Abstract
Backgrounds
The inflammatory biomarker “C-reactive protein to albumin ratio (CAR)” has been reported to significantly correlate to a variety of human cancers. However, there are conflicting results regarding the prognostic value of CAR in colorectal cancer. Previous studies mainly assessed patients in Eastern countries, so their findings may not be applicable to the Western population. Therefore, this updated meta-analysis aimed to investigate the prognostic value of pre-treatment CAR and outcomes of patients with colorectal cancer.
Methods
We conducted a systematic search for eligible literature until October 31, 2020, using PubMed and Embase databases. Studies assessing pre-treatment CAR and outcomes of colorectal cancer were included. Outcome measures included overall survival, disease-free survival, progression-free survival, and clinicopathological features. The pooled hazard ratios (HR) with 95% confidence intervals (CI) were used as effective values.
Results
A total of 15 studies involving 6329 patients were included in this study. The pooled results indicated that a high pre-treatment CAR was associated with poor overall survival (HR 2.028, 95% CI 1.808−2.275, p < 0.001) and poor disease-free survival/progression-free survival (HR 1.768, 95% CI 1.321–2.365, p < 0.001). Subgroup analysis revealed a constant prognostic value of the pre-treatment CAR despite different study regions, sample size, cancer stage, treatment methods, or the cut-off value used. We also noted a correlation between high pre-treatment CAR and old age, male sex, colon cancer, advanced stage (III/IV), large tumor size, poor differentiation, elevated carcinoembryonic antigen levels, neutrophil-to-lymphocyte ratio, and the modified Glasgow prognostic score.
Conclusions
High pre-treatment CAR was associated with poor overall survival, disease-free survival, and progression-free survival in colorectal cancer. It can serve as a prognostic marker for colorectal cancer in clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-021-02253-y.
Keywords: C-reactive protein to albumin ratio, Colorectal cancer, Meta-analysis, Overall survival, Disease-free survival
Introduction
Colorectal cancer (CRC) is the third most common malignancy worldwide. According to global statistics, 1.93 million new cases were diagnosed in 2020 [1]. Despite advances in treatment, including surgical skills, chemotherapy regimens, and the use of biological agents, the rate of death due to CRC remains higher than that due to other cancers, with an estimated 930,000 deaths and CRC was the second-leading cause of cancer-related deaths in 2020 [1]. Because of the heterogeneous nature of CRC, the treatment strategy and outcomes are diverse, even for tumors with the same stage [2]. A 2018 study that assessed the global patterns and trends in CRC demonstrated a diversity in incidence and a mortality rate up to 10-fold worldwide, with distinct gradients across different levels of human development [3]. Determining the prognostic factors for CRC is a critical issue for making treatment plans for CRC and establishing guidance on how intensely the patient should be followed after index treatment.
It is well known that cancer-related inflammation can aid tumor proliferation, angiogenesis, progression, metastasis, and resistance to chemotherapy [4, 5]. Based on this concept, several serum inflammatory markers have been reported as prognostic biomarkers in different cancer types [6]. Either the solitary or combined use of neutrophils, lymphocytes, monocytes, platelets, C-reactive protein (CRP), and albumin has been associated with CRC survival [7–11]. These markers can be further classified into cellular and protein components. CRP is an acute-phase protein regulated by several pro-inflammatory cytokines such as tumor necrosis factor-alpha, interleukin-1, and interleukin-6 [12]. Albumin is recognized as a nutritional status parameter and is associated with chronic inflammation [13, 14]. The utility of these two protein markers has been classically reported as the Glasgow prognostic score (GPS) and modified Glasgow prognostic score (mGPS). GPS and mGPS have been reported as independent prognostic factors for CRC [15, 16]. Recently, a novel inflammation-based marker composed of the CRP-to-albumin ratio (CAR) was proposed to predict the outcome of patients with severe sepsis [17] and served as a prognostic factor for a variety of human malignancies [18]. However, there are conflicting results regarding the prognostic value of CAR in CRC. Ishizuka et al. noted that the overall survival had significantly increased in the low pre-treatment CAR subgroup compared to that of the high pre-treatment CAR subgroup in their study of 627 patients with colorectal cancer who had undergone elective surgery (HR 2.596, P < 0.001) [19]. On the contrary, Zhou et al. demonstrated that the dynamic change in CAR pre- and post-surgery was strongly associated with the overall survival in patients with CRC who had undergone surgery; however, if only the pre-operative CAR data was used, it did not significantly affect the overall survival (HR 1.59, 95% CIs 0.86–2.91) [20]. Previous studies analyzing the correlation between pre-treatment CAR and survival in CRC mainly came from East Asia; therefore, evidence about the application of this biomarker in the Western countries is lacking. Thus, we conducted this updated meta-analysis to assess the association between the pre-treatment value of CAR and outcomes in CRC patients by reviewing the findings of all recently published studies. Moreover, we aimed to identify the clinicopathological features associated with a high CAR.
