Abstract
Venetoclax (VEN) plus azacitidine has become the first-line therapy for elderly patients with acute myeloid leukemia (AML), and has a complete remission (CR) plus CR with incomplete recovery of hemogram rate of ≥70%. However, the 3-year survival rate of these patients is < 40% due to relapse caused by acquired VEN resistance, and this remains the greatest obstacle for the maintenance of long-term remission in VEN-sensitive patients. The underlying mechanism of acquired VEN resistance in AML remains largely unknown. Therefore, in the current study, nine AML patients with acquired VEN resistance were retrospectively analyzed. Our results showed that the known VEN resistance-associated BCL2 mutation was not present in our cohort, indicating that, in contrast to chronic lymphocytic leukemia, this BCL2 mutation is dispensable for acquired VEN resistance in AML. Instead, we found that reconstructed existing mutations, especially dominant mutation conversion (e.g., expanded FLT3-ITD), rather than newly emerged mutations (e.g., TP53 mutation), mainly contributed to VEN resistance in AML. According to our results, the combination of precise mutational monitoring and advanced interventions with targeted therapy or chemotherapy are potential strategies to prevent and even overcome acquired VEN resistance in AML.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40364-021-00288-7.
Keywords: Venetoclax, Acquired resistance, Acute myeloid leukemia
To the Editor
VEN + AZA has become the first-line therapy for elderly patients with AML, and CR + CRi rates of ≥70% have been achieved [1, 2]. Despite this, the 3-year survival rate of patients who receive VEN + AZA is < 40%, mainly due to acquired VEN-R [3]. However, the underlying mechanisms of VEN-R and the status of BCL2Mut in AML, remain largely unknown [4–6].
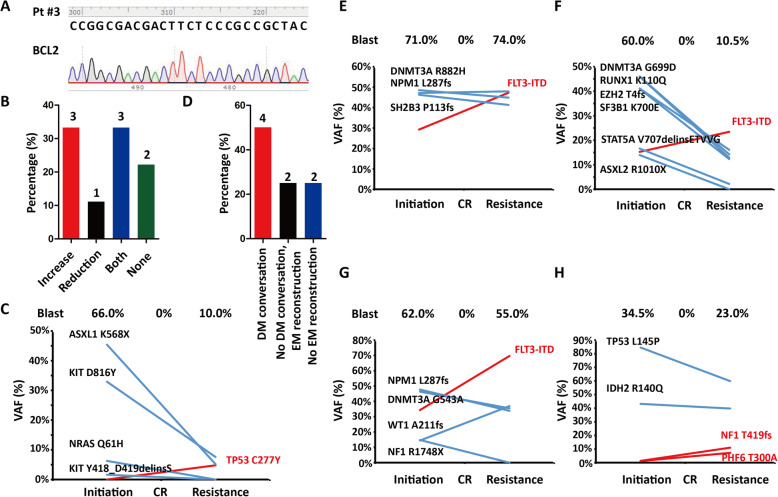
To address this question, we retrospectively analyzed nine elderly AML patients with acquired VEN-R at our center from July 1, 2018 until June 30, 2020 (Table 1). BCL2Mut was detected by PCR combined with Sanger sequencing at VEN-I and VEN-R, but no VEN-R-associated BCL2Mut was identified (Fig. 1a) [6–9]. Due to the relatively low resolution of Sanger sequencing, these samples were then submitted to TES (Novaseq platform, Illumina), in which 236 recurrently mutated genes in hematological malignancies were included. The average raw sequencing depth on target per sample was ≥1000, and a VAF ≥1% was considered significant. As VEN-R-associated BCL2Mut was consistently negative, BCL2Mut was considered dispensable for acquired VEN-R in AML.
Table 1.
Basic characteristics of patients with acquired VEN-R AML in our cohort
| Characteristics | Value |
|---|---|
| Patients (N) | 9 |
| Male/Female (N) | 5/4 |
| Age (year) | 73 (68–78) |
| De novo/Secondary (N) | 8/1 |
| FAB: M0/M1/M4/M5 (N) | 2/1/3/3 |
| Karyotype: normal/abnormal (N) | 4/5 |
| Bone marrow blast at venetoclax initiation (%) | 62 (23–92) |
| Molecular feature at venetoclax initiation (N) | |
| AML1-ETO | 1 |
| NPM1 mutation | 3 |
| FLT3-ITD | 4 |
| DNMT3A mutation | 4 |
| TP53 mutation | 1 |
| ASXL1 mutation | 2 |
| RUNX1 mutation | 2 |
| Bone marrow blast at venetoclax resistance (%) | 10.5 (6–74) |
| Molecular feature at venetoclax resistance (N) | |
| AML1-ETO | 1 |
| NPM1 mutation | 2 |
| FLT3-ITD | 3 |
| DNMT3A mutation | 3 |
| TP53 mutation | 2 |
| ASXL1 mutation | 2 |
| RUNX1 mutation | 1 |
| Cycles from venetoclax initiation to resistance (N) | 3 (3–15) |
Fig. 1.
