Abstract
An accelerating basic science literature is providing key insights into the mechanisms by which spinal neuropeptide Y (NPY) inhibits chronic pain. A key target of pain inhibition is the Gi-coupled neuropeptide Y1 receptor (Y1). Y1 is located in key sites of pain transmission, including the peptidergic subpopulation of primary afferent neurons and a dense subpopulation of small, excitatory, glutamatergic/somatostatinergic interneurons (Y1-INs) that are densely expressed in the dorsal horn, particularly in superficial lamina I-II. Selective ablation of spinal Y1-INs with an NPY-conjugated saporin neurotoxin attenuates the development of peripheral nerve injury-induced mechanical and cold hypersensitivity. Conversely, conditional knockdown of NPY expression or intrathecal administration of Y1 antagonists reinstates hypersensitivity in models of chronic latent pain sensitization. These and other results indicate that spinal NPY release and the consequent inhibition of pain facilitatory Y1-INs represent an important mechanism of endogenous analgesia. This mechanism can be mimicked with exogenous pharmacological approaches (e.g. intrathecal administration of Y1 agonists) to inhibit mechanical and thermal hypersensitivity and spinal neuron activity in rodent models of neuropathic, inflammatory, and postoperative pain. Pharmacological activation of Y1 also inhibits mechanical- and histamine-induced itch. These immunohistochemical, pharmacological, and cell type-directed lesioning data, in combination with recent transcriptomic findings, point to Y1-INs as a promising therapeutic target for the development of spinally directed NPY-Y1 agonists to treat both chronic pain and itch.
Keywords: Neuropeptide Y, NPY, Npy1r, Y1, neuropeptides, pain, neuropathic pain, inflammatory pain, dorsal horn, mechanical itch, chemical itch, neurotransmitters, spinal cord
Neuropeptide Y (NPY) is a 36 amino acid peptide first described in 1982 (Tatemoto et al., 1982). It is highly expressed throughout the body and regulates a wide variety of physiological processes that include food intake, emotional regulation, and cardiovascular function (Brothers and Wahlestedt, 2010). Most species express at least 4 of the different receptor subtypes that bind NPY: Y1, Y2, Y4, and Y5. All NPY receptor subtypes are Gi protein-coupled receptors, so NPY agonists decrease the production of cyclic adenosine monophosphate (cAMP) thus dampening intracellular signaling (Brothers and Wahlestedt, 2010; Brumovsky et al., 2007).
A growing body of preclinical evidence implicates spinal NPY in the modulation of chronic pain and itch. NPY and its cognate Y1 and Y2 receptors are located in the spinal cord across a wide variety of species, including humans (Allen et al., 1984; Gibson et al., 1984). In vitro quantitative receptor autoradiography demonstrates the highest density of NPY binding sites in superficial laminae I-II of the dorsal horn (Kar and Quirion, 1992), a key relay of noxious sensations from the periphery to the brain. In the following review, we emphasize recent transcriptomic, immunohistochemical, pharmacological, and directed lesioning data that implicates an excitatory Y1-IN population in the superficial dorsal horn that drives spinal pain transmission. We also discuss emerging evidence that supports an important contribution of NPY interneurons and Y1-INs to the gating of mechanical and chemical itch.
1. Y1 and Y1-IN anatomy and physiology in the dorsal horn of the spinal cord:
1.1. Expression of NPY receptors in the dorsal root ganglion
Y1 receptor expression is found in the somatic plasmalemma of small, unmyelinated, calcitonin gene-related peptide (CGRP)-expressing, peptidergic neurons in the dorsal root ganglion (DRG) (Taylor et al., 2014; Zhang et al., 1994). Y1 and CGRP colocalize extensively in the DRG soma, and it is becoming increasingly apparent that Y1 is trafficked to central terminals in the dorsal horn only to a limited degree. First, CGRP-positive primary afferent terminals rarely (if-ever) co-express Y1 receptor-like immunoreactivity in the substantia gelatinosa (Taylor et al., 2014; Brumovsky et al., 2002; Zhang et al., 1994); this could be due to extremely sparse expression or limitations in currently available detection tools. Second, despite the clear finding that sciatic nerve ligation robustly downregulates Y1 receptor expression in the DRG and slightly alters Y1 expression in the dorsal horn (proposed to be an effect on Y1-INs and not central terminals) (Brumovsky et al., 2004), other forms of peripheral nerve injury, including dorsal rhizotomy, sciatic nerve transection, or spared nerve injury, cause little to no change in Y1 immunoreactivity in the superficial dorsal horn (Nelson et al., 2019; Nazli and Morris, 2000; Zhang et al., 1994). Thus, it seems that Y1 expression is robust in the soma of CGRP-expressing DRG neurons but minimal at their central terminals. This is consistent with the idea that NPY acts at Y2 rather than Y1 receptors on the central terminals of primary afferents to inhibit SP release (Duggan et al., 1991) and electrical stimulus-evoked EPSCs (Moran et al., 2004).
Y2 receptor-like immunoreactivity is found in approximately 10% of rat lumbar DRG neurons, most of which are small-to-medium sized, peptidergic, thinly myelinated, putative A-nociceptors (Brumovsky et al., 2005). In support of this characterization, an Npy2rChR2 mouse line found the majority (75%) of ChR2 expression in lumbar DRG neurons that co-express neurofilament- heavy polypeptide (Nefh), a marker of myelinated neurons, CGRP, and the nerve growth factor receptor TRKA, a peptidergic nociceptor marker, altogether indicating that Y2 neurons are a population of A-fibers that mediate pain (Arcourt et al., 2017). However, an earlier characterization of an Npy2reGFP mouse line found eGFP-positive afferents that formed lanceolate endings around hair follicles: this is characteristic of rapidly adapting, low-threshold, Aß-mechanoreceptors that mediate light touch somatosensation (Li et al., 2011). This discrepancy is now partially resolved with a comprehensive analysis of Npy2r-expressing DRG neurons from three separate single-cell RNA sequencing gene expression datasets. These datasets demonstrate that Npy2r is expressed in 90% of Nppb/Sst neurons (implicated in the transduction of histaminergic itch), 38% of all A-nociceptors, and 17% of all C-nociceptors; less than 10% Npy2r expression is detected in other cell types including Aß-mechanoreceptors (Ma et al., 2020). Together, these results indicate that Y2 receptors are most heavily expressed in small, peptidergic, thinly myelinated, A-fiber nociceptors that contribute to pain (Chen et al., 2019) and unmyelinated Nppb/Sst afferents implicated in itch (Ma et al., 2020; Huang et al., 2018).
1.2. Localization of Y1-INs and NPY receptors in the spinal cord
To date, at least seven distinct populations of Y1-INs have been identified in the rat lumbar spinal cord (for a more detailed review see (Brumovsky et al., 2006, 2007)), the most abundant of which are referred to as Type 1 Y1-INs (Brumovsky et al., 2006). Type 1 Y1-INs are densely packed within the superficial dorsal horn, particularly in outer lamina II (Hökfelt et al., 2007; Brumovsky et al., 2006). In rat, Y1-INs do not colocalize with the PKCγ interneurons that demarcate the lamina II-III border but instead lie in a tight band just dorsal to them (Nelson et al., 2019). Brumovsky and Hökfelt’s comprehensive analysis of Y1 immunolabeling in the dorsal horn of the rat awaits replication in the mouse.
The vast majority of Y1-INs in outer lamina II are small, fusiform-like shaped, bipolar cells with dendrites extending in the rostral-caudal but not dorsal-ventral axes (Brumovsky et al., 2006). Of the five morphological classes of lamina II interneurons described by Grudt and Perl (Grudt and Perl, 2002), we note that Y1-IN morphology closely matches the “central cell” class. Central cells are restricted to lamina II with a fairly dense dendritic arbor that extends moderately in the rostral-caudal direction and limited dorsal-ventral branching. Additionally, a central cell (and fusiform)-like morphology was noted for ~2/3 of identified lamina II Y1-Cre lineage neurons in the mouse (Acton et al., 2019). Finally, electrophysiological recordings found the majority of neurons that responded to NPY application exhibited a morphology characteristic of central or radial cells (~77%) and only rarely exhibited the morphological characteristics of vertical cells (~8%) (Melnick, 2012). Taken together, these data indicate that most Y1-INs in lamina II of the dorsal horn demonstrate a central cell-like morphology.
Y2 receptors are rarely detected in spinal cord interneurons, as: 1) Y2 receptor-like immunoreactivity in the dorsal horn is restricted to central terminals and never colocalizes with a somatic marker (Brumovsky et al., 2005); 2) dorsal rhizotomy completely eliminates the expression of Y2 receptor immunoreactivity in the dorsal horn (Brumovsky et al., 2005); 3) Npy2reGFP BAC transgenic mice express minimal to no adult GFP expression in the dorsal horn (GENSAT, Stock No: 011016-UCD); and 4) a single cell RNAseq analysis of the dorsal horn reported only a very sparse (and possibly insignificant or nonexistent) expression of Npy2r in the GABA10 subpopulation of GABAergic interneurons (Häring et al., 2018). This extremely sparse expression of Y2 in dorsal horn neurons is in stark contrast to the robust expression of Y1 on Y1-INs.
1.3. Neurophysiological responses of superficial dorsal horn neurons to NPY receptor agonists
Whole cell recordings in the rat spinal cord slice consistently indicate that bath application of NPY inhibits both presynaptic and postsynaptic components of excitatory neurotransmission in the dorsal horn (Table 1). Interrogation of presynaptic mechanisms with voltage-clamp recordings revealed that Y2- but not Y1-selective agonists inhibit the frequency but not amplitude of TTX-resistant miniature excitatory postsynaptic currents (mEPSCs) (Moran et al., 2004); likewise, Y2 but not Y1-selective antagonists blocked the ability of NPY itself to inhibit mEPSC frequency (Melnick, 2012; Moran et al., 2004). Also indicative of a presynaptic action was the ability of NPY to change paired-pulse ratio (Moran et al., 2004). However, further studies are needed in molecularly and/or morphologically identified neurons, as one study could not confirm presynaptic effects of NPY on mEPSCs, possibly due to the tremendous heterogeneity of unclassified substantia gelatinosa neurons (Miyakawa et al., 2005). Further studies are needed to profile subsets of Y1-INs based on molecular, morphological, and/or electrophysiological characteristics. One exciting approach uses transgenic reporter mice to better elucidate intrinsic and evoked firing patterns and perhaps reveal functionally distinct subclasses of Y1-INs. Such an approach has been used to study calretinin-, substance P, and GRP -expressing interneuron populations in the dorsal horn, for example (Dickie et al., 2019; Smith et al., 2015). Albeit, the current consensus is that Y2 inhibition of presynaptic boutons reduces the release of pronociceptive neurotransmitters (Smith et al., 2007). This is most likely from the central terminals of primary afferent neurons, as Y2 expression has not been found on local interneurons or the terminals of supraspinal neurons (see 1.2).
Table 1.
