S14, an essential ribosomal protein, may have evolved to adapt bacteria to zinc-limited environments by replacement of a zinc-binding motif with a zinc-independent sequence. It was expected that the bacterial ribosome would be tolerant to replacement of S14 because of the previous prediction that the spread of C− type S14 involved horizontal gene transfer.
KEYWORDS: ribosome, ribosomal protein S14, zinc, Bacillus subtilis
ABSTRACT
Ribosomal protein S14 can be classified into three types. The first, the C+ type has a Zn2+ binding motif and is ancestral. The second and third are the C− short and C− long types, neither of which contain a Zn2+ binding motif and which are ca. 90 residues and 100 residues in length, respectively. In the present study, the C+ type S14 from Bacillus subtilis ribosomes (S14BsC+) were completely replaced by the heterologous C− long type of S14 from Escherichia coli (S14Ec) or Synechococcus elongatus (S14Se). Surprisingly, S14Ec and S14Se were incorporated fully into 70S ribosomes in B. subtilis. However, the growth rates as well as the sporulation efficiency of the mutants harboring heterologous S14 were significantly decreased. In these mutants, the polysome fraction was decreased and the 30S and 50S subunits accumulated unusually, indicating that cellular translational activity of these mutants was decreased. In vitro analysis showed a reduction in the translational activity of the 70S ribosome fraction purified from these mutants. The abundance of ribosomal proteins S2 and S3 in the 30S fraction in these mutants was reduced while that of S14 was not significantly decreased. It seems likely that binding of heterologous S14 changes the structure of the 30S subunit, which causes a decrease in the assembly efficiency of S2 and S3, which are located near the binding site of S14. Moreover, we found that S3 from S. elongatus cannot function in B. subtilis unless S14Se is present.
IMPORTANCE S14, an essential ribosomal protein, may have evolved to adapt bacteria to zinc-limited environments by replacement of a zinc-binding motif with a zinc-independent sequence. It was expected that the bacterial ribosome would be tolerant to replacement of S14 because of the previous prediction that the spread of C− type S14 involved horizontal gene transfer. In this study, we completely replaced the C+ type of S14 in B. subtilis ribosome with the heterologous C− long type of S14 and characterized the resulting chimeric ribosomes. Our results suggest that the B. subtilis ribosome is permissive for the replacement of S14, but coevolution of S3 might be required to utilize the C− long type of S14 more effectively.
INTRODUCTION
The bacterial 70S ribosome is a complex macromolecule that is composed of small (30S) and large (50S) subunits. The small subunit comprises the 16S rRNA and more than 20 proteins, while the large subunit comprises the 23S and 5S rRNAs and more than 30 proteins (1, 2). The molecular mechanisms of translation have been elucidated in detail by a combination of various approaches (3–7). The small subunit associates with the mRNA and the anticodon stem-loop of the bound tRNA and engages in ensuring the fidelity of translation by checking for correct pairing between the codon and anticodon (8–12). The large subunit associates with the acceptor arms of the tRNA and catalyzes the formation of a peptide bond between the amino acid attached to the tRNA in the A-site and the nascent peptide chain bound to the tRNA in the P-site (13, 14). Therefore, the coordinated action of these subunits is essential for protein synthesis by the ribosome. The ribosomal proteins that constitute these subunits play important roles in translation. For instance, ribosomal protein L1, which is localized to the stalk region near the E-site (7, 15), plays a critical role in the translocation of the newly deacylated tRNA from the P to the E site (16) and contributes to translation initiation with elongation factor P (17).
Ribosomal protein S14 is an important target for understanding the molecular mechanisms of translation. In addition, S14 is an interesting ribosomal protein with respect to discussion of ribosome evolution. S14 can be classified into three types (18, 19) (Fig. 1A). The first, the C+ type (S14C+), has a Zn2+ binding motif containing four conserved cysteines, which is 61-amino-acid (aa) residues or less in length. The second and third are the C− short and C− long types, both of which lack a Zn2+ binding motif and which are ca. 90 residues and 100 residues in length, respectively. The C+ type of S14 is present in eukaryotes, archaea, and several bacterial phyla, whereas the C− types are present in many bacteria (Fig. 1A). Because the C+ type of S14 is found in eukaryotes and archaea, the C+ type is believed to be the ancestral molecule (18, 19) (Fig. 1A). Indeed, some of the cysteine residues, which form the Zn2+ binding motif, are present in several C− type S14s (19).
FIG 1.

Phylogenetic tree and alignment of ribosomal protein S14. (A) Protein sequences, available from the NCBI database (https://www.ncbi.nlm.nih.gov/protein), were aligned, and a phylogenetic tree was constructed by the maximum likelihood method and Le-Gascuel model (59) with the use of MEGA X software (52). Bootstrapping was performed with 1,000 replicates. The scale bar (0.50) indicates the number of changes per site. “[B]” after a species name indicates that the species have both types (C+ and C−) of S14. (B) Alignment of the amino acid sequences of S14 from B. subtilis, E. coli, and S. elongatus. Identical and similar amino acids are highlighted with yellow and blue, respectively. Cysteines forming the zinc-binding motif and remnants of this motif in sequences that do not have all four conserved cysteines are shown in bold.
The majority of bacteria have only one class of S14, although several bacteria harbor two (19) (Fig. 1A). The Gram-positive model organism Bacillus subtilis has the C+ type (RpsN, designated S14BsC+) and the C− short type (YhzA, designated as S14BsC−). The transcription of the gene encoding S14BsC− is repressed by Zur (20), which is a zinc-sensing metalloregulatory protein that regulates the transcription of the zinc uptake machinery (21–23). Although almost all B. subtilis ribosomes bind S14BsC+ in zinc-sufficient conditions, such as in LB medium, when the cellular concentration of Zn2+ is decreased, S14BsC− is expressed following derepression from Zur and can be incorporated into newly synthesized ribosomes (20). Because S14BsC+, a zinc-binding protein, is probably inactivated by zinc limitation, S14BsC− is thought to form a fail-safe system for ribosomes in B. subtilis in zinc-deficient environmental conditions (20).
Ribosomal proteins which contribute to translation, an essential function for all living cells, are highly conserved (24). Several reports have shown that heterologous ribosomal proteins can partially bind to the ribosome of a species in the place of the corresponding naturally occurring ribosomal protein and can sometimes function in the cells (25–28). With respect to the ribosomal protein S14, it has been reported that S14 from Thermus thermophilus, when overexpressed in Escherichia coli, partially bound to the E. coli ribosome (29). This previous study showed that the incorporation of the C+ type of S14 from T. thermophilus into the E. coli ribosome, which normally contains the C− long type of S14, had little effect on the formation of the 70S ribosome or the growth rate. However, the matter was complicated by the homologous C− long type S14 of E. coli remaining in the cells (29). Therefore, it appears that the ancestral C+ type of S14 from T. thermophilus can function normally in E. coli even though it normally only contains the C− long type, which is probably a type of S14 that evolved later. However, whether C− long type S14 can be utilized by ribosomes that mainly use the C+ type of S14 has not been investigated. In addition, complete replacement of an essential ribosomal protein like S14 has not previously been researched in vivo, although partial replacement of ribosomal proteins or complete exchange of nonessential ribosomal protein has been reported in previous studies (25–30).
Although several processes for the evolution of S14 can be hypothesized, bacteria have necessarily experienced the replacement of the C+ type of S14 with the C− type of S14. When the C+ type of S14 was replaced during evolution, the C− type S14 must have been immediately functional in the ribosome because S14 is essential for cell proliferation both in E. coli and B. subtilis (31–33) and probably in almost all bacteria. In this context, it is noteworthy that one of the possible evolutionary processes that could result in in situ gene replacement of the C+ type of S14 with the C− long type has been predicted by comparative genomics (19). Therefore, it might be predicted that the bacterial ribosome is tolerant to replacement of S14 and this permissiveness allows dynamic evolution of S14. In this study, we have attempted to completely replace the S14BsC+ in B. subtilis with the heterologous S14 from E. coli or Synechococcus elongatus PCC7942 to investigate whether B. subtilis ribosomes, which mainly use the C+ type of S14, can utilize the C− long type of S14. We have characterized the resulting chimeric ribosomes to evaluate the fitness of the C− long type of S14 in B. subtilis and finally discuss the possibility of coevolution of S14 and S3.
RESULTS
S14Ec and S14Se can bind to the B. subtilis ribosome and function in the cell.