Mechanism of acquired VEN-R in AML. a No BCL2Mut was found in patients with acquired VEN-R AML. b Changes in the types of mutational genes in our AML cohort according to VEN-R. c The acquired TP53 mutation played a dominant role in the relapse of Pt #8. d Reconstructed existing mutations (EM), especially conversed dominant mutation (DM), were important in acquired VEN-R. e-g Expanded FLT3-ITD-mediated acquired VEN-R in Pt #3 (e), #6 (f), and #7 (g). h The proportion of reconstructed existing mutations in Pt #1
Regarding the difference in the mutational landscape between VEN-I and VEN-R (Supplementary Table 1), the spectrum was skewed in 7/9 patients: 3/7 exhibited a reduction in mutated genes, 1/7 exhibited an increase, and 3/7 showed a reduction in some mutated genes and an increase in others (Fig. 1b). As TP53 mutation has been demonstrated to confer AML VEN-R [10], newly emerged TP53 mutation definitely contributed to VEN-R as shown in Pt #8 (Fig. 1c). However, newly emerged mutations in the remaining three patients had relatively low VAFs compared to the dominant mutations, which indicated that these mutations existed in sub-clones and played a minor role in acquired VEN-R.
We next addressed the proportion of reconstructed existing mutations. Excluding Pt #9 without the molecular relapse, 6/8 patients exhibited reconstructed existing mutations, and 4/8 patients showed dominant mutational conversion (Fig. 1d). FLT3-ITD is the most common mutation in AML [11], but whether it affects VEN sensitivity remains controversial [1]. In Pt #3, #6, and #7, the VAF of FLT3-ITD increased, and it had ranged from a minor mutation at VEN-I to the most common mutation at VEN-R (Fig. 1e–g). Although FLT3-ITD was totally absent from Pt #5, FLT3-ITD still conferred VEN-R for AML in Pt #3, Pt #6, and Pt #7. In Pt #1, IDH2R140Q and TP53L145P mutations were the dominant mutations across the entire treatment course; however, their VAFs decreased, while those of NF1T419fs and PHF6T300A mutations gradually increased with AML progression. These findings indicate that minor mutations can expand and possibly contribute to VEN-R (Fig. 1h).
Although VEN-associated BCL2Mut has been identified in CLL, it was not detected in our AML cohort. There are several possible explanations. First, there was short duration exposure to VEN in AML (AML vs. CLL [months], 5 [3-9] vs. 36[6.5–73]) [12]; second, combination therapy with AZA in AML may have eradicated the emerged BCL2Mut at an early stage; and third, the standard dose of VEN (400 mg/qd) used in AML patients was not reached in 27% of CLL patients. Theoretically, BCL2Mut may have mediated VEN-R in patients with AML as the duration of exposure increased, but in reality, combination therapy at a standard dose made the possibility of emerged BCL2Mut much lower than in CLL. BCL2Mut was still negative in our two cases with ≥1-year exposure duration. In contrast to BCL2Mut, we found that clonal evolution, including newly emerged mutations and reconstructed existing mutations, mainly contributed to VEN-R in AML. For example, newly emerged TP53 mutation or expanded FLT3-ITD could mediate acquired VEN-R in AML, which was also reported by DiNardo [4]. Interestingly, acquired TP53 mutation also mediated VEN-R in CLL independent of BCL2Mut, and it was more common than in AML. Furthermore, reconstructed existing mutations, especially dominant mutation conversion, appear to be more important than newly emerged mutations in acquired VEN-R. More aggressive clinical strategies are required to overcome this mechanism in acquired VEN-R in AML. In our cohort, three patients with AML with expanded FLT3-ITD-mediated acquired VEN-R possibly benefited from dynamic monitoring of FLT3-ITD and early addition of an FLT3 inhibitor to prolong the response to VEN. Therefore, the combination of precise mutational monitoring and advanced interventions with targeted therapy or chemotherapy is key to preventing and overcoming acquired VEN-R in AML.
Supplementary Information
Additional file 1: Table S1. Differences in the mutational landscape between patients with VEN-I and VEN-R AML.
Acknowledgements
We thanked all members of the key laboratory of diagnosis and treatment in hematologic malignancies of Zhejiang and bone marrow morphology laboratory of the First Affiliated Hospital to Zhejiang University College of Medicine for technical support to diagnosis.
