Abstract
Introduction
This study aimed to investigate cognitive aging trajectories, the associated sociodemographic characteristics, and the association of these trajectories with dementia.
Methods
Generally healthy older adults (n = 19,114) were followed for up to 7 years, with regular cognitive assessments. Group‐based trajectory modeling identified distinct cognitive trajectories.
Results
Four to seven trajectories were identified per cognitive domain. Stable trajectories were observed across domains. Improvement in verbal fluency and minor psychomotor slowing were common. Substantial decline in global cognition and episodic memory were observed in a small proportion of individuals. Older, less educated participants and men were more common in lower‐functioning trajectories (p < .001). The highest proportions of dementia cases were in trajectories with major decline in global cognition (56.9%) and memory (33.2%).
Discussion
Inter‐individual variability in cognitive trajectories was observed across all domains. Some individuals appear resilient to cognitive decline even with advancing age. Further research into factors promoting cognitive resilience is needed.
Keywords: cognitive function, dementia, longitudinal, older adults, prospective
1. INTRODUCTION
Changes in cognitive function are common as individuals age, but there is considerable variability between individuals and across cognitive domains. 1 , 2 Cognitive function can be categorized into domains such as learning and memory, processing speed, attention, visuospatial and psychomotor function, language, and executive function. Often, there is some loss in cognitive function, such as memory and processing speed, with aging. 1 However, even minor deficits can affect an individual's daily functioning, such as their capacity to drive, manage finances, and to understand instructions. 3 On the other hand, some cognitive domains, such as vocabulary, may remain stable even with advanced age. 1 Therefore, a better understanding of the natural process and variability of cognitive aging within the general population is needed.
The majority of studies investigating changes in cognitive function in late life have focused on individuals who already exhibit cognitive decline through diagnosis of mild cognitive impairment or incident dementia. 4 Other studies have examined cognitive change between only two timepoints, 5 investigating the extent of cognitive decline. Longitudinal cognitive data, collected at multiple timepoints, enable the patterns or trajectories of cognitive function over time to be determined within a population sample.
A systematic review of 37 studies found that cognitive aging in late life may involve an extended period of stability, decline, and even slight improvement over time, and there is evidence of significant inter‐individual variability. 2 The majority of studies have been restricted to a global measure of cognitive function, most commonly the Mini‐Mental State Examination (MMSE), which has floor and ceiling effects and is insensitive to subtle cognitive change. 6 , 7 Furthermore, it remains unclear to what extent sociodemographic characteristics (i.e., age, education) are associated with cognitive trajectories. Meanwhile, although cognitive decline is associated with dementia, it is uncertain how changes in different cognitive domains in late life predict the development of dementia.
Using a large cohort of generally healthy older adults from Australia and the United States, the present study aimed to characterize how cognitive function changes over time using four cognitive tests, and to describe the most common distinct cognitive trajectories. The second aim was to investigate sociodemographic characteristics associated with trajectory classes, and to determine how incidence risk of dementia differs by trajectory.
2. METHODS
2.1. Participants
This study used data obtained from the ASPREE (Aspirin in Reducing Events in the Elderly) clinical trial: full details of the study have been published previously. 8 In brief, 19,114 adults aged 65+ years (Black and Hispanics) or 70+ years (all other ethno‐racial groups) were recruited from Australia and the United States. At baseline, participants were without recognized cardiovascular disease, diagnosed dementia, or a modified MMSE (3MS) score < 78. Participants were followed for a median of 4.7 years. All participants provided written informed consent.
2.2. Cognition and dementia assessment
The cognitive battery included the 3MS examination for global cognitive function, 9 single letter Controlled Oral Word Association Test (COWAT‐F) for vocabulary and executive function, 10 Hopkins Verbal Learning Test—Revised (HVLT‐R) delayed recall task for episodic memory, 11 and Symbol Digit Modalities Test (SDMT) for attention and psychomotor speed. 12 Seven waves of cognitive data collected at baseline and over follow‐up (year 1, 3, 4, 5, 6, 7, or close‐out visit) were used. Three composite scores, including a global, an executive function, and a memory composite score, were generated using z‐scores of the above cognitive tests (components of the composite scores are shown in the supporting information). Dementia was a secondary endpoint in the ASPREE trial, and was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV). 13
HIGHLIGHTS
There is inter‐individual heterogeneity of cognitive aging across all domains.
High‐functioning and stable trajectories were observed in most domains.
Rapid decline in global cognition and episodic memory was associated with dementia.
Improvement in verbal fluency and minor psychomotor slowing were common at old age.
Some individuals may be resilient to cognitive decline with advancing age.
RESEARCH IN CONTEXT
Systematic review: The authors performed a systematic review via MEDLINE and EMBASE. Findings highlighted the need for further studies to investigate population heterogeneity of cognitive trajectories across multiple cognitive domains, in a large representative sample of community‐dwelling older individuals.
Interpretation: Our findings identified considerable inter‐individual variability in cognitive aging. However, patterns of cognitive trajectories differed across cognitive domains. Slight improvements in verbal fluency and minor decline in psychomotor speed were common. Substantial decline in global cognition and episodic memory were evident for a small proportion of individuals with significantly increased risk of dementia. The majority of individuals maintained stable cognitive trajectories over time.
Future directions: A proportion of individuals appeared to maintain their cognitive function at high levels over time, even with advancing age. Further research into the characteristics of these “successful cognitive agers” who appear resilient to cognitive decline, might help identify novel resilient factors.
2.3. Statistical analysis
Full details of statistical analysis are presented in supporting information. In brief, trajectories were identified using group‐based trajectory modeling (GBTM), which is a semi‐parametric technique that identifies the latent subgroups with distinct trajectories of an indicator within a heterogeneous population based on the longitudinal patterns of the indicator. It subsequently assigns each participant into the most likely subgroup that produced the maximum posterior probability. Participants in the same group share homogenous patterns of the trajectory. GBTM was conducted separately for the four cognitive tests and three composite scores.
Participants included in the analyses were required to have cognitive data at baseline and at one or more subsequent study visit. Year was used as the time metric. Sociodemographic characteristics (age, sex, ethnicity, education), length of follow‐up, and incidence risk of dementia were compared between population subgroups (based on the assigned trajectory class of participants). All statistical analyses were performed using Stata version 16.0 (Stata Corp.).