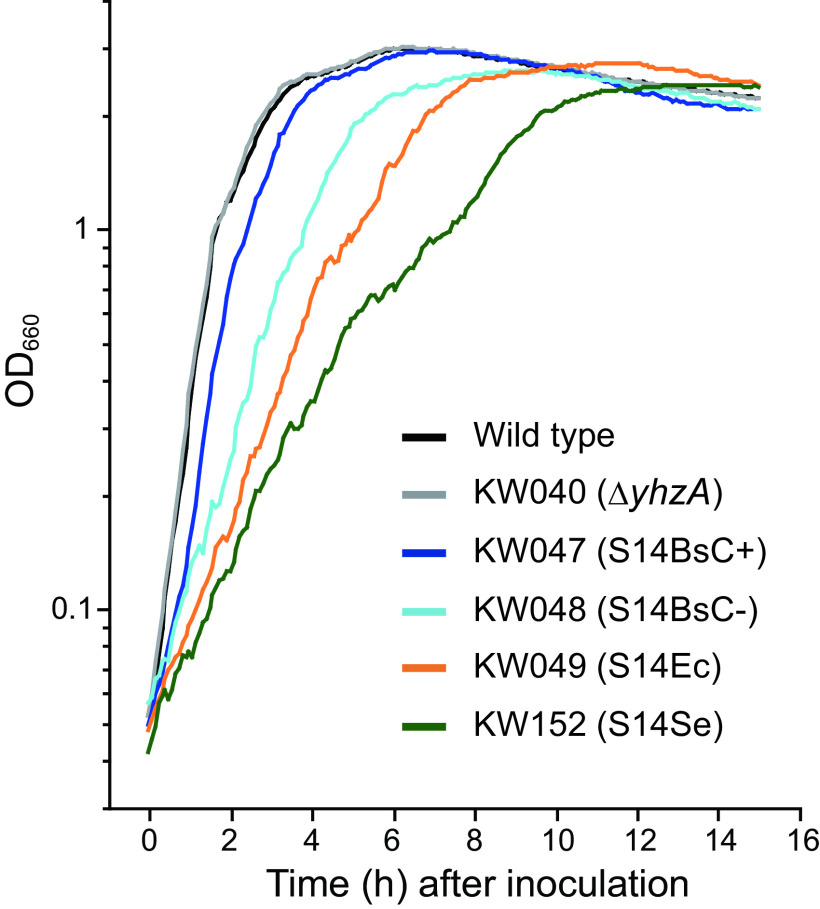
The C− long type of S14 from E. coli, a Gram-negative model organism, or from S. elongatus, a photosynthetic bacterium, was investigated for its ability to function in the B. subtilis ribosome in place of S14BsC+. Because S14BsC− barely expresses in the conditions used in this study (20), the effect of replacement of S14 can be regarded as the result of the replacement of S14BsC+ by heterologous S14. S14BsC+ contains a zinc-binding motif (CXXC-12X-CXXC) between residues 24 and 43, while the motif is lacking in both S14Ec and S14Se (Fig. 1B). S14BsC−, S14Ec, and S14Se are 28 aa, 40 aa, and 39 aa longer than S14BsC+, respectively (Fig. 1B). S14BsC−, S14Ec, and S14Se share 45%, 39%, and 40% identity with S14BsC+, respectively. Figure S1A in the supplemental material shows an overview of the replacement of the gene encoding S14BsC+ (rpsNBs) in the B. subtilis genome by genes encoding heterologous S14. Similarly, we constructed strains in which the gene encoding S14BsC+ or S14BsC− was inserted into the amyE locus. Initially, the gene encoding S14BsC− (yhzA) was deleted to avoid complementation of S14BsC+ by S14BsC−, in spite of an almost total lack of expression of S14BsC− in the conditions used in this study (20). Subsequently, genes encoding individual S14 proteins (rpsNBs, yhzA, rpsNEc, and rpsNSe) were inserted into the aprE locus. The transcription of these genes is regulated by the Pspac promoter, allowing expression to be controlled by addition of IPTG to the medium. Finally, rpsNBs was replaced by the cat gene in conditions that induced expression of each S14 gene (Fig. S1A). We successfully constructed these strains (Table 1) and confirmed that their proliferation was completely dependent on the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to the medium (Fig. S1B). However, the growth rate of the strain harboring only Pspac-rpsNSe S14Se was extremely high, meaning that it was impossible to analyze the ribosomes (Fig. S1C). We, therefore, inserted the gene encoding S14Se (rpsNSe) under the control of the strong promoter, PtrnS, in addition to the Pspac-rpsNSe in the aprE locus (Fig. S1D). Thus, it was demonstrated that both S14Ec and S14Se can complement the function of S14BsC+ at least in part. In the present study, we use the resulting strain (KW152) to investigate the effect of replacement of S14BsC+ by S14Se. The growth rate of the strain harboring the Pspac-rpsNBs (KW047) was not reduced significantly, whereas that of the strain containing Pspac-yhzA (KW048) was moderately decreased compared to that of the wild type (Fig. 2 and Table 2). Notably, the replacement of S14BsC+ by S14Ec (KW049) or S14Se (KW152) caused severe reduction in the growth rate (Fig. 2 and Table 2). The variation of growth rate of these strains suggests, in part, different effects of complementation of S14BsC+ with individual S14 proteins.
TABLE 1.
Bacillus subtilis strains used in the study
| Strain | Genotype (characteristic[s]) | Source or reference |
|---|---|---|
| 168W | Laboratory stock | |
| 168 | trpC2 | Laboratory stock |
| RIK821 | trpC2 ΔyhzA::cat | 21 |
| RIK838 | trpC2 aprE::Pspac-rpsNBs spc | 20 |
| RIK840 | trpC2 aprE::Pspac-yhzA spc | 20 |
| RIK839 | trpC2 ΔrpsNBs::catpt1 aprE::Pspac-rpsN spc | 20 |
| KW040 | ΔyhzA | This study |
| KW041 | ΔyhzA aprE::Pspac-rpsNBs spc | This study |
| KW042 | ΔyhzA aprE::Pspac-yhzA spc | This study |
| KW043 | ΔyhzA aprE::Pspac-rpsNEc spc | This study |
| KW044 | ΔyhzA aprE::Pspac-rpsNSe spc | This study |
| KW047 | ΔyhzA ΔrpsNBs::catpt1 aprE::Pspac-rpsNBs spc | This study |
| KW048 | ΔyhzA ΔrpsNBs::catpt1 aprE::Pspac-yhzA spc | This study |
| KW049 | ΔyhzA ΔrpsNBs::catpt1 aprE::Pspac-rpsNEc spc | This study |
| KW051 | ΔyhzA ΔrpsNBs::catpt1 aprE::Pspac-rpsNEc spc Δspo0E::erm | This study |
| KW151 | ΔyhzA aprE::Pspac-rpsNSe spc PtrnS-rpsNSe kan | This study |
| KW152 | ΔyhzA ΔrpsNBs::catpt1 aprE::Pspac-rpsNSe spc PtrnS-rpsNSe kan | This study |
| KW153 | ΔyhzA ΔrpsNBs::catpt1 aprE::Pspac-rpsNSe spc PtrnS-rpsNSe kan Δspo0E::erm | This study |
| KW156 | ΔyhzA amyE::Phyperspanc-rpsCBs-rpsBBs erm | This study |
| KW157 | ΔyhzA amyE::Phyperspanc-rpsCBs-rpsBBs erm ΔrpsCBs::catpt1 | This study |
| KW158 | ΔyhzA amyE::Phyperspanc-rpsCBs-rpsBBs erm PtrnS-rpsNSe kan ΔrpsCBs::rpsCSe | This study |
| KW159 | ΔyhzA ΔrpsNBs::catpt1 amyE::Phyperspanc-rpsCBs-rpsBBs erm PtrnS-rpsNSe kan ΔrpsCBs::rpsCSe | This study |
FIG 2.
Effect of the replacement of S14BsC+ by heterologous S14 on the growth rate. Cells were grown in LB at 37°C, and the optical density at 660 nm was measured.
TABLE 2.
Doubling times of mutants containing different S14 proteins
| Strain | Doubling time (min)a |
|---|---|
| Wild type | 21 ± 0.8 |
| KW040 (ΔS14BsC−) | 21 ± 0.7 |
| KW047 (S14BsC+) | 25 ± 1 |
| KW048 (S14BsC−) | 43 ± 2 |
| KW049 (S14Ec) | 59 ± 3 |
| KW152 (S14Se) | 76 ± 3 |
Means from three independent experiments (±standard deviation) are shown.