Chronological studies of NPY receptor interventions on spinal cord physiology, pain, and itch
| Model | Species | Sex | NPY Receptor Intervention | Outcome Measure | Key Results | Reference |
|---|---|---|---|---|---|---|
| Naïve | Rat | Male | Intrathecal (i.t.) NPY | Mean arterial blood pressure (MAP) and heart rate (HR) | NPY dose-dependently decreased MAP and HR in normotensive and hypotensive rats. | Chen et al., 1988 |
| Naïve | Rat | Male | i.t. NPY | MAP and HR, multiunit activity recordings from exposed renal sympathetic nerve | NPY dose-dependently decreased MAP, slightly decreased HR, and decreased renal sympathetic nerve activity. | Chen et al., 1990 |
| Naïve | Cat | Not Stated (NS) | Microinjection of NPY into the dorsal horn (DH) | Evoked release of immunoreactive substance P upon electrical stimulation of unmyelinated primary afferents of the tibial nerve | NPY reduced the evoked release of immunoreactive substance P. | Duggan et al., 1991 |
| Naïve | Rat | Male | i.t. NPY | Heat: hotplate (HP) withdrawal (w/d) latency, Mechanical: pressure threshold | NPY dose-dependently increased the HP w/d latency but had no effect on mechanical pressure. | Hua et al., 1991 |
| Naïve | Rat | Male | i.t. NPY, Y2 agonistic fragments, or Y1 agonist | MAP | NPY and Y2 agonistic fragments but not Y1 agonist dose-dependently decreased MAP. | Chen and Westfall, 1993 |
| Neuropathic Pain: sciatic nerve transection | Rat | Female | i.t. NPY | Flexor reflex | NPY dose-dependently depressed flexor reflex in naïve and injured rats but the antinociceptive effect was stronger and longer lasting after nerve transection. | Xu et al., 1994 |
| Neuropathic Pain: partial sciatic nerve ligation | Rat | NS | i.t. NPY, Y1 or Y2 agonists, or a nonselective NPY antagonist | Mechanical: pressure threshold | NPY or Y1 agonist but not Y2 agonist increased nerve injury-induced mechanical hypersensitivity. The nonselective NPY antagonist attenuated the nerve injury-induced hyperalgesia. | White, 1997 |
| Naïve | Rat and Cat | Rat: Males; Cat: NS | None | DH release of immunoreactive NPY | Neither electrical stimulation of peripheral nerves nor noxious mechanical or heat stimulation changed the extensive spontaneous NPY release observed in the DH. | Mark et al., 1997 |
| Neuropathic Pain: chronic constriction of the sciatic nerve | Rat | Male | None | DH release of immunoreactive NPY | CCI extended the zone of spontaneous ipsilateral release of NPY into the deep DH. Electrical stimulation of the injured nerves produced widespread NPY release. | Mark et al., 1998 |
| Inflammatory Pain: hindpaw injection of carrageenan | Rat | Male and Female | i.t. NPY | Flexor reflex | NPY dose-dependently produced brief reflex facilitation at low doses and long-lasting depression at high doses in naïve and injured rats. The reflex facilitation was greater in carrageenan injected animals. | Xu et al., 1998 |
| Neuropathic Pain: sciatic nerve transection | Rat | Female | i.t. Y1 or Y2 agonist | Flexor reflex | Y1 agonist dose-dependently produced reflex depression in naïve and injured rats. Y2 agonist did not produce reflex depression in naïve rats but dose-dependently produced reflex depression in sciatic nerve transected rats. | Xu et al., 1999 |
| Neuropathic Pain: chronic constriction of the sciatic nerve | Rat | Male | None | DH release of immunoreactive NPY | Total conduction block of the injured nerve did not change spontaneous NPY release in the ipsilateral DH. | Colvin and Duggan, 2001 |
| Inflammatory Pain: hindpaw injection of formalin, intraperitoneal injection of acetic acid or MgSO4; Neuropathic Pain: partial sciatic nerve ligation | Mouse | Male | Global knockout of Npy1r; i.t. NPY | Heat: HP w/d latency, tail flick latency; Mechanical: von Frey (vF) w/d threshold; Inflammatory Pain: number of events (lifting, shaking, licking and biting of the paw), number of abdominal stretches | Npy1r knockout increased sensitivity to heat and mechanical stimuli, hyperalgesia in the Phase I but not Phase II response to formalin, visceral hyperalgesia, and peripheral nerve injury-induced mechanical hypersensitivity. Npy1r knockout abolished the antinociceptive effect of NPY on heat hypersensitivity. | Naveilhan et al., 2001 |
| Inflammatory Pain: hindpaw injection of carrageenan or CFA | Rat | Male | i.t. NPY or Y1 or Y2 antagonists | Heat: HP w/d latency, radiant heat w/d latency; Motor Coordination: latency to fall on an accelerating rotarod | NPY dose-dependently increased response latency to heat stimuli, inhibited carrageenan- and CFA-induced heat hypersensitivity, and did not alter motor coordination. The antinociceptive effects were abolished by a Y1 but not Y2 antagonist. A Y1 antagonist enhanced CFA-induced heat hypersensitivity. | Taiwo and Taylor, 2002 |
| Naïve | Rat | NS | NPY or Y1 agonist on spinal cord (SC) slices | Electrophysiology: blind whole-cell patch-clamp recordings in voltage clamp, recording eEPSCs and eIPSCs, recording mEPSCs and mIPSCs, paired-pulse stimulation | NPY acts via Y1 to suppress inhibitory transmission by pre- and postsynaptic mechanisms. NPY acts via Y2 to suppress excitatory transmission exclusively by a presynaptic mechanism. NPY activates an inwardly rectifying K+ conductance in DH neurons. | Moran et al., 2004 |
| Inflammatory Pain: hindpaw injection of formalin | Rat | Male | i.t. NPY or Y1 or Y2 antagonists | Inflammatory Pain: licking duration and the number of flinch events in the formalin assay; MAP and HR | NPY dose-dependently inhibited formalin-induced nocifensive responses that were partially blocked with a Y1 antagonist. NPY dose-dependently increased MAP that was prevented with a Y2 but not Y1 antagonist. | Mahinda et al., 2004 |
| Naive | Rat | Male | NPY, Y1 agonist, or Y1 antagonist on SC slices | Capsaicin-evoked immunoreactive CGRP release from lumbar DH | NPY or Y1 agonist reduced CGRP exocytosis from central terminals of capsaicin sensitive afferent fibers and was partially blocked by a Y1 antagonist. | Gibbs et al., 2004 |
| Naïve | Rat | Male | NPY, Y1 agonist, or Y1 antagonist on SC slices | Electrophysiology: whole-cell patch-clamp recordings in voltage clamp, recording mEPSCs and mIPSCs, recording dorsal root A- or C- fiber-evoked EPSCs | NPY or Y1 agonist induced a potassium-dependent outward current, membrane hyperpolarization, and a suppression of dorsal root stimulation-evoked action potentials. NPY-induced responses were blocked by a Y1 antagonist. | Miyakawa et al., 2005 |
| Naïve | Mouse | Male | Global knockout of Npy1r | Mechanical: vF w/d threshold | Npy1r knockout mice demonstrated profound mechanical hypersensitivity. | Shi et al., 2006 |
| Inflammatory Pain: hindpaw injection of CFA; Neuropathic Pain: partial sciatic nerve ligation | Mouse | Male | Global knockout of Npy1r; i.t. NPY or Y1 antagonist | Heat: HP w/d latency, radiant heat w/d latency; Mechanical: vF w/d threshold; Motor Coordination: latency to fall on an accelerating rotarod | Npy1r knockout produced heat hypersensitivity and longer lasting CFA-induced heat hyperalgesia. Npy1r knockout or a Y1 antagonist prevented the antinociceptive effect of NPY after CFA or partial sciatic nerve ligation. NPY and Y1 antagonist did not alter motor performance. | Kuphal et al., 2008 |
| Inflammatory Pain: hindpaw injection of formalin; Neuropathic Pain: spared nerve injury (SNI, spared sural nerve) | Rat | Male | i.t. NPY or Y1 or Y2 antagonists | Inflammatory Pain: licking duration and the number of flinch events; Cold: acetone w/d duration; Mechanical: vF w/d threshold, tactile stimulus-evoked Fos expression in superficial DH | NPY reduced formalin-induced behaviors, dose-dependently reduced SNI-induced mechanical and cold hypersensitivities, and reduced both formalin- and SNI-induced tactile stimulus-evoked Fos expression. SNI-induced mechanical hypersensitivity and Fos expression were blocked by a Y1 or Y2 antagonist. | Intondi et al., 2008 |
| Inflammatory Pain: hindpaw injection of formalin | Rat | Male and Female | i.t. NPY-saporin to selectively ablate Y1-INs | Heat: HP w/d latency and time licking and guarding hindpaws; Inflammatory Pain: time licking, lifting and biting, and number and time course of responses | NPY-saporin reduced SC but not DRG Y1 immunoreactivity. NPY-saporin elevated reflex w/d thresholds and reduced time spent licking/guarding paws to only low (44°C) heat. NPY-saporin reduced formalin-induced nocifensive behaviors. | Wiley et al., 2009 |
| Inflammatory Pain: hindpaw injection of CFA, latent sensitization after CFA; Neuropathic Pain: SNI, CpxSx (spared tibial nerve), latent sensitization after CpxSx | Mouse | Male | Conditional doxycycline-induced global knockdown of NPY, or intrathecal Y1 or Y2 antagonists | Heat: HP w/d latency, radiant heat w/d latency; Cold: acetone w/d duration; Mechanical: vF w/d threshold; Motor Coordination: latency to fall on an accelerating rotarod; Ambulatory activity: exploration in an open field | NPY knockdown did not alter baseline sensory thresholds, motor coordination, or ambulatory activity but increased the intensity and duration of SNI-induced mechanical and cold hypersensitivity. Following the resolution of CFA- and CpxSx-induced behavioral hypersensitivities, NPY knockdown or Y1 or Y2 antagonists reinstated behavioral hypersensitivities. Y2 antagonist increased Fos and pERK in the DH 14 days after CFA. | Solway et al., 2011 |
| Inflammatory Pain: hindpaw injection of CFA | Rat | Female | i.t. NPY-saporin to selectively ablate Y1-INs | Cold: 10°C cold plate (CP), thermal preference assay (two chamber 15°C vs. 45°C); Affective: feeding interference (overcome a 10°C floor plate to consume a sweet solution), and an escape task (climb onto a shelf to avoid a 10°C floor plate) | NPY-saporin reduced cold aversion on thermal preference and escape tasks, was analgesic to noxious heat on the escape task, and reduced CFA-induced hypersensitivity to cold temperatures experienced on the CP, thermal preference, feeding interference, and escape tasks. | Lemons and Wiley, 2012 |
| Naïve | Rat | NS | NPY, Y1 or Y2 antagonists, or Y1 or Y2 agonists on SC slices | Electrophysiology: blind whole-cell patch-clamp recordings in voltage clamp, recording mEPSCs and mIPSCs; Morphological Categorization: post hoc | NPY or Y1 agonist induced a hyperpolarizing potassium conductance and outward current in primarily central- or radial-like neurons that was abolished by a Y1 but not Y2 antagonist. Y2 agonist had no effect on DH neurons. NPY moderately reduced the frequency of both mEPSCs and mIPSCs via Y1 and Y2. | Melnick, 2012 |
| Inflammatory Pain: hindpaw plantar incision | Rat | Male | i.t. NPY, or Y1 or Y2 antagonists | Hindpaw guarding behavior; Mechanical: vF w/d threshold; Heat: radiant heat w/d latency | NPY reduced incision-induced guarding behavior and heat and mechanical hypersensitivity. The antinociceptive NPY effects were abolished with a Y1 but not Y2 antagonist. | Yalamuri et al., 2013 |
| Inflammatory Pain: hindpaw injection of carrageenan or CFA | Rat and Mouse | Male | Superfusion of slices with Y1 agonist; i.t. NPY or Y1 agonist | NK1R internalization; Y1 agonist stimulated [35S]GTPγS binding; In vivo microdialysis of DH substance P release; Mechanical: vF w/d threshold, pin prick w/d duration; Cold: acetone w/d duration; Heat: radiant heat w/d latency | Y1 agonist decreased dorsal root stimulation-evoked NK1R internalization. CFA increased the affinity of coupling between Y1 and activated G-proteins, but its efficacy was reduced by roughly half. NPY reduced capsaicin-evoked substance P release in vivo. NPY or Y1 agonist inhibited CFA- and carrageenan-induced NK1R internalization and behavioral nociception. | Taylor et al., 2014 |