Methods
Search strategy
This study was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis [21]. A comprehensive search was conducted using PubMed and Embase databases from the earliest records to October 2020. The search terms were as follows: (C-reactive protein to albumin ratio OR C-reactive protein/albumin ratio OR C-reactive protein albumin ratio OR CRP/albumin ratio) AND (colorectal cancer OR rectal cancer OR colon cancer) AND (survival OR outcome OR mortality). The bibliographies of the included trials and related review articles were manually reviewed for potential missing studies. The protocol for this systematic review was registered on INPLASY (https://inplasy.com/), and the registration number is INPLASY202140103. The protocol is available in full on the inplasy.com (10.37766/inplasy2021.4.0103).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) either retrospective or prospective studies reporting the association of pre-treatment CAR and the outcome of CRC patients, (2) participants having pathologically confirmed CRC, (3) CAR data before treatment, (4) include survival outcomes such as overall survival (OS) and/or disease-free survival (DFS)/progression-free survival (PFS) for analysis, (5) provide sufficient information for the extraction of the hazard ratio (HR) and associated 95% confidence intervals (CIs), and (6) studies published in English or Chinese. The exclusion criteria were as follows: (1) lack of sufficient data for extracting HR and the associated 95% CIs; (2) no evaluation of survival outcome; (3) use of post-treatment CAR data; (4) case reports, review articles, or conference abstracts; and (5) studies published in languages other than English or Chinese.
Data extraction and quality assessment
Two independent reviewers examined all retrieved articles and extracted the data using a predetermined form. The following information was extracted: the first author’s name, year of publication, country of study, tumor location, sample size, gender, mean age, cancer stage, type of treatment, the cut-off value of CAR, outcome measures, mean follow-up times, confounding factors of CAR, and analyzed models. Two reviewers evaluated the methodological quality of the enrolled studies independently, according to the Newcastle–Ottawa Quality Assessment Scale (NOS). The NOS contains nine items in three categories: participant selection (four items), comparability (two items), and exposure (three items). A study can be scored a maximum of 1 point for items in the selection and exposure domains and 2 points for the comparability domain [22]. A study with NOS scores of 7 or higher was defined as a high-quality study. Any discrepancies between the two reviewers were resolved through discussion.
Statistical analysis
All statistical analyses were performed using Comprehensive Meta-Analysis software, version 3 (Biostat, Englewood, NJ, USA). The HRs and associated 95% CIs were directly extracted from the study while reporting survival analysis of OS or PFS/DFS and pooling the prognostic value of high pre-treatment CAR. The clinicopathological features associated with high CAR were also extracted from the studies if a study reported a correlation between CAR and clinicopathological factors. The heterogeneity between studies was determined using the Cochran Q-test and I2 statistics. I2 ≥ 50% indicated considerable heterogeneity. A random-effects model was employed to pool the HRs and ORs in this meta-analysis. Funnel plots, Begg’s test, and Egger’s test were used to examine potential publication bias. Statistical significance was defined as p-values < 0.05, except for the determination of publication bias, which was employed at p < 0.1. To evaluate the influence of publication bias, we used the trim-and-fill method. A sensitivity analysis was performed to confirm the robustness of the pooled results by removing each study individually. Moreover, subgroup analysis was performed to investigate the heterogeneity between eligible studies with the following features: study region (Western or Eastern), sample sizes (< 200 or ≥ 200), stage (stage I–III or stage IV), treatment methods (surgery, chemotherapy, or liver resection only), the cut-off value of CAR (< 0.1 or ≥ 0.1), and study method (multivariate or univariate analysis).
Results
Study selection and characteristics
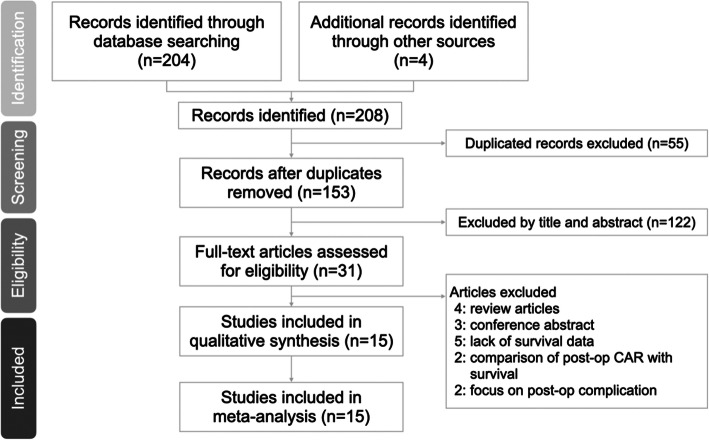
A total of 208 articles were identified based on an online database search and manual search, of which 55 duplicated records were removed. By reviewing the titles and abstracts of the articles, 122 articles were removed. After assessing full-text articles, 16 articles were excluded owing to lack of data to measure survival. Finally, 15 eligible articles were included in the meta-analysis. A flow diagram is shown in Fig. 1.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses flow diagram to search and identify included studies
All included studies were published between 2016 and 2020. The sample sizes of the included studies ranged from 40 to 1303, with 6329 as the total number of enrolled patients. Of these studies, nine were from Japan [19, 23–30], three were from Britain [31–33], two were from China [20, 34], and one was from Korea [35]. The cut-off value of pre-treatment CAR ranged from 0.0278 to 0.6712. There were diverse treatment methods, including surgical resection, chemotherapy regimens, and multidisciplinary treatment. Nine studies consisted of mixed diseases, while five studies consisted only of metastatic disease. The HRs and their 95% CIs were directly extracted from studies on OS and DFS/PFS. The detailed characteristics of the eligible studies are summarized in Table 1 (the confounding factors of CAR are summarized in Additional Table S1).
Table 1.
