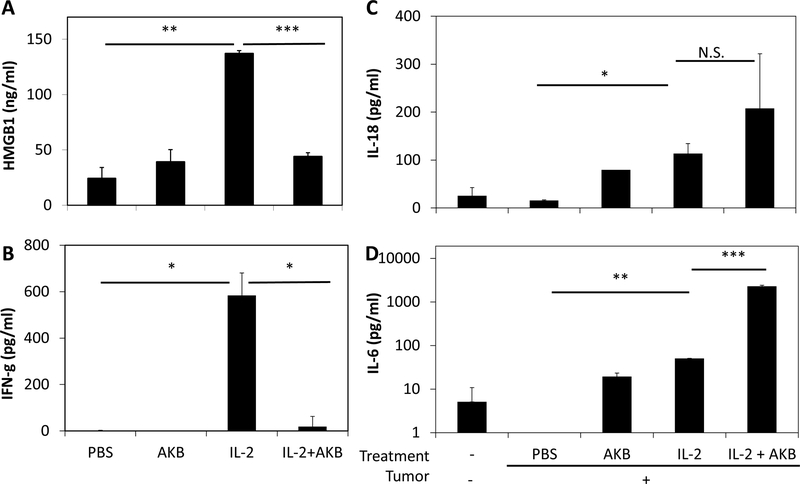
IL-2 induced systemic toxicity, at least if not all, partially results from excessive abundance of various cytokines released into the local microenvironment and circulated systemically. This overwhelming cytokine storm leads to exaggerated immune responses [19]. The precise reason for that is not fully understood but may be caused by an exaggerated response triggered by IL-2 and release of tumor necrosis factor (TNF), interleukin-1 (IL-1) and high mobility group box 1(HMGB1), and an array of other cytokines and chemokines. This so-called cytokine storm could give rise to tissue dysfunction and emergent systemic multi-organ failure. During the past decades, deeper understanding of the biology of inflammation revealed that specific cytokines are sufficient pathogenic mediators of diseases and that selectively targeting such mediators, including HMGB1, TNF, interferon-γ (IFN-γ), IL-1 and IL-6, could attenuate the major clinical signs and symptoms of inflammation[20]. To address the question if AKB 9778 has effects on cytokine release, we examined serum level of several representative cytokines following therapy. HMGB1 is a crucial effector molecule in many pathological settings from acute sepsis to sterile inflammation during trauma, as well as in cancer or chronic inflammatory diseases[21]. It is the central to both innate and adaptive immunity[20,22]. Results from our group suggest that HMGB1 is the key regulator of IL-2-triggered toxicity (Liang X., Li G. and Lotze MT., unpublished data). In the hepatic metastasis model, compared with the PBS control group, serum HMGB1 levels were dramatically increased in animals receiving HDIL-2 (p<0.004) (Figure 2A). Conversely, combination of AKB-9778 and IL-2 significantly reduced serum HMGB1 to the basal level (p<0.001 compared to HDIL-2 alone). Consistently, HDIL-2 significantly increased the level of IFN-γ in serum, whereas AKB-9778 inhibited it (Figure 2B). Besides, IL-18 slightly increased (Figure 2C), consistent with previous observation with IL-2 plus chloroquine (CQ) administration[17]. Interestingly, IL-6 level was substantially elevated AKB (Figure 2D), which might be a sign of improved anti-tumor effect. IL-2 and IL-1β were barely detectable (data not shown). Collectively, the “cytokine storm” associated with the systemic toxicity of HDIL-2 were at least partially inhibited by AKB-9778 administration.
Figure 2. AKB-9778 modulates inflammatory cytokine release by HDIL-2.
On day 4 following IL-2 injection, two hours following the last dose of IL-2 injection, two mice from each group were sacrificed and serum was harvested. Serum HMGB1 (A), IFN-g (B), IL-18 (C) and IL-6 (D) levels were measured by ELISA. Results shown are representative of two independent experiments. *p<0.05, **p<0.01, ***p<0.001, N.S. not significant.