| Inflammatory Pain: hindpaw injection of CFA; Mechanical Itch: touch-evoked itch test; Chemical Itch: intradermal pruritogens | Mouse | NS | Ablation or chemogenetic inhibition of SC NPY-Cre lineage interneurons | Mechanical: vF w/d threshold, responsiveness to light brush, pressure; Cold: acetone w/d duration, CP w/d latency; Heat: HP w/d latency, radiant heat w/d latency; Motor Coordination: latency to fall on accelerating rotarod; Mechanical Itch: alloknesis score from vF stimulation at nape; Chemical Itch: number of scratching and wiping behaviors and bouts | Ablation or inhibition of SC NPY-Cre lineage neurons induced spontaneous scratching behaviors and skin lesions, increased mechanical but not chemical itch, but did not alter cold, heat, or mechanical sensitivity, motor coordination, or CFA-induced mechanical hypersensitivity. | Bourane et al., 2015 |
| Neuropathic Pain: chronic constriction injury (CCI) | Rat | Male | i.t. Y1 agonist | Mechanical: vF w/d threshold; Cold: acetone w/d duration | Y1 agonist dose-dependently reduced behavioral signs of CCI-induced mechanical and cold hypersensitivity. | Malet et al., 2017 |
| Mechanical Itch: touch-evoked itch test; Chemical Itch: intradermal pruritogens | Mouse | NS | i.t. NPY, Y1 agonist, or Y1 antagonist | Mechanical Itch: scratch and shake episode frequency and duration from vF stimulation at nape; Chemical Itch: scratch and shake episode frequency and duration | Y1 agonist reduced shake episode frequency and duration induced by nape stimulation. NPY and Y1 agonist reduced the duration of compound 48/80- and histamine- but not chloroquine-induced scratching. | Gao et al., 2018 |
| Bone Cancer Pain: intra-tibial infusion of MRMT1 - Luc2 cancer cells | Rat | Male and Female | i.t. NPY, or Y1 or Y2 antagonists | Limb use test and limb weight-bearing assay | NPY restored limb function and weight bearing and these antihyperalgesic effects were blocked by Y1 or Y2 antagonists. | Diaz-delCastillo et al., 2018 |
| Inflammatory Pain: hindpaw plantar incision | Rat | Male | i.t. NPY | Mechanical: vF w/d threshold; hindpaw guarding behavior; Heat: radiant heat w/d latency | NPY reduced incision-induced hindpaw guarding and thermal hyperalgesia. | Gupta et al., 2018 |
| Spontaneous Itch: non-evoked acute scratching behavior | Mouse | Male | i.t. Y1 agonist or Y1 or Y2 antagonists | Mechanical: vF w/d threshold; Heat: radiant heat w/d latency; Motor Coordination: latency to fall on an accelerating rotarod; Spontaneous Itch: number of scratching bouts | Y2 antagonist induced spontaneous pain-related scratching and mechanical but not thermal hypersensitivity. Y1 antagonist did not induce spontaneous scratching or mechanical or thermal hypersensitivity and alleviated Y2 antagonist-induced pain behaviors. Y2 antagonist but not Y1 agonist reduced motor coordination at high doses. | Chen et al., 2019 |
| Inflammatory Pain: hindpaw injection of formalin or capsaicin; Spontaneous Itch: non-evoked acute scratching behavior; Mechanical Itch: touch-evoked itch test; Chemical Itch: intradermal pruritogens | Mouse | Male and Female | SC and hindbrain Npy1r knockout; Ablation of SC NPY-Cre lineage neurons; Ablation or chemogenetic activation and inhibition of SC Y1-Cre lineage interneurons | Mechanical: vF w/d threshold, responsiveness to light brush, pressure threshold, pin prick w/d duration; Heat: HP w/d latency, radiant heat w/d latency; Inflammatory Pain: time spent licking, flinching, and biting; Spontaneous Itch: number of spontaneous scratching bouts; Mechanical Itch: alloknesis score from vF stimulation at nape; Chemical Itch: scratching bouts, duration, and rate | Y1-Cre lineage neuron ablation prevented NPY-Cre lineage neuron ablation-induced itch, enhanced mechanical but not chemical itch, and reduced vF thresholds without affecting other mechanical, heat, or inflammatory pain behavioral responses. Activation of Y1-Cre lineage neurons reduced vF thresholds and increased mechanical and spontaneous itch. SC Npy1r knockout reduced vF thresholds but had no effect on other mechanical, heat, acute pain, or chemical pruritogen-induced behavioral responses. | Acton et al., 2019 |
| Neuropathic Pain: SNI | Rat | Male | Y1 internalization in SC slices as an in situ measure of NPY release | Y1 internalization was quantified by visually counting Y1-INs in laminae I-II and classifying them as either with or without internalization; Mechanical: vF w/d threshold, and non-noxious- or noxious-evoked Y1 internalization. | NPY, capsaicin, NMDA, and high K+ induced Y1 internalization in DH neurons. Electrical stimulation of the DH frequency-dependently induced NPY release and was decreased by a Y1 antagonist. Dorsal root immersion in capsaicin induced NPY release and it was blocked by CNQX. Nerve injury increased Y1 internalization induced by DH stimulation and in vivo internalization induced by noxious and non-noxious stimuli. | Marvizon et al., 2019 |
| Inflammatory Pain: Latent sensitization after intraplantar CFA | Mice | Male | i.t. Y1 antagonist | Mechanical: vF w/d threshold | Y1 antagonist reinstated mechanical hypersensitivity following the resolution of CFA-induced inflammation and this was prevented in AC1 knockout mice or with administration of a pharmacological blocker to the N-methyl-D-aspartate receptor (NMDAR), adenylyl cyclase type 1 (AC1), protein kinase A (PKA), transient receptor potential cation channel A1 (TRPA1), channel V1 (TRPV1), or exchange protein activated by cAMP (Epac1 or Epac2). | Fu et al., 2019 |
| Neuropathic Pain: SNI | Rat | Male | i.t. NPY, or NPY-saporin | Mechanical: vF w/d threshold, pressure threshold, pin prick w/d duration, tactile stimulus-evoked Fos expression in superficial DH; Cold: acetone w/d duration; Heat: HP w/d latency, radiant heat w/d latency; Motor Coordination: latency to fall on an accelerating rotarod; Ambulatory Activity: exploration in an open field | NPY-saporin dose-dependently attenuated the development of SNI-induced mechanical and cold hypersensitivity, but did not change normal mechanical or thermal thresholds, motor coordination, or locomotor activity. NPY reduced neuropathic mechanical hypersensitivity and light touch-evoked c-Fos expression in DH Y1-INs. | Nelson et al., 2019 |
| Neuropathic Pain: Latent sensitization after CpxSx | Mouse | Male | Doxycycline-induced global knockdown of NPY, or intrathecal Y1 antagonist | Mechanical: vF w/d threshold; Cold: acetone w/d duration; Affective Pain: 3-chamber conditioned place preference and avoidance assay | Y1 antagonist or NPY knockdown induced conditioned place aversion (CPA). Y1 antagonist reinstated mechanical and cold hypersensitivity following the resolution of CpxSx-induced hypersensitivity and reinstatement and CPA were prevented in AC1 knockout mice or with an NMDA receptor antagonist, AC1 inhibitor, or TRPV1 or TRPA1 channel blockers. | Fu et al., 2020 |
| Mechanical Itch: touch-evoked itch test; Chemical Itch: intradermal pruritogens; Atopic Dermatitis-like Itch: application of dust mite extract | Mouse | Male and Female | i.t. Y2 agonist or Y2 antagonist | Mechanical Itch: scratch and shake episode frequency and duration from vF stimulation at nape; Chemical Itch: scratch and shake episode frequency and duration; Atopic Dermatitis- like Itch: scratch and shake episode frequency and duration | Y2 agonist reduced the frequency and duration of compound 48/80-induced scratching and the duration of IL-31- and histamine-induced scratching. Y2 agonist did not reduce scratching induced by SLIGRL, chloroquine, topical dust mite extract, or mechanical itch induced by vF at nape. | Ma et al., 2020 |
| Inflammatory Pain: intra-articular injection of dilute formalin | Rat | Male | i.t. Y1 agonist, Y1 antagonist, or NPY-saporin | Mechanical: Paw Elevation Time (PET)-rats are placed on a revolving cylinder for 1min and scored for the total time that the hindpaw is not in contact with the cylinder surface | NPY-saporin and Y1 agonist reduced formalin-induced PET and conversely Y1 antagonist increased the PET. | Souza-Silva et al., 2020 |
In stark contrast to the view that Y2 mediates the ability of NPY to presynaptically inhibit central terminals, the prevailing view is that Y1 mediates the ability of NPY to postsynaptically inhibit spinal interneurons. Bath administration of NPY or the Y1 receptor-specific agonist [Leu31,Pro34]-NPY consistently produced an outward current and membrane hyperpolarization in spinal interneurons that can be blocked with Y1 but not Y2 antagonists (Melnick, 2012; Miyakawa et al., 2005). The NPY-induced outward current was blocked with either barium ions or cesium ions + TEA, consistent with activation of K+ channels (Melnick, 2012; Miyakawa et al., 2005). The NPY current was also blocked with GDP-β-S (Melnick, 2012; Miyakawa et al., 2005), and current-voltage curves revealed that NPY generated inward rectification in some neurons (Miyakawa et al., 2005; Moran et al., 2004), suggesting an NPY-mediated activation of Gi-protein coupled inwardly rectifying K+ (GIRK) channels. However, NPY activated an inwardly rectifying potassium conductance in only a subset (26%) of cells (Moran et al., 2004); the low percentage may reflect the fact that recordings were conducted in unidentified neurons that may or may not express Y1. Therefore, it is through GIRK channels that NPY is thought to hyperpolarize excitatory Y1-INs and thus block action potential discharge, leading to the occlusion of spinal transmission of nociceptive signals from the central terminal of primary afferent neurons. Further evidence for this comes from voltage-clamp studies showing that NPY inhibits eEPSCs and dorsal root stimulation-evoked action potentials (Miyakawa et al., 2005; Moran et al., 2004). These data provide the framework for the simplest and most prominent postsynaptic mechanism of NPY analgesia (Figure 1).
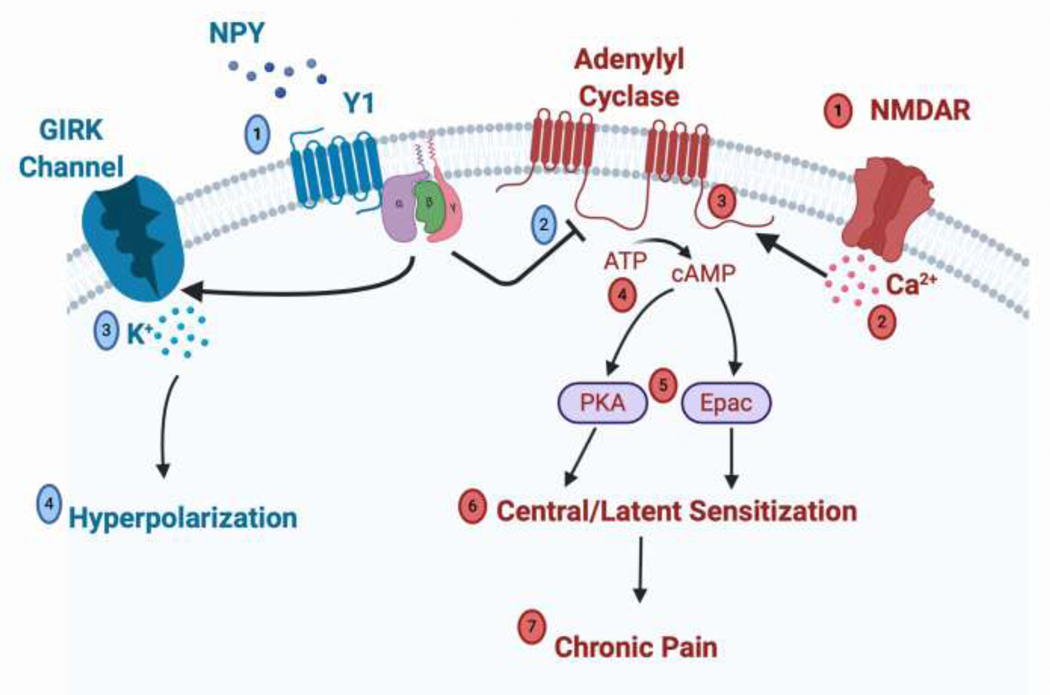
Figure 1: An Intracellular Signaling Mechanism in Spinal Y1-INs for the Endogenous or Pharmacological Inhibition of Chronic Pain by NPY.
Injury induced sensitization and pain (denoted in red). Tissue injury leads to the opening of N-methyl-D-aspartate receptors (NMDAR) (1) and subsequent calcium influx into the cell (2). Increased calcium stimulates adenylyl cyclase type 1 (AC1) (3) to catalyze the conversion of adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP) (4). Increased cytosolic cAMP serves as a second messenger that activates protein kinase A (PKA) and exchange protein activated by cAMP (Epac) (5) leading to long term potentiation and increased neuronal responsiveness to nociceptive inputs that are characteristic of central and latent sensitization (6). Central/latent sensitization in Y1-INs leads to the development and maintenance of chronic pain (7). Endogenous or exogenous NPY inhibition of pain (denoted in blue). NPY binds to Y1 Gi protein-coupled receptors on Y1-INs (1), and Gi protein activation leads to potent inhibition of AC1 that significantly decreases cytosolic cAMP levels (2). Additionally, activation of Gi promotes the opening of gated inwardly rectifying potassium channels (GIRK) and potassium influx (3) that lead to hyperpolarization of Y1-INs (4). Thus Y1- selective ligand binding acts as a therapeutic in order to promote anti-hyperalgesia and hold chronic pain in a state of remission.