Summary of the retrieved studies investigating pre-treatment C-reactive protein to albumin ratio on survival in colorectal cancer patients
| Author | Year | Country | Tumor Location | Sample size | Gender, M:F | Age (years) | Stage | Treatment | Cut-off value | Outcome | HR | 95% CI | Follow-up time | Analysis | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ishizuka | 2016 | Japan | CRC | 627 | 400:227 | NA | 0–IV | Surgery | 0.038 | OS | 2.596 | 1.603–4.204 | NA | MV | 8 |
| Shibutani | 2016 | Japan | CRC | 99 | 57:42 | 63 (27–86) | IV | Chemotherapy | 0.183 | OS | 1.866 | 1.057–3.295 | 2.6–73.2 | MV | 8 |
| Shibutani | 2016 | Japan | CRC | 705 | 411:294 | 68 (26-90) | I–III | Surgery | 0.0278 |
DFS CSS |
1.503 1.672 |
1.054–2.143 1.012–2.764 |
NA | MV | 8 |
| Solaini | 2016 | UK | CRLM | 194 | 125:69 | 66 (59-73) | IV | Liver resection | 0.133 | OS | 2.04 | 1.26–3.3 | NA | UV | 7 |
| Haruki | 2017 | Japan | CRLM | 106 | 76:30 | 64.5 (39-87) | IV | Liver resection | 0.04 |
OS DFS |
2.559 1.731 |
1.420–4.613 1.087–2.758 |
NA | MV | 8 |
| Ide | 2017 | Japan | Rectum | 115 | 82:33 | 64 (33–83) | I–III | Surgery | 0.049 |
OS DFS |
5.09 4.98 |
2.31–11.58 2.34–11.14 |
65 (2–189) | MV | 8 |
| Dolan | 2018 | UK | Colon | 801 | 430:371 | NA | I–III | Surgery | 0.22 |
OS CSS |
1.84 1.76 |
1.49–2.26 1.31–2.35 |
NA | MV | 7 |
| Climent | 2019 | Ireland | CRC | 566 | 260:306 | 69.9 ± 12.3 | I–III | Surgery | 0.46 |
OS DFS |
1.84 1.73 |
1.1–3.1 0.8–3.3 |
NA | UV | 6 |
| Shibutani | 2019 | Japan | CRC | 40 | 25:15 | NA | IV | Chemotherapy | 0.122 |
OS PFS |
6.478 4.525 |
1.374–30.537 0.826–24.794 |
NA | MV | 8 |
| Sakamoto | 2020 | Japan | CRLM | 184 | 121:63 | 63.1 (25-94) | IV | Liver resection | 0.093 |
OS PFS |
2.82 1.62 |
1.63–4.72 1.02–2.49 |
NA | MV | 8 |
| Son | 2020 | Korea | CRC | 789 | 486:283 | NA | I–III | Surgery | 0.14 | OS | 1.97 | 1.47–2.64 | 91 (67–115) | UV | 7 |
| Suzuki | 2020 | Japan | Colon | 1303 | 689:614 | 65 (26-93) | II–III | Surgery | 0.02558 |
OS DFS |
1.8 1.28 |
1.24–2.68 0.99–1.66 |
60.2 (3–106) | MV | 8 |
| Ni | 2016 | china | CRC | 148 | 97:51 | NA | IV | Chemotherapy | 0.6712 | OS | 2.243 | 1.450–3.470 | NA | MV | 8 |
| Zhou | 2018 | china | CRC | 516 | 331:185 | NA | I–IV | Surgery | 0.07/0.08 |
OS DFS |
1.59 1.05 |
0.86–2.91 0.71–1.56 |
21.72 (2.11–118.72) | MV | 8 |
| Tominaga | 2016 | Japan | Colon | 136 | 79:57 | NA | III | Chemotherapy | 0.1 | DFS | 4.43 | 1.94–10.15 | NA | UV | 7 |
CRC colorectal cancer, CRLM colorectal cancer liver metastasis, MV multivariate, UV univariate, OS overall survival, DRS disease-free survival, CSS cancer-specific survival, PFS progression-free survival
Quality analysis
The quality of the eligible articles was evaluated by NOS. As shown in additional Table S2, the NOS scores of the included studies ranged from 7 to 8 and were regarded as high quality.
Meta-analysis of the effect of CAR on overall survival
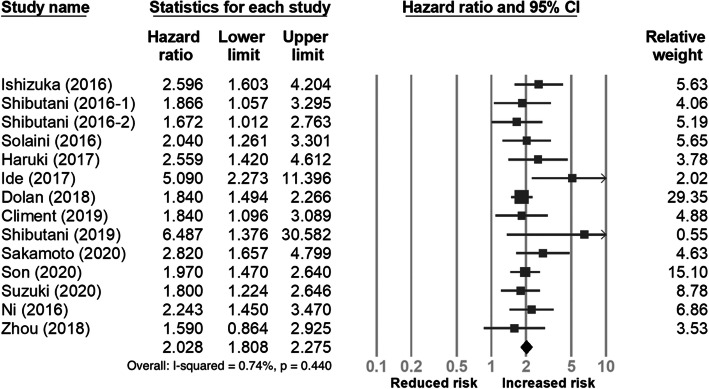
Fourteen studies [18, 19, 23–29, 31–35] with a total of 6193 participants reported the association of pre-treatment CAR with overall survival. As shown in Fig. 2, the meta-analysis indicated that a high pre-treatment CAR was associated with poor OS (HR 2.028, 95% CI 1.808−2.275). For further evaluation of the prognostic value of CAR on OS, we conducted subgroup analysis using predefined features, and the results are summarized in Table 2. A relatively more significant prognostic effect on OS was observed in studies from the Eastern region, with sample size < 200, stage IV CRC, chemotherapy or liver resection, or using a cut-off value of CAR < 0.1.