AKB-9778 further enhanced immune cell infiltration within the liver
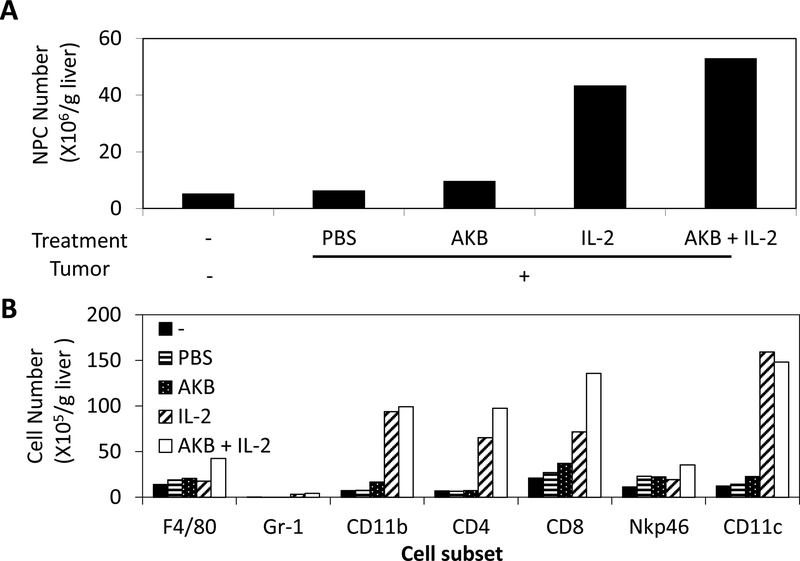
Administration of HDIL-2 and AKB 9778 assisted in immune cells to migrate and infiltrate into liver. Two hours following the last dose of HDIL-2 twice daily for five days, liver non-parenchymal cells were isolated, counted and analyzed by flow cytometry. Livers were pooled from animals in order to get the number of non-parenchymal cells more accurately. Consistent with our previous studies, intrahepatic leukocyte numbers stayed around the same following intra-portal infusion of tumor cells[17], but were increased following HDIL-2 administration (Figure 3A). Flow cytometric analysis demonstrated that HDIL-2 increased CD11b+, CD4+, CD8+, and CD11c+ cells (Figure 3B). Notably, co-administration of AKB-9778 further enhanced this effect (50% CD4+ T, 90% CD8+ T, and 84% NKp46+ Natural Killer (NK) cells), correlating with improved anti-tumor effect (Figure 1B).
Figure 3. AKB-9778 promotes immune cell migration and infiltration into the liver.
Two hours following the last dose of HDIL-2, liver non-parenchymal (NPC) cells were isolated and collected together from 3 mice per group in order to get accurate number. NPC cells were counted (A), followed by staining with individual antibodies and analyzed by flow cytometry (B). Individual markers were chosen for specific cell subsets among immune cell population.
AKB-9778 reduced plasma Ang2 levels in IL-2-treated mice and affected tube formation in endothelial cells
Others have suggested that AKB-9778 selectively increased pTie2 levels in the endothelial cells in a time/dose-dependent manner, as do the levels of pAKT and pERK1/2[15] (Cao M. and Yi T., Cleveland Clinic, unpublished data). Tie2 is a receptor protein tyrosine kinase and can activate the AKT signaling cascade, essential for endothelial cell function and viability[8].