Like mu opioid receptor analgesic drugs, Y1 agonists suppress not only excitatory but also inhibitory synaptic events in substantia gelatinosa neurons. For example, either NPY or the Y1 agonist [F7,P34]NPY effectively and reversibly attenuated eIPSCs that were evoked by dorsal root stimulation (Smith et al., 2007; Moran et al., 2004). To reconcile this finding with the clear anti-hyperalgesic effects of NPY, we proposed a second mechanism of NPY analgesia: NPY/Y1 inhibits the spinal release of inhibitory neurotransmitters onto inhibitory neurons, e.g. disinhibition of pain inhibition (Smith et al., 2007); however, this hypothesis requires demonstration that Y1 agonists will preferentially decrease eIPSCs in inhibitory neurons. A third and more likely explanation is that Y1 agonists dampen excitatory inputs from Y1-INs to many classes of dorsal horn interneurons, including inhibitory ones. Thus, reducing excitatory Y1-IN inputs to both excitatory and inhibitory neurons dampens net excitatory and inhibitory transmission. Y1-, excitatory neuron-, and inhibitory neuron-Cre and reporter mouse lines are now available to further explore the dorsal horn microcircuitry in response to application of Y1 agonists. Such mice will allow targeted neuronal recordings and advance our understanding of spinal Y1 agonist mechanisms of action in Y1-IN subpopulations and across excitatory and inhibitory neuron populations.
2. Immunohistochemical and genetic identification of excitatory Y1-IN subpopulations
2.1. Immunohistochemical evidence of Y1-INs as a subpopulation of excitatory interneurons
Tomas Hökfelt and colleagues were the first to demonstrate in rat dorsal horn that Y1-INs are predominantly excitatory (Zhang et al., 1999). This was based on the extensive co-expression of Y1 and somatostatin immunoreactivity; somatostatin is almost exclusively expressed in glutamatergic and not GABAergic interneurons (Chamessian et al., 2018; Duan et al., 2014). The large-scale transcriptomic analyses discussed in section 2.4 are consistent with the detection of Npy1r in excitatory, somatostatinergic interneurons in the superficial dorsal horn, but immunohistochemical co-labeling had been difficult to interpret due to the intense plexus of dendritic and terminal staining that surrounds Y1-immunopositive cells. Therefore, to enhance Y1 resolution within dorsal horn neurons, we first injected NPY by the intrathecal route to promote receptor internalization, thereby concentrating the Y1 signal within the cell soma. With this new method, we reported that rat Y1-INs colocalize not only with somatostatin, but also with immunoreactivity for calbindin (~36% colocalization) and calretinin (~19% colocalization) (Nelson et al., 2019), two additional markers of excitatory interneuron populations implicated in nociceptive transmission (Petitjean et al., 2019; Smith et al., 2015, 2019; Peirs et al., 2015; Craig et al., 2002). Future immunohistochemical studies with newly established and largely nonoverlapping neurochemical markers of excitatory interneurons in superficial dorsal horn, such as substance P, Neuropeptide FF, and cholecystokinin (Dickie et al., 2019; Gutierrez-Mecinas et al., 2019; Todd, 2017), will enable the further segregation of Y1-INs into distinct subpopulations.
2.2. Y1-Cre lineage neurons are a subpopulation of excitatory interneurons
The above findings have been difficult to replicate in the mouse due to the absence of a specific Y1 antibody that robustly stains mouse spinal cord, leading some to turn to Y1-Cre lineage neurons characterized in a Y1-Cre x tdTomato reporter mouse line. This approach reveals colocalization with two markers of excitatory interneurons that have been implicated in the transduction of mechanical stimuli: cMaf+ (~35% colocalization): and retinoid-related orphan receptor alpha (RORα) (~10% colocalization), as well as gastrin releasing peptide (GRP)-expressing neurons (~15% colocalization) and neurokinin 1 receptor (NK1R)-expressing interneurons, located in both superficial (lamina I) and deep (lamina III-IV) dorsal horn (~10% colocalization). Colocalization was not detected with either gastrin releasing peptide receptor (GRPR)-expressing interneurons or cells that express the astrocytic markers s100β or glial fibrillary acidic protein (GFAP) (Acton et al., 2019). A cautionary note with interpretation of this data, however, is that the Y1-Cre lineage captured an extremely large population of dorsal horn neurons (~40% of total NeuN+ dorsal horn neurons). Many of these likely represent neurons that exhibit transient Npy1r expression in development, as ~55% of adult Y1-Cre lineage neurons contained Npy1r mRNA. A large population of deeper neurons (lamina IIi – lamina III) was also labeled with tdTomato yet found to be Npy1r negative (Acton et al., 2019). Against this backdrop, we note that c-Maf is predominately expressed in the interneurons of laminae III-IV (Hu et al., 2012) while Y1 is predominately expressed in superficial dorsal horn (Nelson et al., 2019; Brumovsky et al., 2006, 2007). Furthermore, both single-cell and single-nucleosome transcriptional profiling indicate that the Npy1r and Maf genes are in distinct clusters of dorsal horn interneurons (Häring et al., 2018; Sathyamurthy et al., 2018). These data suggest that any colocalization of Y1-Cre lineage neurons with c-Maf represents an artifact of transient expression during development. This can be tested with double-label fluorescence in situ hybridization in dorsal horn of the adult mouse.
2.3. Developmental fate transcription factors indicate that Y1-INs are excitatory interneurons
Tlx1/3 is a transcription factor that determines glutamatergic cell fate in dorsal horn interneurons (Guo et al., 2012; Xu et al., 2008; Cheng et al., 2005). In situ hybridization of Npy1r mRNA with antibodies against Tlx3 established that the majority of dorsal horn superficial Y1-INs are glutamatergic neurons. Additionally, Tlx1/3 knockout almost completely abolished Npy1r expression in mouse dorsal horn (Guo et al., 2012). Perhaps most convincingly, we show here for the first time that Y1-immunoreactivity densely colocalizes with Tlx3- immunoreactivity in the lumbar dorsal horn of rats (Figure 2). Another transcription factor that is downstream from Tlx1/3, Lmx1b, is also found almost exclusively in excitatory spinal neurons and is extensively colocalized with Y1-Cre lineage neurons in mice (Acton et al., 2019). Conversely, Pax2 is a transcription factor that determines inhibitory cell fate in dorsal horn interneurons (Huang et al., 2008), and Pax2 immunoreactivity does not colocalize with Y1-INs in rat (Nelson et al., 2019) (Figure 2) or Y1-Cre lineage neurons in mouse (Acton et al., 2019). Together these data indicate that almost all Y1-INs in dorsal horn are glutamatergic.
Figure 2: Y1-INs colocalize with excitatory Tlx3 but not inhibitory Pax2 immunoreactivity in rat dorsal horn.
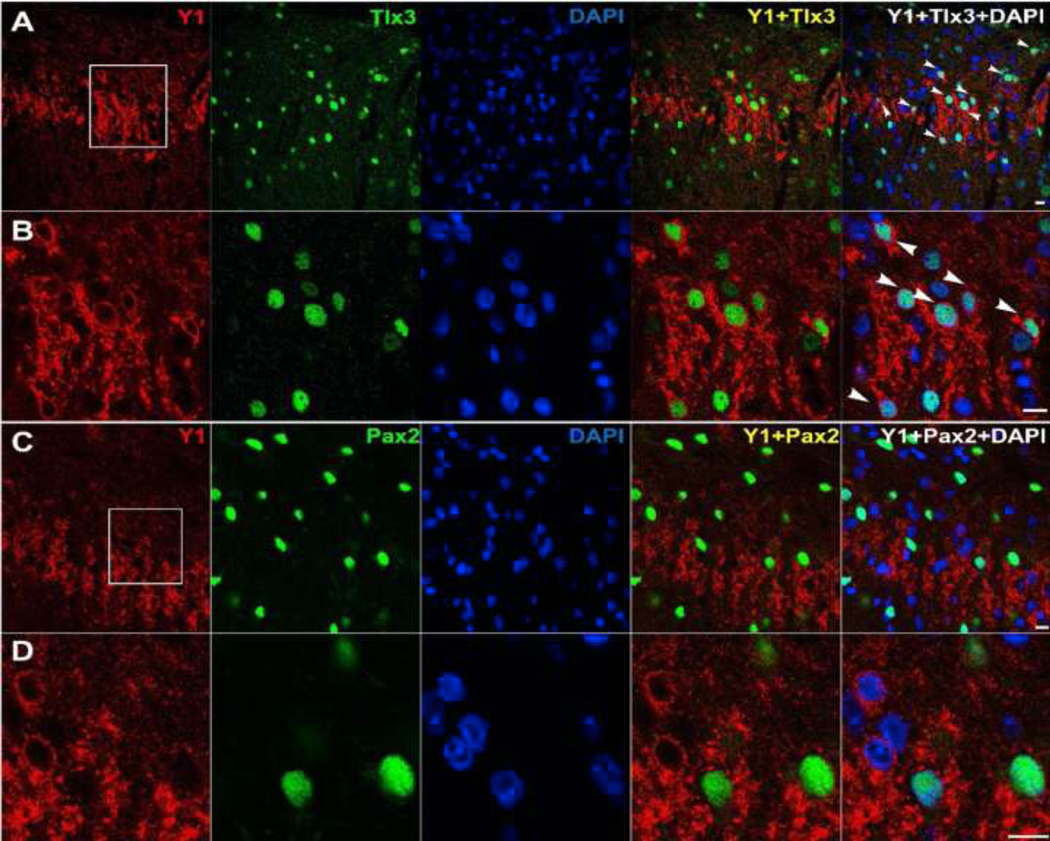
98.96% of Y1-INs in lamina II colocalize with the excitatory neuronal marker Tlx3 (A, B). In contrast, 3.39% of Y1-INs colocalize with the inhibitory neuronal marker Pax2 (C, D). Panels B and D show magnified images from their respective regions of interest. Arrows indicate instances of colabeling. Scale bars: 10 μm. All images are courtesy of Dr. Weisi Fu. Images A and B are published here for the first time. Images C and D are reproduced with permission from (Nelson et al., 2019). Methods are described in detail in (Nelson et al., 2019). Briefly, male Sprague-Dawley rats (Charles Rivers Laboratories) were perfused and L4-L6 transverse spinal cord sections were collected. Primary antibodies: rabbit anti-NPY Y1 receptor (1: 5,000, courtesy of Janice Urban), and guinea pig anti-Tlx3 (1: 10,000, courtesy of Carmen Birchmeier), goat anti-Pax-2 (R&D systems, 1: 1,000, AF3364). To help with identification of Y1-INs, two successive intrathecal injections of 30µg NPY were performed in naïve rats, separated by 1h to promote Y1 receptor internalization (Nelson et al., 2019).
2.4. Transcriptomic sub-classification of Y1-IN subpopulations
High-throughput, unbiased, transcriptomic analyses have revolutionized the characterization of interneuron populations in the dorsal horn, as pioneered by the laboratories of Ariel Levine and Patrik Ernfors. In the first dorsal horn transcriptomics study, Sathyamurthy et al. used single-nucleus transcriptional profiling and identified Npy1r-expressing neurons as one of the 43 cluster types (DE-2) to be selectively enriched. DE-2 is an excitatory interneuron cluster defined by the expression not only of Npy1r but also by the expression of both neuropeptide genes Grp (codes for gastrin releasing peptide) and Sst (codes for somatostatin) (Sathyamurthy et al., 2018). This is interesting because, as described in 2.1, Y1-INs extensively colocalize with somatostatin-like immunoreactivity in the superficial dorsal horn (Nelson et al., 2019; Zhang et al., 1999). Somatostatin-expressing interneurons (SST-INs) are excitatory dorsal horn neurons predominately found in outer lamina II that receive direct C-fiber inputs (Duan et al., 2014). Transcriptional profiling of SST-INs identified Npy1r as a significantly enriched gene (Chamessian et al., 2018). Because of the importance of the somatostatin and gastrin releasing peptides in spinal pain and itch transmission, we felt it was important to rigorously confirm the presence of Sst and Grp in Y1-INs. To this end, we conducted in situ hybridization for Grp and Sst in the lumbar spinal cord of Npy1reGFP BAC transgenic mice. As illustrated by the previously unpublished data of Figure 3, our results point to Y1eGFP neurons containing extensive Grp and Sst mRNA, as predicted by the transcriptomic data.
