Abstract
Background
Myeloid sarcoma (MS) is a very rare condition, develops both in patients with other hematological neoplasms, and as isolated tumor. MS of the gynecologic tract is extremely rare. An available literature data about diagnosis and management of MS is summarized in the article. The role of chemotherapy, radiation therapy, surgery and bone marrow transplantation in the treatment is discussed. Polychemotherapy and allogeneic bone marrow transplantation were suggested to be the optimal treatment strategy of MS of the gynecological tract. The use of new targeted agents results in promising clinical data.
Case presentation
We are presenting a rare clinical case of a MS of the uterine cervix with concomitant bone marrow involvement and describe all the peculiarities of the clinical course, diagnosis, and treatment. The patient received chemotherapy followed by allogeneic bone marrow transplantation. The pre-transplant therapy allowed us to perform allogeneic bone marrow transplantation with the deepest response possible: complete PET-negative and MRD-negative remission of the disease.
Conclusions
MS remains a subject of discussion regarding its diagnostic and therapeutic aspects. The use of novel targeting agents can be perspective option for patient with extramedullary disease.
Keywords: Myeloid sarcoma of the uterine cervix, Venetoclax, Hypomethylating agent, Stem cell transplantation, Acute myeloid leukemia
Background
MS (also known as chloroma or granulocytic sarcoma) is a very rare condition characterized by proliferation of immature myeloid cells in extramedullary sites [1]. Chloroma most often develops in patients with acute myeloid leukemia (AML), other myeloproliferative neoplasms, or myelodysplastic syndromes, but it can be also presented as an isolated mass. MS, associated with AML, may precede it, develop during the onset or relapse of disease [2–4]. Untreated isolated MS usually leads to AML at a median of 7 months (range of 1 to 2) [5]. Although MS has been reported in various parts of the body, the most common sites are lymph nodes, soft tissues, and bones [5]. MS of the gynecologic tract, in particular the cervix, is rare (up to 5.8–22.7% of all localizations). Vaginal bleeding is a very common presenting symptom of MS of the cervix or uterus [6, 7]. Diagnosis of MS is difficult, especially in the absence of bone marrow involvement, and is based on a combination of clinical features, radiological investigations, and tissue biopsy [1, 8, 9]. Immunohistochemical analysis is an important part of MS diagnostics, which allows establishing the correct diagnosis in 96% of cases [5, 8]. FDG-PET/CT is a useful tool to estimate the extent of the lesion [10]. Treatment of MS with AML (Cytarabine-based) protocols (regardless of bone marrow involvement) is the most reasonable approach [1]. Successful use of targeted agents such as Gemtuzumab ozogamicin, hypomethylating agents (Decitabine and 5-azacitidine), and BCL-2 inhibitors (Venetoclax) has also been described [11–14]. Surgical and radiation therapies are accepted treatment methods, but their roles in the treatment algorithm are not well-defined. Surgical intervention should be considered before the systemic treatment in patients with symptoms of mass effect or when excision biopsy was necessary to establish the diagnosis [1]. Radiation is used in addition to chemotherapy in patients with isolated MS, who do not achieve complete regression of the tumor mass after polychemotherapy, as well as palliative option when compression symptoms (superior vena cava syndrome, spinal cord roots compression) are present [1, 11, 15, 16]. The hematopoietic stem cell transplantation, is an important therapeutic option which can be used in patients with and without bone marrow involvement [1, 8, 17]. In this article, we present a rare clinical case of an MS of the uterine cervix with concomitant bone marrow involvement.
Case presentation
A 49-year-old female with ECOG Performance Score of 0 presented with complaints of profuse metrorrhagia following period of amenorrhea. There was no history of weight loss, fever, or night sweats. A speculum examination showed an exophytic tumor originating from the uterine cervix with contact bleeding. The histological and immunohistochemical study of the biopsy showed that the biopsied tissue was diffuse infiltrated by polymorphous cells with positivity for MPO, LCA, CD99, CD4, CD117, CD15, BCL-2, and negativity for lymphoid, epithelial, and neuroendocrine markers (MCК, CК7, ALK, CD10, CD20, CD3, CD30, CD34, chromogranin A, synaptophysin, desmin, Fli-1, S-100, PAX-4, CD56, CD7) (Fig. 1). The Ki67 was expressed in 40% of the cells.
Fig. 1.