We next examined whether these heterologous S14 molecules can bind to the B. subtilis 70S ribosome. Ribosomal proteins were prepared from 70S ribosomes and analyzed by Radical-free and highly reducing (RFHR) two-dimensional (2D) gel electrophoresis (Fig. 3). The 70S ribosome prepared from wild-type cells contained S14BsC+, and novel protein spots were detected on a 2D gel of ribosomal proteins prepared from cells harboring S14Ec or S14Se (Fig. 3). These novel protein spots were identified as S14Ec and S14Se by peptide mass fingerprinting. Although the protein spot corresponding to S14BsC− was difficult to observe on a 2D gel because of overlap with the spot corresponding to S20, which has a similar pI and molecular weight (MW) (S14BsC−, pI of 11.2 and MW of 10 kDa; S20, pI of 11.2 and MW of 9.4 kDa), it has previously been demonstrated that S14BsC−, expressed from the Pspac promoter, could bind to the ribosome (20). Image analysis shows that the relative intensity of protein spots corresponding to S14BsC+, S14Ec, and S14Se were 1.9%, 2.0%, and 2.0%, respectively, when total intensity of ribosomal protein spots was defined as 100% (see Table S2 in the supplemental material). These results indicate that the S14Ec and S14Se were incorporated fully into the B. subtilis 70S ribosome.
FIG 3.
Component proteins of 70S ribosomes prepared from cells containing heterologous S14. The upper panel shows whole 2D gel of ribosomal proteins from 70S ribosomes from wild type. The areas of the two-dimensional gels containing the spot corresponding to each S14 were extracted from gel images (lower panels). Spots corresponding to S14 are indicated by closed arrowheads, and the absence of the protein spot corresponding to S14Bs upon replacement is indicated by open arrowheads.
The replacement of S14 reduces translational activity of B. subtilis.
We next investigated the effect of replacement of S14 on the function of the B. subtilis ribosome. To determine the state of ribosomes in the cells, cell lysates were analyzed by sucrose density gradient sedimentation. As shown in Fig. 4, in the KW049 (S14Ec) and KW152 (S14Se) cells, a decrease in the polysome fraction as well as accumulation of the 30S and 50S subunits was observed. Although similar trends are also observed in the KW048 (S14BsC−) cells, the change in the ribosome pattern was less than that for the other mutants only containing heterologous S14. Polysomes, which consist of active 70S ribosomes bound to an mRNA, are abundant in cells with high translational activity. Thus, reduction in the polysome fraction and accumulation of inactive subunits in the lysates from mutants indicates that the cellular translational activity was decreased upon replacement of S14BsC+ by S14Ec or S14Se. These results prompted us to evaluate the translational activity of the 70S ribosome fraction purified from these mutants in vitro. In these experiments, the translational activity of prepared ribosomes toward gfp mRNA was determined by measurement of the amount of synthesized green fluorescent protein (GFP). Note that the purified 70S ribosomes used in this analysis harbored all ribosomal proteins, which was confirmed by 2D gel analysis (Fig. 3). As expected, the translational activities of ribosome fractions containing S14Ec or S14Se were significantly decreased (S14Ec, 59%; S14Se, 42%; relative to the wild-type ribosome), while that of ribosomes containing S14BsC− was slightly decreased (82% relative to the wild-type ribosome) (Fig. 5). The reductions in the translational activity of a ribosome fraction containing heterologous S14 as well as S14BsC− are consistent with the result from the sucrose density gradient sedimentation experiments.
FIG 4.
Decrease in the polysome fraction and accumulation of the 30S and 50S subunits in cells harboring S14Ec or S14Se. Crude cell extracts were sedimented through a 10 to 40% sucrose gradient as described in Materials and Methods. The 30S, 50S, 70S, and polysome peaks are indicated in each individual profile. Abs, absorbance.
FIG 5.
In vitro translation analysis using 70S ribosomes containing heterologous S14. The relative amount of GFP, which was synthesized by each 70S ribosome, is shown as relative activity where the amount of GFP synthesized by wild-type 70S is defined as 1. The 70S ribosomes were purified from wild-type, KW047 (S14BsC+), KW048 (S14BsC−), KW049 (S14Ec), and KW152 (S14Se) cells.
To explore the contributing factors to the reduction in the translational activity upon replacement of S14, component proteins of the 30S subunit that accumulated unusually in the KW049 (S14Ec) and KW152 (S14Se) cells were analyzed by 2D electrophoresis (Fig. 6A). The results showed that the relative amounts both of S2 and S3 in the 30S fraction purified from the KW049 and KW152 cells were decreased. It was difficult to prepare the 30S subunit to high purity because the 30S fractions from mutants were prepared in normal Mg2+ concentration, which maintains 70S ribosomes. Therefore, several protein spots from the 50S subunit contaminated the 30S fraction of mutant cells. In contrast, when the 30S subunit was prepared from the wild type, 70S ribosomes were dissociated artificially into 30S and 50S subunits by centrifugation of lysate in low-Mg2+ concentration because wild-type cells contain very small amounts of subunits in normal conditions. Thus, spots corresponding to proteins other than the components of the 30S subunit were barely detected in the 2D gel of 30S components from the wild type. Therefore, the total intensity of protein spots was calculated from species that are present only in the 30S subunit, and the relative intensity of each protein spot was calculated. The data indicate that S14Ec and S14Se fully bound to the 30S subunit because the relative intensities of these heterologous S14 spots were similar to those of the S14BsC+ (Table S2). In contrast, the relative intensities both of S2 and S3 spots were decreased in the 30S fractions prepared from KW049 and KW152 cells (Table 2). It is, thus, likely that the rate of incorporation of S2 and S3 by the 30S subunit containing heterologous S14 is reduced.
FIG 6.

Effect of the replacement of S14BsC+ by heterologous S14 on the assembly of the 30S subunit. (A) Two-dimensional electrophoresis analysis of the component proteins of each 30S subunit prepared from cells containing heterologous S14. Ribosomal protein spots are indicated by closed arrowheads, and the absence of the protein spot corresponding to S14Bs by replacement is indicated by open arrowheads. (B) Comparison of ribbon diagram of S14BsC+ (cyan) with that of S14BsC− (yellow), S14Ec (green), and S14Se (magenta). The cysteine residues that form the zinc-binding motif of S14BsC+ are highlighted in red. The protein structures of S14BsC+, S14BsC−, S14Ec, and S14Se were predicted by SWISS-MODEL (http://swissmodel.expasy.org/) (60) using the solved structure of B. subtilis ribosome (Protein Data Bank [PDB] accession number 5NJT), Mycobacterium smegmatis C− ribosome (PDB accession number 6DZI), E. coli ribosome (PDB accession number 3J9Y), and chloroplast ribosome of Spinacia oleracea (PDB accession number 6ERI) as the template. An image of the resulting structure was generated using PyMOL (www.pymol.org) (61). The N-terminal extrusion is highlighted with a dotted circle (see details in text). (C) Location of S2 (cyan), S3 (green), and S14 (red) in the B. subtilis 70S ribosome are shown using the solved structure (PDB accession number 5NJT). The 16S rRNA in the 30S subunit is shown in blue.
The sporulation of B. subtilis is inhibited at an early stage by replacement of S14.
The replacement of S14BsC+ by heterologous S14 decreased not only the growth rate but also the sporulation efficiency (Table 3). In cells containing S14BsC+, more than 80% of viable cells formed heat-resistant spores 24 h after inoculation when cells were grown in sporulation medium, 2× Schaeffe's-glucose (2× SG). The sporulation frequency of KW048 (S14BsC−) cells decreased by half compared to that of wild-type cells. Furthermore, KW049 (S14Ec) and KW152 (S14Se) cells were also severely defective in forming heat-resistant spores. Note that the extension of the cultivation time did not increase the number of spores formed by KW049 and KW152 cells (data not shown). At the initiation stage of B. subtilis sporulation, cells divide asymmetrically, and chromosomal DNA is concentrated in the forespore (34). In wild-type cells, asymmetric septation and concentration of chromosomal DNA were detected 5 h after inoculation into sporulation medium (Fig. 7A). In contrast, in the KW049 and KW152 cells, asymmetric septation was not observed even 24 h after inoculation (Fig. 7A). These results show that replacement of S14BsC+ by heterologous S14 causes a defect in the initiation stage of sporulation. Thus, we next evaluated the level of Spo0A in these mutants. Phosphorylation of Spo0A, the master transcriptional regulator of sporulation, governs the decision to initiate sporulation (35–37). In wild-type cells, the level of Spo0A increased 4 h after inoculation into sporulation medium (Fig. 7B). In the KW048 cells, the amount of Spo0A increased 6 h after inoculation, although the level of Spo0A was lower than that in the wild type (Fig. 7B). However, the increase in the level of Spo0A in the KW049 and KW152 cells was significantly delayed and reduced, even after taking into consideration the slow growth (Fig. 7B and C). Moreover, in the KW152 cells, Spo0A was barely detectible even 10 h after inoculation (Fig. 7B). These results indicate that the decrease in the Spo0A levels is one of the causes for the sporulation defect in the mutants harboring heterologous S14. The accumulation of sufficient Spo0A requires phosphorylation of Spo0A (Spo0A∼P) because transcription of spo0A is fully activated by Spo0A∼P (38). To investigate whether the stabilization of Spo0A∼P, which induces expression of spo0A and sporulation genes, could rescue the sporulation defect caused by replacement of S14, the spo0E gene, encoding the phosphatase that dephosphorylates Spo0A∼P, was deleted in KW049 and KW152 strains. The deletion of the spo0E gene in these strains significantly improved the expression level of spo0A as well as sporulation frequency, although the growth rate of neither KW049 nor KW152 was restored (Fig. 7B and C and Table 3). In particular, the sporulation frequency of the strain harboring S14Ec was improved to the same level as that of the KW048 (S14BsC−) strain. However, the sporulation frequency of the strain harboring S14Se did not fully recover, indicating that the defect in sporulation in the KW152 cells is not only a function of a reduction in the Spo0A level.