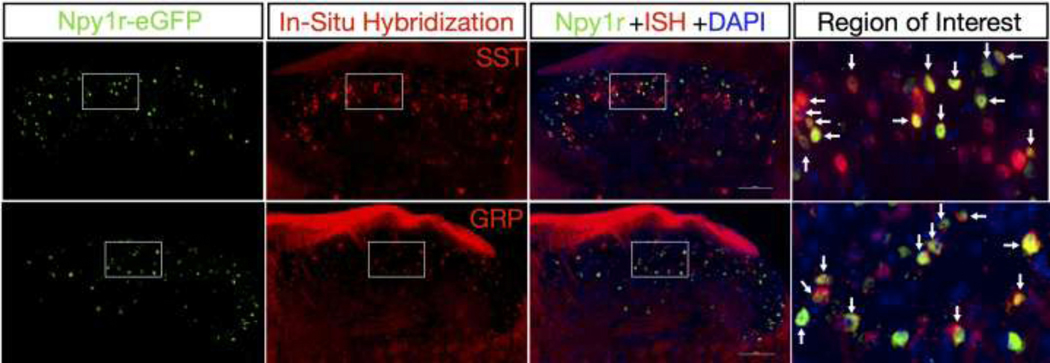
Figure 3: Npy1reGFP Interneurons Colocalize with Grp and Sst mRNA.
New data showing double staining of Npy1reGFP interneurons from BAC transgenic mice with Sst or Grp mRNA by in situ hybridization. Right panels show magnified images from their respective insets. Arrows indicate instances of colabeling. Scale bars: 100 μm. Brief description of experimental methods: 8-week-old male Npy1reGFP mice (MMRRC, 010554-UCD) were transcardially perfused with ice cold 1x PBS followed by 10% buffered formalin and spinal cords were extracted, post-fixed in 10% formalin overnight at 4°C, and then stored in 30% sucrose at 4°C. 20μm thick L3-L4 floating spinal cord sections were obtained on a vibrating microtome and mounted on Superfrost Plus Microscope slides. Slides underwent pretreatment for in situ hybridization (ISH) consisting of 10min Xylene bath, 4min 100% ethanol bath, and 2min RNAscope® H2O2 treatment. Slides were washed in deionized H2O and allowed to dry overnight. The following day the ISH protocol for RNAscope Fluorescent v2 Assay (Advanced Cell Diagnostics) was followed for hybridization to marker probes (RNAscope® Probe- Mm-Grp-C2, 317861-C2; RNAscope® Probe- Mm-Sst-C2, 404631-C2). Upon completion of ISH, slides were blocked for 1h with 3% normal goat serum and incubated overnight at 4°C with chicken anti-GFP primary antibody (Abcam, 1:1,000, ab13970) to restore the GFP fluorescence (which had diminished during fixation). The following day slides were incubated in goat anti-chicken Alexa Fluor 488 secondary antibody (Invitrogen, 1:1,000, A-11039) for 1h, and coverslipped with VECTASHIELD HardSet Antifade Mounting Medium with DAPI. All images were captured on a Nikon Eclipse Ti2 microscope using a 40x objective and analyzed using NIS- Elements Advanced Research software v5.02.
In the second transcriptomics study, Haring et al used unbiased single-cell RNA-sequencing of dorsal horn and also found Npy1r to be selectively enriched in excitatory neurons, namely the glutamatergic clusters Glut2, Glut8, and Glut9 (Häring et al., 2018). The Glut2 cluster represents Cck-expressing neurons that are predicted to participate in allodynia or pathological pain but not acute pain transmission (Häring et al., 2018). The Glut8 and Glut9 clusters were located in the superficial dorsal horn, consistent with the known expression pattern of the Y1 receptor. Noxious heat increased immediate early gene expression within both Glut8 and Glut9 excitatory neurons and noxious cold activated Glut9 neurons, supporting the idea that the NPY-Y1 system contributes to the spinal transmission of noxious heat and cold (Lemons and Wiley, 2012; Taiwo and Taylor, 2002; Hua et al., 1991).
Interestingly, results from both transcriptomics studies found an extremely large overlap between Npy1r and Nmur2, the gene that encodes the neuromedin U receptor 2. In both electrophysiological and behavioral experiments, neuromedin U via the neuromedin U receptor 2 has been implicated in nociception (Torres et al., 2007; Cao et al., 2003; Yu et al., 2003). This colocalization between Npy1r and Nmur2 is novel, and like other genes that significantly overlap with the Y1-IN population, such as Car12, Reln, Npff, Grp, and Cck (Häring et al., 2018; Sathyamurthy et al., 2018), remains to be further explored. Together these large-scale transcriptomic analyses indicate that Npy1r is detected in excitatory, somatostatinergic interneurons in the superficial dorsal horn. Future studies should investigate the overlap of adult Npy1r with candidate gene targets such as Nmur2, Car12, and Grp to uncover potentially distinct Y1-IN subpopulations of dorsal horn interneurons.
3. Pharmacological or endogenous engagement of NPY-Y1 signaling reduces behavioral signs of chronic pain
The pioneering studies of Yaksh and colleagues found that intrathecal administration of NPY dose-dependently increased noxious hotplate thresholds in rats (Hua et al., 1991). This was confirmed a decade later and found to be specific to the heat modality, as NPY or Y1-selective agonists do not change baseline mechanical withdrawal thresholds in rats (Taiwo and Taylor, 2002). Additionally, early studies from Tomas Hökfelt and colleagues found that intrathecal NPY profoundly decreases the nociceptive flexor reflex in naïve rats and in models of nerve injury and acute inflammation (Xu et al., 1994, 1998, 1999). Since these early behavioral studies, an established body of preclinical evidence across a variety of injury conditions as far ranging as bone cancer-induced pain (Diaz-delCastillo et al., 2018) indicates that intrathecal administration of NPY acts through Y1 receptors to dose-dependently reduce behavioral (and spinal molecular) markers of spinal nociceptive transmission, including not just heat but also cold and mechanical hypersensitivity (Table 1).
3.1. Global and selective spinal/hindbrain Npy1r knockout
Global germ line Npy1r knockout mice display abnormally enhanced behavioral reflex responses to noxious heat, visceral chemical, and non-noxious mechanical stimuli as compared to genetic controls (Shi et al., 2006; Naveilhan et al., 2001). They also develop a more pronounced hypersensitivity in response to inflammation or peripheral nerve injury (Kuphal et al., 2008; Naveilhan et al., 2001). However, Y1 receptors are expressed throughout the body and so Goulding and colleagues ablated Npy1r specifically in spinal cord and hindbrain neurons (including the medulla, pons, and cerebellum) by crossing Npy1r-Flox mice with Lbx1-Cre mice (Acton et al., 2019). In contrast to germ line deletion, these mice exhibited hypersensitivity to von Frey hairs but not to other sensory stimuli including light brush, noxious pinprick, noxious pressure, noxious heat, capsaicin, or chemical pruritogens. These studies suggest that spinal/hindbrain Y1 contributes to a tonic inhibition of responsiveness to non-noxious punctate mechanical stimulation, while Y1 receptors located elsewhere contribute to the tonic inhibition of responsiveness to dynamic brush, noxious mechanical stimulation, noxious heat, and chemical pain.
In contrast to the reduced thresholds observed in Npy1r deletion mutant mice, intrathecal administration of Y1 antagonists did not change von Frey mechanical withdrawal thresholds in naïve or sham-injured mice (Fu et al., 2020; Chen et al., 2019; Solway et al., 2011). One possible explanation for this discrepancy is that while intrathecal injection likely restricts Y1 antagonism to the spinal cord (and perhaps the DRG), the interruption of Y1 function after Npy1r knockout extends to the medulla, where NPY can exert not only pronociceptive but also antinociceptive actions. On one hand, microinjection of NPY into the nucleus gracilis reduced von Frey thresholds in the uninjured rat (Ossipov et al., 2002), and microinjection of NPY Y1 antagonists into the nucleus gracilis or the cisterna magna reversed mechanical hypersensitivity in rats with spinal nerve ligation (Fukuoka and Noguchi, 2015; Ossipov et al., 2002). On the other hand, injection of NPY into the cisterna magna dose-dependently reversed mechanical hypersensitivity in rats with a variant of the spared nerve injury (SNI) model (Jung et al., 2009). Thus, NPY in the nucleus gracilis is pronociceptive but the pro- or antinociceptive role of NPY in the cisterna magna after peripheral nerve injury remains unclear. Furthermore, injection of NPY into the rostral ventral medulla (RVM) reduced behavioral signs of hypersensitivity in models of chronic neuropathic and inflammatory pain (Cleary et al., 2014; Taylor et al., 2007). The RVM contains two classes of pain modulatory neurons: the pain facilitatory ON-cells, and the pain inhibitory OFF-cells (Chen and Heinricher, 2019). Y1-immunoreactivity is found on both ON- and OFF-cells (Cleary et al., 2014). Perhaps the loss of Npy1r in spinal/hindbrain and global Npy1r knockout mice results in an overall net increase in ON-cell activity in the RVM that is responsible for the hyperalgesia to light punctate touch. Future studies could test this hypothesis with conditional knockout of Npy1r in specific regions of the CNS, for example with injection of AAV-Cre into the RVM or spinal cord of adult Npy1r-Flox mice, followed by the determination of mechanical threshold.
3.2. Spinally-directed NPY in models of neuropathic pain
Intrathecal NPY or [Leu31,Pro34]-NPY dose-dependently reduced mechanical and cold hypersensitivity in rats with SNI or chronic constriction injury (CCI) (Malet et al., 2017; Intondi et al., 2008), and reduced hind paw withdrawal latency to heat in CD1 mice following partial sciatic nerve ligation (Kuphal et al., 2008). After SNI in rats, NPY reduced the immunohistochemical expression of non-noxious, tactile stimulus-induced Fos, a marker of neuronal activation, in the superficial dorsal horn. These reductions in neuropathic pain-like behavior and Fos expression were both reversed with BIB03304 (Intondi et al., 2008). Subsequent studies reported that intrathecal NPY reduced Fos expression within Y1-INs, indicating that Y1 retains the capacity to inhibit spinal pain transmission after nerve injury (Nelson et al., 2019). Taken together, these data in both rats and mice promote the spinal Y1 receptor as a compelling target for analgesic drug development to treat traumatic nerve injury-induced neuropathic pain.
3.3. Spinally-directed NPY in models of inflammatory pain
Intrathecal NPY or [Leu31,Pro34]-NPY produced anti-hyperalgesic effects in rats in the intraplantar CFA model of inflammatory pain (Taylor et al., 2014; Taiwo and Taylor, 2002), the intraplantar formalin model of ongoing pain (Mahinda and Taylor, 2004), the hindpaw plantar incision model of postoperative pain (Gupta et al., 2018; Yalamuri et al., 2013), and the intra-knee joint formalin model of joint inflammation (Souza-Silva et al., 2020). The Y1 antagonist BIBO3304 prevented these antihyperalgesic effects, and the inhibitory effects of intrathecal NPY on mechanical and heat hyperalgesia in the CFA model of inflammation were lost in Npy1r knockout mice (Kuphal et al., 2008). These data promote the spinal Y1 receptor as a compelling target for analgesic drug development to treat inflammatory pain.
3.4. Safety profile after intrathecal administration of NPY
The above studies indicate a strong basic science rationale for the development of spinally-directed Y1-selective agonists for the treatment of chronic pain, but would they be safe in a therapeutic setting? On the one hand, intrathecal administration of antihyperalgesic doses of NPY or Y1-selective agonists disrupts neither motor locomotion nor touch sensitivity in non-injured rodents (Chen et al., 2019; Malet et al., 2017; Solway et al., 2011; Kuphal et al., 2008; Taiwo and Taylor, 2002). On the other hand, NPY contributes to cardiovascular regulation (Tan et al., 2018), and surface application of NPY to the spinal cord changes blood flow (Chen et al., 1988, 1990). Similarly, intrathecal NPY induces vasoconstriction that results in a transient decrease in blood flow to the spinal cord and increases in mean arterial pressure and heart rate (Mahinda and Taylor, 2004; Xu et al., 1999). However, this effect was transient and could be prevented with a Y1 agonist (Chen and Westfall, 1993) or pre-administration of a selective Y2 antagonist (Mahinda and Taylor, 2004). If Y2 is indeed the primary mediator of cardiovascular side effects following intrathecal NPY, then the potential risks for adverse cardiovascular effects may be avoided with the use of analgesic doses of intrathecal Y1-selective agonists.
Target specificity will be key in the development of a spinally-directed Y1-selective analgesic for chronic pain. In contrast to the clear antihyperalgesic actions of NPY when targeted to the spinal cord (Table 1) or multiple brain areas (Alhadeff et al., 2018; Mellado et al., 1996), other studies indicate that Y1 receptor activation in the nucleus gracilus (Fukuoka and Noguchi, 2015; Ossipov et al., 2002) and Y2 receptor activation in the DRG (Sapunar et al., 2011; Tracey et al., 1995) produce hyperalgesia. These pronociceptive actions of NPY in the DRG and nucleus gracilis can be avoided with targeted administration of Y1 agonists to the spinal cord with the implantation of chronic intrathecal catheters, a method commonly engaged for the management of chronic pain (Knight et al., 2007).
