Abstract
The search for new, robust, and reproducible biomarkers for Alzheimer's disease (AD) diagnosis is a challenge. We recently reported that salivary lactoferrin (Lf) could be presented as new biomarker candidate for AD, being both non‐invasive and cost‐effective, as well as having appropriate diagnostic performance for the clinical detection of AD subjects. Saliva is an attractive sample type for biomarker‐based testing approaches for several other diseases; however, its composition may change under certain circumstances. It is therefore critical to maintain a consistent salivary handling protocol, considering possible extrinsic factors that may influence salivary Lf concentration. In this work, we analyzed salivary Lf concentration under different handling conditions and donor‐dependent factors including age, inter‐diurnal variations, physical activity, and pharmacological treatments. Our aim was to evaluate the influence of such conditions on salivary Lf concentration. In conclusion, we found that most of these extrinsic factors should be considered in the future when using Lf as a predictive biomarker for AD.
Keywords: aging, Alzheimer's disease, biomarker, circadian rhythm, exercise, lactoferrin, measurement, regulatory factors, reproducibility, saliva, treatments
1. NARRATIVE
In searching for possible robust biomarkers for Alzheimer's disease (AD), researchers worldwide unanimously agreed to establish consistent criteria. These criteria include samples that are easy to collect, of high sensitivity and specificity, inexpensive in commercial test format, with clear cut‐off values, and having reproducible results over time. We recently reported the potential of salivary lactoferrin (Lf) as a candidate biomarker for AD as it meets the above‐mentioned criteria. By examining two independent cohorts, our results showed that reduced salivary Lf levels are specific for AD. This potential biomarker displayed accurate parameters with very high sensitivity and specificity, combined with being non‐invasive and cost‐effective features for a good biomarker. 1 Salivary biomarkers are promising candidates for the diagnosis and monitoring of neurological diseases. 2 , 3 , 4 It has been shown that Lf is useful in the diagnosis and management of other diseases, including periodontitis, inflammation, and gastrointestinal diseases, 5 , 6 as well as in salivary gland carcinoma. 7 Although saliva is the source of an attractive field for biomarker‐based testing for several diseases, it is important to consider factors that may influence salivary Lf concentration. 8 This is because saliva composition may change under certain circumstances, including sampling processing, and many environmental and lifestyle factors. 9 To confirm the hypothesis that salivary Lf is suitable as a diagnostic biomarker for AD, key aspects that must be taken into account are debated here before the use of this biomarker in future clinical practice. One key point is the storage and maintenance of salivary samples to avoid changes in protein concentration between the collection time and the time when the analysis is carried out.
Saliva flow rate may vary depending upon collection time during the day, 10 therefore research should be carried out minimizing the possible circadian variability on salivary Lf production. Another key aspect that we must consider is donor age as it may influence salivary Lf levels. Among the elderly population, susceptibility to infections is increased and the main reason could be a reduced adaptive immunity response. 11 Because salivary Lf is an important innate‐immune defense element, we sought to determine whether healthy aging correlates with optimal salivary Lf production.
Physical activity may also influence Lf levels. Epidemiologic data suggest that regular physical activity may slow the rate of cognitive decline and thus delay or prevent dementia. 12 , 13 Acute exercise with moderate intensity has been shown to increase antimicrobial protein concentration in saliva and particularly Lf levels (when levels are compared before and immediately after exercise). 14 Noticeably, 1 hour after finishing exercise, salivary Lf levels returned to the initial values registered before exercise. 14
RESEARCH IN CONTEXT
Systematic review: The association between “salivary lactoferrin (Lf)” and “Alzheimer's disease (AD) biomarkers” and the terms: “pre‐analytical handling procedures” (including temperature storage and freezing/thawing cycles), “regulatory extrinsic factors” (including aging, circadian rhythm, exercise, and AD medications) and “standardized protocols,” was reviewed in the literature. Lately, there have been intensified efforts in searching for minimally or non‐invasive peripheral markers for the early diagnosis of AD, focused on blood and/or saliva. We have recently showed that salivary Lf discriminates between patients with mild cognitive impairment (MCI) and AD and control subjects.