Fig. 2.
Forest plot of the correlation between the C-reactive protein to albumin ratio and overall survival in patients with colorectal cancer
Table 2.
Subgroup analysis of overall survival
| Subgroups | No. of studies | Pooled HR (95% CI) | Heterogeneity | |
|---|---|---|---|---|
| I2 (%) | p value | |||
| Region | ||||
| Eastern | 11 | 2.164 (1.838–2.549) | 13.78 | 0.313 |
| Western | 3 | 1.867 (1.560–2.33) | 0 | 0.927 |
| Sample sizes | ||||
| < 200 | 7 | 2.473 (1.964–3.115) | 8.535 | 0.363 |
| ≥ 200 | 7 | 1.885 (1.649–2.154) | 0 | 0.878 |
| Stage | ||||
| I–III | 6 | 1.926 (1.627–2.281) | 19.71 | 0.285 |
| Include IV | 8 | 2.273 (1.872–2.761) | 0 | 0.682 |
| Treatment | ||||
| Surgery | 8 | 1.952 (1.680–2.268) | 13.5 | 0.325 |
| Chemotherapy | 3 | 2.223 (1.539–3.210) | 8.89 | 0.333 |
| Liver resection | 3 | 2.412 (1.778–3.273) | 0 | 0.658 |
| Cut-off value | ||||
| ≥ 0.1 | 8 | 1.924 (1.682–2.200) | 0 | 0.842 |
| < 0.1 | 6 | 2.382 (1.812–3.129) | 34.427 | 0.178 |
| Analysis method | ||||
| MV | 11 | 2.122 (1.798–2.503) | 22.476 | 0.229 |
| UV | 3 | 1.960 (1.564–2.455) | 0 | 0.958 |
CI confidence interval, HR hazard ratio, MV multivariate, UV univariate
Sensitivity analysis of CAR on overall survival and publication bias
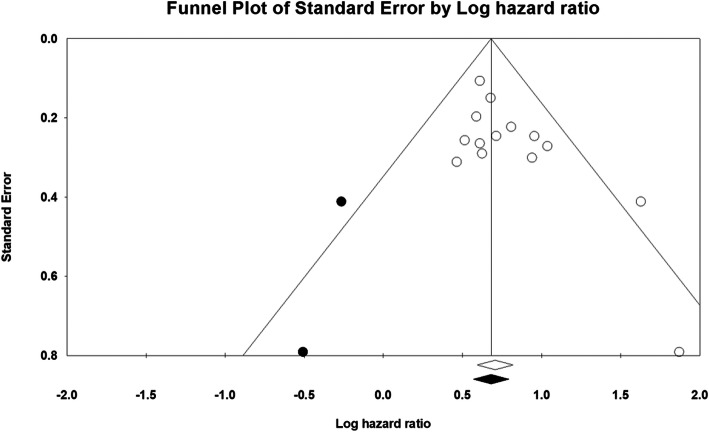
Sensitivity analysis revealed no substantial impact on the pooled results after removing one study (Additional Figure S1). Publication bias potentially existed, as Begg’s test result was 0.080 and Egger’s test result was 0.022. There were two missing studies in the funnel plot using the trim-and-fill analysis. However, no significant alteration in the pooled result was found after inputting these two studies (HR 2.001, 95% CI 1.733−2.322). The funnel plot is shown in Fig. 3.
Fig. 3.
Filled funnel plots for publication bias test of overall survival, the open circles are the real studies; the closed circles are the “filled” studies; the open diamond is the mean effect size of the real studies; the closed diamond is the mean effect size adding the “filled” studies
Meta-analysis of the effect of CAR on disease- and progression-free survival
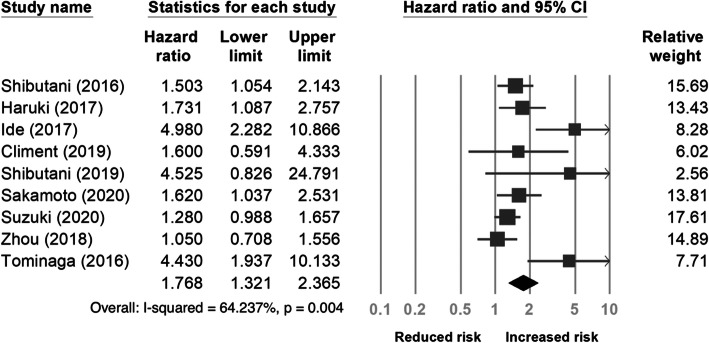
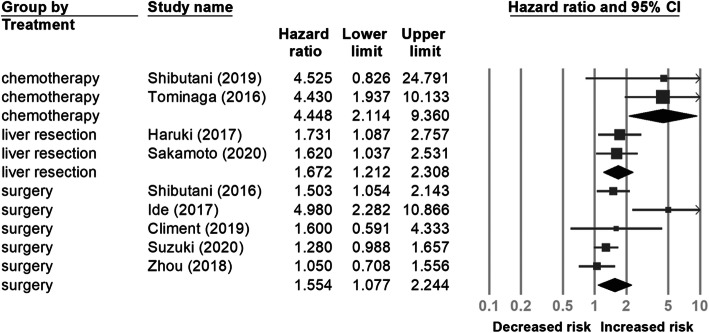
Nine studies [20, 24–30, 33] with a total of 3671 patients provided data on pre-treatment CAR and DFS/PFS. As shown in Fig. 4, the pooled HR showed that a high pre-treatment CAR was associated with poor DFS/PFS (HR 1.768, 95% CI 1.321−2.365). In the subgroup analysis (Table 3), a significant association between high pre-treatment CAR and worse DFS/PFS was observed in the chemotherapy subgroup (HR 4.448, 95% CI 2.114−9.360) (Fig. 5).