3.5. Injury engages an endogenous spinal NPY-Y1 signaling cascade that opposes nociception
NPY Y1 receptor antagonists exert minimal if any effect on thermal or mechanical paw withdrawal thresholds in non-injured animals, indicating an absence of a tonic NPY-Y1 inhibitory control of nociception (Chen et al., 2019; Solway et al., 2011). In stark contrast, painful injury induces the development of a robust, compensatory mechanism of NPY antihyperalgesia in the dorsal horn. In the setting of inflammatory pain, for example, intraplantar CFA increased the affinity of functional Y1 receptor-coupling to activated G-proteins in the lumbar dorsal horn (Taylor et al., 2014). This suggests that inflammation augments the functionality of receptor-G protein interactions, leading to the amplification of intracellular signaling. This may explain how after CFA, NPY can effectively reduce the noxious mechanical stimulus-evoked release of substance P from the central terminals of primary afferent neurons, an effect not observed in uninjured controls (Taylor et al., 2014). In the setting of neuropathic pain, peripheral nerve injury induced a massive de novo expression of NPY in neurons in the DRG after damage to their axons (Magnussen et al., 2015; Wakisaka et al., 1991). Peripheral nerve injury also increased the spontaneous release of NPY in the dorsal horn (Colvin and Duggan, 2001; Mark et al., 1998) that was not abolished by total anesthesia-induced conduction block of the injured nerve, suggesting that most NPY release is coming from local dorsal horn NPY-inhibitory interneurons and not the injured primary afferents (Colvin and Duggan, 2001). In support of this idea, peripheral nerve injury increased mechanical stimulus-evoked NPY release from dorsal horn neurons in vivo (Marvizon et al., 2019); this study overcame the notoriously difficult assessment of spinal NPY concentrations (Mark et al., 1997) with the use of a robust assay of Y1 internalization as a proxy for NPY release (Marvizon et al., 2019). Together these results suggest that NPY release in the dorsal horn following peripheral nerve injury stems from dorsal horn interneuron release. So then what might the massive injury-induced de novo expression of NPY in the DRG be doing? A small body of evidence indicates that NPY causes neurite outgrowth from nerve-injured DRG fibers (White and Mansfield, 1996), suggesting that NPY contributes to the repair and regrowth of damaged neurons. Indeed, NPY-induced repair is commonly observed in the central nervous system (Decressac and Barker, 2012). Future studies in DRG conditional NPY knockout mice may elucidate the contribution of NPY to nerve injury-induced pain and axonal repair and reinnervation in the dorsal horn. Taken together, these results from both inflammatory and neuropathic injury are consistent with a homeostatic process by which injury-induced pronociceptive neurotransmission in the dorsal horn is counterbalanced by: 1) Enhanced NPY release from dorsal horn neurons → 2) Enhanced Y1-Gi protein coupling → 3) Suppressed release of pronociceptive neurotransmitters, including substance P, from the central terminals of primary afferent neurons.
Tissue or nerve injury sensitizes dorsal horn neurons in the spinal cord, leading to an increase in the intensity and duration of pain (Latremoliere and Woolf, 2009; Ji et al., 2003). This central sensitization can facilitate the protective aspects of acute pain, but failure to resolve can lead to chronic pain. With the development of a latent form of central sensitization (latent sensitization or LS), sensitization is kept in remission by an opposing mechanism that typically includes activation of inhibitory G-protein coupled receptors, such as Y1 (Taylor and Corder, 2014). LS can persist for at least months, even after the appearance of complete recovery from tissue injury and the re-establishment of normal pain thresholds (Corder et al., 2013). LS can be revealed upon disruption of pain inhibitory GPCRs, including spinal NPY signaling. For example, in CFA or peripheral nerve injury models, conditional knockdown of NPY in NPYtet -transgenic mice or intrathecal administration of BIBO3304 leads to a rapid, robust, and repeatable reinstatement not only of mechanical and thermal hypersensitivity (Fu et al., 2019, 2020; Solway et al., 2011) but also the affective component of pain as determined with conditioned place preference and aversion assays (Fu et al., 2020). These results indicate that the NPY-Y1 system contributes to a powerful endogenous analgesia mechanism whereby animals naturally recover from inflammatory- or nerve injury-induced hyperalgesia. The development of a pathologically high set point in the equilibrium between pronociceptive and antinociceptive processes (an allostatic state) may have important implications: failures in endogenous inhibitory NPY signaling could increase the ratio of excitation/inhibition, thereby unleashing LS to drive the transition from acute to chronic pain states (Figure 1).
The pronociceptive signaling pathways associated with LS are being revealed with the use of a four-step approach: 1) induce inflammatory or neuropathic pain, 2) allow pain resolution, 3) interrupt a putative signaling mechanism with pharmacological antagonist or genetic deletion, and 4) test for reinstatement of hyperalgesia and affective pain. With this approach, we could prevent BIBO3304- or genetic NPY knockdown-induced pain reinstatement by spinal blockade of N-methyl-D-aspartate receptors (NMDAR) with intrathecal administration of MK-801, adenylyl cyclase type 1 (AC1) with intrathecal NB001 or in AC1 knockout mice, protein kinase A (PKA) with intrathecal H89, exchange protein activated by cAMP (Epac) with intrathecal ESI-09, TRPA1 with intrathecal HC030031, or TRPV1 with intrathecal AMG9801 (Fu et al., 2019, 2020). As illustrated in the flow diagram of Figure 1, we have proposed that injury promotes a pronociceptive NMDAR→AC1→cAMP signaling cascade of LS that involves PKA and Epac1/2 and is kept in remission by tonic NPY-Y1 inhibition. New treatments for chronic pain might either mimic endogenous NPY analgesia or inhibit AC1, PKA, or Epac.
4. Y1-INs mediate chronic pain
Section 3 indicates that spinal nociceptive processing can be inhibited with intrathecal administration of a Y1 agonist or endogenous release of NPY within the dorsal horn. Since Y1 receptors are predominantly located on glutamatergic neurons (Nelson et al., 2019), the consequent activation of inhibitory G-proteins and decreases in intracellular signaling (Brumovsky et al., 2007) and hyperpolarization (Melnick, 2012; Smith et al., 2007) may reduce the net pain excitation:inhibition ratio, tipping the balance towards pain relief. But what is the relative contribution of each of the two key Y1-expressing substrates of excitatory neurotransmission in the dorsal horn, Y1-INs and the central terminals of peptidergic primary afferent neurons? An emerging body of literature has begun to address this question, with an initial focus on two methods to selectively ablate Y1-INs: intrathecal administration of NPY conjugated to the saporin neurotoxin (NPY-saporin) and site-specific conditional knockout of Y1-Cre lineage neurons.
4.1. Selective ablation of spinal Y1-INs with NPY-saporin
NPY-saporin is an NPY-conjugated ribosomal toxin that is selectively endocytosed and internalized within Y1-expressing neurons (Lemons and Wiley, 2012; Wiley et al., 2009). As with other peptide-saporin conjugates, intrathecal administration of NPY-saporin selectively ablates dorsal horn Y1-INs, while sparing other subpopulations of neurons (including those expressing the mu opiate receptor or the neurokinin-1 receptor) as well as Y1-expressing DRG neurons (Nelson et al., 2019; Wiley et al., 2009). There is considerable evidence that intrathecal neuropeptide-saporin conjugates do not produce toxicity in DRG neurons either because they are not internalized into primary afferent terminals, or, if internalized, are not axonally transported to cell bodies in the DRG (Wiley et al., 2009; Kline and Wiley, 2008; Wiley and Kline, 2000).
Acute nociceptive reflexes and pain in the absence of injury.
When tested in the hotplate test (48–56 °C), a conventional assay of nocifensive reflexive withdrawal, intrathecal NPY saporin did not change paw withdrawal latency (Nelson et al., 2019; Wiley et al., 2009). NPY-saporin also failed to change the hindpaw withdrawal response to noxious radiant heat (Hargreaves’ test), noxious pin prick, and von Frey hairs (Nelson et al., 2019). On the other hand, NPY-saporin did decrease nocifensive responses thought to engage supraspinal pain modulatory systems. For example, NPY-saporin reduced hindpaw licking and guarding in a 44 °C hotplate assay of affective pain, reduced ongoing nociception during both phases of the response to intraplantar injection of dilute formalin, and reduced aversion to a 10 °C cold plate (Lemons and Wiley, 2012; Wiley et al., 2009). NPY saporin did not alter motor control in uninjured rats as assessed by time on an accelerating rotarod or general activity measures in an open field arena (Nelson et al., 2019). Taken together, these data indicate that Y1-INs contribute to the affective components of acute pain transmission without impinging upon motor coordination or the protective nocireflexive components of acute pain (Table 1).
Ablation of Y1-INs with NPY-saporin did not alter responsiveness to non-noxious von Frey filaments or noxious pinprick (Nelson et al., 2019). In contrast, ablation of excitatory SST-INs decreased mechanical sensitivity to non-noxious von Frey filaments and abolished responsiveness to noxious pinprick (Duan et al., 2014). Furthermore, hindbrain and spinal cord conditional knockout of Sst from SST-INs increased mechanical sensitivity to von Frey filaments (Huang et al., 2018). Somatostatin is well recognized as a pain inhibitory peptide that hyperpolarizes dorsal horn interneurons (Jiang et al., 2003; Kim et al., 2002; Murase et al., 1982). Thus, excitatory SST-INs have a pronociceptive role in mechanosensation and the inhibitory somatostatin peptide has a complimentary antinociceptive role in mechanotransduction, together indicating that SST-INs are critical for baseline mechanosensitivity. This might seem to be inconsistent with the fact that Y1-INs contain extensive somatostatin mRNA (Figure 3) and ablation of Y1-INs does not affect mechanical sensitivity at baseline. However, the Y1-IN population is smaller than the SST-IN population (~60% of excitatory interneurons in laminae I-II of dorsal horn) (Gutierrez-Mecinas et al., 2016). The SST-IN population is transcriptomically, immunohistochemically, morphologically, and electrophysiologically heterogenous and overlaps with numerous classes of excitatory dorsal horn interneurons exclusive of the Y1-IN population (Peirs et al., 2020; Chamessian et al., 2018; Häring et al., 2018; Todd, 2017; Gutierrez-Mecinas et al., 2016; Duan et al., 2014). Conversely, Y1-INs are a smaller subclass of excitatory dorsal horn interneurons that often express Grp mRNA (Figure 3) (Sathyamurthy et al., 2018), and neither ablation nor chemogenetic inhibition of dorsal horn GRP-Cre interneurons altered responsiveness to von Frey filament or pin prick stimulation (Albisetti et al., 2019) We suggest that it is a Grp- or Npy1r-negative subpopulation of SST-INs that regulates baseline mechanosensitivity. Future work can utilize results from high-throughput transcriptomic datasets to develop refined Flp- and Cre-mouse lines. This will allow more selective targeting of increasingly smaller dorsal horn neuronal populations to better identify microcircuits responsible for individual behaviors.
Tissue and nerve injury models of persistent pain:
Intrathecal administration of NPY-saporin reduced several operant and cognitive measures of CFA-induced allodynia, including responsiveness to cold temperatures in a thermal preference assay (two chamber 15 °C vs. 45 °C) , feeding interference (overcome a 10 °C floor plate to consume a sweet solution), and an escape task (climb onto a shelf to avoid a 10 °C floor plate), but did not interfere with systemic morphine-induced analgesia (Lemons and Wiley, 2012). Similarly in the SNI model of neuropathic pain, NPY-saporin dose-dependently reduced the development of mechanical allodynia (hindpaw withdrawal response to von Frey filaments), mechanical hyperalgesia (response to blunt pin), and cold allodynia (hindpaw withdrawal response duration to acetone droplet evaporation) (Nelson et al., 2019). Together, these directed lesion studies support the idea that the Y1-IN subpopulation of dorsal horn neurons is necessary for the maintenance of both mechanical and cold modalities of nociceptive transmission in chronic pain states.