Interpretation: We found that handling conditions and donor‐dependent factors including age, between‐day variations, physical activity, and medication, may influence salivary Lf concentration. These results support the recommendation to maintain a consistent salivary handling protocol, considering possible extrinsic factors that may impact salivary Lf concentration. All these extrinsic factors must be considered for future validation of salivary Lf as an AD biomarker.
Future direction: These recommendations provide a checklist for standardizing collection protocols to establish large cohorts of well‐characterized samples in biomarker research studies. Future works should use the suggested standardized procedures as prerequisite ensuring data replication, and guaranteeing that Lf levels are not compromised by pre‐analytical factors.
The utility of salivary Lf as a diagnostic biomarker for AD will depend upon the impact on its concentration of currently used treatments. In particular, the National Institute for Health and Care Excellence (NICE) recommends the use of acetylcholinesterase (AChE) inhibitors for patients with mild‐to‐moderate AD and memantine for severe AD. AChE mediates acetylcholine catabolism and salivary protein secretion may be mediated by acetylcholine release under autonomic nervous system control. 15 Hence, AChE inhibitors may influence the Lf levels by increasing acetylcholine levels.
The present work aimed to study the effects of different pre‐analytical handling procedures (including storage at different temperatures and the freezing/thawing cycles) and regulatory extrinsic factors (including aging, circadian rhythm, exercise, and AD medications) that may influence salivary Lf production in donors. We found that extrinsic factors such as temperature and consecutive freezing/thawing cycles affect Lf concentration in salivary samples. However, it is important to note that 4°C sample maintenance of up to 10 days while maintaining less than three freezing/thawing cycles does not significantly modify Lf concentration. This condition facilitates sample transport and analysis making the process easier and more cost‐effective. Therefore, we recommend a standardized collection and storage protocol to replicate studies and to ensure that the statistical power is not compromised by preanalytical factors. We recommend freezing saliva samples after collection at –80°C into aliquots to ensure the long‐term stability of Lf. Small aliquot volumes are optimal to avoid freezing/thawing cycles by requiring only one aliquot to be thawed and maintained at 4°C for subsequent analysis of Lf levels.
For biomarkers that are influenced by circadian rhythms, the collection time is important. However, we did not find any significant diurnal variation impact on salivary Lf levels. As it often is difficult to accomplish standardization of sample acquisition time in daily clinical practice, a more rigorous study collecting samples for evaluation every 8 hours during a day should be performed. Meanwhile, to avoid possible modifications of salivary Lf levels in collected samples, we recommend carrying out the collection at the same time of the day with every single donor.
Extrinsic factors such as age, moderate physical activity, and medical treatments may modify salivary Lf production in donors. Although salivary Lf levels may vary with age, these variations do not occur in subjects within the same range of age. Therefore, to compare Lf levels between healthy and AD subjects we recommend collecting samples within age‐matched grouped donors for comparison. Information on the age at sampling is necessary to allow comparisons with the age reference value. As salivary Lf levels undergo an acute increase after exercise and these high levels remain for at least for 1 hour, we, recommend collection of samples during rest or at least 1 hour after completion of any intense or moderate physical activity, to avoid false increases in Lf levels. Medication can influence the reference range of biomarkers, so this clinical information must be provided with each sample. Treatment with AChE inhibitors may affect salivary Lf levels increasing its concentration and this could be a consequence of treatment itself. To confirm that AChE inhibitor treatment increases salivary Lf levels, we recommend carrying out future longitudinal studies analyzing salivary Lf levels in treated or untreated patients separately, facilitating disease progression monitoring.