3. RESULTS
3.1. Trajectory modeling
After excluding participants whose cognitive data were missing at baseline or available only at one follow‐up timepoint, the number of participants included for analysis was 18,016 for 3MS, 17,971 for COWAT‐F, 17,873 for HVLT‐R, and 17,883 for SDMT (Figure S1 in supporting information). The sample size available for the three composite scores—the global, executive function, and memory composite score was 17,724, 17,861, and 17,835, respectively. Of the participants included in the analysis, the number with cognitive data at each study visit is summarized in Table S1 in supporting information.
From the trajectory modeling of the four cognitive tests, between four and seven classes were identified, depending on the test. The full process of model selection and the assessment of model adequacy are shown, respectively, in Tables S2 and S3 in supporting information. The trajectory plots of the four cognitive tests are presented in Figure 1, with the parameters of these trajectories summarized in Table S4 in supporting information. Distinct trajectory classes were given subjective labels according to visual features of the mean trajectory, specifically the baseline level and slope of change.
FIGURE 1.
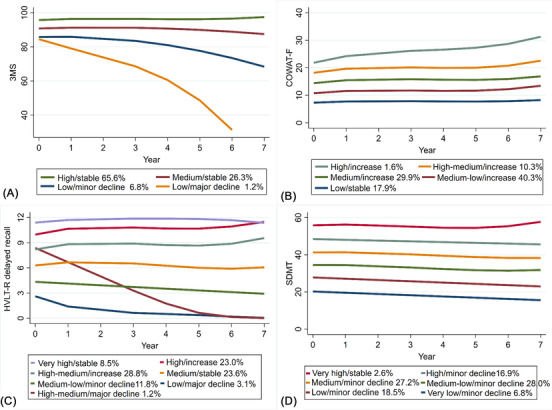
Trajectory plots of individual cognitive tests (A) 3MS (n = 18,016), (B) COWAT‐F (n = 17,971), (C) HVLT‐R delayed recall (n = 17,837), (D) SDMT (n = 17,883). 3MS, modified Mini‐Mental State Examination; COWAT‐F, Controlled Oral Word Association Test‐F; HVLT‐R, Hopkins Verbal Learning Test–Revised (delayed recall); SDMT, Symbol Digit Modalities Test. Note: (1) The x‐axis denotes the year of cognitive assessments conducted at baseline as well as 1, 3, 4, 5, 6, and 7 years of follow‐up. The y‐axis denotes the raw scores of the cognitive tests. (2) The percentages refer to the proportions of participants assigned into the corresponding classes
The model for 3MS scores over time identified four classes (Figure 1A), including a high‐functioning class with the highest baseline and slight improvement that accounted for the majority of participants (high/stable: 65.6%), and a medium‐functioning class that started at a lower baseline and remained quite stable over time (medium/stable: 26.3%). The two lower‐functioning classes had the lowest initial score, of which one had only gradual decline (low/minor decline: 6.8%) while the other dropped substantially (low/major decline: 1.2%).
The five classes detected for the COWAT‐F differed from one another on the basis of baseline score, but all remained relatively stable over time with some improvements in performance, and the degree of improvement increased progressively from the class with the lowest baseline to the highest class (Figure 1B). However, most participants fell into the lowest three classes which together accounted for 88.1% of the study sample (medium/increase: 29.9%, medium‐low/increase: 40.3%, low/stable: 17.9%).
Seven classes were identified for the HVLT‐R delayed recall (Figure 1C), including two classes starting at the highest level (very high/stable: 8.5%, high/increase: 23.0%) and another two that had medium baseline scores (high‐medium/increase: 28.8%, medium/stable: 23.6%), all either increasing or remaining stable over time. Two classes starting at lower baselines showed decline over time (medium‐low/minor decline: 11.8%, low/major decline: 3.1%), and one class was seen with a relatively high baseline score that declined substantially (high‐medium/major decline: 1.2%).
For SDMT (Figure 1D), the six classes identified were roughly parallel and remained relatively stable from baseline across follow‐up with minor decline, except for the highest and smallest class (very high/stable, 2.6%).
Trajectories of the three composite scores are presented in Figure S2 in supporting information and their parameters are presented in Table S4. There were seven classes observed in the global composite score; these followed a pattern such that trajectories with lower baselines tended to have higher rates of decline (Figure S2a). Five classes (class 1–5), accounted for 91.3% of the study sample and were all stable over time, but in the lowest two classes at baseline there was minor to substantial decline (class 6: 6.8%, class 7: 2.1%). For the executive function composite score (Figure S2b), the model identified seven classes which were similar in their change over time, with slight decline observed only in the lowest two classes (class 6: 10.2%, class 7: 2.8%). There was a total of eight classes for the memory composite score (Figure S2c), with a number of different patterns. The two most frequent classes were high performing and stable over time (class 1: 43.8%, class 2: 31.9%). Four classes declined over time, with different patterns and to varying degrees. For example, class 3 (5.2%) and class 8 (2.2%) both displayed minor declines, but class 8 had the lowest baseline among the eight classes. Class 6 (4.4%) and class 7 (2.2%) both had medium baseline and rapid decline as well as similar endpoints, but the shapes of these two trajectories were different, with class 6 being more curvilinear. There was improvement observed in class 4 (7.0%) and class 5 (3.3%), with class 5 starting from a lower baseline than class 4.
The observed trajectories of cognitive scores for each individual within the classes were also plotted along with the class‐specific mean trajectories, with the results shown in Figure 2 for the four cognitive tests and Figure S3 in supporting information for the three composite scores. The sensitivity analysis using the dropout model did not materially change the results of the basic models (Table S5, Figures S4 and S5 in supporting information).
FIGURE 2.
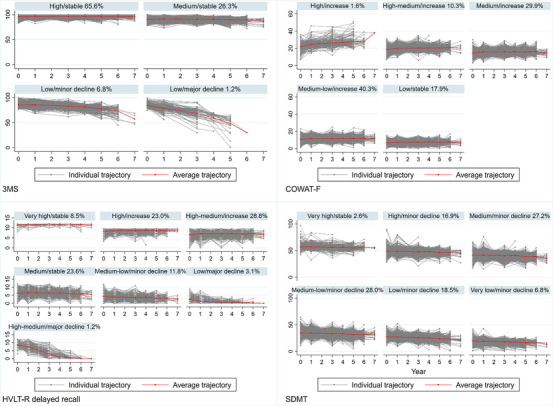
Individual trajectories of individual cognitive tests by class: 3MS (n = 18,016), COWAT‐F (n = 17,971), HVLT‐R delayed recall (n = 17,837), SDMT (n = 17,883). 3MS, modified Mini‐Mental State Examination; COWAT‐F, Controlled Oral Word Association Test‐F; HVLT‐R, Hopkins Verbal Learning Test–Revised (delayed recall); SDMT, Symbol Digit Modalities Test. Note: (1) The x‐axis denotes the year of cognitive assessments conducted at baseline as well as 1, 3, 4, 5, 6, and 7 years of follow‐up. The y‐axis denotes the raw scores of the cognitive tests. (2) The percentages refer to the proportions of participants assigned into the corresponding classes. (3) Trajectories were generated by connecting the observed cognitive data at each timepoint
3.2. Baseline characteristics
Table S6 in supporting information shows the baseline sociodemographic and cognitive scores of the individuals who participated in ASPREE. The majority of the participants were aged 70 to 74 years (55.5%), were female (56.4%), and White Australian (85.6%), and received >12 years of education (54.8%).