Fig. 4.
Forest plot of the correlation between the C-reactive protein to albumin ratio and disease-free survival/progression-free survival in patients with colorectal cancer
Table 3.
Subgroup analysis of disease-free survival/progression-free survival
| Subgroups | No. of studies | Pooled HR (95% CI) | Heterogeneity | |
|---|---|---|---|---|
| I2 (%) | p value | |||
| Region | ||||
| Eastern | 8 | 1.793 (1.314–2.447) | 68.69 | 0.002 |
| Western | 1 | 1.600 (0.591–4.333) | 0 | 1 |
| Sample sizes | ||||
| < 200 | 5 | 2.646 (1.599–4.378) | 61.822 | 0.033 |
| ≥ 200 | 4 | 1.289 (1.075–1.546) | 0 | 0.584 |
| Stage | ||||
| I–III | 5 | 2.115 (1.297–3.449) | 76.339 | 0.002 |
| Include IV | 4 | 1.477 (1.044–2.089) | 40.865 | 0.167 |
| Treatment | ||||
| Surgery | 5 | 1.554 (1.077–2.244) | 68.94 | 0.012 |
| Chemotherapy | 2 | 4.448 (2.114–9.360) | 0 | 0.982 |
| Liver resection | 2 | 1.672 (1.212–2.308) | 0 | 0.84 |
| Cut-off value | ||||
| ≥ 0.1 | 4 | 2.269 (1.215–4.240) | 55.379 | 0.081 |
| < 0.1 | 5 | 1.613 (1.129–2.304) | 71.106 | 0.008 |
| Analysis method | ||||
| MV | 7 | 1.610 (2.214–2.135) | 61.628 | 0.016 |
| UV | 2 | 2.769 (1.024–7.490) | 57.907 | 0.123 |
CI confidence interval, HR hazard ratio, MV multivariate, UV univariate
Fig. 5.
Forest plot for subgroup analysis of the correlation between the C-reactive protein to albumin ratio and disease-free survival/progression-free survival in patients with colorectal cancer according to treatment type
Sensitivity analysis of the effect of CAR on disease- and progression-free survival
The sensitivity analysis also revealed no significant influence on the pooled results after omitting one study (Additional Figure S2).
Clinicopathological characteristics associated with a high CAR
A total of 11 variables were surveyed in this meta-analysis to determine the association between clinicopathological characteristics and high pre-treatment CAR. Variables included age, sex, tumor location, tumor sidedness, pathological differentiation, stage, tumor size, lymphatic invasion, pre-treatment CEA, mGPS, and NLR. The details of the clinicopathological factors associated with a high pre-treatment CAR are summarized in Additional Table S3. The results revealed that a high pre-treatment CAR was associated with older age (odds ratio [OR] 1.470, p = 0.007), male sex (OR 1.452, p = 0.001), location (colon vs. rectum, OR 1.724, p = 0.008), differentiation (poor vs. moderate/high, OR 1.611, p = 0.002), stage (III/IV vs. I/II, OR 2.255, p < 0.001), high CEA (OR 2.111, p = 0.042), tumor size (> 50 mm vs. < 50 mm, OR 3.687, p < 0.001), high NLR (OR 2.452, p = 0.002), and advanced mGPS (1 vs. 0, OR 21.405, p < 0.001). Conversely, no association was found between high pre-treatment CAR and tumor sidedness or lymphatic invasion. The details of the relationship between high pre-treatment CAR and clinicopathological characteristics are summarized in Table 4.
Table 4.
Meta-analysis of the association between the C-reactive protein to albumin ratio and clinicopathological characteristics
| Characteristic | No. of studies | OR (95% CI) | p value | Heterogeneity | |
|---|---|---|---|---|---|
| I2 (%) | p value | ||||
| Age (> median vs. < median) | 4 | 1.470 (1.028–2.102) | 0.007 | 15.853 | 0.312 |
| Gender (male vs. female) | 7 | 1.452 (1.167–1.807) | 0.001 | 18.219 | 0.291 |
| Location (colon vs. rectum) | 5 | 1.724 (1.156–2.570) | 0.008 | 35.14 | 0.187 |
| Location (right vs. left) | 3 | 1.106 (0.586–2.089) | 0.756 | 49.317 | 0.139 |
| Differentiation (poor vs. mod./well) | 5 | 1.611 (1.195–2.172) | 0.002 | 0 | 0.587 |
| Stage (III/IV vs. I/II) | 2 | 2.255 (1.642–3.095) | < 0.001 | 0 | 0.688 |
| pre-treatment CEA (high vs. low)a | 3 | 2.111 (1.028–4.337) | 0.042 | 85.982 | 0.001 |
| Tumor size (≥ 50 mm vs. < 50 mm) | 1 | 3.687 (2.608–5.211) | < 0.001 | - | - |
| Lymphatic invasion (yes vs. no) | 4 | 1.020 (0.659–1.579) | 0.929 | 79.615 | 0.002 |
| NLR (high vs. low)b | 2 | 2.452 (1.381–4.354) | 0.002 | 42.753 | 0.186 |
| mGPS (1 vs. 0) | 5 | 21.405 (6.468–70.835) | < 0.001 | 66.499 | 0.018 |
CEA carcinoembryonic antigen, CI confidence interval, mGPS modified Glasgow prognostic score, NLR neutrophil-to-lymphocyte ratio, OR odds ratio
aTwo studies used CEA 5 ng/mL as the cut-off value, and one study used CEA 8.7 ng/mL as the cut-off value
bThe cut-off values were chosen as 3.0 and 2.9, respectively
Discussion
We conducted this systematic review and meta-analysis with a total of 6329 participants to determine the prognostic value of CAR in CRC patients. This study included the most recent literature from both Eastern and Western countries. Our study showed that high pre-treatment CAR was associated with the poor survival, either overall or disease-free, of CRC patients. A double death risk is observed more in high pre-treatment CAR patients than in those with low pre-treatment CAR. Moreover, up to four-fold of the progression risk is observed in stage IV CRC patients receiving chemotherapy. The subgroup analysis revealed the constant effect of high pre-treatment CAR on survival, including stratification by region, sample size, stage, treatment type, or CAR cut-off value.