4.2. Selective ablation of spinal Y1-Cre lineage INs with intersectional genetics
Goulding and colleagues restricted the expression of diphtheria toxin receptors or inhibitory designer receptors to Y1-Cre lineage neurons in the spinal cord and then applied diphtheria toxin or clozapine-N-oxide (Acton et al., 2019). The resulting ablation or inhibition of Y1-Cre lineage neurons did not alter responsiveness to noxious stimulation, be it cutaneous heat or mechanical pressure or hindpaw injection of capsaicin or formalin. Contrary to the NPY-saporin studies, however, ablation or silencing of Y1-Cre lineage neurons in the adult mouse reduced sensitivity to light punctate touch using von Frey filaments (Acton et al., 2019). A probable explanation for this discrepancy is that in addition to the tight band of somatostatin- and Y1-INs in adult superficial dorsal horn (Nelson et al., 2019; Duan et al., 2014), the Y1-Cre lineage captures additional populations of neurons that appear to represent transient developmental expression of Y1 in the low-threshold mechanosensor-recipient zone (LTMR-RZ) in deeper laminae. Because the LTMR-RZ processes input from low-threshold mechanoreceptors (ie. Aß fibers) (Moehring et al., 2018; Abraira and Ginty, 2013), their ablation, rather than those of the Y1-INs expressed in the adult, likely explains the reduced sensitivity to light punctate touch. Still, this study sets the stage for important studies to assess the role of Y1-INs in chronic pain states. One such study could be the intraspinal administration of recombinant adeno-associated viruses (rAAVs) carrying inhibitory chemical- or opsin-gated channels into the spinal cords of Y1-Cre mice (Haenraets et al., 2017, 2018). This approach will allow the selective manipulation of adult Y1-INs and avoids the transient developmental expression of Y1 seen in the Y1-Cre lineage studies. Future studies might then probe inhibition of Y1-INs using chemogenetics or optogenetics in both naïve and chronic pain states. Such studies would complement and extend the NPY-saporin studies in clarifying the role that YI-INs play in the development and maintenance of chronic pain.
5. Where do Y1-INs fit within the dorsal horn microcircuitry of chronic pain?
It is becoming increasingly clear that different injury conditions engage distinct dorsal horn spinal microcircuits to mediate allodynia (Peirs and Seal, 2016; Peirs et al., 2015). Accordingly, Y1 agonists may inhibit pain differently depending on the type of injury, e.g. inflammation vs. nerve injury, as we previously hypothesized (Smith et al., 2007).
Inflammatory pain:
In the context of inflammation, Y1 agonists likely prevent C- and A-fiber primary afferent nociceptive transmission from being propagated to lamina I nociceptive projection neurons in the dorsal horn. This effect may occur via both pre- and postsynaptic mechanisms. First, Y1 agonists may hyperpolarize Y1-INs that are the postsynaptic targets of C-fibers (Miyakawa et al., 2005). One such postsynaptic target may be calretinin-positive Y1-INs; dorsal horn interneurons that express calretinin have been increasingly implicated in inflammatory pain (Peirs et al., 2015), and we find that there is ~19% colocalization between calretinin- and Y1- immmunoreactive neurons in the rat dorsal horn (Nelson et al., 2019). Second, Y1 agonists may act presynaptically on peptidergic, capsaicin-sensitive primary afferent terminals that contain Y1 receptors to reduce primary afferent transmitter release (Taylor et al., 2014; Gibbs et al., 2004), although this remains controversial; Moran and Smith reported that unlike Y2 agonists, the Y1 agonist F7P34NPY did not suppress excitatory transmission at the central terminal of presynaptic primary afferent neurons (Moran et al., 2004). Third, Y1 agonists may act collectively at both pre- and postsynaptic targets to reduce inflammatory hyperalgesia.
Neuropathic pain:
The dorsal horn circuitry of mechanical allodynia after peripheral nerve injury is believed to involve the propagation of low-threshold input from Aß-fibers. For example, selective pharmacological inhibition of A-fibers suppressed mechanical allodynia after chemotherapy, nerve injury, and diabetic neuropathy, while inhibition of C-fibers did not (Xu et al., 2015). Because Y1 is exclusively found in small, peptidergic DRG neurons and only sparsely expressed at their central terminals, we suggest that Y1-INs in neuropathic pain circuits are inhibited by Y1 agonists at a site that is downstream of Aß-fiber input. As illustrated in Figure 4, the propagation of low-threshold input from Aß-fibers is thought to activate disinhibited PKCγ interneurons in inner lamina II that transmit light touch in a dorsally-directed microcircuit to lamina I projection neurons (Todd, 2017; Peirs and Seal, 2016; Petitjean et al., 2015; Lu et al., 2013). This circuit begins with PKCγ interneurons that first synapse onto excitatory transient central cells, which in turn synapse onto excitatory vertical cells, that then target lamina I pain projection neurons (Lu et al., 2013). We hypothesize that Y1-INs are a subset of these transient central cells, based on both their morphology and their firing patterns in response to NPY, as discussed in section 1.2. Furthermore, the only transient central cell population that has been identified to date is the GRP population (Dickie et al., 2019), and both single nucleosome RNA-sequencing (Sathyamurthy et al., 2018) and the in situ hybridization results of Figure 3 demonstrate significant overlap between Npy1r and Grp. Taken together, these results provide the premise for our hypothesis that spinally-directed Y1 agonists prevent the propagation of light-touch information to spinal projection neurons by inhibiting Y1-IN transient central cells (Nelson, 2019).
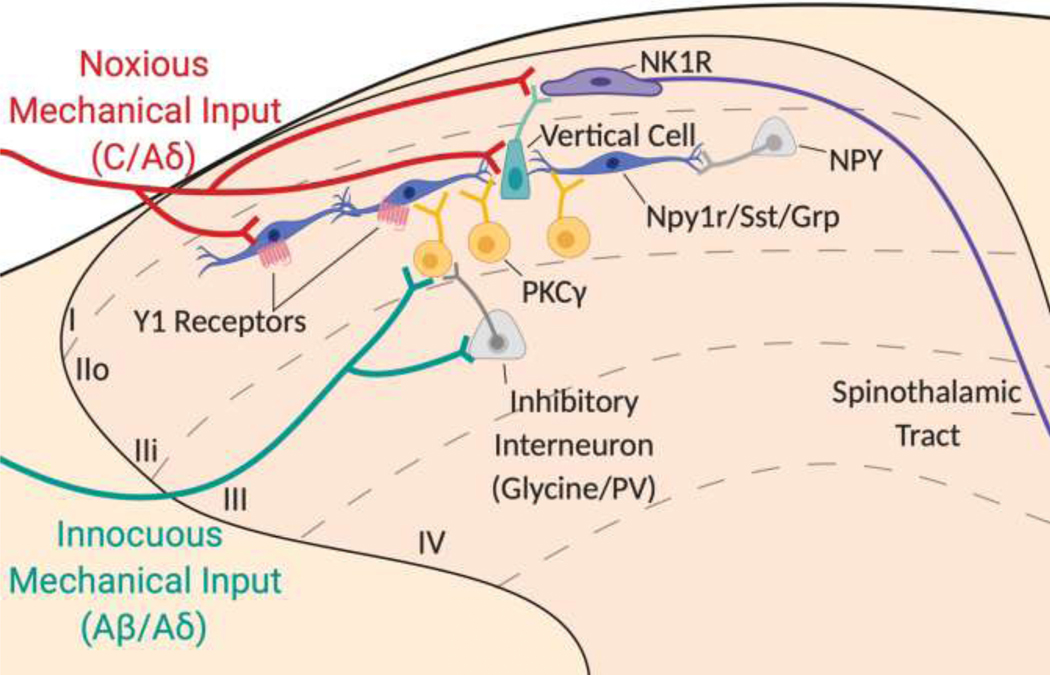
Figure 4: Hypothetical Contribution of the Y1-IN in the Ascending Dorsal Horn Microcircuit for Mechanical Pain.
Noxious mechanical inputs activate C/Aδ nociceptors (red) that project into the superficial laminae of the dorsal horn and synapse onto Y1-INs (dark blue), projection neurons that express the neurokinin receptor type 1 (NK1R) (purple), and/or vertical cells (green). Inhibitory NPY interneurons (light grey) may “gate” some of these nociceptive inputs at the Y1-IN. Innocuous mechanical inputs activate Aß/Aδ myelinated afferents (green) that project into deeper laminae of the dorsal horn and synapse onto interneurons marked by the expression of protein kinase C γ (PKCγ) (yellow). However, feedforward inhibition via inhibitory glycinergic and parvalbumin (PV) interneurons (light grey) prevents the activation of PKCγ interneurons. In the context of neuropathic pain, feedforward inhibition onto PKCγ interneurons is lost and innocuous light touch inputs are able to activate a dorsally-directed microcircuit from PKCγ interneurons onto transient central cells (Npy1r/SST/GRP neurons) that synapse onto vertical cells, ultimately leading to the activation of ascending NK1R interneurons that travel via the anterolateral tracts in the contralateral spinal cord to be processed via higher order pain centers such as the lateral parabrachial nucleus.
6. NPY and Y1-INs in itch
A rapidly emerging body of literature implicates NPY signaling at Y1-INs in the regulation of both mechanical and chemical itch (Table 1).
6.1. Intrathecal NPY inhibits mechanical and chemical itch
Mechanical Itch:
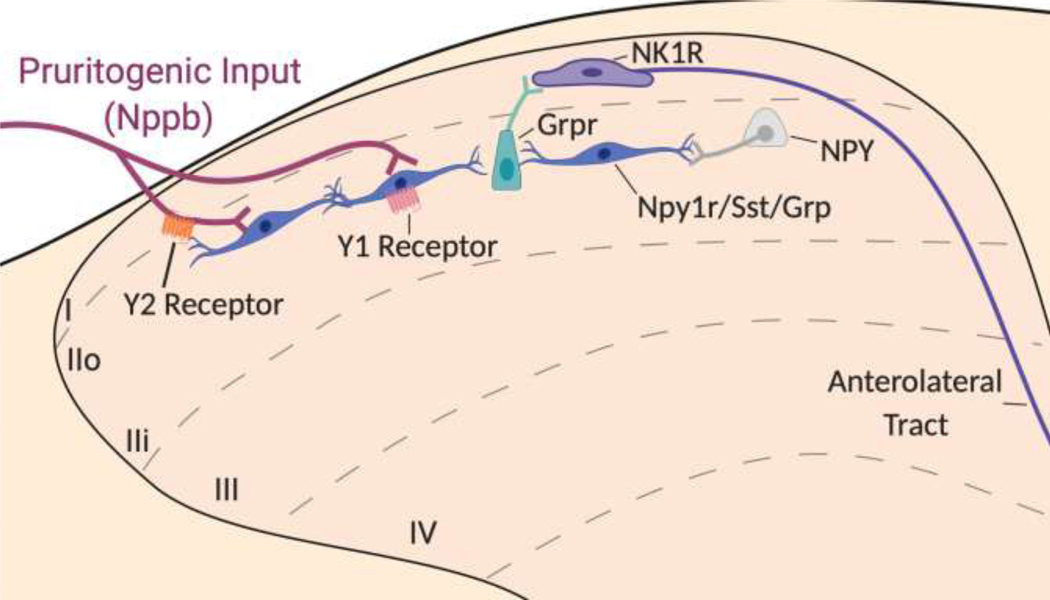
Bourane et al. reported that the application of a 0.07 gram von Frey filament to the shaved nape of the neck in mice produced directed scratching behavior, thus establishing a new mouse model of mechanical itch (thought to represent the tickle-like experience of an insect walking on exposed skin) (Bourane et al., 2015). Intrathecal administration of the Y1-selective agonist [Leu31,Pro34]-NPY reduced filament-induced scratching, indicating that exogenous administration of NPY acts at Y1 to dampen mechanical itch (Acton et al., 2019; Gao et al., 2018).
Chemical Itch:
Intradermal injection of pruritogenic compounds, such as histamine or the mast cell degranulator 48/80, increases duration and frequency of scratching bouts. Intrathecal [Leu31,Pro34]-NPY reduced the duration of scratching behavior but not the frequency of scratching bouts induced by either 48/80 or histamine (Gao et al., 2018). Similarly, intrathecal administration of the Y2 agonist, peptide YY (PYY)3–36, also reduced 48/80- and histamine-induced scratching (Ma et al., 2020). These data indicate that exogenous administration of NPY acts at Y1 and Y2 receptors to dampen chemical itch. The chemical itch circuit involves a specialized relay from periphery to spinal cord dorsal horn. Neuropeptide natriuretic polypeptide b (Nppb) is expressed in somatostatin positive DRG neurons that respond to chemical pruritogenic stimuli in the periphery (Huang et al., 2018). NPPB-expressing primary afferents subsequently activate GRP neurons in the spinal cord that in turn activate gastrin releasing peptide receptor-expressing INs (GRPR-INs) and begin the supraspinal transmission of itch (Jakobsson et al., 2019; Pagani et al., 2019; Solinski et al., 2019; Mishra and Hoon, 2013). Y1 agonists may inhibit chemical itch transmission via hyperpolarization of interneurons coexpressing Y1, SST, and GRP in the superficial dorsal horn (Jakobsson et al., 2019) (Figure 5). Additionally, ~90% of NPPB-expressing DRG neurons coexpress Y2 (Ma et al., 2020). We speculate that intrathecal NPY may prevent the release of pruritogenic neurotransmitters from Nppb-expressing central terminals via Y2 activation and suggest that NPY may represent a therapeutic target for chronic itch at both Y1 and Y2 receptors (Figure 5).