In conclusion, all these extrinsic factors must be considered for future validation of salivary Lf as an AD biomarker. In summary, we recommend the above‐proposed procedures for handling and maintaining samples for all research groups or labs focused on the validation of salivary Lf levels as a biomarker for AD. Additionally, we encourage consideration of variations in salivary Lf levels that might be related to age, lifestyle, and ongoing patient treatments. These recommendations provide a checklist for standardizing collection to establish large cohorts of well‐characterized samples in biomarker research studies. The suggested standardized protocols are a prerequisite to ensuring data reproducibility, similarly they also guarantee that the statistical power gained by increasing the numbers of saliva samples is not compromised by pre‐analytical factors.
2. CONSOLIDATED RESULTS AND STUDY DESIGN
We have analyzed Lf concentration in salivary samples under different storage conditions by enzyme‐linked immunosorbent assays (ELISAs). Then, we evaluated possible variations in Lf concentration in saliva samples from donors (healthy subjects and AD patients) in different experimental conditions, varying circadian rhythm, age, exercise, and AD medication (AChE inhibitors, and memantine). Additionally, total protein content in saliva samples was evaluated according to whether the patients were under treatment or not. In all cases, salivary Lf concentration was measured using the Lf human ELISA kit (ab200015, Abcam) and total protein content was estimated by the bicinchoninic acid (BCA) method (Pierce). For all measurements, except for the analysis of Lf concentration after several freezing/thawing cycles, saliva samples underwent one freezing/thawing cycle only. Finally, statistical analysis for multiple comparisons was calculated by one‐way analysis of variance followed by Fisher's least significant difference correction. Student's t‐test was used for single pairwise comparisons. Statistical analysis and exponential curve fitting were performed using GraphPad Prism 6.01 (GraphPad Software). Data were generated from a minimum of three independent measurements by triplicate. All data are expressed as mean ± standard error of the mean (SEM) or ± standard deviation (SD) when appropriate. Grubbs outlier filter was used for all data. In all cases, statistical significance was set at P < .05 (*P < .05, **P < .01, ***P < .001; ****P < .0001, #P < .05, ##P < .01).
As we previously mentioned, sample processing and storage procedures are the main pre‐analytic factors that may influence salivary Lf concentration. Sample storage temperature and time after thawing may give different concentration values of salivary Lf. To analyze possible variations over time on salivary protein concentration after thawing, we analyzed salivary Lf concentration at different timepoints. The samples were stored at –80°C immediately after collection and the analysis was carried out starting the day when the samples were thawed (timepoint zero), and at 5, 10, and 15 days thereafter. The stability of 4°C stored salivary samples was evaluated during these 15 days. At these timepoints, bench‐top sample temperature was maintained at 4°C. We found that the Lf concentration of salivary samples was highest at baseline (–80°C). Salivary Lf concentration remained stable during the first 10 days and significantly decreased by day 15 after thawing (≈30%; Figure 1A). Samples that underwent no, or only one freezing/thawing cycle, did not show differences in salivary protein concentration (Figure 1B). After two freezing/thawing cycles, Lf concentration in saliva progressively decreased, with a significant reduction from the third cycle (Figure 1C). Such concentration reduction may have been due to protein denaturalization.
FIGURE 1.