Additionally, a high pre-treatment CAR correlated with many advanced clinicopathological features, such as older age, advanced tumor size, stage, elevated CEA and NLR levels, and prominence more in the colon than in the rectum. In addition to these factors, a high level of CA-199 [19] and an increased tumor depth [23] were reported to be correlated with a high pre-treatment CAR. Consequently, CAR can be used as a prognostic biomarker for patients with CRC. Assessing CAR before treatment is essential not only for its prognostic value but also for stratifying high-risk patients.
It is well known that inflammation plays a critical role in tumorigenesis. Malignancies can trigger an intrinsic inflammatory response that affects the tumor cells’ microenvironment in tumor progression and metastasis [36]. Therefore, several inflammation-related markers have been studied for use in cancer treatment. In recent decades, the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, GPS, and mGPS have been widely used to predict CRC outcomes [6, 15, 16]. GPS and mGPS are composed of CRP and albumin, both of which may reflect the underlying inflammatory condition and nutritional status. Because only three scores characterize GPS and mGPS, most patients are categorized to have scores 0 or 1, typically considered to have a better prognosis than score 2. However, by using CAR, which has a quantitative nature with a continuous range of values, we can further divide the GPS/mGPS group with CAR. Ishizuka et al. found a superior overall survival in the low CAR subgroup than in the high CAR subgroup for CRC patients with GPS 0 and GPS 1, respectively [19].
In addition to the predictive role of prognosis in patients with CRC, there are several CAR utilities in clinical practice. A multicenter study conducted by Hashimoto et al. found that high pre-operative CAR was an independent predictor of postoperative complications (HR 2.864, p = 0.029) in patients aged 85 years or older who underwent primary tumor resection for CRC [37].
Shibutani et al. found that the normalization of CAR at 8 weeks after initiating chemotherapy for stage IV CRC patients tended to have a better OS than the persistently high CAR subgroup [23]. A study conducted by Zhou et al. also found that dynamic changes in inflammatory markers, including CAR, are associated with OS [20]. Moreover, Tominaga et al. investigated 136 patients with stage III CRC receiving adjuvant chemotherapy and found a significant increase in the risk of severe side effects of chemotherapy (HR 7.06, 95% CI 2.51–19.88, p < 0.01) in patients with high pre-treatment CAR [30]. In addition to pre-treatment CAR usage, Ge et al. found that high postoperative CAR on POD-3 had a higher positive predictive value for postoperative complications than CRP alone [38]. Another study conducted by Matsuoka et al. found the survival benefit of adjuvant chemotherapy in stage III CRC patients with high postoperative CAR after curative surgery but no superior outcome of adjuvant chemotherapy in the low postoperative CAR subgroup [39].
As an acute-phase protein, CRP is mediated by many pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α [12]. These cytokines suppress the synthesis of albumin under inflammatory conditions [13, 14]. Thus, as a combination of these two proteins, CAR may reflect the severity of inflammation, which is believed to be correlated with tumor progression. This updated meta-analysis summarized the most recent studies and indicated the prognostic value of pre-treatment CAR for OS and DFS/PFS. Thus, CAR data should be used for both, checking the pre-treatment status of patients with CRC and other cancers, and observing the dynamic changes in CAR values during and post-treatment. More studies involving CAR are warranted to gain a complete understanding of its utility in CRC treatment.
Limitations of this meta-analysis
This study has some limitations. First, all of the included studies were retrospective in design, the existence of co-morbidities influenced the CRP and albumin values, and selection bias potentially existed. Second, most of the included studies originated in the Eastern region. The pooled results may not be as consistent as they are in the Western region. Third, the CAR cut-off value varied between each study; therefore, we could not determine the most reliable clinical practice value. Fourth, we could only extract HRs and 95% CIs directly for the meta-analysis from each study. The existence of missing data may reduce the overall accuracy of the pooled results. Finally, publication bias did exist in this systemic review and may attributed to the following reasons. We only included the articles in English and Chinese although we did not set any limitation of language during the searching process. Unpublished papers with non-significant results may exist; however, there was no significant alteration in the pooled result after adding two missing studies in the funnel plot using the trim-and-fill analysis. We believe this meta-analysis is still reliable.
Conclusion
This meta-analysis indicates that elevated pre-treatment CAR is correlated with poor OS and DFS/PFS in patients with CRC. CAR is a reliable biomarker in clinical practice. It is a predictor of prognosis and stratified high-risk patients from the existing classification.