3.3. Sociodemographic characteristics and incident dementia according to trajectory classes
Tables 1‐4 show the sociodemographic characteristics, randomization group, the number/proportion of incident dementia cases, and the length of follow‐up, according to trajectory classes of the four cognitive tests. Results of three composite scores are shown in Tables S7‐S9 in supporting information.
TABLE 1.
Characteristics by cognitive trajectory class of modified Mini‐Mental State Examination (3MS; n = 18,016)
| Class, N (%) | |||||
|---|---|---|---|---|---|
| Baseline characteristics | High/stable 11, 824 (65.6) | Medium/stable 4744 (26.3) | Low/minor decline 1232 (6.8) | Low/major decline 216 (1.2) | P‐value* |
| Age, years | <.001 | ||||
| 65–69 a | 299 (2.5) | 153 (3.2) | 29 (2.4) | 7 (3.2) | |
| 65–74 | 7275 (61.5) | 2297 (48.4) | 482 (39.1) | 57 (26.4) | |
| 75–84 | 4001 (33.8) | 2053 (43.3) | 595 (48.3) | 115 (53.2) | |
| ≥85 | 249 (2.1) | 241 (5.1) | 126 (10.2) | 37 (17.1) | |
| Sex | <.001 | ||||
| Male | 4690 (39.7) | 2423 (51.1) | 672 (54.6) | 106 (49.1) | |
| Female | 7134 (60.3) | 2321 (48.9) | 560 (45.4) | 110 (50.9) | |
| Ethnicity | <.001 | ||||
| Australian White | 10,245 (86.7) | 4068 (85.8) | 1028 (83.4) | 174 (80.6) | |
| US White | 833 (7.0) | 148 (3.1) | 39 (3.2) | 12 (5.6) | |
| Black | 394 (3.3) | 275 (5.8) | 85 (6.9) | 18 (8.3) | |
| Hispanic/Latino | 229 (1.9) | 154 (3.3) | 45 (3.7) | 8 (3.7) | |
| Other | 123 (1.0) | 99 (2.1) | 35 (2.8) | 4 (1.9) | |
| Education, years | <.001 | ||||
| < 12 | 4559 (38.6) | 2692 (56.8) | 745 (60.5) | 123 (56.9) | |
| 12–15 | 3540 (29.9) | 1275 (26.9) | 349 (28.3) | 59 (27.3) | |
| ≥16 | 3725 (31.5) | 777 (16.4) | 138 (11.2) | 34 (15.7) | |
| Randomization group | .443 | ||||
| Aspirin group | 5853 (49.5) | 2374 (50.0) | 626 (50.8) | 117 (54.2) | |
| Placebo group | 5971 (50.5) | 2370 (50.0) | 606 (49.2) | 99 (45.8) | |
| Incident dementia | <.001 | ||||
| Yes | 31 (0.3) | 129 (2.7) | 264 (21.4) | 123 (56.9) | |
| No | 11,793 (99.7) | 4615 (97.3) | 968 (78.6) | 93 (43.1) | |
| Median (inter‐quartile range) | |||||
| Length of follow‐up, years | 4 (3‐5) | 3 (3‐5) | 3 (3‐5) | 3 (1‐3) | <.001 |
| Number of cognitive assessments | 4 (3‐4) | 3 (3‐4) | 3 (3‐4) | 3 (2‐3) | <.001 |
Only includes U.S. Black or Hispanic/Latino individuals, who were eligible to enroll from 65 years and over (all other individuals needed to be aged at least 70 years to be eligible).
P‐values were based on chi‐squared tests or Fisher's exact tests for categorical variables, and Kruskal‐Wallis H‐tests for length of follow‐up.
Note: The labels of the trajectory classes correspond to those shown in Figure 1A.
TABLE 4.
Characteristics by cognitive trajectory class of Symbol Digit Modalities Test (SDMT; n = 17,883)
| Class, N (%) | |||||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics | Very high/stable 456 (2.6) | High/minor decline 3025 (16.9) | Medium/minor decline 4869 (27.2) | Medium‐low/minor decline 5002 (28.0) | Low/minor decline 3314 (18.5) | Very low/minor decline 1217 (6.8) | P‐value* |
| Age, years | <.001 | ||||||
| 65–69 a | 13 (2.9) | 106 (3.5) | 133 (2.7) | 119 (2.4) | 83 (2.5) | 29 (2.4) | |
| 65–74 | 371 (81.4) | 2166 (71.6) | 3096 (63.6) | 2718 (54.3) | 1363 (41.1) | 328 (27.0) | |
| 75–84 | 71 (15.6) | 739 (24.4) | 1567 (32.2) | 1998 (39.9) | 1650 (49.8) | 689 (56.6) | |
| ≥85 | 1 (0.2) | 14 (0.5) | 73 (1.5) | 167 (3.3) | 218 (6.6) | 171 (14.1) | |
| Sex | <.001 | ||||||
| Male | 130 (28.5) | 1034 (34.2) | 1971 (40.5) | 2370 (47.4) | 1673 (50.5) | 652 (53.6) | |
| Female | 326 (71.5) | 1991 (65.8) | 2898 (59.5) | 2632 (52.6) | 1641 (49.5) | 565 (46.4) | |
| Ethnicity | <.001 | ||||||
| Australian White | 372 (81.6) | 2532 (83.7) | 4198 (86.2) | 4386 (87.7) | 2887 (87.1) | 1,022 (84.0) | |
| US White | 59 (12.9) | 280 (9.3) | 324 (6.7) | 217 (4.3) | 118 (3.6) | 27 (2.2) | |
| Black | 12 (2.6) | 120 (4.0) | 166 (3.4) | 204 (4.1) | 167 (5.0) | 97 (8.0) | |
| Hispanic/Latino | 5 (1.1) | 50 (1.7) | 104 (2.1) | 125 (2.5) | 98 (3.0) | 52 (4.3) | |