TABLE 3.
Sporulation frequency of mutants containing different S14 proteins
| Strain | CFU ml−1a |
Frequency (%)a | |
|---|---|---|---|
| Total | Spores | ||
| Wild type | 7.4 × 108 | 6.3 × 108 | 87 ± 6.7 |
| (ΔS14BsC−) | 8.4 × 108 | 6.6 × 108 | 79 ± 2.5 |
| S14BsC+ | 6.3 × 108 | 5.1 × 108 | 81 ± 4.4 |
| S14BsC− | 6.4 × 108 | 2.7 × 108 | 42 ± 6.4 |
| S14Ec | 5.0 × 108 | 1.1 × 106 | 0.22 ± 0.1 |
| S14Se | 5.7 × 108 | 4.4 × 103 | 0.00064 ± 0.00011 |
| S14Ec Δspo0E | 7.2 × 108 | 3.3 × 108 | 46 ± 4.7 |
| S14Se Δspo0E | 6.3 × 108 | 1.7 × 106 | 0.29 ± 0.076 |
Means of three independent experiments (±standard deviation for sporulation frequency).
FIG 7.
The replacement of S14BsC+ by heterologous S14 causes a sporulation defect. (A) Microscopic observation of wild-type and mutants harboring S14Ec or S14Se cells. Differential interference contrast and fluorescence images of FM4-64 stained (to visualize membranes) and DAPI stained (to visualize chromosomes) cells grown for indicated times at 37°C in sporulation medium. The arrowheads in the micrographs indicate prespores. Bars indicate 10 μm. (B) Cells were grown in sporulation medium at 37°C and were collected at the indicated times. Crude cell extracts were subjected to Western blot analysis using antisera against Spo0A. (C) Cells were grown in sporulation medium at 37°C, and the optical density at 600 nm was measured.
DISCUSSION
S14 may have evolved to adapt bacteria to zinc-limited environments by replacement of a zinc-binding motif with a zinc-independent sequence. Bacteria that possessed only the ancestral C+ type S14 obtained the C− type S14 by horizontal gene transfer or gene duplication followed by evolution to the C− type (18, 19). With respect to this, it is intriguing to consider how the bacterial ribosome accommodated the recently gained C− type molecule and what changes in the ribosome were necessary.
Comparative analysis of structures of S14 show that the C-terminal region of S14BsC+, which contains the zinc-binding motif, and that of S14Ec and S14Se are very similar, despite the lack of the zinc-binding motif in S14Ec and S14Se (Fig. 6B). In contrast, the protrusion of the N-terminal extended region of S14Ec and S14Se can be seen when the structures of S14BsC+ and heterologous S14 are merged (Fig. 6B). Despite the remarkable differences in protein size relative to S14Bs, S14Ec and S14Se can function in B. subtilis. Although we cannot exclude the possibility that the actual assembly efficiency of heterologous S14s into the 30S subunit is lower than that of S14BsC+ because degradation of immature 30S subunit lacking S14 could not be evaluated, S14Ec and S14Se were fully integrated into the 30S subunit (Fig. 6A). Consistent with this observation, these heterologous S14s bound sufficiently well to the 70S ribosome (Fig. 3). The C-terminal region of S14 is important for binding to the ribosome. It has been reported that mutation of the zinc-binding motif of S14 from T. thermophilus reduces the degree of incorporation into the E. coli ribosome relative to wild-type S14 of T. thermophilus (29). Therefore, it seems likely that, during evolution, additional amino acids have been added to the N-terminal extended region of S14 to maintain the structure of this important region in the absence of zinc. This hypothesis is supported by a previous report that showed that a molecule containing a deletion of the N-terminal extended region (S14BsC−) could not complement S14BsC+ function (20).
Although S14Ec and S14Se, which harbor a structurally conserved C-terminal region, bind fully to the B. subtilis ribosome, these heterologous S14 molecules do not completely complement the function of S14BsC+. It is noteworthy that the assembly efficiency of S2 and S3 into a 30S subunit containing heterologous S14 was significantly decreased (Fig. 6A; see also Table S2 in the supplemental material). In the 30S subunit, S14 and S3 are located close to each other (Fig. 6C). As mentioned above, the N-terminal region of S14Ec and S14Se protrude from the structure of S14BsC+ (Fig. 6B). Thus, the extended region of heterologous S14 is likely to cause a reduction in the assembly efficiency of S3 into the 30S subunit following structural alteration of the neighboring site, which forms the S14-S3 binding region in the 30S subunit. As a result, the assembly efficiency of S2, which is located near S3 in the 30S subunit, is also likely to be decreased. In fact, in vitro reconstitution studies have shown that binding of S14 to the ribosome is required for assembly of S3 and S2 into the 30S subunit (40, 41). Moreover, we found previously that repression of the expression of S14BsC+ causes reduction in the amount both of S2 and S3 in the 30S ribosomal fraction in B. subtilis (20).
Our in vitro assay indicated that the translational activity of the ribosome fraction containing S14Ec or S14Se was markedly decreased (Fig. 5). Initiation of translation requires binding of the 30S initiation complex and the 50S subunit to form the active 70S ribosome (42). In the KW049 (S14Ec) and KW152 (S14Se) cells, 30S subunits, which are unable to coassemble with 50S, aberrantly accumulated, probably due to reduction in the assembly efficiency of S3 and S2. However, at least 37% of 30S subunits (see Table S2; relative intensity of S2 in 30S from KW049 cells is divided by that of 30S from wild-type cells), which aberrantly accumulated in KW049 cells, contained S3 and S2. Thus, it is likely that even in the mature 30S subunit containing heterologous S14, the assembly efficiency of the 30S and 50S subunits is decreased. In addition, it is possible that heterologous S14 does not completely fit the translation system of B. subtilis even if the 70S ribosome is formed. A previous study showed decreased peptidyl transferase activity of ribosomes containing S14 with a mutation in the Zn2+ binding motif (29). Therefore, structural alteration of sites neighboring S14, caused by introducing heterologous S14, may also affect activity of translation elongation. However, it is clear that heterologous S14 reduces the cellular translational activity of B. subtilis.
Although the effect of the replacement of S14BsC+ by S14BsC− was much lower than the replacement of S14BsC+ by heterologous S14 in cells containing S14BsC−, the amount of 30S and 50S subunits was moderately increased, and the translational activity of the 70S ribosome fraction containing S14BsC− was slightly decreased compared to the wild type. These effects on the ribosomes manifested in a decrease in the growth rate of the KW048 (S14BsC−) cells. We have previously observed that the growth rate of the strain lacking S14BsC+, but which has inducible S14BsC− (under the control of the Pspac promoter), was decreased and also found that overexpression of S14BsC− (the protein level was 1.7-fold higher than when S14BsC− was induced by Pspac promoter) did not improve the growth rate of the strain (20). Thus, these effects, which were caused by replacement of S14BsC+ by S14BsC−, were probably not due to insufficient expression of S14BsC−. These results combined with those obtained previously demonstrate that the B. subtilis ribosome does not completely utilize the C− short type of S14. However, in zinc-deficient conditions, which are not suitable for rapid growth, B. subtilis may use the C− short type of S14 to repress the cellular translational activity to some extent.