Supplementary Information
Additional file 1: Figure S1. Sensitivity analysis of C-reactive protein to albumin ratio and overall survival in patients with colorectal cancer
Additional file 2: Figure S2. Sensitivity analysis of C-reactive protein to albumin ratio and disease-free survival / progression-free survival in patients with colorectal cancer
Additional file 3: Table S1. The confounding factors extracted from studies investigating pre-treatment C-reactive protein to albumin ratio on survival in colorectal cancer patients
Additional file 4: Table S2. Scores of the eligible studies according to the Newcastle-Ottawa Scale
Additional file 5: Table S3. The detail of retrieving clinicao-pathology factors associated with high pre-treatment CAR
Acknowledgements
Not applicable
Abbreviations
- CEA
Carcinoembryonic antigen
- CAR
C-reactive protein to albumin ratio
- CRC
Colorectal cancer
- CI
Confidence interval
- DFS
Disease-free survival
- GPS
Glasgow prognostic score
- HR
Hazard ratio
- mGPS
Modified Glasgow prognostic score
- NLR
Neutrophil-to-lymphocyte ratio
- NOS
Newcastle–Ottawa Quality Assessment Scale
- OS
Overall survival
- PFS
Progression-free survival
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
Authors’ contributions
CL and YY contributed equally to this work. CL and JY conceived the study design. CL and YL collected and analyzed the data, and CL and YC drafted the manuscript. YH and JC reviewed and contributed to the revision of the manuscript. The authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study did not require ethical approval because it did not include data linked to individual patient information.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chun-Kai Liao and Yen-Lin Yu contributed equally to this work.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: cancer today. Lyon: International Agency for Research on Cancer; 2020. [Google Scholar]
- 2.Molinari C, Marisi G, Passardi A, Matteucci L, De Maio G, Ulivi P. Heterogeneity in colorectal cancer: a challenge for personalized medicine? Int J Mol Sci. 2018;19(12):3733. doi: 10.3390/ijms19123733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 6.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. doi: 10.1097/mco.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 7.Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ, Lee JH. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17(3):216–222. doi: 10.3109/1354750x.2012.656705. [DOI] [PubMed] [Google Scholar]
- 8.Read JA, Choy ST, Beale PJ, Clarke SJ. Evaluation of nutritional and inflammatory status of advanced colorectal cancer patients and its correlation with survival. Nutr Cancer. 2006;55(1):78–85. doi: 10.1207/s15327914nc5501_10. [DOI] [PubMed] [Google Scholar]
- 9.Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2017;265(3):539–546. doi: 10.1097/sla.0000000000001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo HD, Kim K, Kim J. Association between preoperative C-reactive protein level and colorectal cancer survival: a meta-analysis. Cancer Causes Control. 2015;26(11):1661–1670. doi: 10.1007/s10552-015-0663-8. [DOI] [PubMed] [Google Scholar]
- 11.Gupta D, Lis CG. Pre-treatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9(1):69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakazaki H. Preoperative and postoperative cytokines in patients with cancer. Cancer. 1992;70(3):709–713. doi: 10.1002/1097-0142(19920801)70:3<709::aid-cncr2820700328>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. doi: 10.1207/s15327914nc392_8. [DOI] [PubMed] [Google Scholar]
- 14.Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. 2005;39(4):S143–S146. doi: 10.1097/01.mcg.0000155514.17715.39. [DOI] [PubMed] [Google Scholar]
- 15.Choi KW, Hong SW, Chang YG, Lee WY, Lee B, Paik IW, Lee H. Inflammation-based score (Glasgow prognostic score) as an independent prognostic factor in colorectal cancer patients. Ann Surg Treat Res. 2014;86(6):309–313. doi: 10.4174/astr.2014.86.6.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He L, Li H, Cai J, Chen L, Yao J, Zhang Y, Xu W, Geng L, Yang M, Chen P, Zheng J, Yang Y, Gong S. Prognostic value of the Glasgow prognostic score or modified Glasgow prognostic score for patients with colorectal cancer receiving various treatments: a systematic review and meta-analysis. Cell Physiol Biochem. 2018;51(3):1237–1249. doi: 10.1159/000495500. [DOI] [PubMed] [Google Scholar]
- 17.Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One. 2013;8(3):e59321. doi: 10.1371/journal.pone.0059321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu HJ, Ma Y, Deng F, Ju WB, Sun XY, Wang H. The prognostic value of C-reactive protein/albumin ratio in human malignancies: an updated meta-analysis. Onco Targets Ther. 2017;10:3059–3070. doi: 10.2147/OTT.S137002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. 2016;23(3):900–907. doi: 10.1245/s10434-015-4948-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhou ZQ, Pang S, Yu XC, Xue Q, Jiang HY, Liang XJ, Liu L. Predictive values of postoperative and dynamic changes of inflammation indexes in survival of patients with resected colorectal cancer. Curr Med Sci. 2018;38(5):798–708. doi: 10.1007/s11596-018-1946-6. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 31 Oct 2020.