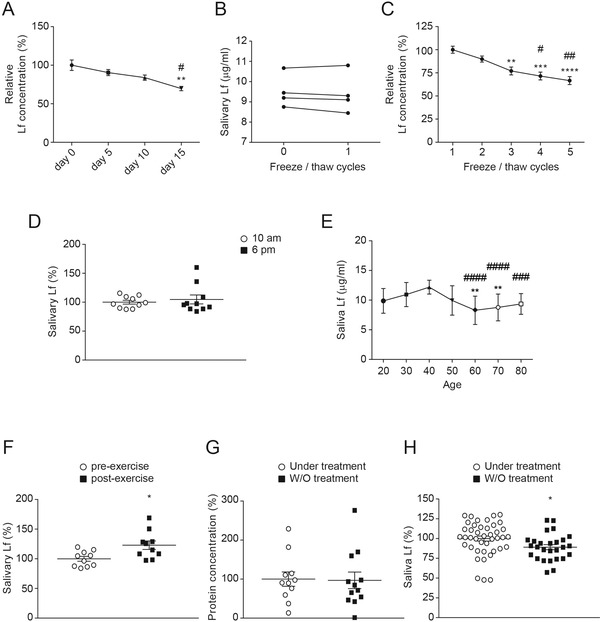
Salivary lactoferrin (Lf) concentration changes. A, Relative Lf concentration in salivary samples collected from four subjects (mean age 66.9 years) stored at 4°C for 15 days. Samples were frozen at –80°C after collection. Then, they were thawed and maintained at 4°C. Values after thawing were compared to the baseline levels from samples stored at –80°C stated as 100%. Data are expressed in percentage and are shown as mean ± standard error of the mean (SEM). Differences between groups were assessed using one‐way analysis of variance (ANOVA) followed by the Bonferroni test. **P < .01 versus day 0; #P < .05 versus day 5. B, Effect of freezing/thawing on salivary Lf concentration. Salivary Lf concentration in four subjects’ samples (mean age 66.9 years) that had not been subjected to any freeze/thaw cycle (point 0) compared to samples from the same subjects that had been subjected to one freeze–thaw cycle (point 1). Connecting lines between point 0 and point 1 represent Lf values from the same subject. Data show individual values of Lf concentration. C, Relative salivary Lf concentration in samples from eight subjects (mean age 66.9 years) related to the number of freeze–thaw cycles. Salivary Lf levels after only one freeze–thaw cycle were used as the baseline value and stated as 100%. Data are expressed in percentage and are shown as mean ± SEM. Differences between groups were assessed using one‐way ANOVA followed by the Bonferroni test. **P < .01, ***P < .001, ****P < .0001 versus cycle 1; #P < .05, ##P < .01 versus cycle 2. D, Salivary Lf levels in samples collected from 10 patients (mean age 52.5 years) at 10 am and after 8 hours (6 pm). Results showed no significant diurnal or day‐to‐day variation of Lf concentration. Data are shown as mean ± SEM. E, Salivary Lf levels from 20‐ to 80‐year‐old subjects (n = 12 to 37 subjects per group). Data are shown as mean ± standard deviation (SD). Differences between groups were assessed using the Kruskal‐Wallis test. **P < .01 versus 30 y/o; ###P < .001; ####P < .0001 versus 40 y/o. F, Salivary Lf levels in samples collected from 10 subjects (mean age 52.5 years old) before and after physical exercise performance (1 hour training). Saliva was collected at two timepoints: pre‐exercise; and immediately after exercise cessation. Data are shown as mean ± SEM. Differences between groups were assessed using Student's t‐test. **P < .01. G, Total protein concentration in saliva samples collected from Alzheimer's disease (AD) patients (mean age 67.2 years) with (n = 12) or without (n = 12) acetylcholinesterase (AChE) inhibitor treatment. The results showed no significant variation in protein concentration. Data are shown as mean ± SEM. H, Salivary Lf levels from AD patients (mean age 67.2 years) with (n = 42) or without (n = 27) AChE inhibitor treatment. Treated AD patients showed higher salivary Lf levels. Data are shown as mean ± SEM. Differences between groups were assessed using Student's t‐test. *P < .05
We investigated the possible variability in salivary Lf concentration linked to the circadian cycle. Saliva flow rate may vary depending on the collection time during the day. We did not find statistical differences in salivary Lf levels between collected samples from the same subject in the morning and 8 hours later (Figure 1D). Our results are consistent with previous findings in which either non‐significant diurnal or day‐to‐day variation on salivary Lf levels in samples obtained from 20‐ to 25‐year‐old male subjects were reported. 16
It is known that age may influence salivary Lf production and this may be related to impaired innate‐immune defenses making the elderly more susceptible to infections. Therefore, age‐related differences in salivary Lf levels were also analyzed. A gradual reduction in salivary Lf concentration was observed in subjects from their fourth decade onward (Figure 1E).