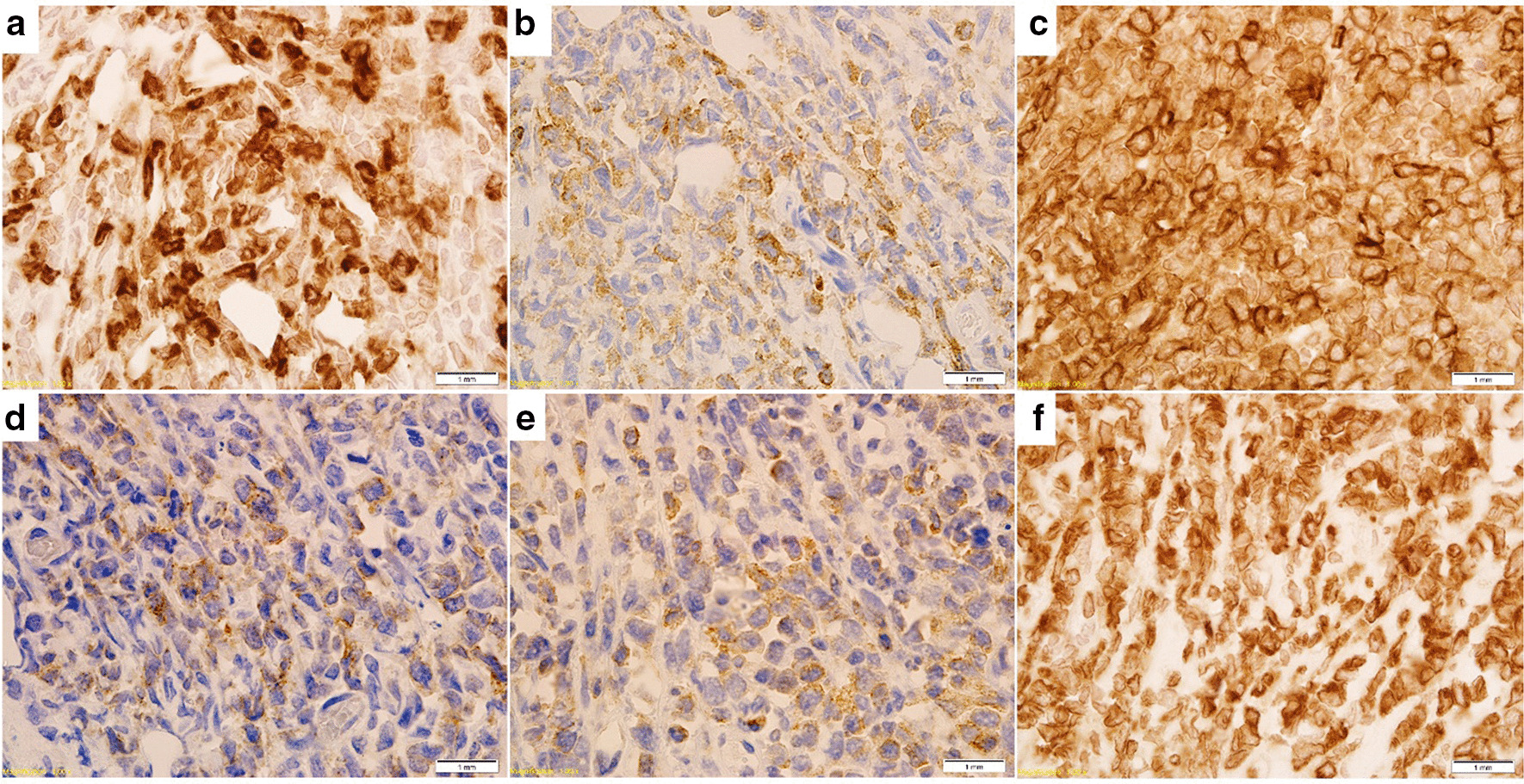
Immunohistochemical study of MS. a Expression of CD15; b expression of CD45; c expression of CD99; d expression of CD117; e expression of myeloperoxidase, f expression of BCL-2
Laboratory studies revealed the following values: hemoglobin 10.9 g/dL, platelet—177 × 109/l, and white-cell count 3500 per cubic millimeter without pathological findings at leucogram. A bone marrow biopsy showed the presence of 16.2% positive MPO pathological blasts. The immunophenotypic analysis showed the expression of CD117, CD13, CD33, CD64, CD4, and MPO. Karyotype was normal—46, XX (20 metaphases were analyzed), while FISH demonstrated inversion 3 (identified the separation of the fusion signal from region 3q26 at 76 out of 200 analyzed interphase nuclei). A mutation in NPM1B was detected by quantitative PCR (737.8% copies). Lumbar puncture with prophylactic intrathecal chemotherapy was made and there was no evidence of CNS involvement. A pelvic magnetic resonance imaging (MRI) with contrast revealed an intrapelvic lymph nodes lesion and 43 × 75 × 76 mm tumor originating from the uterine cervix with invasion of the uterine corpus, parametrium, and pelvic peritoneum. A FDG-PET/CT showed a 71 × 48 mm lesion at the cervix with a standard uptake value maximum (SUVlbm max) of 5.5 (Fig. 2). Other hypermetabolic focuses were noted at intrapelvic lymphatic nodes: along the common iliac vessels (up to 8 mm with SUVlbm max = 2.16 on the right and up to 7 mm with SUVlbm max = 2.2 on the left), and the external iliac vessels (up to 15 × 11 mm with SUVlbm max = 2.6 on the right and 19 × 14 mm with SUVlbm max = 2.9 on the left). These findings were compatible with the diagnosis of MS involving the cervix and corpus of the uterus, parametrium, pelvic lymph nodes, and bone marrow.