- 23.Shibutani M, Maeda K, Nagahara H, Iseki Y, Hirakawa K, Ohira M. The significance of the C-reactive protein to albumin ratio as a marker for predicting survival and monitoring chemotherapeutic effectiveness in patients with unresectable metastatic colorectal cancer. Springerplus. 2016;5(1):1798. doi: 10.1186/s40064-016-3529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal cancer. Anticancer Res. 2016;36:995–901. [PubMed] [Google Scholar]
- 25.Haruki K, Shiba H, Horiuchi T, Sakamoto T, Gocho T. Fujiwara y, et al. Impact of the C-reactive protein to albumin ratio on long-term outcomes after hepatic resection for colorectal liver metastases. Am J Surg. 2017;214(4):752–756. doi: 10.1016/j.amjsurg.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Ide S, Toiyama Y, Okugawa Y, Oki S, Okugawa Y, Yasuda H, Fujikawa H, et al. Clinical significance of C-reactive protein-to-albumin ratio with rectal cancer patient undergoing chemoradiotherapy followed by surgery. Anticancer Res. 2017;37:5797–5704. doi: 10.21873/anticanres.12022. [DOI] [PubMed] [Google Scholar]
- 27.Shibutani M, Nagahara H, Fukuoka T, Iseki Y, Matsutani S, Wang EN, et al. Prognostic significance of the C-reactive protein-to-albumin ratio in patients with metastatic colorectal cancer treated with trifluridine/thymidine phosphorylase inhibitor as later-line chemotherapy. Anticancer Res. 2019;39(2):1051–1057. doi: 10.21873/anticanres.13212. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto Y, Mima K, Imai K, Miyamoto Y, Tokunaga R, Akiyama T, Daitoku N, Hiyoshi Y, Iwatsuki M, Nagai Y, Baba Y, Iwagami S, Yamashita YI, Yoshida N, Baba H. Preoperative C-reactive protein-to-albumin ratio and clinical outcomes after resection of colorectal liver metastases. Surg Oncol. 2020;35:243–248. doi: 10.1016/j.suronc.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S, Akiyoshi T, Oba K, Oba K, Otsuka F, Tominaga T, et al. Comprehensive comparative analysis of prognostic value of systemic inflammatory biomarkers for patients with stage II/III colon cancer. Ann Surg Oncol. 2020;27(3):844–852. doi: 10.1245/s10434-019-07904-9. [DOI] [PubMed] [Google Scholar]
- 30.Tominaga T, Nonaka T, Sumida Y, Hidaka S, Sawai T, Nagayasu T. The C-reactive protein to albumin ratio as a predictor of severe side effects of adjuvant chemotherapy in stage III colorectal cancer patients. PLoS One. 2016;11(12):e0167967. doi: 10.1371/journal.pone.0167967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solaini L, Atmaja BT, Arumugam P, Hutchins RR, Abraham AT, Bhattacharya S, Kocher HM. The role of perioperative inflammatory-based prognostic systems in patients with colorectal liver metastases undergoing surgery. A cohort study. Int J Surg. 2016;36(Pt A):8–12. doi: 10.1016/j.ijsu.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Dolan RD, McSorley ST, Park JH, Watt DG, Roxburgh CS, Horgan PG, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119(1):40–51. doi: 10.1038/s41416-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Climent M, Ryan ÉJ, Stakelum Á, Khaw YL, Creavin B, Lloyd A, Alhassan D, Mohan HM, Kennelly R, Sheahan K, Winter DC. Systemic inflammatory response predicts oncological outcomes in patients undergoing elective surgery for mismatch repair-deficient colorectal cancer. Int J Color Dis. 2019;34(6):1069–1078. doi: 10.1007/s00384-019-03274-6. [DOI] [PubMed] [Google Scholar]
- 34.Ni X, Wu P, Wu J, Ji M, Shao Y, Zhou W, et al. C-reactive protein/albumin ratio as a predictor of survival of metastatic colorectal cancer patients receiving chemotherapy. Int J Clin Exp Pathol. 2016;9:5525–5534. [Google Scholar]
- 35.Son W, Shin SJ, Park SH, Lee SK, Park EJ, Baik SH, et al. Clinical impact of combined modified Glasgow prognostic score and C-reactive protein/albumin ratio in patients with colorectal cancer. Diagnostics. 2020;10:859. doi: 10.3390/diagnostics10110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto S, Tominaga T, Nonaka T, Hamada K, Araki M, Takeshita H, Fukuoka H, Wada H, To K, Komatsu H, Tanaka K, Sawai T, Nagayasu T. The C-reactive protein to albumin ratio predicts postoperative complications in oldest-old patients with colorectal cancer. Int J Color Dis. 2020;35(3):423–431. doi: 10.1007/s00384-019-03491-z. [DOI] [PubMed] [Google Scholar]
- 38.Ge X, Cao Y, Wang H, Yan W, Yang D, Chen G, et al. Diagnostic accuracy of the postoperative ratio of C-reactive protein to albumin for complications after colorectal surgery. World J Surg Oncol. 2017;15:15. doi: 10.1186/s12957-016-1092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuoka H, Ando K, Hu Q, Zaitsu Y, Tsuda Y, Hisamatsu Y, Nakashima Y, Kimura Y, Oki E, Mori M. Postoperative C-reactive protein/albumin ratio is a biomarker of risk of recurrence and need for adjuvant chemotherapy for stage III colorectal cancer. Int J Clin Oncol. 2020;25(7):1318–1326. doi: 10.1007/s10147-020-01672-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Sensitivity analysis of C-reactive protein to albumin ratio and overall survival in patients with colorectal cancer
Additional file 2: Figure S2. Sensitivity analysis of C-reactive protein to albumin ratio and disease-free survival / progression-free survival in patients with colorectal cancer
Additional file 3: Table S1. The confounding factors extracted from studies investigating pre-treatment C-reactive protein to albumin ratio on survival in colorectal cancer patients
Additional file 4: Table S2. Scores of the eligible studies according to the Newcastle-Ottawa Scale
Additional file 5: Table S3. The detail of retrieving clinicao-pathology factors associated with high pre-treatment CAR
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.