The effect of physical activity on salivary Lf levels was also analyzed. We found that after 1 hour of moderate physical activity salivary Lf levels significantly increased. Such increase was evaluated in 10 adult participants compared to their levels analyzed just before the exercise (Figure 1F).
Regarding the possible effects of AChE inhibitor treatment on salivary protein production, no variations were found in total protein content when this was analyzed in mild‐to‐moderate AD patients recruited from our recent study 1 and grouped into medicated and non‐medicated groups (Figure 1G). Specific analysis of salivary Lf levels showed that they were significantly increased in AD patients under AChE inhibitor treatment (Figure 1H). These results may suggest possible specific immunity deregulation in AD with effects on salivary Lf release. 17 Regarding the possible effects of memantine treatment on salivary protein production, we mainly recruited patients with mild to moderate AD and in fewer numbers, patients with severe AD. As a consequence only five AD patients were treated with memantine. We did not find significant differences in total protein content or salivary Lf levels in memantine‐treated patients.
3. DETAILED METHODS AND RESULTS
The lack of standardized pre‐analytical procedures in biomarkers research may cause a huge variability in the results obtained. It is important, therefore, to get a unified pre‐analytical protocol that will help to standardize the specific biomarker measurements establishing global cut‐offs. 18 The present study aimed at checking salivary Lf concentration–affecting factors. This will help to develop unified protocols for pre‐analytical handling of saliva samples obtaining reliable measurements of Lf. Widespread implementation of AD biomarkers in routine clinical practice requires the establishment of standard operating procedures for such biological samples.
It is known sample protein concentration may be affected by several factors including sample handling. Collection and storage conditions must affect the levels of all proteins contained in the sample equally. On the other hand, intrinsic and extrinsic factors affecting donors might have influenced the production of specific proteins contained in the sample. The diagnostic power of a new biomarker may be determined by those factors that can modulate its expression. In this study, we assessed whether salivary Lf concentration in samples from AD patients and healthy donors is affected by the storage conditions and the freezing/thawing cycles after collection. Additionally, we have evaluated the impact of extrinsic factors affecting donors, including circadian rhythm, exercise, age, and medication on salivary Lf production.
Although the biological sample transport between different laboratories could be easier at 4°C, it may have detrimental consequences on Lf‐related results. A slight but non‐significant reduction in Lf concentration was observed in aliquots thawed once and maintained at 4°C for 10 days, compared to a reference sample (aliquot maintained at –80°C, not thawed until analysis). In any case, our findings indicate that Lf concentration remained unaltered in saliva samples stored at 4°C for at least 10 days, but we recommend avoiding 4°C storage above that time.
Freezing and thawing procedures may introduce concentration gradients in biological samples. To avoid such gradients, thorough mixing of the tube contents is necessary. In this regard, several works investigated the biophysical properties of sample‐containing proteins or proteins themselves. 19 , 20 In these studies it was demonstrated that repeated freeze–thaw cycles may affect protein stability and concentration. Authors indicate that using cryoprotectants in the sample buffer would be beneficial but such effect would disappear after four to five freeze–thaw cycles. 19 , 20
The reported reduction of salivary Lf concentration after repeated freeze–thaw cycles highlights the importance of avoiding such freezing–thawing. This would minimize sample degradation that might give misleading Lf concentration values.