Fig. 2.
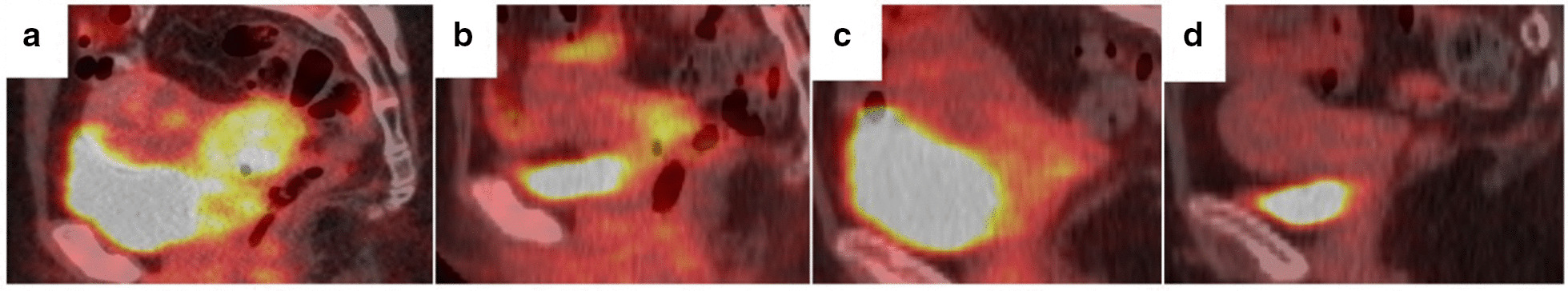
Dynamics of tumor by FDG-PET/CT. a Study at the onset of the disease; b study after course of induction therapy; c study after course of consolidation therapy; d study after course of specific therapy with Venetoclax and 5-azacitidine
The patient received AML type induction chemotherapy with cytosine arabinoside (200 mg/m2/day for 7 days) and daunorubicin (60 mg/m2 for 3 days). The course was complicated by febrile neutropenia and probable invasive mycosis which was successfully treated. The patient achieved complete clinical and hematologic remission (platelet—373 × 109/l, absolute neutrophil count—1360 per cubic millimeter, bone marrow blasts—1%, independence of red cell transfusions) and partial metabolic response (decrease of tumor mass by 80.3%). The consolidation chemotherapy included high-dose cytosine arabinoside (3 g/m2/day). The control PET/CT showed the decrease in tumor mass (by 93% comparing to the initial size), and we were unable to achieve complete remission due to extramedullary lesion. Considering the data available in the literature about the successful use of BCL-2 inhibitors in combination with hypomethylating agents in AML, including extramedullary lesions [13, 14, 18], the patient underwent a course of chemotherapy with Venetoclax and 5-azacitidine (75 mg/m2). Post-chemotherapy examination demonstrated that the patient achieved complete MRD-negative remission (Fig. 2).
The patient underwent allogeneic hematopoietic stem cell transplantation from human leukocyte antigens (HLA)-matched related donor (sister) with myeloablative conditioning regimen (Fludarabine 150 mg/m2, Busulfan 12 mg/kg). Posttransplant period was complicated by sepsis, associated with Klebsiella pneumoniae, and systemic invasive mycosis involving the liver, spleen, and lungs, which were successfully treated. The patient achieved complete donor chimerism (100%) in 6 weeks after bone marrow transplantation. There was no evidence of graft-versus-host disease. The patient remains in complete MRD-negative and PET-negative remission for 4 months after bone marrow transplantation.