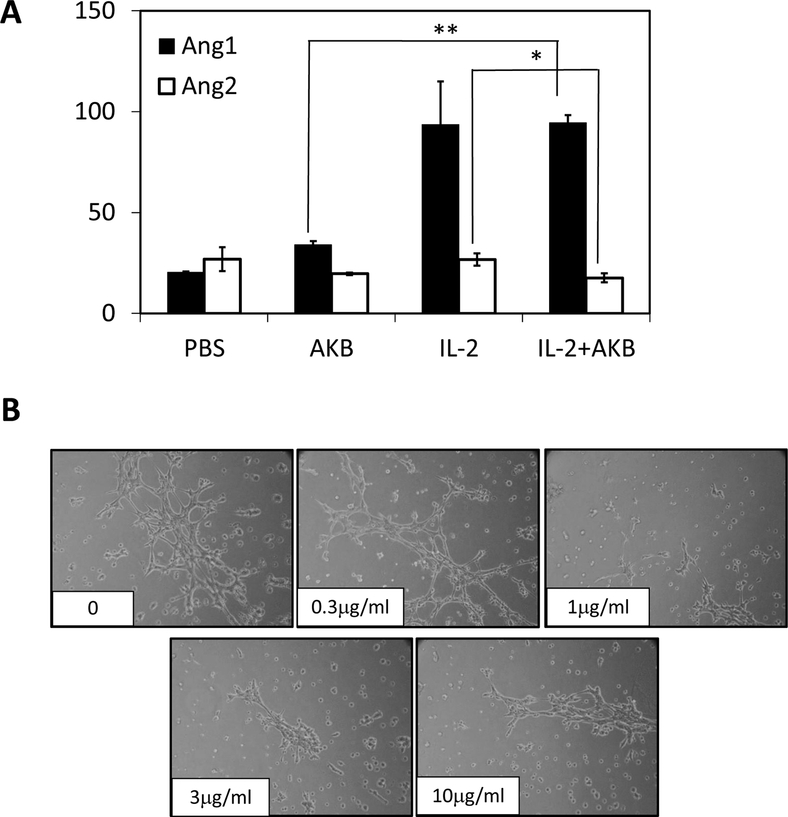
To gain insight into the mechanism of action of AKB-9778 in mitigating VLS, we examined the plasma levels of Ang2 and Ang1 in mice from each group treated with vehicle control, IL-2 or the combination of IL-2/AKB-9778 (Figure 4A). Ang2 protein was evident at substantial level in serum samples from all groups and remained essentially the same during the course of treatment in serum from either PBS or IL-2-treated mice (Figure 4A). However, Ang2 protein decreased gradually in sera of AKB-9778-administered mice in the presence or absence of IL-2, indicating that AKB-9778 may influence pTie2 level by reducing Ang2. Ang1 protein level was increased around 4-fold in both IL-2 alone group and combination group, suggesting IL-2 treatment increased circulating Ang1 in mice, which was not affected by VE-PTP inhibitor treatment.
Figure 4. AKB-9778 reduces plasma Ang2 levels and affects endothelial tube formation.
A) Serum Ang2 and Ang1 levels were measured during the time course of IL-2 +/− AKB-9778 administration. C) Human Dermal Microvascular Endothelial Cells (HDMVECs) were seeded onto solidified growth factor reduced Matrigel in MCDB131-DMEM. Media was supplemented with AKB-9778 at increasing concentrations as shown in the representative images. HDMVEC tube formation was with an Olympus D25 camera attached to a Nikon inverted microscope using a 20x objective. (n=3)
We further evaluated whether AKB-9778 itself affected the functions of immune cells and the viability on endothelial cells and tumor cells in vitro. In the classic 51Cr release assay, there was no apparent impact on splenocyte cytotoxicity, ranging from 10nM to 10μM (Supplementary Fig. S2). Furthermore, the capacities of tumor cell growth were not inhibited by treatment of AKB, indicating AKB didn’t act on tumor cells directly (Supplementary Fig. S3). To address the effect of AKB-9778 on endothelium, we utilized a common assay of angiogenesis, in which endothelial cells (ECs) are placed in a matrix of basement membrane proteins to assess their ability to form tube-like structures with neighboring cells. Specifically, we evaluated EC tube formation on matrigel in the presence of AKB-9778 at increasing concentrations. After four hours of culture on growth factor-reduced matrigel, tube formation was reduced in cells exposed to AKB-9778 at a dose of 1μg/ml and higher (Figure 4C).
DISCUSSION
With durable complete responses in patients with advanced melanoma and renal cancer[1,2], IL-2 continues to hold promise for an improved clinical spectrum of activity. However, its toxicity including the vascular leak syndrome (VLS)[3] and cytokine storm[23] often requires early cessation of treatment, restricting its full potential. Lessening adverse effects to extend the therapeutic index of IL-2 therapy could enhance therapeutic efficacy. A number of agents have been under investigation in combination with IL-2 in prior studies with little clinical benefits identified[6,7].