Abbreviations
- AML
Acute myeloid leukemia
- AZA
Azacitidine
- BCL2Mut
BCL2 mutation
- CLL
Chronic lymphocytic leukemia
- CR
Complete remission
- CRi
Complete remission with incomplete recovery of hemogram
- PCR
Polymerase chain reaction
- Pt
Patient
- TES
Targeted-exome-sequencing
- VAF
Variant allele frequency
- VEN
Venetoclax
- VEN-I
Venetoclax initiation
- VEN-R
Venetoclax resistance
Authors’ contributions
H.-H. Z. and X. Z. designed this study. J.-J. Q., H.-F. W., and Y. Z. collected the clinical materials. Y.-G. W. collected the samples for further sequencing. X. Z. displayed the PCR experiment. X. Z. integrated the data and wrote the manuscript. P.-X. Q., Y.-J. L., and J. J. provided advices for this work. H.-H. Z. revised this manuscript. All authors approved the manuscript. X. Z., J.-J. Q., H.-F. W., and Y.-G. W. were considered contributing equally to this work.
Funding
This study was funded by the National Natural Science Foundation of China (81800199), and the Natural Science Foundation of Zhejiang Province (LY21H080003).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study was approved by the ethical review committees of the First Affiliated Hospital to Zhejiang University College of Medicine.
Consent for publication
Written informed consent was obtained from this patient.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiang Zhang, Jiejing Qian, Huafeng Wang and Yungui Wang contributed equally to this work.
References
- 1.DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, Dohner H, Letai A, Fenaux P, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 3.Pollyea DA, Pratz K, Letai A, Jonas BA, Wei AH, Pullarkat V, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol. 2021;96(2):208–17. [DOI] [PubMed]
- 4.DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, Thijssen R, Pomilio G, Ivey A, Salmon JM, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791–803. doi: 10.1182/blood.2019003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tausch E, Close W, Dolnik A, Bloehdorn J, Chyla B, Bullinger L, Dohner H, Mertens D, Stilgenbauer S. Venetoclax resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica. 2019;104(9):e434–e437. doi: 10.3324/haematol.2019.222588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blombery P, Thompson ER, Nguyen T, Birkinshaw RW, Gong JN, Chen X, McBean M, Thijssen R, Conway T, Anderson MA, Seymour JF, Westerman DA, Czabotar PE, Huang DCS, Roberts AW. Multiple BCL2 mutations cooccurring with Gly101Val emerge in chronic lymphocytic leukemia progression on venetoclax. Blood. 2020;135(10):773–777. doi: 10.1182/blood.2019004205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blombery P, Anderson MA, Gong JN, Thijssen R, Birkinshaw RW, Thompson ER, Teh CE, Nguyen T, Xu Z, Flensburg C, Lew TE, Majewski IJ, Gray DHD, Westerman DA, Tam CS, Seymour JF, Czabotar PE, Huang DCS, Roberts AW. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2019;9(3):342–353. doi: 10.1158/2159-8290.CD-18-1119. [DOI] [PubMed] [Google Scholar]
- 8.Blombery P, Birkinshaw RW, Nguyen T, Gong JN, Thompson ER, Xu Z, Westerman DA, Czabotar PE, Dickinson M, Huang DCS, Seymour JF, Roberts AW. Characterization of a novel venetoclax resistance mutation (BCL2 Phe104Ile) observed in follicular lymphoma. Br J Haematol. 2019;186(6):e188–e191. doi: 10.1111/bjh.16069. [DOI] [PubMed] [Google Scholar]
- 9.Birkinshaw RW, Gong JN, Luo CS, Lio D, White CA, Anderson MA, Blombery P, Lessene G, Majewski IJ, Thijssen R, Roberts AW, Huang DCS, Colman PM, Czabotar PE. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat Commun. 2019;10(1):2385. doi: 10.1038/s41467-019-10363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nechiporuk T, Kurtz SE, Nikolova O, Liu T, Jones CL, D'Alessandro A, Culp-Hill R, d'Almeida A, Joshi SK, Rosenberg M, Tognon CE, Danilov AV, Druker BJ, Chang BH, McWeeney SK, Tyner JW. The TP53 apoptotic network is a primary mediator of resistance to BCL2 inhibition in AML cells. Cancer Discov. 2019;9(7):910–925. doi: 10.1158/2159-8290.CD-19-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research N, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, Hoadley K, Triche TJ, Jr, Laird PW, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coutre S, Choi M, Furman RR, Eradat H, Heffner L, Jones JA, Chyla B, Zhou L, Agarwal S, Waskiewicz T, Verdugo M, Humerickhouse RA, Potluri J, Wierda WG, Davids MS. Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood. 2018;131(15):1704–1711. doi: 10.1182/blood-2017-06-788133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Differences in the mutational landscape between patients with VEN-I and VEN-R AML.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.