Discussion
MS involving the female reproductive system occurs rather rarely. We were able to identify 57 cases of leukemic cervical tumors in published articles from 2002 to 2019 (Table 1). Patients often complain of vaginal bleeding at the onset of disease [6, 7], it was the first symptom in the above case. FDG-PET/CT appears to be the best imaging option to assess the presence of extramedullary AML and effectively used to both search for other possible lesions and monitoring the response to therapy [19–21]. MS may often be misdiagnosed for malignant lymphoma, small cell carcinoma, and undifferentiated tumor, so performing immunohistochemistry study with epithelial, neuroendocrine, myeloid, and lymphoid markers it is highly recommended [1, 2, 8, 21, 22]. Determining the markers, characteristic for myeloid cells (MPO, LCA) and the absence of lymphoid (CD20, CD10, CD3, CD30, CD7), epithelial (MCK, CK7), neuroendocrine (chromogranin A, synaptophysin), and other (ALK, desmin, Fli-1, S-100) markers allowed us to decide on the diagnosis. Additionally, the high expression of BCL-2 was found in the biopsy sampling.
Table 1.
Literature review of myeloid sarcoma involving the gynecologic tract
| No | Authors, country, year of study | Number of cases (n) | Affected region | Concurrent AML | Treatment | % (n) | Duration of observation (months) |
|---|---|---|---|---|---|---|---|
| 1 [6] | B. Pathak et al., Canada, 2005 | 25 |
Cervix—76% (n = 19) Uterine corpus—8% (n = 2) External genital organs—4% (n = 1) ≥ 2 gynecologic sites—4% (n = 1) Not stated—8% (n = 2) |
48% (n = 12) | CT | 28 (7) | 7.7 |
| RT | 8 (2) | 5 | |||||
| SI | 8 (2) | 3 | |||||
| CT + RT | 24 (6) | 13.75 | |||||
| CT + SI | 8 (2) | 20.5 | |||||
| RT + SI | 4 (1) | 1.75 | |||||
| Without treatment | 16 (4) | < 1 | |||||
| 2 [40] | R.M. Kahn et al., USA, 2019 | 1 | ≥ 2 gynecologic sites (uterine corpus, fallopian tubes, left ovary) | Yes | CT + SI | 3 | |
| 3 [12] | G.Modi et al., India, 2004 | 1 | External genital organs (vagina) | No | Hypomethylating therapy with decitabine | > 4 | |
| 4 [41] | J.-A. Hernández et al., Spain, 2002 | 2 | Cervix, left mesosalpinx, ovaries | 100% (n = 2) | CT + SI + autoBMT | 10 | |
| External genital organs (vagina) | CT + autoBMT | 10 | |||||
| 5 [42] | M. Ucar, M. Guryildirim, Turkey, 2014 | 1 | Breast, cervix, uterus | Yes | CT | > 2 | |
| 6 [43] | M.G. Garcia et al., USA, Spain, 2006 | 11 |
Cervix—27.3% (n = 3) Ovary—18.2% (n = 2) Uterine corpus—9% (n = 1) External genital organs—9% (n = 1) ≥ 2 gynecologic sites—36.5% (n = 4) |
36.5% (n = 4) | CT | 64 (7) | 40 |
| RT | 9 (1) | 5 | |||||
| CT + alloBMT | 9 (1) | > 6 | |||||
| SI | 73 (8) | - | |||||
| 9 |
Cervix—11% (n = 1) Ovary—89% (n = 8) |
22% (n = 2) | CT | 33 (3) | 15 | ||
| CT + alloBMT | 11 (1) | 10 | |||||
| 7 [44] | S.C.H. Kim et al., France, 2010 | 1 | Cervix | No | CT + RT | > 72 | |
| 8 [45] | H. Gill et al., China, 2012 | 1 | Cervix, left pelvic cavity | No | CT | 16 | |
| 9 [7] | W. Gui et al., China, 2019 | 2 | Cervix, external genital organs (vagina), intrapelvic lymph nodes | No | SI + CT | 21 | |
| Cervix, uterus, intrapelvic lymph nodes | Yes | CT | 26 | ||||
| 10 [46] | A.S. Weingertner et al., France, 2009 | 1 | Cervix, intrapelvic lymph nodes | Yes | CT | > 3 | |
| 11 [47] | Y. Yu et al., China, 2015 | 1 | External genital organs (vulva, vagina), cervix | No | CT | 4 | |
| 12 [48] | H. Bao et al., China, 2019 | 1 | External genital organs | Yes | SI + CT + alloBMT | > 6 | |
CT chemotherapy, RT radiotherapy, SI surgical intervention, autoBMT autologous bone marrow transplantation, alloBMT allogeneic bone marrow transplantation
Certain AML genetic abnormalities, like t(8;21), inv(16) and t(9;11), mutations in NPM1, NRAS, and DNMT3A, are associated with a higher incidence of extramedullary disease [1, 3, 5, 23]. Apart from a mutation in NPM1B, we also identified inversion of chromosome 3, which is strongly associated with poor prognosis in AML patients [24]. No association of chromosome 3 inversion with the extramedullary lesion was found in the literature.