| Other | 8 (1.8) | 43 (1.4) | 77 (1.6) | 70 (1.4) | 44 (1.3) | 19 (1.6) | |
| Education, years | <.001 | ||||||
| < 12 | 110 (24.1) | 896 (29.6) | 1849 (38.0) | 2418 (48.3) | 1916 (57.8) | 856 (70.3) | |
| 12–15 | 134 (29.4) | 943 (31.2) | 1478 (30.4) | 1468 (29.4) | 906 (27.3) | 254 (20.9) | |
| ≥16 | 212 (46.5) | 1186 (39.2) | 1542 (31.7) | 1116 (22.3) | 492 (14.9) | 107 (8.8) | |
| Randomization group | <0.001 | ||||||
| Aspirin group | 214 (46.9) | 1513 (50.0) | 2391 (49.1) | 2506 (50.1) | 1681 (50.7) | 603 (49.6) | |
| Placebo group | 242 (53.1) | 1512 (50.0) | 2478 (50.9) | 2496 (49.9) | 1633 (49.3) | 614 (50.4) | |
| Incident dementia | <.001 | ||||||
| Yes | 0 (0.0) | 9 (0.3) | 39 (0.8) | 101 (2.0) | 201 (6.1) | 188 (15.5) | |
| No | 456 (100.0) | 3016 (99.7) | 4830 (99.2) | 4901 (98.0) | 3113 (93.9) | 1029 (84.5) | |
| Median (inter‐quartile range) | |||||||
| Length of follow‐up, years | 4 (3‐5) | 4 (3‐5) | 3 (3‐5) | 3 (3‐5) | 3 (3‐5) | 3 (1‐5) | <.001 |
| Number of cognitive assessments | 4 (3‐4) | 3 (3‐4) | 3 (3‐4) | 3 (3‐4) | 3 (3‐4) | 3 (2‐4) | <.001 |
Only includes U.S. Black or Hispanic/Latino individuals, who were eligible to enroll from 65 years and over (all other individuals needed to be aged at least 70 years to be eligible).
P‐values were based on chi‐squared tests or Fisher's exact tests for categorical variables, and Kruskal‐Wallis H‐tests for length of follow‐up.
Note: The labels of the trajectory classes correspond to those shown in Figure 1D.
TABLE 2.
Characteristics by cognitive trajectory class of Controlled Oral Word Association Test‐F (COWAT‐F; n = 17,971)
| Class, N (%) | ||||||
|---|---|---|---|---|---|---|
| Baseline characteristics | High/increase 285 (1.6) | High‐medium/increase 1844 (10.3) | Medium/increase 5371 (29.9) | Medium‐low/increase 7250 (40.3) | Low/stable 3221 (17.9) | P‐value* |
| Age, years | <.001 | |||||
| 65–69 a | 0 (0.0) | 30 (1.6) | 118 (2.2) | 231 (3.2) | 109 (3.4) | |
| 65–74 | 180 (63.2) | 1146 (62.2) | 3087 (57.5) | 4035 (55.7) | 1636 (50.8) | |
| 75–84 | 99 (34.7) | 608 (33.0) | 1986 (37.0) | 2720 (37.5) | 1333 (41.4) | |
| ≥85 | 6 (2.1) | 60 (3.3) | 180 (3.4) | 264 (3.6) | 143 (4.4) | |
| Sex | <.001 | |||||
| Male | 81 (28.4) | 621 (33.7) | 2080 (38.7) | 3312 (45.7) | 1778 (55.2) | |
| Female | 204 (71.6) | 1223 (66.3) | 3291 (61.3) | 3938 (54.3) | 1443 (44.8) | |
| Ethnicity | <.001 | |||||
| Australian White | 269 (94.4) | 1657 (89.9) | 4637 (86.3) | 6187 (85.3) | 2722 (84.5) | |
| US White | 10 (3.5) | 105 (5.7) | 377 (7.0) | 396 (5.5) | 142 (4.4) | |
| Black | 0 (0.0) | 41 (2.2) | 177 (3.3) | 365 (5.0) | 189 (5.9) | |
| Hispanic/Latino | 2 (0.7) | 22 (1.2) | 102 (1.9) | 197 (2.7) | 113 (3.5) | |
| Other | 4 (1.4) | 19 (1.0) | 78 (1.5) | 105 (1.5) | 55 (1.7) | |
| Education, years | <.001 | |||||
| < 12 | 63 (22.1) | 510 (27.7) | 1995 (37.1) | 3565 (49.2) | 1964 (61.0) | |
| 12–15 | 88 (30.9) | 533 (28.9) | 1645 (30.6) | 2098 (28.9) | 845 (26.2) | |
| ≥16 | 134 (47.0) | 801 (43.4) | 1731 (32.2) | 1587 (21.9) | 412 (12.8) | |
| Randomization group | .487 | |||||
| Aspirin group | 138 (48.4) | 934 (50.7) | 2624 (48.9) | 3621 (49.9) | 1628 (50.5) | |
| Placebo group | 147 (51.6) | 910 (49.3) | 2747 (51.1) | 3629 (50.1) | 1593 (49.5) | |
| Incident dementia | <.001 | |||||
| Yes | 5 (1.8) | 21 (1.1) | 98 (1.8) | 205 (2.8) | 216 (6.7) | |
| No | 280 (98.2) | 1823 (98.9) | 5273 (98.2) | 7045 (97.2) | 3005 (93.3) | |
| Median (inter‐quartile range) | ||||||
| Length of follow‐up, years | 4 (3‐5) | 4 (3‐5) | 3 (3‐5) | 3 (3‐5) | 3 (3‐5) | <.001 |
| Number of cognitive assessments | 4 (3‐4) | 4 (3‐4) | 3 (3‐4) | 3 (3‐4) | 3 (3‐4) | <.001 |
Only includes U.S. Black or Hispanic/Latino individuals, who were eligible to enroll from 65 years and over (all other individuals needed to be aged at least 70 years to be eligible).
P‐values were based on chi‐squared tests or Fisher's exact tests for categorical variables, and Kruskal‐Wallis H‐tests for length of follow‐up.
Note: The labels of the trajectory classes correspond to those shown in Figure 1B.
TABLE 3.