Other possible influences, including lifestyle and exposure to environmental factors, may affect salivary Lf production. Several studies showed that salivary Lf concentration might be affected by circadian rhythms and physical activity 14 , 21 , 22 or environmental and lifestyle factors, including habits like smoking and medication intake. 23 , 24 , 25 Our results show no between‐day related differences on salivary Lf concentration. Although many studies show that protein release and metabolic pathways may respond instantaneously to changes in circadian clocks, our findings are consistent with other studies in which researchers did not find significant between‐day differences in concentration of Lf in saliva 16 and tears. 26
Here we show that salivary Lf concentration reduces with age, being significant in the fourth decade. Various researchers observed reduced Lf and peroxidase activity in healthy elderly subjects, thus representing a challenge for oral tissues due to imbalances in salivary antimicrobial agents. 27 , 28 , 29 Histological studies carried out on salivary glands have shown that the proportional volume of acinar cell secretion was reduced with age. 30 This was considered one of the major causes of dry mouth. 31 Acinar cells in the salivary glands secrete Lf. Thus, histological changes affecting the salivary gland may result in overall salivary gland hypofunction and reduced Lf levels.
We also found that physically active compared to sedentary subjects might benefit from increased immunological protection mechanisms at different levels. Several studies reported that salivary levels of antibacterial proteins/immunological agents were higher after exercise in physically active people compared to those observed in sedentary subjects. These observations highlight that regular exercise/training improves immune/inflammatory status. 14 , 32 , 33 , 34 Because physical activity and exercise result in increased salivary Lf levels, we propose that physically active people may have greater protection against infections by exercise‐modulated changes to the immune/inflammatory response. This observation reinforces the hypothesis that physical activity intervention could be a low‐cost and low‐risk protective factor for AD 36 and thus, we encourage regular physical activity intervention for patients with AD.
The impact of currently used AD treatments on salivary Lf concentration must be also explored. We estimated total protein content in collected saliva samples from AD patients, recruited from our recent study. 1 Subjects were grouped as patients under treatment or not under treatment with AChE inhibitors, and patients under treatment with or not under treatment with memantine, which are drugs currently used for AD treatment. Although the secretion of salivary proteins is mediated by acetylcholine release under autonomic nervous system control, 15 no changes were found in total protein concentration in saliva samples collected from AD patients, with or without AChE inhibitor treatment. However, salivary Lf levels were significantly increased in AD patients under AChE inhibitors treatment suggesting a protein‐specific effect. This could be explained as treatment with AChE inhibitors increases acetylcholine levels, thus upregulating Lf release in salivary glands. Such upregulation was reported in a study in which it was demonstrated that vasoactive intestinal peptide, which is released along with acetylcholine by parasympathetic nerves, stimulates Lf release in submucosal glands. 35 Stimulation of parasympathetic neurons leads to the release of acetylcholine, which acts upon muscarinic receptors on salivary glands. 15 AChE catalyzes the breakdown of acetylcholine, thus enzyme inactivation leads to acetylcholine accumulation, resulting in augmented muscarinic receptor‐mediated stimulation of Lf secretion. Regarding memantine, we did not find differences in salivary Lf concentration between samples from memantine‐treated compared to samples from non‐memantine–treated patients.
All of these differences are important factors to be considered and investigated in future multicenter studies. Our present results provide interesting and convincing evidence showing salivary Lf variability depends on extrinsic factors. Also, our work further supports the idea that salivary Lf be considered a sensitive and non‐invasive biomarker for AD.
ETHICS
Approval of the study was obtained from the local Research Ethics Review Committee from Hospital 12 de Octubre Research Institute in Madrid (Spain). Written informed consent was given from all participants or representatives. Subjects’ consent was obtained according to the Declaration of Helsinki, and approval came from the research ethics committee of the abovementioned institution.
CONFLICTS OF INTEREST
Dr. Eva Carro and Dr. Gorka Orive are co‐founders of GEROA Diagnostics. No other disclosures are reported.
ACKNOWLEDGMENTS
We are grateful to the patients and donors without whom these works would not have been possible. We thank Robert Jenkins for editorial assistance. This study was supported by Dr. Carro's grants from Instituto de Salud Carlos III (FIS18/00118), FEDER, Comunidad de Madrid (S2017/BMD‐3700; NEUROMETAB‐CM), and CIBERNED (PI2016/01). The funders had no role in the conceptualization, study design, data collection analysis, and preparation of this manuscript.