The replacement of S14BsC+ by heterologous S14 also affected sporulation (Table 3; Fig. 7). The sporulation defect of mutants harboring heterologous S14 was probably not caused solely by the decreased growth rate because the sporulation frequency of the ΔrpmH (L34) mutant, which also shows a growth defect (doubling time, 70.5 min) similar in severity to that of KW152 (S14Se), was almost the same as that of the wild type (43). In previous work, a defect in sporulation has also been observed in ribosomal protein mutants, such as in the ΔrplA (L1) mutant, where sporulation frequency was less than 0.01% (33). Similar to the KW152 cells, in ΔrplA mutant cells, the level of Spo0A did not increase even 10 h after inoculation into sporulation medium (43). Thus, B. subtilis may repress the initiation of sporulation by sensing aberrant ribosomes. In the present study, deletion of Spo0E that dephosphorylates and hence inactivates Spo0A (44) increased the level of phosphorylated, active Spo0A, and also increased sporulation frequency of the mutants harboring heterologous S14. Therefore, the replacement of S14BsC+ with heterologous S14 probably inhibits initiation of sporulation via blocking the phosphorylation of Spo0A with a subsequent decrease in the expression level of spo0A. However, the sporulation frequency of the KW152 (S14Se) did not fully recover upon deletion of Spo0E. It, therefore, seems likely that an insufficient level of translational activity means the KW152 cells cannot synthesize sufficient amounts of the proteins required for sporulation even if Spo0A∼P activates the transcription of sporulation initiation genes. In contrast, the sporulation frequency of cells harboring S14BsC− was not severely decreased (Table 3), indicating that S14BsC− can complement the function of S14BsC+ at least in terms of sporulation. Further investigation into the reasons why S14BsC− allows efficient sporulation but heterologous S14 does not will provide valuable information about the role of ribosome in sporulation initiation.
In the present study, we have completely replaced S14BsC+ with S14Ec and S14Se. To our knowledge, this is the first example of complete replacement of an essential ribosomal protein with heterologous ribosomal proteins. The results described here suggest that bacteria are permissive to the replacement of S14, which supports the previous prediction that the C− type S14 spread by horizontal gene transfer (18, 19). However, the change of S14, especially the difference in conformation of the N-terminal extension, reduces the assembly efficiency of S3 and S2 and affects ribosome functions. It seems likely that coevolution of S3 was required for the C− type S14 to function effectively in the ribosome. The expansion of the protein size of S3 both in E. coli (S3Ec, 223 aa) and S. elongatus (S3Se, 244 aa) compared to B. subtilis (S3Bs, 218 aa) also supports this hypothesis. To test this hypothesis, we attempted to replace the S3Bs with S3Se and found that S3Se cannot function in the presence of S14Bs, whereas it can function in conditions in which S14Se is expressed alone (Fig. 8). This result suggests that S3Se functions only in an S14Se-binding ribosome. Moreover, the expression of S3Bs slightly affected the growth of cells that utilize S14Se and S3Se (Fig. 8). Therefore, it seems likely that S3Se is more compatible with an S14Se-binding ribosome than S3Bs even in the B. subtilis ribosome. Further investigation using the chimeric ribosomes constructed in this study and in other species, such as T. thermophilus, which has only the C+ type of S14, and Streptomyces coelicolor, which has both the C+ type and C− long type of S14, will reveal more about the coevolution of ribosomal proteins.
FIG 8.
The replacement of S3Bs by S3Se in the presence of S14BsC+ and/or S14Se. (A) Strains KW158 (presence of S14Bs and S14Se) and KW159 (presence of only S14Se) were streaked onto an LB plate without (left plate) or with (right plate) 1 mM IPTG. KW158 grew only when S3Bs and S2Bs were expressed, whereas KW159 did not require the induction of S3Bs expression. (B) Cells grown on the LB plate containing 1 mM IPTG were inoculated into LB medium without or with 1 mM IPTG and cultured at 37°C, and the optical density at 600 nm was measured.
MATERIALS AND METHODS
Media and culture conditions.
LB medium (45), LB agar, and 2× Schaeffer’s sporulation medium supplemented with 0.1% glucose (2× SG) (46) were used. The culture conditions and media for preparing competent cells have been described previously (47). When required, 5 μg ml−1 chloramphenicol, 5 μg ml−1 kanamycin, 100 μg ml−1 spectinomycin, and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) were added to the media. In Fig. 3, growth curves of B. subtilis cells were generated by automatically measuring the optical density at 660 nm (OD660) value of each culture every 5 min using a TVS062CA incubator (Advantec).
Bacterial strains.
All of the B. subtilis strains used in this study are isogenic with B. subtilis strain 168. For all primers used in the study, see Table S1 in the supplemental material. To remove the cat gene from strain RIK821 (ΔyhzA::cat trpC2) (33), PCR DNAs, trp+, and ΔyhzA were simultaneously used to transform RIK821. The transformants that showed Trp+ and chloramphenicol-sensitive (Cms) phenotypes were selected to obtain the strain KW040 (ΔyhzA). The trp+ DNA was amplified by PCR using the primers trpDF and hisCR and 168W chromosomal DNA as the template. To obtain the ΔyhzA mutant DNA, upstream and downstream regions of yhzA were amplified by PCR using the appropriate primers (yhzAuF and yhzAuR for the upstream region and yhzAdF and yhzAdR for the downstream region). The two resultant fragments were then ligated and amplified by PCR using the primers yhzAuF and yhzAdR. To obtain the strains KW041 (ΔyhzA aprE::Pspac-rpsNBs spc) and KW042 (ΔyhzA aprE::Pspac-yhzA spc), KW040 was transformed by chromosomal DNA of RIK838 (aprE::Pspac-rpsNBs spc) and RIK840 (aprE::Pspac-yhzA spc), respectively, and spectinomycin-resistant (Spcr) transformants were selected. The strain KW043 (ΔyhzA aprE::Pspac-rpsNEc spc) was constructed as follows. A SalI-EcoRI-digested PCR fragment, amplified using the primers rpsNEcF and prsNEcR and E. coli K12 chromosomal DNA, was cloned into pAPNC213 (48). The recombinant plasmid was linearized by ScaI and used to transform the strain KW040, and Spcr transformants were selected. To obtain the strain KW044 (ΔyhzA aprE::Pspac-rpsNSe spc), the rpsNSe region of the S. elongatus PCC7942 genome was amplified by the primers rpsNSeF and rpsNSeR, and upstream and downstream regions of rpsNBs inserted into the aprE (KW041) were amplified by PCR using the appropriate primers (rpsNSeUF and rpsNSeUR for the upstream region and rpsNSeDF and rpsNSeDR for the downstream region). These three PCR fragments were then ligated and amplified by PCR using the primers rpsNSeUF and rpsNSeDR. The resulting PCR DNA was used to transform the strain KW040, and Spcr transformants were selected. For disruption of rpsNBs in the original locus, KW041, KW042, and KW043 were transformed by ΔrpsNBs::catpt1 DNA, which was amplified from the ΔrpsNBs::catpt1 region of the RIK839 genome (20), by PCR using primers ΔrpsNCmF and ΔrpsNCmR, and Cmr transformants were selected on solid medium containing 1 mM IPTG. The resulting strains were designated as KW047 (ΔyhzA ΔrpsNBs::catpt1 aprE::Pspac-rpsNBs spc), KW048 (ΔyhzA ΔrpsNBs::catpt1 aprE::Pspac-yhzA spc), and KW049 (ΔyhzA ΔrpsNBs::catpt1 aprE::Pspac-rpsNEc spc). To insert rpsNSe under the PtrnS of the KW044 genome, upstream and downstream regions of PtrnS were amplified by PCR using the appropriate primers (PtrnSUF and PtrnSUR for the upstream region and PtrnSDF and PtrnSDR for the downstream region). The rpsNSe region of the S. elongatus genome and the kanamycin-resistant (Kmr) gene of pDG148 were amplified by PCR using PtrnSNSeF and PtrnSNSeR as well as PtrnSKmF and PtrnSKmR, respectively. These four amplified PCR DNAs were then ligated and amplified by PCR using the primers PtrnSUF and PtrnSDR. The resulting PCR DNA was used to transform the strain KW044, and Kmr transformants were selected. The resulting strain was designated as KW151 (ΔyhzA aprE::Pspac-rpsNSe spc PtrnS-rpsNSe kan). To obtain KW152 (ΔyhzA ΔrpsNBs::catpt1 aprE::Pspac-rpsNSe spc PtrnS-rpsNSe kan), KW151 was transformed using ΔrpsNBs::catpt1 DNA, and Cmr transformants were selected. For construction of the strains KW051, KW049 was transformed by Δspo0E::erm DNA, which was