Characteristics by cognitive trajectory class of Hopkins Verbal Learning Test–Revised (HVLT‐R) delayed recall (n = 17,873)
| Class, N (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline characteristics | Very high/stable 1518 (8.5) | High/increase 4095 (23.0) | High‐medium/increase 5139 (28.8) | Medium/stable 4211 (23.6) | Medium‐low/minor decline 2112 (11.8) | Low/minor decline 554 (3.1) | High‐medium/major decline 208 (1.2) | P‐value* |
| Age, years | <0.001 | |||||||
| 65–69 a | 33 (2.2) | 101 (2.5) | 175 (3.4) | 124 (2.9) | 41 (1.9) | 10 (1.8) | 4 (1.9) | |
| 65–74 | 1060 (69.8) | 2693 (65.8) | 2975 (57.9) | 2131 (50.6) | 897 (42.5) | 173 (31.2) | 67 (32.2) | |
| 75–84 | 407 (26.8) | 1244 (30.4) | 1873 (36.5) | 1751 (41.6) | 1,017 (48.2) | 297 (53.6) | 118 (56.7) | |
| ≥85 | 18 (1.2) | 57 (1.4) | 116 (2.3) | 205 (4.9) | 157 (7.4) | 74 (13.4) | 19 (9.1) | |
| Sex | <0.001 | |||||||
| Male | 462 (30.4) | 1445 (35.3) | 2127 (41.4) | 2159 (51.3) | 1,231 (58.3) | 317 (57.2) | 75 (36.1) | |
| Female | 1,056 (69.6) | 2,650 (64.7) | 3012 (58.6) | 2052 (48.7) | 881 (41.7) | 237 (42.8) | 133 (63.9) | |
| Ethnicity | <.001 | |||||||
| Australian White | 1354 (89.2) | 3511 (85.7) | 4376 (85.2) | 3592 (85.3) | 1862 (88.2) | 486 (87.7) | 165 (79.3) | |
| US White | 92 (6.1) | 320 (7.8) | 304 (5.9) | 192 (4.6) | 69 (3.3) | 29 (5.2) | 20 (9.6) | |
| Black | 27 (1.8) | 131 (3.2) | 231 (4.5) | 221 (5.3) | 111 (5.3) | 32 (5.8) | 18 (8.7) | |
| Hispanic/Latino | 24 (1.6) | 73 (1.8) | 159 (3.1) | 131 (3.1) | 41 (1.9) | 5 (0.9) | 3 (1.4) | |
| Other | 21 (1.4) | 60 (1.5) | 69 (1.3) | 75 (1.8) | 29 (1.4) | 2 (0.4) | 2 (1.0) | |
| Education, years | <.001 | |||||||
| < 12 | 450 (29.6) | 1501 (36.7) | 2353 (45.8) | 2177 (51.7) | 1,155 (54.7) | 307 (55.4) | 95 (45.7) | |
| 12–15 | 456 (30.0) | 1205 (29.4) | 1502 (29.2) | 1194 (28.4) | 602 (28.5) | 151 (27.3) | 68 (32.7) | |
| ≥16 | 612 (40.3) | 1389 (33.9) | 1284 (25.0) | 840 (19.9) | 355 (16.8) | 96 (17.3) | 45 (21.6) | |
| Randomization group | .435 | |||||||
| Aspirin group | 777 (51.2) | 2029 (49.6) | 2519 (49.0) | 2082 (49.4) | 1090 (51.6) | 271 (48.9) | 107 (51.4) | |
| Placebo group | 741 (48.8) | 2066 (50.4) | 2620 (51.0) | 2129 (50.6) | 1022 (48.4) | 283 (51.1) | 101 (48.6) | |
| Incident dementia | <.001 | |||||||
| Yes | 0 (0.0) | 5 (0.1) | 35 (0.7) | 90 (2.1) | 160 (7.6) | 174 (31.4) | 69 (33.2) | |
| No | 1518 (100.0) | 4090 (99.9) | 5104 (99.3) | 4121 (97.9) | 1952 (92.4) | 380 (68.6) | 139 (66.8) | |
| Median (inter‐quartile range) | ||||||||
| Length of follow‐up, years | 5 (3‐5) | 4 (3‐5) | 3 (3‐5) | 3 (3‐5) | 3 (3‐5) | 3 (1‐5) | 4 (3‐5) | <.001 |
| Number of cognitive assessments | 4 (3‐4) | 4 (3‐4) | 3 (3‐4) | 3 (3‐4) | 3 (2‐4) | 3 (2‐4) | 4 (3‐4) | <.001 |
Only includes U.S. Black or Hispanic/Latino individuals, who were eligible to enroll from 65 years and over (all other individuals needed to be aged at least 70 years to be eligible).
P‐values were based on chi‐squared tests or Fisher's exact tests for categorical variables, and Kruskal‐Wallis H‐tests for length of follow‐up.
Note: The labels of the trajectory classes correspond to those shown in Figure 1C.
Generally, participants in the lower‐functioning classes with either lower baseline level or greater decline were more likely to be older, male, and with a lower education level. We did not observe any between‐class difference in the randomization to aspirin versus placebo, for any of the cognitive tests, which aligns with the main findings of the trial. 14
For all tests, there was a significant difference in incident dementia cases across the classes. This was most noticeable for the 3MS, where more than half of the participants in the low/major decline class developed dementia during follow‐up. For HVLT‐R delayed recall, the two classes with major decline (starting at medium and low, respectively) also had a substantial proportion of individuals with incident dementia (31.4% and 33.2%, respectively). Similar patterns were seen for the global and memory composite scores. On the other hand, the differences in the proportion of individuals with incident dementia were less marked for COWAT (1.8% to 6.7%), SDMT (0% to 15.5%), and the executive function composite score (0% to 18.1%).
4. DISCUSSION
In this study, we explored the trajectories of cognitive function in late life and the underlying heterogeneity at a population level, using a sample of initially healthy older adults. We identified substantial heterogeneity in cognitive change across individuals, with multiple subgroups displaying hierarchically distinct trajectories, and these varied across the domains assessed. Our findings also suggest that for a large proportion of individuals, the process of cognitive aging is relatively stable over the maximum 7 years of follow‐up. However, in a small proportion of individuals cognitive change was dynamic. In general, moderate to substantial decline in global cognition and delayed recall was observed in a small proportion of participants, and this corresponded to a high proportion of individuals with incident dementia. On the other hand, the majority of participants had only slight decline in SDMT and small improvements of varying degrees in COWAT‐F over time. Overall, a pattern of increasing age, lower education, and a lower proportion of women was observed from the highest to lowest trajectory class, but this was not consistent across all domains.
There are several strengths in our study. With more than 17,000 participants, this is so far the largest study investigating the inter‐individual variability in late‐life cognitive trajectories. In addition, we used four cognitive tests in this study, enabling an investigation into the variability of the trajectories across multiple domains comparably in the same population, which has seldom been investigated previously. 15 , 16 , 17 , 18 Further, we set minimum inclusion criteria related to cognitive data availability to reduce the risk of selection bias, while also performing sensitivity analyses to ensure that the results were not significantly affected by non‐random dropouts.