The genetic assessment of extramedullary tumor is indeed, especially in a case of isolated disease [21], although these data are lacking in our case as in the majority of others, presented in Table 1.
MS is a systemic disease and AML-type regimens are most often recommended as initial therapy as stated in National Comprehensive Cancer Network and European Leukemia Net (2017) guidelines [19, 24]. Earlier studies suggested, MS had had the worse prognosis, in comparison to AML without extramedullary disease [25, 26]. Currently, the majority of authors consider that extramedullary AML is not per se associated with poor prognosis [16, 21].
Standard cytarabine-containing regimens, such as «7 + 3», HDAC (high doses of cytarabine), are used for eligible patients [15, 16, 19, 24]. This regimen was applied to our patient. Bone marrow MRD-negative response was obtained, that looked surprising: usually patients with chromosome 3 abnormality are resistant to chemotherapy: CR—31%, 5-year OS—5.7% ± 3%; EFS—0%; RFS-4.3% ± 4% [27]. Nevertheless, failure of metabolic response in extramedullary disease was registered according to the control PET/CT. Reasons for this discordant are unclear. Cunningham et al. attribute them to gene or microenvironmental deregulations [20, 28, 29], but that is continued to be studied.
In patients with chemotherapy resistant MS, radiation therapy is standardly recommended as second line of treatment [1, 11, 15, 16, 21]. Delay in systemic therapy often results to early relapse in patient with chromosome 3 inversion [27, 30, 31]. Thus, we thought about alternative regimens that could be used instead of or as an addition to irradiation.
Recently registered medicines such as anti-CD33 monoclonal antibody [32, 33], tyrosine kinase inhibitors (for FIP1L1-PDGFR [34, 35], and FLT3-ITD [36]), and DNA methyltransferase inhibitors [12] offer the possibility to continue systemic therapy for patients with MS.
The expression of CD33 was not studied in the biopsied tissue, targets for FLT-3 inhibitors were lacking in our patient. Targeting of BCL-2, involving mitochondrial pathway of apoptosis, has emerged as an efficacious and well-tolerated clinical strategy. It is based on founded on myeloblast function rather than on genetics [37, 38]. Regarding MS, overexpression of BCL-2 was found by Wang et al. [39]. Worth mentioning, that the tumor tissue of our patient was revealed to hyperexpression of BCL-2.
Combination of Venetoclax with 5-azacytidine was described as a very promising regimen in several case reports as well as the retrospective study of the small number of patients [13, 14]. This therapeutic option resulted in complete metabolic response and MRD-negative complete remission. Regimen was well tolerable without unusual toxicities. The prompt response of the extramedullary tumor to the combination of Venetoclax with 5-azacytidine allowed us to proceed to allogeneic hematopoietic SCT.
Allogeneic SCT is believed to improve OS in patients both with isolated MS and concurrent AML [8, 17]. Transplantation is usually recommended as consolidative strategy of extramedullary AML based on the disease risk (by cytogenetics or molecular data) and in case of resistance [16, 21]. Patient has been in complete remission until the present.
We report for the first-time successful treatment of uterine cervix MS with Venetoclax-based regimen is described. Of note, this is the first description MS of the uterine cervix with concurrent bone marrow involvement with chromosome 3 inversion.
Conclusion
MS of the gynecologic tract is a very rare and often misdiagnosed hematologic malignancy, whose diagnostics and therapeutic aspects remain a subject of discussion. MS treatment standard is AML-type chemotherapy. Postremission therapy is dependent upon a number of factors: extent of disease, risk profile, and performance status. The use of Venetoclax with hypomethylating agent has shown good results in our clinical case. Adding this combination to standard treatment regimens in patients with extramedullary AML might become a promising clinical option. The pre-transplant therapy allowed us to perform allogeneic bone marrow transplantation with the deepest response possible: complete PET-negative and MRD-negative remission of the disease.
Hematopoietic stem-cell transplantation remains an important therapeutic option.
Acknowledgements
Not applicable.