amplified from the Δspo0E::erm region of the RIK737 genome (49) by PCR using primers spo0EF and spo0ER, and erythromycin-resistant (Emr) transformants were selected. To obtain KW153, Δspo0E::erm DNA and ΔrpsNBs::catpt1 DNA were simultaneously used for transforming KW151, and Emr Cmr transformants were selected. Strain KW158 (ΔyhzA amyE::Phyperspanc-rpsCBs-rpsBBs erm PtrnS-rpsNSe kan ΔrpsCBs::rpsCSe) and KW159 (ΔyhzA ΔrpsNBs::catpt1 amyE::Phyperspanc-rpsCBs-rpsBBs erm PtrnS-rpsNSe kan ΔrpsCBs::rpsCSe) were constructed as follows. To amplify the rpsCBs and rpsBBs, oligonucleotide primers rpsCBsF and rpsCBsR as well as rpsBBsF and rpsBBsR were used, respectively. The resulting fragments were simultaneously used as the template and amplified the joint fragment using primers rpsCBsF and rpsBBsR. A HindIII-SphI-digested PCR fragment was cloned into pDR111 (50). The recombinant plasmid was linearized by BamHI and used to transform the strain KW040, and Emr transformants were selected. To replace the rpsC gene in the resulting strain (KW156) with catpt1, oligonucleotide primers were used to amplify the upstream (ΔrpsCuF and ΔrpsCuR) and downstream (ΔrpsCdF and ΔrpsCdR) regions of the rpsC gene. Next, the cat gene of pCBB31 (51) without promoter and terminator region (catpt1) was amplified by PCR using the primers ΔrpsCcF and ΔrpsCcR. Another PCR amplification, in which all three abovementioned amplified fragments were added as the DNA template, was performed using the primers ΔrpsCuF and ΔrpsCdR. The resulting fragment was used to transform strain KW156, and Cmr transformants were selected on solid medium containing 1 mM IPTG. To replace the ΔrpsCBs::cat region of the resulting strain (KW157) with rpsCSe, KW157 was transformed with ΔrpsCBs::rpsCSe and PtrnS-rpsNSe spc DNA fragments, and Spcr Cms transformants were selected in the presence of 1 mM IPTG. The resulting strain was designated as KW158. The ΔrpsCBs::rpsCSe DNA fragment was constructed as follows. Oligonucleotide primers were used to amplify the upstream (rpsCuF and rpsCuR) and downstream (rpsCdF and rpsCdR) regions of the rpsCBs gene. Next, the rpsCSe gene of the S. elongatus PCC7942 genome was amplified using the primers rpsCSeF and rpsCSeR. Another PCR amplification, in which all three abovementioned amplified fragments were added as the DNA template, was performed using the primers rpsCuF and rpsCdR. The PtrnS-rpsNSe spc DNA fragment was amplified using primer PtrnSUF and PtrnSDR with genomic DNA of KW151. To replace the rpsN gene of KW158 by catpt1, the KW156 was transformed with the PCR DNA of the ΔrpsNBs::catpt1 region of the RIK839 genome, which was amplified using primers ΔrpsNCmF and ΔrpsNCmR (20), and Cmr transformants were selected on solid medium containing 1 mM IPTG. The resulting strain was designated as KW159.
Evolutionary analysis by maximum likelihood method.
The evolutionary history was inferred using the maximum likelihood method and Le-Gascuel model (52). The tree with the highest log likelihood (−4,894.94) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial trees for the heuristic search were obtained automatically by applying neighbor-joining and BioNJ algorithms to a matrix of pairwise distances estimated using a Jones-Taylor-Thornton (JTT) model and then selecting the topology with superior log likelihood value. A discrete gamma distribution was used to model evolutionary rate differences among sites (5 categories [+G, parameter = 1.9182]). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 4.37% sites). Bootstrapping was performed with 1,000 replicates. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 54 amino acid sequences of S14. There were a total of 103 positions in the final data set. The protein sequences of S29 from eukaryotes, corresponding to the S14 of bacteria, were used. Evolutionary analyses were conducted in MEGA X (52).
Preparation of 70S ribosomes.
The 70S ribosomes were prepared as described previously (53). In brief, B. subtilis cells were grown in LB medium at 37°C with shaking to exponential phase (OD600, ∼0.4) and harvested. The cells were disrupted by passage through a French pressure cell, and cell debris was removed by centrifugation. The resulting cell lysate was centrifuged, resulting in the S100 pellet fraction. One hundred A260 units of the resulting fraction were layered onto 10 to 40% sucrose density gradients, which were subjected to centrifugation at 4°C for 17 h at 67,000 × g (Hitachi P28ST2 rotor). Samples were collected with a Piston Gradient Fractionator (BioComp), and the A260 of each fraction was determined to obtain the 70S fraction. The 70S fraction was diluted with buffer I (1:2 dilutions) and centrifuged at 65,000 × g for 17 h at 4°C. The resulting 70S ribosome pellet was resuspended with buffer I [20 mM Tris-HCl (pH 7.6), 15 mM (CH3COO)2Mg, 100 mM CH3COONH4, 0.1 mM dithiothreitol (DTT), and 2 mM phenylmethylsulfonyl fluoride (PMSF)] and stored at −80°C until required.
Two-dimensional gel electrophoresis.
The RFHR two-dimensional gel electrophoresis was performed essentially according to the published procedures (54, 55) using a modified electrophoresis apparatus (Nippon Eido). One-step CBB staining solution (Bio Craft) was used to stain the gels. Intensities of protein spots were determined using ImageJ software (http://imagej.nih.gov/ij/).
Protein identification by mass spectrometry.
Protein spots on the 2D gel were excised and washed twice with distilled water. After reduction with 100 mM DTT and alkylation with 100 mM iodoacetamide, the proteins were hydrolyzed with trypsin at 30°C for 18 h. Peptides were extracted from the gel pieces and desalted using ZipTip C18 pipette tips (Millipore). The resulting samples were analyzed by nanoscale liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS) systems (DiNa high-pressure liquid chromatography [HPLC] system; KYA tech Corporation/QSTAR XL Applied Biosystems). Peptide mass fingerprint data were compared with the E. coli sequence database or S. elongatus sequence database using the MASCOT search engine (Matrix Science, London). Search parameters were as follows: peptide mass tolerance, ±0.2 Da; fragment mass tolerance, ±0.2 Da; missed cleavage, 1; variable modification, carbamidomethylation of Cys residues and oxidation of Met residues. S14Ec and S14Se gave ion scores of 344 and 187, respectively.
Sucrose density gradient sedimentation analysis.
B. subtilis cells were grown in LB medium at 37°C with shaking to the exponential phase (OD600, ∼0.4) and harvested. Sucrose density gradient sedimentation analysis was performed as described previously (31). Briefly, the cells were disrupted by passage through a French pressure cell, and cell debris was removed by centrifugation. Aliquots of extract (10 A260 units) were layered onto 10 to 40% sucrose density gradients, which were subjected to centrifugation at 65,000 × g for 17.5 h at 4°C (Hitachi P40ST rotor). Samples were collected with a Piston Gradient Fractionator (BioComp), and absorbance profiles were monitored at 254 nm using a Bio-Mini UV monitor (ATTO, Japan).
In vitro analysis for translational activity of 70S ribosome fraction.
In vitro translation assays were performed as described previously with some modifications (56). For preparation of the S100 fraction, B. subtilis cells were grown in super-rich medium to late exponential phase (OD600 = 1.0). Before harvesting the cells, KCl (1 M) was added to the culture. The cells were washed with Tris-magnesium-ammonium-glycerol (TMAG) buffer (10 mM Tris-HCl [pH 7.7], 10 mM magnesium acetate, 30 mM ammonium acetate, 10% glycerol, 0.1 mM DTT) and lysed in TMAG buffer supplemented with 2 mM PMSF and 75 U of DNase I by passage through a French pressure cell. After removing cell debris, the lysate was centrifuged at 30,000 × g for 30 min at 4°C. The supernatant was centrifuged at 200,000 × g for 100 min at 4°C. The upper half of the supernatant was collected as the S100 fraction and dialyzed against TMAG buffer. tRNAs were isolated from B. subtilis cells and grown in super-rich medium using an ISOGEN reagent (Nippon Gene) according to the manufacturer’s instructions. GFP mRNA was used as the template for in vitro translation experiments. A DNA fragment containing a T7 RNA polymerase promoter and GFP coding sequence was amplified by PCR. The PCR product was subjected to in vitro transcription using a ScriptMAX Thermo T7 transcription kit (Toyobo) at 37°C for 2 h. The synthesized mRNA was purified using a MicroSpin G-25 column (GE Healthcare).