The trajectories of 3MS score followed a typical pattern—the lower the baseline, the greater the rate of decline, and therefore the trajectories did not intersect. This pattern is consistent with previous studies of the trajectories of global cognition, 2 suggesting that the level of cognitive function, in the absence of disease, remains relatively stable over adulthood and into later life. The 3MS is a global cognitive measure that covers a variety of cognitive domains. It is considered to be superior to the MMSE with a refined scoring scale that means it is less susceptible to ceiling effects and has improved validity and reliability. 9 , 19 The 3MS has been widely used to screen for dementia, 19 and this is supported by our findings showing that baseline 3MS, even though all participants had a score above 77, had the best performance of all tests in distinguishing incident dementia. Our results suggest that the two higher classes with 3MS scores > 90 at baseline did not have substantial decline over follow‐up, while the two classes with lower baseline scores (around 85) experienced decline. This decline reached what could be considered clinically significant (< 78) 45 by the second and sixth annual visit for the major and minor decliners, respectively. They were also at an increased likelihood of having dementia compared to the general population of older adults in Australia and the United States, according to nationally representative data from these two countries. 20 , 21 Our results looking at the global composite score largely align with findings from the 3MS, although a greater number of classes were identified, probably reflecting the greater variability of the data using z‐scores. 22
In terms of HVLT‐R delayed recall, the classes were largely distinguished by their baseline level. The four classes with a score of 6 or above at baseline remained relatively constant during follow‐up, while the two lower classes with baseline scores at around 3 to 4 experienced some decline. However, there was one class starting at relatively high initial function with a baseline score of 8.4 that showed a sharp decline (≈1.7 annually) during follow‐up. Given that a 5‐point drop on the HVLT‐R is equivalent to a 1.5 standard deviation decline, 11 this level of decline is substantial and would be highly clinically significant. A similar finding was also reported in one study that investigated the trajectories of delayed recall using a sample of 219 subjects who were cognitively normal and aged 60+ years at baseline. 23 Unsurprisingly, individuals in this class in our study were most likely to reach the dementia endpoint during the trial, followed by those in the lowest class with moderate decline. The memory composite score partitioned the study sample to a larger extent, identifying more distinct trajectories and less within‐class variation. This might be a reason the incidence risk of dementia was more stratified by the memory composite score than HVLT‐R delayed recall. Memory deficit is a central diagnostic criterion of DSM‐IV dementia. 24 Indeed, impairment of episodic memory—including verbal learning and memory—has been consistently observed in patients of Alzheimer's disease (AD), and is the most pronounced symptom in many studies. 25 , 26 , 27 The findings of our study further indicate that memory loss might be one of the earliest signs of severe cognitive impairment at the pre‐symptomatic stage, because the delayed recall trajectories of those who are most susceptible to dementia in this study have already deviated from others a few years prior to diagnosis.
Language ability, especially vocabulary, is known to remain generally intact or even improve in late life, being less influenced by aging. 1 However, verbal fluency incorporates both vocabulary (an aspect of semantic memory) and executive function, and the two subtypes—semantic fluency and phonemic fluency—differ in task demands and clinical implications. 28 Compared to semantic fluency, phonemic fluency has been reported to be more preserved in aging and less impaired in individuals with dementia. 29 , 30 This is consistent with our findings, as all COWAT‐F trajectories appeared to be relatively stable over time, with even some evidence of improvement, particularly for individuals with high baseline scores. This improvement probably reflects practice effects. 31 Executive function is largely reflected by phonemic fluency, especially in letter‐based tasks, because examinees must retrieve words from their phonetic storage that can be difficult to associate with personal experiences. 28 , 32 Therefore, the improvement might be a result of certain word‐retrieval strategies proactively generated by high‐functioning individuals. A deficit of phonemic fluency was reported to be less prominent than that of semantic fluency or other domains in AD, 27 and less evident as well when converted into standard scores. 33 This aligns with our finding that the lower‐functioning classes of COWAT‐F could not remarkably distinguish the incident dementia cases.
In terms of psychomotor speed, most SDMT trajectories in this study showed moderate decline to a similar degree, which is partially in line with most previous findings from the general population. A number of cross‐sectional studies reported slower speed of information processing in older age groups compared to younger counterparts across countries and ethnicities. 34 , 35 , 36 , 37 In contrast, some other studies failed to observe an association between age and SDMT performance in young and middle‐aged adults, 12 , 38 which potentially indicates that psychomotor slowing might only present in late life. Indeed, linear decline in psychomotor speed was observed in longitudinal studies when the participants were followed‐up into their late adulthood. 39 , 40 Our findings further suggest that gradual psychomotor slowing might be considerably prevalent or even inevitable after the age of 70, regardless of the level of functioning at an early stage, which is possibly in part due to age‐related changes in white matter. 41 Slowed psychomotor speed has also been found to be associated with dementia in previous studies, and used as a marker for cognitive impairment. 42 , 43 In the current study, the SDMT trajectories with differences mainly at baseline stratified the incidence risk of dementia to a moderate degree, with those starting lower being more likely to reach this endpoint. Because there was not substantial variance across the SDMT trajectories in the changes over follow‐up, it is worth investigating the potential role of psychomotor speed in dementia screening, especially the thresholds that can be used cross‐sectionally for specific age groups.
Generally, individuals in the lower‐functioning classes across all of the cognitive domains were more likely to be older, male, and with lower levels of education. This is largely consistent with previous studies 44 as well as the findings regarding the baseline cognitive data from this sample. 11 , 45 , 46 Among cognitively healthy individuals, women generally outperform men in episodic memory at old age, especially in verbal‐based recall. 47 , 48 However, women are found to be at a higher risk of AD than men 20 , 49 and once diagnosed, usually have greater impairment in memory function. 47 This might help explain why women were in the majority in both the highest performing and in the most rapidly declining classes of episodic memory (HVLT‐R delayed recall). This also aligns with the findings that the lowest class in 3MS and the global composite score showed a slightly higher proportion of women. However, we cannot rule out the possibility that some other factors, such as lifestyles and socioeconomic factors, might have been associated with the sharp decline in cognitive function.