Abbreviations
- AML
Acute myeloid leukemia
- BCL-2
B-cell lymphoma 2
- CD
Cluster of differentiation
- CNS
Central nervous system
- CR
Complete remission
- DNA
Deoxyribonucleic acid
- ECOG
Eastern Cooperative Oncology Group
- EFS
Event-free survival
- FDG-PET/CT
Fluorodeoxyglucose positron emission tomography/computed tomography
- FISH
Fluorescence in situ hybridization
- FLT3
FMS-like tyrosine kinase 3
- HLA
Human leukocyte antigens
- LCA
Leucocyte common antigen
- MCK
Multi-cytokeratin
- MPO
Myeloperoxidase
- MRD
Minimal residual disease
- MRI
Magnetic resonance imaging
- MS
Myeloid sarcoma
- NPM1B
Nucleophosmin 1B
- OS
Overall survival
- PCR
Polymerase chain reaction
- FDG-PET/CT
Positron emission tomography–computed tomography
- RFS
Relapse-free survival
- SCT
Stem cell transplantation
- SUVlbm max
Standard uptake value maximum
Authors' contributions
AS managed the patient, analyzed this case report, literature findings and prepared the manuscript. LG was attending doctor of this patient, took part in writing the Case Presentation and Discussion section. DZ, IB, VI, YA and SE were hematologists involved in the management of the patient, worked on writing the Case Presentation and analyzing the literature. DM and Darina Z were hematologists who performing the allogeneic transplantation and wrote the section describing the course and results of transplantation. YM, KB, TN and AP were doctors involved in the diagnostical part of management of the patient, performed the morphological, cytogenetic and molecular studies and wrote the diagnostic part of Case Presentation and Discussion section. DR was the radiologist, who analyzed the PET/CT-results and prepared images with PET/CT results with commentaries. RG was the pathologist, analyzing biopsy and prepared histologic images with commentaries for this article. AZ contributed in critical revision of the manuscript. All listed authors meet the ICMJE criteria. We attest that all authors contributed significantly to the creation of this manuscript, each having fulfilled criteria as established by the ICMJE. All authors read and approved the manuscript.
Funding
No funding was received for this work. This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2020-901).
Availability of data and materials
All data related to this case report are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Almond L, Charalampakis M, Ford S, et al. Myeloid sarcoma: presentation, diagnosis, and treatment. Clin Lymphoma Myeloma Leuk. 2017;17(5):263–267. doi: 10.1016/j.clml.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow S. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2017. pp. 167–168. [Google Scholar]
- 3.Kawamoto K, Miyoshi H, Yoshida N, et al. Clinicopathological, cytogenetic, and prognostic analysis of 131 myeloid sarcoma patients. Am J Surg Pathol. 2016;40(11):1473–1483. doi: 10.1097/PAS.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 4.Campidelli C, Agostinelli C, Stitson R, et al. Myeloid sarcoma: extramedullary manifestation of myeloid disorders. Am J Clin Pathol. 2009;132(3):426–437. doi: 10.1309/AJCP1ZA7HYZKAZHS. [DOI] [PubMed] [Google Scholar]
- 5.Byrd JC, Edenfield WJ, Shields DJ, et al. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol. 1995;13(7):1800–1816. doi: 10.1200/JCO.1995.13.7.1800. [DOI] [PubMed] [Google Scholar]
- 6.Pathak B, Bruchim I, Brisson ML, et al. Granulocytic sarcoma presenting as tumors of the cervix. Gynecol Oncol. 2005;98(3):493–497. doi: 10.1016/j.ygyno.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Gui W, Li J, Zhang Z, et al. Primary hematological malignancy of the uterine cervix: a case report. Oncol Lett. 2019;18(3):3337–3341. doi: 10.3892/ol.2019.10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21(2):340–350. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]
- 9.Sharma V, Dora T, Patel M, et al. Case report of diffuse large B cell lymphoma of uterine cervix treated at a semiurban cancer centre in North India. Case Rep Hematol. 2016;2016:3042531. doi: 10.1155/2016/3042531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aschoff P, Häntschel M, Oksüz M, et al. Integrated FDG-PET/CT for detection, therapy monitoring and follow-up of granulocytic sarcoma. Initial Results Nuklearmedizin. 2009;48(5):185–191. doi: 10.3413/nukmed-0236. [DOI] [PubMed] [Google Scholar]