In this study, we used a reliable murine hepatic metastatic colorectal cancer model developed previously by our group[17]. This model emulates tumor metastases more closely and is more applicable to IL-2 therapy, given the fact that IL-2 has been approved for patients with metastatic renal cancer and melanoma. We found targeting VE-PTP with a selective small molecule inhibitor, AKB-9778, augmented anti-tumor effects induced by IL-2 (Figure 1B), associated with enhanced immune cell infiltrates (Figure 3A and 3B), demonstrating the therapeutic potential for this compound as an adjuvant of IL-2 therapy. Considering the essential regulation of Tie2 signaling pathway in endothelial cells maintenance and vascular integrity[8], as well as its minimal impact on tumor viability (Supplementary S3), its anti-tumor action might result from modulation of the tumor vasculature, possibly allowing more effector immune cells to transmigrate and infiltrate within the liver. Alternatively it could delay tumor growth and reduce metastasis by normalizingof the structure and function of tumor vessels [15]. Genetic depletion of endothelial VE-PTP in mice induced tumor blood vessel enlargement, although it had no apparent impact on tumor growth under those experimental conditions[24]. Pharmacological VE-PTP inhibition, decreased growth of small tumors as well as metastatic progression by a vascular stabilizing effect[15]. What’s more, the capacity of AKB-9778 to reduce circulating Ang2 levels might be unique in the developed vasculature and could contribute to delayed tumor growth in light of the growth promoting activity of the angiopoietin [25,26]. Given that Ang2 can regulate tumor cells[25,26] and myeloid cells[27], modulation of Ang2 and/or Tie2 signaling in the non-endothelial cells might also be involved if VE-PTP expression is verified. Further mechanistic evaluations of AKB-9778 in other tumor models are warranted. In particular, assessment of AKB-9778 effects on tumor blood vessels could provide insights into the model of action for the inhibitor, especially in the setting of IL-2 therapy.
In addition to anti-tumor activity, targeting VE-PTP with AKB-9778 also contributed to mitigating VLS, as featured by lessened pulmonary edema (Figure 1C). The consequences were likely attributable to various effects. First, AKB-9778 increased pTie2 levels (and down-stream pATK) in endothelial cells (Cao M. and Yi T., unpublished data), which occupies a crucial role in endothelial viability[8]. AKB-9778 was also capable of diminishing the activity of the Tie2-antagonist Ang2 as demonstrated by the reduced levels of Ang2 in serum treated with AKB-9778+/−IL-2 (Figure 4A). In light of the fact that Ang2 was primarily released by stressed endothelial cells[28] that require Tie2 signals for viability[8], AKB-9778 might prevent Ang2 release by endothelial cells via activating the Tie2 signaling cascade to reduce endothelial stress. Additionally, AKB-9778 appeared to reduce endothelial tube formation in vitro, suggesting that it potentially can affect the function of the endothelium in vivo. During angiogenesis, destabilization of capillaries allows for migration of endothelial cells out of the vessel to promote neovascularization elsewhere, which can contribute to diminished permeability. Ang2 mediates blood vessel destabilization [29] and is stored in EC Weibel-Palade bodies[28]. Thus, the effects of AKB-9778 on endothelial cell angiogenic behavior may infer a benefit in attenuating vascular leak. Given that each of the effects could be beneficial, the combination may account for the efficacy of targeting VE-PTP in mitigating VLS in mice.
Apart from VLS, cytokine storm was also relieved with a spectrum of decreased cytokine in serum (Figure 2A–B), which might limit endothelial stress, and the resultant adventitial inflammatory response at non-tumor sites. Furthermore, active immune cells recruited within the tumor environment could locally secrete the pro-inflammatory cytokines rather than systemically. Among those cytokines, HMGB1 is critical in the pathogenesis of many inflammatory states following tissue damage or injury, and is found in the serum from patients including those with cancer as well as other settings[21,30,31]. We have previously shown that HMGB1 was released at very high levels following HDIL-2 treatment[17]. It is a potentially candidate molecule mediating the development of the ‘systemic autophagic syndrome’, a phenomenon we termed for the adverse effects of IL-2. HMGB1 level was significantly decreased in mice treated with AKB-9778, shedding light on the possibilities that AKB-9778 limited organ dysfunction and tumor growth by decreasing HMGB1. The detailed mechanism(s) by which AKB-9778 reduced HMGB1 level needs further investigation.