For in vitro translation experiments, a reaction mixture was assembled containing 0.1 mg of the S100 fraction, 55 mM HEPES-KOH (pH 7.5), 210 mM potassium acetate, 27.5 mM ammonium acetate, 10.7 mM magnesium acetate, 68 μM folinic acid, 1 mM DTT, and 1 mM PMSF. Samples were preincubated at 30°C for 15 min to remove endogenous mRNA. Then, 1.2 mM ATP, 0.8 mM GTP, 80 mM creatine phosphate, 25 μg creatine kinase, 10 U of RNase inhibitor, 0.4 mM each amino acid, 15 μg of tRNA, 100 μg of the in vitro-transcribed mRNA, and 3 A260 units of 70S ribosomes that were prepared as described above were added, and the samples were further incubated at 30°C for 2 h. The resulting GFP was analyzed by SDS-PAGE and detected by Western blotting using an anti-GFP antibody (MBL Japan). The intensity of the immunoblot band corresponding to GFP was determined using ImageJ software (http://imagej.nih.gov/ij/).
Preparation of 30S ribosomal subunits.
B. subtilis cells were grown in LB medium at 37°C with shaking to the exponential phase (OD600, ∼0.4) and harvested. The 30S ribosomal fraction was prepared as described previously with a slight modification (20). In brief, cells were disrupted by passage through a French pressure cell. After removal of cell debris by centrifugation, aliquots of extracts were layered onto sucrose density gradients of 10 to 40% in buffer I containing 15 mM (for preparation of 30S from KW049 and KW152) or 1 mM (for preparation of 30S from wild-type cells) MgSO4 and then centrifuged at 65,000 × g for 17 h at 4°C (Hitachi P28ST2 rotor; 200 A260 units per tube). Fractions containing 30S subunits were identified by a peak in absorbance at 260 nm. The fraction(s) was diluted with buffer I (1:2 dilutions) and centrifuged at 65,000 × g for 17 h at 4°C. The resulting 30S ribosome pellet was resuspended with buffer I and stored at −80°C until required.
Sporulation assay.
B. subtilis cells were grown in 2× SG medium for 24 h at 37°C with shaking. Heat-resistant spores were counted by heating the cells at 80°C for 10 min, plating them on LB agar plates, and then incubating the plates at 37°C for 24 h.
Microscopic imaging.
B. subtilis cells were grown in 2× SG medium at 37°C with shaking. At the indicated times, 500 μl of the culture was removed and subjected to centrifugation at 12,000 × g for 1 min. The cell pellet was resuspended in 40 μl of culture supernatant, and then FM4-64 (Invitrogen) and 4′,6-diamidino-2-phenylindole (DAPI) (Wako Pure Chemical Industries) were added to final concentrations of 10 μg/ml and 5 μg/ml, respectively. The cell suspension was mounted on a microscope slide, coated with poly-l-lysine to fix the cells, and differential interference contrast and fluorescence images were obtained with a LSM 800 confocal fluorescence microscope (Carl Zeiss).
Western blot analysis.
Western blot analysis was performed according to a previously described method (57). Aliquots (15 μg of protein) of crude cell extracts were loaded onto a sodium dodecyl sulfate-polyacrylamide gel (10 to 20% gradient) and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore Co., Japan). This membrane was then used in the Western blot assay using antisera (1:10,000 dilution) against Spo0A (58).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-Aid for Scientific Research (C) (26450101, 15K07013, and 19K06483 to G.A., Y.K.-Y., and A.S., respectively), Grant-in-Aid for Young Scientists (B) (17K15253, 17H05451, and 23770157 to G.A., S.W., and Y.K.-Y., respectively), the Advanced Low Carbon Technology Research and Development Program (ALCA) of the Japan Science and Technology Agency (JST) (to S.W.), Noda Institute for Scientific Research GRANT (to A.S.), and Strategic Research Foundation Grant-aided Project for Private Universities (S1201003 to F.K. and Y.K.-Y.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Kurland CG. 1972. Structure and function of the bacterial ribosome. Annu Rev Biochem 41:377–408. 10.1146/annurev.bi.41.070172.002113. [DOI] [PubMed] [Google Scholar]
- 2.Nomura M. 1970. Bacterial ribosome. Bacteriol Rev 34:228–277. 10.1128/BR.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao YG, Selmer M, Dunham CM, Weixlbaumer A, Kelley AC, Ramakrishnan V. 2009. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science 326:694–699. 10.1126/science.1179709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmeing TM, Ramakrishnan V. 2009. What recent ribosome structures have revealed about the mechanism of translation. Nature 461:1234–1242. 10.1038/nature08403. [DOI] [PubMed] [Google Scholar]
- 5.Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FV, Weir JR, Ramakrishnan V. 2009. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 326:688–694. 10.1126/science.1179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takyar S, Hickerson RP, Noller HF. 2005. mRNA helicase activity of the ribosome. Cell 120:49–58. 10.1016/j.cell.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. 2001. Crystal structure of the ribosome at 5.5 Å resolution. Science 292:883–896. 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 8.Jenner L, Demeshkina N, Yusupova G, Yusupov M. 2010. Structural rearrangements of the ribosome at the tRNA proofreading step. Nat Struct Mol Biol 17:1072–1078. 10.1038/nsmb.1880. [DOI] [PubMed] [Google Scholar]
- 9.Ogle JM, Brodersen DE, Clemons WM, Jr, Tarry MJ, Carter AP, Ramakrishnan V. 2001. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 292:897–902. 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- 10.Schluenzen F, Tocilj A, Zarivach R, Harms J, Gluehmann M, Janell D, Bashan A, Bartels H, Agmon I, Franceschi F, Yonath A. 2000. Structure of functionally activated small ribosomal subunit at 3.3 Å resolution. Cell 102:615–623. 10.1016/s0092-8674(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 11.Wimberly BT, Brodersen DE, Clemons WM, Jr, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V. 2000. Structure of the 30S ribosomal subunit. Nature 407:327–339. 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- 12.Yusupova GZ, Yusupov MM, Cate JH, Noller HF. 2001. The path of messenger RNA through the ribosome. Cell 106:233–241. 10.1016/s0092-8674(01)00435-4. [DOI] [PubMed] [Google Scholar]
- 13.Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289:905–920. 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- 14.Nissen P, Hansen J, Ban N, Moore PB, Steitz TA. 2000. The structural basis of ribosome activity in peptide bond synthesis. Science 289:920–930. 10.1126/science.289.5481.920. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal RK, Lata RK, Frank J. 1999. Conformational variability in Escherichia coli 70S ribosome as revealed by 3D cryo-electron microscopy. Int J Biochem Cell Biol 31:243–254. 10.1016/s1357-2725(98)00149-6. [DOI] [PubMed] [Google Scholar]
- 16.Fei J, Kosuri P, MacDougall DD, Gonzalez RL. 2008. Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation. Mol Cell 30:348–359. 10.1016/j.molcel.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Blaha G, Stanley RE, Steitz TA. 2009. Formation of the first peptide bond: the structure of EF-P bound to the 70S ribosome. Science 325:966–970. 10.1126/science.1175800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brochier C, Philippe H, Moreira D. 2000. The evolutionary history of ribosomal protein RpS14: horizontal gene transfer at the heart of the ribosome. Trend Genet 16:529–533. 10.1016/S0168-9525(00)02142-9. [DOI] [PubMed] [Google Scholar]
- 19.Makarova KS, Ponomarev VA, Koonin EV. 2001. Two C or not two C: recurrent disruption of Zn-ribbons, gene duplication, lineage-specific gene loss, and horizontal gene transfer in evolution of bacterial ribosomal proteins. Genome Biol 2:RESEARCH 0033. 10.1186/gb-2001-2-9-research0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natori Y, Nanamiya H, Akanuma G, Kosono S, Kudo T, Ochi K, Kawamura F. 2007. A fail-safe system for the ribosome under zinc-limiting conditions in Bacillus subtilis. Mol Microbiol 63:294–307. 10.1111/j.1365-2958.2006.05513.x. [DOI] [PubMed] [Google Scholar]