- 11.Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2(5):309–316. doi: 10.1177/2040620711410774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modi G, Madabhavi I, Panchal H, et al. Primary vaginal myeloid sarcoma: a rare case report and review of the literature. Case Rep Obstet Gynecol. 2015;2015:957490. doi: 10.1155/2015/957490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otoukesh S, Zhang J, Nakamura R, et al. The efficacy of venetoclax and hypomethylating agents in acute myeloid leukemia with extramedullary involvement [published online ahead of print, 2020 Mar 19]. Leuk Lymphoma. 2020;1–4. 10.1080/10428194.2020.1742908 [DOI] [PubMed]
- 14.Kanate AS, Vos J, Chargualaf MJ. Venetoclax for refractory myeloid sarcoma. J Oncol Pract. 2019;15(7):413–415. doi: 10.1200/JOP.18.00753. [DOI] [PubMed] [Google Scholar]
- 15.Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118(14):3785–3793. doi: 10.1182/blood-2011-04-347229. [DOI] [PubMed] [Google Scholar]
- 16.Shahin O, Ravandi F. Myeloid sarcoma. Curr Opin Hematol. 2020;27(2):88–94. doi: 10.1097/MOH.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 17.Chevallier P, Labopin M, Cornelissen J, et al. Allogeneic hematopoietic stem cell transplantation for isolated and leukemic myeloid sarcoma in adults: a report from the Acute Leukemia Working Party of the European group for Blood and Marrow Transplantation. Haematologica. 2011;96(9):1391–1394. doi: 10.3324/haematol.2011.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachowiez C, DiNardo CD, Konopleva M. Venetoclax in acute myeloid leukemia—current and future directions. Leuk Lymphoma. 2020;61(6):1313–1322. doi: 10.1080/10428194.2020.1719098. [DOI] [PubMed] [Google Scholar]
- 19.Pollyea DA, Bixby D, Perl A, et al. NCCN guidelines insights: acute myeloid leukemia, version 2.2021: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw 2021;19(1):14, 45, 64. [DOI] [PubMed]
- 20.Cunningham I. Post-transplant leukemia relapse in organs: biology and behavior in 585 reports. Crit Rev Oncol/Hematol. 2021;157:103170. doi: 10.1016/j.critrevonc.2020.103170. [DOI] [PubMed] [Google Scholar]
- 21.Shallis RM, Gale RP, Lazarus HM, et al. Myeloid sarcoma, chloroma, or extramedullary acute myeloid leukemia tumor: a tale of misnomers, controversy and the unresolved. Blood Rev. 2020 doi: 10.1016/j.blre.2020.100773. [DOI] [PubMed] [Google Scholar]
- 22.Neiman RS, Barcos M, Berard C, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981;48(6):1426–1437. doi: 10.1002/1097-0142(19810915)48:6<1426::aid-cncr2820480626>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Kashofer K, Gornicec M, Lind K, et al. Detection of prognostically relevant mutations and translocations in myeloid sarcoma by next generation sequencing. Leuk Lymphoma. 2018;59(2):501–504. doi: 10.1080/10428194.2017.1339879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma. 2006;47(12):2527–2541. doi: 10.1080/10428190600967196. [DOI] [PubMed] [Google Scholar]
- 26.Byrd JC, Weiss RB, Arthur DC, et al. Extramedullary leukemia adversely affects hematologic complete remission rate and overall survival in patients with t(8;21)(q22;q22): results from Cancer and Leukemia Group B 8461. J Clin Oncol. 1997;15(2):466–475. doi: 10.1200/JCO.1997.15.2.466. [DOI] [PubMed] [Google Scholar]
- 27.Lugthart S, Gröschel S, Beverloo HB, et al. Clinical, molecular, and prognostic significance of WHO type inv(3)(q21q26.2)/t(3;3)(q21;q26.2) and various other 3q abnormalities in acute myeloid leukemia. J Clin Oncol. 2010;28(24):3890–3898. doi: 10.1200/JCO.2010.29.2771. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham I. A basis for updating our approach to resistant acute leukemia. Am J Hematol. 2012;87:251–257. doi: 10.1002/ajh.22256. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham I, Hamele-Bena D, Guo Y, et al. Extramedullary leukemia behaving as solid cancer: clinical, histologic, and genetic clues to chemoresistance in organ sites. Am J Hematol. 2019;94:1200–1207. doi: 10.1002/ajh.25594. [DOI] [PubMed] [Google Scholar]
- 30.Sitges M, Boluda B, Garrido A, et al. Acute myeloid leukemia with inv(3)(q21.3q26.2)/t(3;3)(q21.3;q26.2): Study of 61 patients treated with intensive protocols. Eur J Haematol. 2020;105(2):138–147. doi: 10.1111/ejh.13417. [DOI] [PubMed] [Google Scholar]