Bartolome F, Orive G, Carro E Standardizing salivary lactoferrin measurements to obtain a robust diagnostic biomarker for Alzheimer's disease. Alzheimer's Dement. 2021;13:e12173. 10.1002/dad2.12173
REFERENCES
- 1. González‐Sánchez M, Bartolome F, Antequera D, et al. Decreased salivary lactoferrin levels are specific to Alzheimer's disease. EBioMedicine. 2020;57:102834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farah R, Haraty H, Salame Z, Fares Y, Ojcius DM. Said Sadier N. Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomed J. 2018;41:63‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gleerup HS, Hasselbalch SG, Simonsen AH. Biomarkers for Alzheimer's disease in saliva: a systematic review. Dis Markers. 2019:4761054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olsen I, Singhrao SK. Low levels of salivary lactoferrin may affect oral dysbiosis and contribute to Alzheimer's disease: a hypothesis. Med Hypotheses. 2021;146:110393. [DOI] [PubMed] [Google Scholar]
- 5. Glimvall P, Wickström C, Jansson H. Elevated levels of salivary lactoferrin, a marker for chronic periodontitis?. J Periodontal Res. 2012;47:655‐660. [DOI] [PubMed] [Google Scholar]
- 6. Janšáková K, Escudier M, Tóthová Ľ, Proctor G. Salivary changes in oxidative stress related to inflammation in oral and gastrointestinal diseases. Oral Dis. 2021;27:280‐289. [DOI] [PubMed] [Google Scholar]
- 7. Seifert G, Caselitz J. Markers of oral and salivary gland tumors: immunocytochemical investigations. Cancer Detect Prev. 1985;8:23‐34. [PubMed] [Google Scholar]
- 8. Dupree EJ, Darie CC. Examination of a non‐invasive biomarker for the diagnosis of prodromal Alzheimer's disease and Alzheimer's disease dementia. EBioMedicine. 2020;57:102882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ide M, Saruta J, To M, et al. Relationship between salivary immunoglobulin a, lactoferrin and lysozyme flow rates and lifestyle factors in Japanese children: a cross‐sectional study. Acta Odontol Scand. 2016;74:576‐583. [DOI] [PubMed] [Google Scholar]
- 10. Proctor GB. The physiology of salivary secretion. Periodontol 2000. 2016;70:11‐25. [DOI] [PubMed] [Google Scholar]
- 11. Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fratiglioni L, Paillard‐Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343‐353. [DOI] [PubMed] [Google Scholar]
- 13. Rolland Y, Abellan van Kan G, Vellas B. Physical activity and Alzheimer's disease: from prevention to therapeutic perspectives. J Am Med Dir Assoc. 2008;9:390‐405. [DOI] [PubMed] [Google Scholar]
- 14. Gillum T, Kuennen M, McKenna Z, Castillo M, Jordan‐Patterson A, Bohnert C. Exercise increases lactoferrin, but decreases lysozyme in salivary granulocytes. Eur J Appl Physiol. 2017;117:1047‐1051. [DOI] [PubMed] [Google Scholar]
- 15. Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3‐18. [DOI] [PubMed] [Google Scholar]
- 16. Mukherjee S, Crawford JM, McClear N, Tsang A. A longitudinal study of unsaturated iron‐binding capacity and lactoferrin in unstimulated parotid saliva. Biol Trace Elem Res. 1997;57:1‐8. [DOI] [PubMed] [Google Scholar]
- 17. Bermejo‐Pareja F, del Ser T, Valentí M, de la Fuente M, Bartolome F, Carro E. Salivary lactoferrin as biomarker for Alzheimer's disease: Brain‐immunity interactions. Alzheimer's & Dementia. 2020;16:1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hansson O, Mikulskis A, Fagan AM, et al. The impact of preanalytical variables on measuring cerebrospinal fluid biomarkers for Alzheimer's disease diagnosis: a review. Alzheimers Dement. 2018;14:1313‐1333. [DOI] [PubMed] [Google Scholar]