There are some limitations to this study that need to be acknowledged. The study sample is comprised of generally healthy older adults, as the eligibility criteria excluded individuals with dementia or other severe comorbidities. However, the recruitment of the participants was mainly through primary care physicians in Australia or clinical trial centers in the United States, and therefore, the participants should be broadly representative of individuals who have reached older age in relatively good health. Another limitation is that the data only concern the cognitive change during a specific period, with participants aged 65 years or above, and the majority between 70 and 75 years, who were followed for an average of almost 5 years. Thus, it is not possible to infer how these trajectories will evolve over a longer time frame. Two trajectories with sharp decline were identified for the 3MS and HVLT‐R delayed recall, both with the highest proportion of incident dementia cases. However, it remains unknown why several participants were also in these classes who did not reach the dementia endpoint during the follow‐up. Possible explanations include the continuation of pre‐existing neurodegenerative diseases other than dementia, and the terminal cognitive decline prior to death. 50 However, future studies are needed to investigate and validate the cause of such rapid decline.
5. CONCLUSION
The results of this study indicate that, in relatively healthy older adults, the variability of cognitive change was observed across individuals in all cognitive domains assessed. Most individuals were able to maintain their cognitive function at a relatively stable level over the follow‐up period. Slight improvement in phonemic fluency and moderate decline in psychomotor speed were common. Accelerated decline was observed in only a small proportion of individuals in delayed recall and global cognitive function, and this was most common in individuals that developed incident dementia. Therefore, although gradual cognitive decline is often thought to be an expected component of aging in many individuals, 1 normal cognitive aging can also be characterized as the maintenance of cognitive function over time, with some individuals continuing to perform at very high levels, even with advancing age.
CONFLICTS OF INTEREST
Anne M. Murray reports receiving consulting fees from Alkahest, Inc. and grants from the National Institute on Aging. Raj C. Shah reports grants for clinical research regarding dementia and Alzheimer's disease from the National Institutes of Health, the Centers for Medicare and Medicaid Services, the Department of Defense, and the Illinois Department of Public Health; being a non‐compensated member of the Board of Directors of the Alzheimer's Association–Illinois Chapter; and being a site principal investigator or sub‐investigator for clinical trials and research studies for which his institution (Rush University Medical Center) is sponsored (Amylyx Pharmaceuticals, Inc.; Eli Lilly & Co., Inc.; Genentech, Inc.; Lundbeck, Inc.; Merck & Co, Inc.; Navidea Biopharmaceuticals; Novartis Pharmaceuticals, Inc.; Roche Holdings AG; and Takeda Development Center Americas, Inc.). Robyn L. Woods, Rory Wolfe, Elsdon Storey, Trevor T. J. Chong, John J. McNeil, and Suzanne G. Orchard report no competing interests.
FUNDING INFORMATION
This work was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (U01AG029824); the National Health and Medical Research Council (NHMRC) of Australia (334047 and 1127060); Monash University (Australia); and the Victorian Cancer Agency (Australia). Joanne Ryan is funded by an NHMRC Dementia Research Leader Fellowship (APP1135727). Zimu Wu is funded by a Research Training Program scholarship, awarded by Monash University and the Australian government. The funding bodies were not involved in study design; collection, analysis, and interpretation of the data; the writing of the manuscript; or in the decision to submit the article for publication.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
The authors acknowledge the significant contribution of the dedicated and skilled staff in Australia and the United States to the ASPREE clinical trial. The authors are also most grateful to the ASPREE participants, who so willingly volunteered for this study, and the medical staff and clinics who cared for the participants.
Wu Zimu, Woods RobynL, Wolfe Rory, et al., the ASPREE Investigator Group . Trajectories of cognitive function in community‐dwelling older adults: A longitudinal study of population heterogeneity. Alzheimer's Dement. 2021;13:e12180. 10.1002/dad2.12180
REFERENCES
- 1. Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, Phyo AZZ, Al‐harbi T, Woods RL, Ryan J. Distinct cognitive trajectories in late life and associated predictors and outcomes: a systematic review. J Alzheimers Dis Rep. 2020:459‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anstey KJ, Wood J. Chronological age and age‐related cognitive deficits are associated with an increase in multiple types of driving errors in late life. Neuropsychology. 2011;25(5):613‐621. [DOI] [PubMed] [Google Scholar]
- 4. Peters R, Booth A, Rockwood K, Peters J, D'Este C, Anstey KJ. Combining modifiable risk factors and risk of dementia: a systematic review and meta‐analysis. BMJ Open. 2019;9(1):e022846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta‐analyses. Alzheimer's Dement. 2017;13(4):406‐418. [DOI] [PubMed] [Google Scholar]
- 6. Terrera GM, Brayne C, Matthews F, Group CCSC. One size fits all? Why we need more sophisticated analytical methods in the explanation of trajectories of cognition in older age and their potential risk factors. Int Psychogeriatr. 2010;22(2):291‐299. [DOI] [PubMed] [Google Scholar]
- 7. Howrey BT, Raji MA, Masel MM, Peek MK. Stability in cognitive function over 18 years: prevalence and predictors among older Mexican Americans. Curr Alzheimer Res. 2015;12(7):614‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McNeil JJ, Woods RL, Nelson MR, et al. Baseline characteristics of participants in the ASPREE (Aspirin in reducing events in the elderly) study. J Gerontol A Biol Sci Med Sci. 2019;74(5):748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teng EL, Chui HC. The modified mini‐mental state (3MS) examination. J Clin Psychiatry. 1987;48(8):314‐318. [PubMed] [Google Scholar]
- 10. Ross TP. The reliability of cluster and switch scores for the controlled oral word association test. Arch Clin Neuropsychol. 2003;18(2):153‐164. [PubMed] [Google Scholar]
- 11. Ryan J, Woods RL, Murray AM, et al. Normative performance of older individuals on the Hopkins Verbal Learning Test‐Revised (HVLT‐R) according to ethno‐racial group, gender, age and education level. Clin Neuropsychol. 2020:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheridan LK, Fitzgerald HE, Adams KM, et al. Normative Symbol Digit Modalities Test performance in a community‐based sample. Arch Clin Neuropsychol. 2006;21(1):23‐28. [DOI] [PubMed] [Google Scholar]
- 13. Bell CC. DSM‐IV: diagnostic and statistical manual of mental disorders. JAMA, J Am Med Assoc. 1994;272(10):828‐829. [Google Scholar]
- 14. Ryan J, Storey E, Murray AM, et al. Randomized placebo‐controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology. 2020;95(3):e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teipel SJ, Cavedo E, Lista S, et al. Effect of Alzheimer's disease risk and protective factors on cognitive trajectories in subjective memory complainers: an INSIGHT‐preAD study. Alzheimer's dement. 2018;14(9):1126‐1136. [DOI] [PubMed] [Google Scholar]
- 16. Lin FV, Wang X, Wu R, Rebok GW, Chapman BP. Alzheimer's disease neuroimaging i. identification of successful cognitive aging in the Alzheimer's disease neuroimaging initiative study. J Alzheimers Dis. 2017;59(1):101‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graziane JA, Beer JC, Snitz BE, Chang CC, Ganguli M. Dual trajectories of depression and cognition: a longitudinal population‐based study. Am J Geriatr Psychiatry. 2016;24(5):364‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sha T, Cheng W, Yan Y. Prospective association between sleep‐related factors and the trajectories of cognitive performance in the elderly Chinese population across a 5‐year period cohort study. PLoS One. 2019;14(9):e0222192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cullen B, O'Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78(8):790‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Australian Institute of Health and Welfare 2012. Dementia in Australia. In. Canberra: AIHL; 2012.