How AKB-9778 mitigates VLS has diverse implications in other disorders. Vascular leak is a common pathogenic element in septic shock, infections, inflammations, auto-immune diseases as well as in various vascular conditions[32,33]. Hence, targeting VE-PTP in endothelial cells might be beneficial as interventions or adjuvants for these indications. The main mechanism is likely be direct activation of Tie 2 in endothelial cells resulting in vascular stabilization via several mechanisms including reduction of Ang2 release.
Identification of the VE-PTP inhibitor with pre-clinical activity for VLS and cancer indicates a promising future in developing PTP-related therapeutics. Given the fact that therapeutic PTP inhibitors are in early stages of development [34], our work herein provides pre-clinical insights of targeting VE-PTP to reduce IL-2 toxicity and to improve clinical outcome. VE-PTP inhibitors may allow higher or longer IL-2 dosing and a broader usage of the cytokine and other immuno-therapeutics, for which vascular toxicity is common [35]. The VE-PTP inhibitor AKB-9778 is therefore promising for clinical translation and developing VE-PTP-targeting therapeutics. Recent information [36] suggests that such approaches also limits renal injury secondary to diabetes. In addition [37], Angiopoietin 2 concentration (normal 2.5 +/− 1.0ng/mL) increased in kidney cancer patients during IL-2 treatment to 18.6 +/− 4.9 ng/mL (p < 0.05) during the last day of therapy correlating negatively with forced expiratory volume in 1 s (Spearman r = −0.78, p < 0.0001). Thus efforts to diminish toxicity and enhance efficacy in the setting of planned clinical trials with IL-2 and AKB-9778 are in order.
Supplementary Material
Conflicts of Interest and Sources of Funding:
Work done in support of findings reviewed in this manuscript was aided by the Core Support of the UPCI 3P30CA047904–22S1 (Davidson). This work was also supported by the National Institutes of Health (R01 CA181450 to MTL/HJZ).
Abbreviations:
- VE-PTP
vascular endothelial protein tyrosine phosphatase
- VLS
vascular leak syndrome
- Ang1
angiopoietin 1
- Ang2
angiopoietin 2
- IL-2
interleukin-2
- Tie2
tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2
- pTie-2
phospho-Tie2
- HMGB1
high mobility group box 1
- IFN-γ
interferon-γ
REFERENCES
- 1.Rosenberg SA. Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J. Sci. Am. [Internet]. 6 Suppl 1, S2–7 (2000). Available from: http://www.ncbi.nlm.nih.gov/pubmed/10685650. [PubMed] [Google Scholar]
- 2.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. [Internet]. 17(7), 2105–16 (1999). Available from: http://www.ncbi.nlm.nih.gov/pubmed/10561265. [DOI] [PubMed] [Google Scholar]
- 3.Mier JW, Aronson FR, Numerof RP, Vachino G, Atkins MB. Toxicity of immunotherapy with interleukin-2 and lymphokine-activated killer cells. Pathol. Immunopathol. Res. [Internet]. 7(6), 459–76 (1988). Available from: http://www.ncbi.nlm.nih.gov/pubmed/2976936. [DOI] [PubMed] [Google Scholar]
- 4.Lotze MT, Matory YL, Rayner AA, et al. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer [Internet]. 58(12), 2764–72 (1986). Available from: http://www.ncbi.nlm.nih.gov/pubmed/3490903. [DOI] [PubMed] [Google Scholar]
- 5.Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology [Internet]. 37(2–3), 117–32 (1997). Available from: http://www.ncbi.nlm.nih.gov/pubmed/9403331. [DOI] [PubMed] [Google Scholar]
- 6.Du Bois JS, Trehu EG, Mier JW, et al. Randomized placebo-controlled clinical trial of high-dose interleukin-2 in combination with a soluble p75 tumor necrosis factor receptor immunoglobulin G chimera in patients with advanced melanoma and renal cell carcinoma. J. Clin. Oncol. [Internet]. 15(3), 1052–62 (1997). Available from: http://www.ncbi.nlm.nih.gov/pubmed/9060545. [DOI] [PubMed] [Google Scholar]