- 31.Rogers HJ, Hsi ED. Myeloid Neoplasms with inv(3)(q21q26.2) or t(3;3)(q21;q26.2) Surg Pathol Clin. 2013;6(4):677–692. doi: 10.1016/j.path.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Piccaluga PP, Martinelli G, Rondoni M, et al. Gemtuzumab ozogamicin for relapsed and refractory acute myeloid leukemia and myeloid sarcomas. Leuk Lymphoma. 2004;45(9):1791–1795. doi: 10.1080/1042819042000219485. [DOI] [PubMed] [Google Scholar]
- 33.Zaytsev D, Girshova L, Ivanov V, et al. Rapid efficacy of Gemtuzumab Ozogamicin in refractory AML patients with pulmonary and kidney failure. Biology (Basel) 2020;9(2):28. doi: 10.3390/biology9020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang TC, Chang H, Chuang WY. Complete response of myeloid sarcoma with FIP1L1-PDGFRA-associated myeloproliferative neoplasms to imatinib mesylate monotherapy. Acta Haematol. 2012;128(2):83–87. doi: 10.1159/000338217. [DOI] [PubMed] [Google Scholar]
- 35.Mandelker D, Dal Cin P, Jacene HA, et al. Refractory myeloid sarcoma with a FIP1L1-PDGFRA rearrangement detected by clinical high throughput somatic sequencing. Exp Hematol Oncol. 2015;4:30. doi: 10.1186/s40164-015-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grillo G, Zucchetti E, Forno B, Lotesoriere I, Inzoli E, Cairoli R. Targeted therapy in FLT3-ITD positive myeloid sarcoma: proof of principle. Blood. 2017;130(Supplement 1):5061. [Google Scholar]
- 37.Pollyea DA, Amaya M, Strati P, et al. Venetoclax for AML: changing the treatment paradigm. Blood Adv. 2019;3(24):4326–4335. doi: 10.1182/bloodadvances.2019000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konopleva M, Letai A. BCL-2 inhibition in AML: an unexpected bonus? Blood. 2018;132(10):1007–1012. doi: 10.1182/blood-2018-03-828269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang HQ, Li J. Clinicopathological features of myeloid sarcoma: Report of 39 cases and literature review. Pathol Res Pract. 2016;212(9):817–824. doi: 10.1016/j.prp.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Kahn RM, Gordhandas S, Chapman-Davis E, et al. Acute myeloid leukemia presenting as myeloid sarcoma with a predisposition to the gynecologic tract. Case Rep Oncol Med. 2019;2019:1–5. doi: 10.1155/2019/4189275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernandez J-A, Navarro J-T, Rozman M, et al. Primary myeloid sarcoma of the gynecologic tract: a report of two cases progressing to acute myeloid leukemia. Leuk Lymphoma. 2002;43(11):2151–2153. doi: 10.1080/1042819021000016096. [DOI] [PubMed] [Google Scholar]
- 42.Ucar M, Guryildirim M. Granulocytic sarcoma of the uterus: a rare presentation of extramedullary relapse of AML and importance of MRI. Case Rep Radiol. 2014;2014:1–4. doi: 10.1155/2014/501342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia MG, Deavers MT, Knoblock RJ, et al. Myeloid sarcoma involving the gynecologic tract: a report of 11 cases and review of the literature. Am J Clin Pathol. 2006;125(5):783–790. doi: 10.1309/H9MM-21FP-T7YB-L3PW. [DOI] [PubMed] [Google Scholar]
- 44.Kim SCH, Natarajan-Ame S, Lioure B, et al. Successful treatment of a granulocytic sarcoma of the uterine cervix in complete remission at six-year follow-up. J Oncol. 2010;2010:1–3. doi: 10.1155/2010/812424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill H, Loong F, Mak V, et al. Myeloid sarcoma of the uterine cervix presenting as missed abortion. Arch Gynecol Obstet. 2012;286(5):1339–1341. doi: 10.1007/s00404-012-2454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weingertner AS, Wilt M, Atallah I, et al. Myeloid sarcoma of the uterine cervix as presentation of acute myeloid leukaemia after treatment with low-dose radioiodine for thyroid cancer: a case report and review of the literature. Case Rep Oncol. 2009;2(1):1–6. doi: 10.1159/000191215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y, Qin X, Yan S, et al. Non-leukemic myeloid sarcoma involving the vulva, vagina, and cervix: a case report and literature review. Oncol Targets Ther. 2015;8:3707–3713. doi: 10.2147/OTT.S92815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bao H, Gao J, Chen YH, et al. Rare myeloid sarcoma with KMT2A (MLL)-ELL fusion presenting as a vaginal wall mass. Diagn Pathol. 2019;14(1):26. doi: 10.1186/s13000-019-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data related to this case report are available from the corresponding author on reasonable request.