- 19. Arsiccio A, Giorsello P, Marenco L, Pisano R. Considerations on protein stability during freezing and its impact on the freeze‐drying cycle: a design space approach. J Pharm Sci. 2020;109:464‐475. [DOI] [PubMed] [Google Scholar]
- 20. Lee JE, Kim SY, Shin SY. Effect of repeated freezing and thawing on biomarker stability in plasma and serum samples. Osong Public Health Res Perspect. 2015;6:357‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Killer SC, Svendsen IS, Gleeson M. The influence of hydration status during prolonged endurance exercise on salivary antimicrobial proteins. Eur J Appl Physiol. 2015;115:1887‐1895. [DOI] [PubMed] [Google Scholar]
- 22. Svendsen IS, Hem E, Gleeson M. Effect of acute exercise and hypoxia on markers of systemic and mucosal immunity. Eur J Appl Physiol. 2016;116:1219‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berlutti F, Pantanella F, Natalizi T, et al. Antiviral properties of lactoferrin–a natural immunity molecule. Molecules. 2011;16:6992‐7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nishida N, Yamamoto Y, Tanaka M, et al. Association between involuntary smoking and salivary markers related to periodontitis: a 2‐year longitudinal study. J Periodontol. 2008;79:2233‐2240. [DOI] [PubMed] [Google Scholar]
- 25. Persson L, Bergström J, Ito H, Gustafsson A. Tobacco smoking and neutrophil activity in patients with periodontal disease. J Periodontol. 2001;72:90‐95. [DOI] [PubMed] [Google Scholar]
- 26. Ng V, Cho P, Mak S, Lee A. Variability of tear protein levels in normal young adults: between‐day variation. Graefes Arch Clin Exp Ophthalmol. 2000;238:892‐899. [DOI] [PubMed] [Google Scholar]
- 27. Dodds MW, Johnson DA, Yeh CK. Health benefits of saliva: a review. J Dent. 2005;33:223‐233. [DOI] [PubMed] [Google Scholar]
- 28. Nagler RM. Salivary glands and the aging process: mechanistic aspects, health‐status and medicinal‐efficacy monitoring. Biogerontology. 2004;5:223‐233. [DOI] [PubMed] [Google Scholar]
- 29. Salvolini E, Martarelli D, Di Giorgio R, Mazzanti L, Procaccini M, Curatola G. Age‐related modifications in human unstimulated whole saliva: a biochemical study. Aging (Milano). 2000;12:445‐448. [DOI] [PubMed] [Google Scholar]
- 30. Vissink A, Spijkervet FK, Van Nieuw Amerongen A. Aging and saliva: a review of the literature. Spec Care Dentist. 1996;16:95‐103. [DOI] [PubMed] [Google Scholar]
- 31. Vissink A, Mitchell JB, Baum BJ, et al. Clinical management of salivary gland hypofunction and xerostomia in head‐and‐neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010;78:983‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akimoto T, Kumai Y, Akama T, et al. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br J Sports Med. 2003;37:76‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Santos J, Foster R, Jonckheere AC, et al. Outdoor endurance training with air pollutant exposure versus sedentary lifestyle: a comparison of airway immune responses. Int J Environ Res Public Health. 2019;16:4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shimizu K, Hanaoka Y, Akama T, Kono I. Ageing and free‐living daily physical activity effects on salivary beta‐defensin 2 secretion. J Sports Sci. 2017;35:617‐623. [DOI] [PubMed] [Google Scholar]
- 35. Baraniuk JN, Lundgren JD, Okayama M, et al. Vasoactive intestinal peptide in human nasal mucosa. J Clin Invest. 1990;86:825‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]