- 7.McDermott DF, Trehu EG, Mier JW, et al. A two-part phase I trial of high-dose interleukin 2 in combination with soluble (Chinese hamster ovary) interleukin 1 receptor. Clin. Cancer Res. [Internet]. 4(5), 1203–13 (1998). Available from: http://www.ncbi.nlm.nih.gov/pubmed/9607578. [PubMed] [Google Scholar]
- 8.Peters KG, Kontos CD, Lin PC, et al. Functional significance of Tie2 signaling in the adult vasculature. Recent Prog. Horm. Res. [Internet]. 59, 51–71 (2004). Available from: http://www.ncbi.nlm.nih.gov/pubmed/14749497. [DOI] [PubMed] [Google Scholar]
- 9.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science (80-. ). [Internet]. 277(5322), 55–60 (1997). Available from: http://www.ncbi.nlm.nih.gov/pubmed/9204896. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK, Carmeliet P. SnapShot: Tumor angiogenesis. Cell [Internet]. 149(6), 1408–1408.e1 (2012). Available from: http://www.ncbi.nlm.nih.gov/pubmed/22682256. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher DC, Bhatt RS, Parikh SM, et al. Angiopoietin 2 is a potential mediator of high-dose interleukin 2-induced vascular leak. Clin. Cancer Res. [Internet]. 13(7), 2115–20 (2007). Available from: http://www.ncbi.nlm.nih.gov/pubmed/17404094. [DOI] [PubMed] [Google Scholar]
- 12.Fachinger G, Deutsch U, Risau W. Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2. Oncogene [Internet]. 18(43), 5948–53 (1999). Available from: http://www.ncbi.nlm.nih.gov/pubmed/10557082. [DOI] [PubMed] [Google Scholar]
- 13.Bäumer S, Keller L, Holtmann A, et al. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood [Internet]. 107(12), 4754–62 (2006). Available from: http://www.ncbi.nlm.nih.gov/pubmed/16514057. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez MG, Hughes VC, Pan L, et al. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis. Proc. Natl. Acad. Sci. U. S. A. [Internet]. 104(9), 3243–8 (2007). Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1802730&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goel S, Gupta N, Walcott BP, et al. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J. Natl. Cancer Inst. [Internet]. 105(16), 1188–201 (2013). Available from: http://www.ncbi.nlm.nih.gov/pubmed/23899555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarasinghe KKD, Evdokimov AG, Evidokimov AG, et al. Design and synthesis of potent, non-peptidic inhibitors of HPTPbeta. Bioorg. Med. Chem. Lett. [Internet]. 16(16), 4252–6 (2006). Available from: http://www.ncbi.nlm.nih.gov/pubmed/16759857. [DOI] [PubMed] [Google Scholar]
- 17.Liang X, De Vera ME, Buchser WJ, et al. Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Cancer Res. [Internet]. 72(11), 2791–801 (2012). Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3417121&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang X, Chavez ARDV, Schapiro NE, et al. Ethyl pyruvate administration inhibits hepatic tumor growth. J. Leukoc. Biol. [Internet]. 86(3), 599–607 (2009). Available from: http://www.ncbi.nlm.nih.gov/pubmed/19584311. [DOI] [PubMed] [Google Scholar]
- 19.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. [Internet]. 76(1), 16–32 (2012). Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3294426&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. [Internet]. 29, 139–62 (2011). Available from: http://www.ncbi.nlm.nih.gov/pubmed/21219181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. [Internet]. 28, 367–88 (2010). Available from: http://www.ncbi.nlm.nih.gov/pubmed/20192808. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Liang X, Lotze MT. HMGB1: The Central Cytokine for All Lymphoid Cells. Front. Immunol. [Internet]. 4(March), 68 (2013). Available from: http://www.ncbi.nlm.nih.gov/pubmed/23519706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panelli MC, White R, Foster M, et al. Forecasting the cytokine storm following systemic interleukin (IL)-2 administration. J. Transl. Med. [Internet]. 2(1), 17 (2004). Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=434535&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Huang H, Boland P, et al. Embryonic stem cell tumor model reveals role of vascular endothelial receptor tyrosine phosphatase in regulating Tie2 pathway in tumor angiogenesis. Proc. Natl. Acad. Sci. U. S. A. [Internet]. 106(52), 22399–404 (2009). Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2794035&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helfrich I, Edler L, Sucker A, et al. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin. Cancer Res. [Internet]. 15(4), 1384–92 (2009). Available from: http://www.ncbi.nlm.nih.gov/pubmed/19228739. [DOI] [PubMed] [Google Scholar]
- 26.Hu B, Cheng S-Y. Angiopoietin-2: development of inhibitors for cancer therapy. Curr. Oncol. Rep. [Internet]. 11(2), 111–6 (2009). Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2867109&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Palma M, Naldini L. Tie2-expressing monocytes (TEMs): novel targets and vehicles of anticancer therapy? Biochim. Biophys. Acta [Internet]. 1796(1), 5–10 (2009). Available from: http://www.ncbi.nlm.nih.gov/pubmed/19362584. [DOI] [PubMed] [Google Scholar]
- 28.Fiedler U, Scharpfenecker M, Koidl S, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood [Internet]. 103(11), 4150–6 (2004). Available from: http://www.ncbi.nlm.nih.gov/pubmed/14976056. [DOI] [PubMed] [Google Scholar]
- 29.Jain RK. Molecular regulation of vessel maturation. Nat. Med. [Internet]. 9(6), 685–93 (2003). Available from: http://www.ncbi.nlm.nih.gov/pubmed/12778167. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Tang D, Lotze MT. Ménage à Trois in stress: DAMPs, redox and autophagy. Semin. Cancer Biol. [Internet]. 23(5), 380–90 (2013). Available from: http://www.ncbi.nlm.nih.gov/pubmed/23994764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang D, Kang R, Zeh HJ, Lotze MT. High-mobility group box 1 and cancer. Biochim. Biophys. Acta [Internet]. 1799(1–2), 131–40 (2010). Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2818552&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Heijden M, van Nieuw Amerongen GP, Chedamni S, van Hinsbergh VWM, Johan Groeneveld AB. The angiopoietin-Tie2 system as a therapeutic target in sepsis and acute lung injury. Expert Opin. Ther. Targets [Internet]. 13(1), 39–53 (2009). Available from: http://www.ncbi.nlm.nih.gov/pubmed/19063705. [DOI] [PubMed] [Google Scholar]
- 33.Van Meurs M, Kümpers P, Ligtenberg JJM, Meertens JHJM, Molema G, Zijlstra JG. Bench-to-bedside review: Angiopoietin signalling in critical illness - a future target? Crit. Care [Internet]. 13(2), 207 (2009). Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2689450&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi T, Lindner D. The role and target potential of protein tyrosine phosphatases in cancer. Curr. Oncol. Rep. [Internet]. 10(2), 114–21 (2008). Available from: http://www.ncbi.nlm.nih.gov/pubmed/18377824. [DOI] [PubMed] [Google Scholar]
- 35.Bascon JU. Vascular leak syndrome: a troublesome side effect of immunotherapy, Immunopharmacology, 39/3 (1998) 255. Immunopharmacology [Internet]. 39(3), 255, 257 (1998). Available from: http://www.ncbi.nlm.nih.gov/pubmed/9754911. [PubMed] [Google Scholar]
- 36.Carota IA, Kenig-Kozlovsky Y, Onay T, Scott R, Thomson BR, Souma T, Bartlett CS, Li Y, Procissi D, Ramirez V, Yamaguchi S, Tarjus A, Tanna CE, Li C, Eremina V, Vestweber D, Oladipupo SS, Breyer MD, Quaggin SE. Targeting VE-PTP phosphatase protects the kidney from diabetic injury. J Exp Med. April 1;216(4):936–949. (2019) doi: 10.1084/jem.20180009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gores KM, Delsing AS, Kraus SJ, Powers L, Vaena DA, Milhem MM, Monick M, Doerschug KC. Plasma angiopoietin 2 concentrations are related to impaired lung function and organ failure in a clinical cohort receiving high-dose interleukin 2 therapy. Shock. 2014. August;42(2):115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.