- 21.Outten CE, O'Halloran TV. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492. 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 22.Gaballa A, Helmann JD. 1998. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J Bacteriol 180:5815–5821. 10.1128/JB.180.22.5815-5821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaballa A, Wang T, Ye RW, Helmann JD. 2002. Functional analysis of the Bacillus subtilis Zur regulon. J Bacteriol 184:6508–6514. 10.1128/jb.184.23.6508-6514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts E, Sethi A, Montoya J, Woese CR, Luthey-Schulten Z. 2008. Molecular signatures of ribosomal evolution. Proc Natl Acad Sci U S A 105:13953–13958. 10.1073/pnas.0804861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu XQ, Gillham NW, Boynton JE. 1989. Chloroplast ribosomal protein gene rps12 of Chlamydomonas reinhardtii. Wild-type sequence, mutation to streptomycin resistance and dependence, and function in Escherichia coli. J Biol Chem 264:16100–16108. 10.1016/S0021-9258(18)71592-5. [DOI] [PubMed] [Google Scholar]
- 26.Giese K, Subramanian AR. 1991. Expression and functional assembly into bacterial ribosomes of a nuclear-encoded chloroplast ribosomal protein with a long NH2-terminal extension. FEBS Lett 288:72–76. 10.1016/0014-5793(91)81005-s. [DOI] [PubMed] [Google Scholar]
- 27.Bubunenko MG, Schmidt J, Subramanian AR. 1994. Protein substitution in chloroplast ribosome evolution. A eukaryotic cytosolic protein has replaced its organelle homologue (L23) in spinach. J Mol Biol 240:28–41. 10.1006/jmbi.1994.1415. [DOI] [PubMed] [Google Scholar]
- 28.Weglöhner W, Jünemann R, von Knoblauch K, Subramanian AR. 1997. Different consequences of incorporating chloroplast ribosomal proteins L12 and S18 into the bacterial ribosomes of Escherichia coli. Eur J Biochem 249:383–392. 10.1111/j.1432-1033.1997.00383.x. [DOI] [PubMed] [Google Scholar]
- 29.Xaplanteri MA, Papadopoulos G, Leontiadou F, Choli-Papadopoulou T, Kalpaxis DL. 2007. The contribution of the zinc-finger motif to the function of Thermus thermophilus ribosomal protein S14. J Mol Biol 369:489–497. 10.1016/j.jmb.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 30.Maguire BA, Manuilov AV, Zimmermann RA. 2001. Differential effects of replacing Escherichia coli ribosomal protein L27 with its homologue from Aquifexaeolicus. J Bacteriol 183:6565–6572. 10.1128/JB.183.22.6565-6572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoji S, Dambacher CM, Shajani Z, Williamson JR, Schultz PG. 2011. Systematic chromosomal deletion of bacterial ribosomal protein genes. J Mol Biol 413:751–761. 10.1016/j.jmb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akanuma G, Nanamiya H, Natori Y, Yano K, Suzuki S, Omata S, Ishizuka M, Sekine Y, Kawamura F. 2012. Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation. J Bacteriol 194:6282–6291. 10.1128/JB.01544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins D, Dworkin J. 2012. Recent progress in Bacillus subtilis sporulation. FEMS Microbiol Rev 36:131–148. 10.1111/j.1574-6976.2011.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoch JA. 1993. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol 47:441–465. 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- 36.Stephenson K, Hoch JA. 2002. Evolution of signalling in the sporulation phosphorelay. Mol Microbiol 46:297–304. 10.1046/j.1365-2958.2002.03186.x. [DOI] [PubMed] [Google Scholar]
- 37.Molle V, Fujita M, Jensen ST, Eichenberger P, Gonzalez-Pastor JE, Liu JS, Losick R. 2003. The Spo0A regulon of Bacillus subtilis. Mol Microbiol 50:1683–1701. 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 38.Chastanet A, Losick R. 2011. Just-in-time control of Spo0A synthesis in Bacillus subtilis by multiple regulatory mechanisms. J Bacteriol 193:6366–6374. 10.1128/JB.06057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reference deleted. [Google Scholar]
- 40.Mizushima S, Nomura M. 1970. Assembly mapping of 30S ribosomal proteins from E. coli. Nature 226:1214–1218. 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- 41.Held WA, Ballou B, Mizushima S, Nomura M. 1974. Assembly mapping of 30 S ribosomal proteins from Escherichia coli. Further studies. J Biol Chem 249:3103–3111. 10.1016/S0021-9258(19)42644-6. [DOI] [PubMed] [Google Scholar]
- 42.Gualerzi CO, Pon CL. 2015. Initiation of mRNA translation in bacteria: structural and dynamic aspects. Cell Mol Life Sci 72:4341–4367. 10.1007/s00018-015-2010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akanuma G, Yamazaki K, Yagishi Y, Iizuka Y, Ishizuka M, Kawamura F, Kato-Yamada Y. 2018. Magnesium suppresses defects in the formation of 70S ribosomes as well as in sporulation caused by lack of several individual ribosomal proteins. J Bacteriol 200:e00212-18. 10.1128/JB.00212-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohlsen KL, Grimsley JK, Hoch JA. 1994. Deactivation of the sporulation transcription factor Spo0A by the Spo0E protein phosphatase. Proc Natl Acad Sci U S A 91:1756–1760. 10.1073/pnas.91.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 46.Leighton TJ, Doi RH. 1971. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem 246:3189–3195. 10.1016/S0021-9258(18)62213-6. [DOI] [PubMed] [Google Scholar]
- 47.Ashikaga S, Nanamiya H, Ohashi Y, Kawamura F. 2000. Natural genetic competence in Bacillus subtilis Natto OK2. J Bacteriol 182:2411–2415. 10.1128/jb.182.9.2411-2415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morimoto T, Loh PC, Hirai T, Asai K, Kobayashi K, Moriya S, Ogasawara N. 2002. Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis. Microbiology (Reading) 148:3539–3552. 10.1099/00221287-148-11-3539. [DOI] [PubMed] [Google Scholar]
- 49.Nanamiya H, Takahashi K, Fujita M, Kawamura F. 2000. Deficiency of the initiation events of sporulation in Bacillus subtilis clpP mutant can be suppressed by a lack of the Spo0E protein phosphatase. Biochem Biophys Res Commun 279:229–233. 10.1006/bbrc.2000.3911. [DOI] [PubMed] [Google Scholar]
- 50.Ben-Yehuda S, Rudner DZ, Losick R. 2003. RacA, a bacterial protein that anchorschromosomes to the cell poles. Science 299:532–536. 10.1126/science.1079914. [DOI] [PubMed] [Google Scholar]
- 51.Imamura D, Kobayashi K, Sekiguchi J, Ogasawara N, Takeuchi M, Sato T. 2004. spoIVH (ykvV), a requisite cortex formation gene, is expressed in both sporulating compartments of Bacillus subtilis. J Bacteriol 186:5450–5459. 10.1128/JB.186.16.5450-5459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki S, Akanuma G, Kawamura F. 2015. Purification of 70S ribosomes from Bacillus subtilis. Bio-protocol 5:e1432. 10.21769/BioProtoc.1432. [DOI] [Google Scholar]
- 54.Wada A. 1986. Analysis of Escherichia coli ribosomal proteins by an improved two dimensional gel electrophoresis. I. Detection of four new proteins. J Biochem 100:1583–1594. 10.1093/oxfordjournals.jbchem.a121866. [DOI] [PubMed] [Google Scholar]
- 55.Nanamiya H, Akanuma G, Natori Y, Murayama R, Kosono S, Kudo T, Kobayashi K, Ogasawara N, Park SM, Ochi K, Kawamura F. 2004. Zinc is a key factor in controlling alternation of two types of L31 protein in the Bacillus subtilis ribosome. Mol Microbiol 52:273–283. 10.1111/j.1365-2958.2003.03972.x. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki S, Kondo N, Yoshida M, Nishiyama M, Kosono S. 2019. Dynamic changes in lysine acetylation and succinylation of the elongation factor Tu in Bacillus subtilis. Microbiology (Reading) 165:65–77. 10.1099/mic.0.000737. [DOI] [PubMed] [Google Scholar]
- 57.Nanamiya H, Shiomi E, Ogura M, Tanaka T, Asai K, Kawamura F. 2003. Involvement of ClpX protein in the post-transcriptional regulation of a competence specific transcription factor, ComK protein, of Bacillus subtilis. J Biochem 133:295–302. 10.1093/jb/mvg040. [DOI] [PubMed] [Google Scholar]
- 58.Nanamiya H, Ohashi Y, Asai K, Moriya S, Ogasawara N, Fujita M, Sadaie Y, Kawamura F. 1998. ClpC regulates the fate of a sporulation initiation sigma factor, σH protein, in Bacillus subtilis at elevated temperatures. Mol Microbiol 29:505–513. 10.1046/j.1365-2958.1998.00943.x. [DOI] [PubMed] [Google Scholar]
- 59.Le SQ, Gascuel O. 2008. An improved general amino acid replacement matrix. Mol Biol Evol 25:1307–1320. 10.1093/molbev/msn067. [DOI] [PubMed] [Google Scholar]
- 60.Arnold K, Bordoli L, Kopp J, Schwede T. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201. 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 61.DeLano WL. 2002. The PyMOL molecular graphics system. DeLano Scientific, San Carlos, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.