- 21. Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bergemann TL, Bangirana P, Boivin MJ, Connett JE, Giordani BJ, John CC. Statistical approaches to assess the effects of disease on neurocognitive function over time. J Biom Biostat. 2012;Suppl 7:7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding X, Charnigo RJ, Schmitt FA, Kryscio RJ, Abner EL. Evaluating trajectories of episodic memory in normal cognition and mild cognitive impairment: results from ADNI. PLoS One. 2019;14(2):e0212435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014;30(3):421‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer's disease. Cogn Behav Neurol. 2003;16(4):211‐218. [DOI] [PubMed] [Google Scholar]
- 26. Aretouli E, Brandt J. Episodic memory in dementia: characteristics of new learning that differentiate Alzheimer's, Huntington's, and Parkinson's diseases. Arch Clin Neuropsychol. 2010;25(5):396‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: a meta‐analysis. Neuropsychologia. 2004;42(9):1212‐1222. [DOI] [PubMed] [Google Scholar]
- 28. Shao Z, Janse E, Visser K, Meyer AS. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol. 2014;5:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49(12):1253‐1258. [DOI] [PubMed] [Google Scholar]
- 30. Clark LJ, Gatz M, Zheng L, Chen YL, McCleary C, Mack WJ. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2009;24(6):461‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jutten RJ, Grandoit E, Foldi NS, et al. Lower practice effects as a marker of cognitive performance and dementia risk: a literature review. Alzheimers Dement (Amst). 2020;12(1):e12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo L, Luk G, Bialystok E. Effect of language proficiency and executive control on verbal fluency performance in bilinguals. Cognition. 2010;114(1):29‐41. [DOI] [PubMed] [Google Scholar]
- 33. Monsch AU, Seifritz E, Taylor KI, Ermini‐Funfschilling D, Stahelin HB, Spiegel R. Category fluency is also predominantly affected in Swiss Alzheimer's disease patients. Acta Neurol Scand. 1997;95(2):81‐84. [DOI] [PubMed] [Google Scholar]
- 34. Kiely KM, Butterworth P, Watson N, Wooden M. The Symbol Digit Modalities Test: normative data from a large nationally representative sample of Australians. Arch Clin Neuropsychol. 2014;29(8):767‐775. [DOI] [PubMed] [Google Scholar]
- 35. Ebaid D, Crewther SG, MacCalman K, Brown A, Crewther DP. Cognitive processing speed across the lifespan: beyond the influence of motor speed. Front Aging Neurosci. 2017;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gonzalez HM, Whitfield KE, West BT, Williams DR, Lichtenberg PA, Jackson JS. Modified‐Symbol digit modalities test for African Americans, Caribbean Black Americans, and non‐Latino Whites: nationally representative normative data from the National Survey of American Life. Arch Clin Neuropsychol. 2007;22(5):605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arango‐Lasprilla JC, Rivera D, Rodriguez G, et al. Symbol Digit Modalities Test: normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation. 2015;37(4):625‐638. [DOI] [PubMed] [Google Scholar]
- 38. Pena‐Casanova J, Quinones‐Ubeda S, Quintana‐Aparicio M, et al. Spanish Multicenter Normative Studies (NEURONORMA Project): norms for verbal span, visuospatial span, letter and number sequencing, trail making test, and symbol digit modalities test. Arch Clin Neuropsychol. 2009;24(4):321‐341. [DOI] [PubMed] [Google Scholar]
- 39. Carlson MC, Xue QL, Zhou J, Fried LP. Executive decline and dysfunction precedes declines in memory: the Women's Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2009;64(1):110‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salthouse TA. The processing‐speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403‐428. [DOI] [PubMed] [Google Scholar]
- 41. Kerchner GA, Racine CA, Hale S, et al. Cognitive processing speed in older adults: relationship with white matter integrity. PLoS One. 2012;7(11):e50425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haworth J, Phillips M, Newson M, Rogers PJ, Torrens‐Burton A, Tales A. Measuring information processing speed in mild cognitive impairment: clinical versus research dichotomy. J Alzheimers Dis. 2016;51(1):263‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amieva H, Meillon C, Proust‐Lima C, Dartigues JF. Is low psychomotor speed a marker of brain vulnerability in late life? digit symbol substitution test in the prediction of Alzheimer, Parkinson, Stroke, Disability, and Depression. Dement Geriatr Cogn Disord. 2019;47(4‐6):297‐305. [DOI] [PubMed] [Google Scholar]
- 44. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673‐2734. [DOI] [PubMed] [Google Scholar]
- 45. Ryan J, Woods RL, Britt C, et al. Normative performance of healthy older individuals on the modified mini‐mental state (3MS) examination according to ethno‐racial group, gender, age, and education level. Clin Neuropsychol. 2019;33(4):779‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ryan J, Woods RL, Britt CJ, et al. Normative data for the symbol digit modalities test in Older White Australians and Americans, African‐Americans, and Hispanic/Latinos. J Alzheimers Dis Rep. 2020;4(1):313‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Loprinzi PD, Frith E. The role of sex in memory function: considerations and recommendations in the context of exercise. J Clin Med. 2018;7(6):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Herlitz A, Yonker JE. Sex differences in episodic memory: the influence of intelligence. J Clin Exp Neuropsychol. 2002;24(1):107‐114. [DOI] [PubMed] [Google Scholar]
- 49. Mielke MM. Sex and gender differences in Alzheimer's disease dementia. Psychiatr Times. 2018;35(11):14‐17. [PMC free article] [PubMed] [Google Scholar]
- 50. MacDonald SW, Hultsch DF, Dixon RA. Aging and the shape of cognitive change before death: terminal decline or terminal drop?. J Gerontol B Psychol Sci Soc Sci. 2011;66(3):292‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