Figure 5: Hypothetical Contribution of the NPY Y1 and Y2 Receptors in the Ascending Dorsal Horn Microcircuit for Chemical Itch.
Natriuretic polypeptide b-expressing (Nppb) pruritogenic inputs (maroon) project into the superficial laminae of the dorsal horn and synapse onto Y1-INs (dark blue) that co-express somatostatin (Sst) and gastrin releasing peptide (Grp). The central terminals of the Nppb-expressing pruritogenic inputs express the Y2 receptor (orange). Y1-INs synapse onto interneurons marked by the expression of gastrin releasing peptide receptor (Grpr) which in turn activate ascending itch projection neurons that express the neurokinin receptor type 1 (NK1R) (purple). NPY interneurons (light grey) may “gate” some of these pruritogenic inputs at Y1-INs or Y2- expressing terminals. Thus, endogenous or exogenous NPY can act at either or both NPY receptors to dampen the transmission of chemical itch. This figure is adapted from (Ma et al., 2020; Jakobsson et al., 2019).
6.2. Ablation of spinal NPY-Cre lineage INs induces spontaneous and mechanical itch
NPY-expressing neurons represent approximately one-third of spinal inhibitory interneurons in the superficial dorsal horn (Boyle et al., 2017). Selective ablation of spinal NPY-Cre lineage interneurons induced spontaneous scratching behaviors as well as mechanical (but not chemical) itch (Bourane et al., 2015). These data suggest that NPY-Cre lineage interneurons tonically inhibit spontaneous scratching and mechanically-evoked itch, with the caveat that ablation can lead to confounding compensations that include circuit rearrangements.
6.3. Spinal NPY-Cre ablation-induced itch is attenuated by lesion of spinal Y1-Cre or Ucn3-Cre INs
NPY-Cre interneuron ablation-induced scratching could be prevented with concomitant ablation of Y1-Cre lineage neurons(Acton et al., 2019). By contrast, ablation of somatostatin-Cre (SOM-Cre) lineage neurons had no effect (Acton et al., 2019). These disparate results following Y1-Cre and SOM-Cre ablation are surprising because the vast majority of Y1-INs are somatostatin-positive (Nelson et al., 2019; Sathyamurthy et al., 2018; Zhang et al., 1999) (Figure 3). As described in Section 4.2, one explanation for this discrepancy may be the transient expression of Y1-Cre in the LTMR-RZ. Further studies are needed to determine whether ablation of Y1-Cre neurons in superficial lamina II, or deeper Y1-Cre neurons in the LTMR-RZ, are responsible for loss of filament-induced itch.
Pan et al. reported that NPY-Cre ablation-induced mechanical itch is prevented by concomitant ablation of a small excitatory interneuron population marked by Urocortin 3 expression (Ucn3-INs) (Pan et al., 2019). The Ucn3-INs are found in inner lamina II and outer lamina III of the dorsal horn and receive direct Aß low-threshold mechanosensory input (Pan et al., 2019). Very few Ucn3-INs exhibit Npy1r expression (14.3% colocalization), and in recordings from Ucn3-INs in spinal cord slices, application of [Leu31,Pro34]-NPY did not attenuate action potential induction following current injection (Pan et al., 2019). These results imply that Ucn3-INs exhibit little to no functional Y1, and it is a very small population of Ucn3/Y1-INs that gate mechanical itch, or, more likely, Y1-INs are not involved in gating mechanical itch at all. Further studies, including investigations of a possible Y1-Cre lineage/Ucn3-IN overlap, are warranted to determine if a small population of Ucn3/Y1 interneurons are “gated” via NPY-Cre interneurons and mediate mechanical itch.
7. Clinical significance and therapeutic potential of NPY agents for chronic pain and itch
There is a critical and pressing need to develop safe, nonaddictive, and efficacious analgesic and antipruritic drugs. Thirty years of preclinical research suggest that NPY is potently antihyperalgesic in several models of inflammatory and neuropathic pain, as well as antipruritic in assays of both mechanical and chemical itch (Table 1). NPY likely acts via the Y1 receptor to hyperpolarize Y1-INs, a subclass of excitatory, somatostatinergic interneurons in the superficial dorsal horn that are likely to be essential for both pain and itch. Developing drugs to selectively mimic the body’s endogenous spinal inhibition systems may be highly efficacious in the treatment of pain and itch. Altogether, these results suggest that novel therapeutics for the treatment of chronic pain or itch may include spinally-directed Y1 agonists.
Supplementary Material
Highlights.
Spinally-directed NPY inhibits behavioral and molecular signs of pain and itch
Selective ablation or inhibition of spinal Y1-INs decreases neuropathic pain
Injury engages endogenous NPY-Y1 signaling to prevent transition to chronic pain
Spinal Y1-INs are promising targets for the treatment of chronic pain and itch
8. Acknowledgements
We thank Dr. Weisi Fu for the immunohistochemistry images provided in Figure 3. We thank Drs. Janice Urban and Carmen Birchmeier for the antibodies provided for Figure 3. We thank Dr. Michael Gold for providing insightful feedback for the preparation of this manuscript. Figures 1, 4, and 5 were created with BioRender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraira VE, Ginty DD, 2013. The sensory neurons of touch. Neuron 79, 618–39. 10.1016/j.neuron.2013.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acton D, Ren X, Di Costanzo S, Dalet A, Bourane S, Bertocchi I, Eva C, Goulding M, 2019. Spinal Neuropeptide Y1 Receptor-Expressing Neurons Form an Essential Excitatory Pathway for Mechanical Itch. Cell Rep. 28, 625–639. 10.1016/j.celrep.2019.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albisetti GW, Pagani M, Platonova E, Hösli L, Johannssen HC, Fritschy J-M, Wildner H, Zeilhofer HU, 2019. Dorsal Horn Gastrin-Releasing Peptide Expressing Neurons Transmit Spinal Itch But Not Pain Signals. J. Neurosci 39, 2238–2250. 10.1523/JNEUROSCI.2559-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, Guo C, Hantman AW, De Jonghe BC, Betley JN, 2018. A Neural Circuit for the Suppression of Pain by a Competing Need State. Cell 173, 140–152. 10.1016/j.cell.2018.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JM, Gibson SJ, Adrian TE, Polak JM, Bloom SR, 1984. Neuropeptide Y in human spinal cord. Brain Res. 308, 145–148. 10.1016/0006-8993(84)90926-0 [DOI] [PubMed] [Google Scholar]
- Arcourt A, Gorham L, Dhandapani R, Birchmeier C, Heppenstall PA, Lechner SG, Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, 2017. Touch Receptor-Derived Sensory Information Alleviates Acute Pain Signaling and Fine-Tunes Nociceptive Reflex Coordination Article Touch Receptor-Derived Sensory Information Alleviates Acute Pain Signaling and Fine-Tunes Nociceptive Reflex Coordination. Neuron 93, 179–193. 10.1016/j.neuron.2016.11.027 [DOI] [PubMed] [Google Scholar]
- Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, Goulding M, 2015. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350, 550–554. 10.1126/science.aac8653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle KA, Gutierrez-Mecinas M, Polgár E, Mooney N, O’Connor E, Furuta T, Watanabe M, Todd AJ, 2017. A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 363, 120–133. 10.1016/j.neuroscience.2017.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers SP, Wahlestedt C, 2010. Therapeutic potential of neuropeptide y (NPY) receptor ligands. EMBO Mol. Med. 2, 429–439. 10.1002/emmm.201000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky P, Hofstetter C, Olson L, Ohning G, Villar M, Hökfelt T, 2006. The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord. Neuroscience 138, 1361–1376. 10.1016/j.neuroscience.2005.11.069 [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Shi TS, Landry M, Villar MJ, Hökfelt T, 2007. Neuropeptide tyrosine and pain. Trends Pharmacol. Sci. 28, 93–102. 10.1016/j.tips.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Stanic D, Shuster S, Herzog H, Villar M, Hökfelt T, 2005. Neuropeptide Y2 receptor protein is present in peptidergic and nonpeptidergic primary sensory neurons of the mouse. J. Comp. Neurol 489, 328–348. 10.1002/cne.20639 [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Bergman E, Liu HX, Hökfelt T, Villar MJ, 2004. Effect of a graded single constriction of the rat sciatic nerve on pain behavior and expression of immunoreactive NPY and NPY Y1 receptor in DRG neurons and spinal cord. Brain Res. 1006, 87–99. 10.1016/j.brainres.2003.09.085 [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Shi TJ, Matsuda H, Kopp J, Villar MJ, Hökfelt T, 2002. NPY Y1 receptors are present in axonal processes of DRG neurons. Exp. Neurol 174, 1–10. 10.1006/exnr.2001.7845 [DOI] [PubMed] [Google Scholar]
- Cao CQ, Yu XH, Dray A, Filosa A, Perkins MN, 2003. A pro-nociceptive role of neuromedin U in adult mice. Pain 104, 609–616. 10.1016/S0304-3959(03)00118-0 [DOI] [PubMed] [Google Scholar]
- Chamessian A, Young M, Qadri Y, Berta T, Ji RR, Van De Ven T, 2018. Transcriptional Profiling of Somatostatin Interneurons in the Spinal Dorsal Horn. Sci. Rep 8, 1–16. 10.1038/s41598-018-25110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QL, Heinricher MM, 2019. Descending Control Mechanisms and Chronic Pain. Curr. Rheumatol. Rep 21, 1–7. 10.1007/s11926-019-0813-1 [DOI] [PubMed] [Google Scholar]
- Chen S, Liu XY, Jiao Y, Chen ZF, Yu W, 2019. NPY2R signaling gates spontaneous and mechanical, but not thermal, pain transmission. Mol. Pain 15, 1–7. 10.1177/1744806919887830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Henderson K, Beinfeld MC, Westfall TC, 1988. Alterations in blood pressure of normotensive and hypertensive rats following intrathecal injections of neuropeptide Y. J. Cardiovasc. Pharmacol 12, 473–478. 10.1097/00005344-198810000-00014 [DOI] [PubMed] [Google Scholar]
- Chen X, Knuepfer MM, Westfall TC, 1990. Hemodynamic and sympathetic effects of spinal administration of neuropeptide Y in rats. Am. J. Physiol. - Hear. Circ. Physiol 259, H1674–H1680. 10.1152/ajpheart.1990.259.6.h1674 [DOI] [PubMed] [Google Scholar]
- Chen X, Westfall TC, 1993. Depressor effect of intrathecal neuropeptide Y (NPY) is mediated by Y2 subtype of NPY receptors. J. Cardiovasc. Pharmacol 21, 720–724. 10.1097/00005344-199305000-00005 [DOI] [PubMed] [Google Scholar]
- Cheng L, Samad OA, Xu Y, Mizuguchi R, Luo P, Shirasawa S, Goulding M, Ma Q, 2005. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat. Neurosci 8, 1510–1515. 10.1038/nn1569 [DOI] [PubMed] [Google Scholar]
- Cleary DR, Roeder Z, Elkhatib R, Heinricher MM, 2014. Neuropeptide Y in the rostral ventromedial medulla reverses inflammatory and nerve injury hyperalgesia in rats via non-selective excitation of local neurons. Neuroscience 271, 149–159. 10.1016/j.neuroscience.2014.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin LA, Duggan AW, 2001. The effect of conduction block on the spinal release of immunoreactive-neuropeptide Y (ir-NPY) in the neuropathic rat. Pain 91, 235–240. 10.1016/S0304-3959(00)00438-3 [DOI] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK, 2013. Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 341, 1394–1399. 10.1126/science.1239403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Zhang ET, Blomqvist A, 2002. Association of spinothalamic lamina I neurons and their ascending axons with calbindin-immunoreactivity in monkey and human. Pain 97, 105–115. 10.1016/S0304-3959(02)00009-X [DOI] [PubMed] [Google Scholar]
- Decressac M, Barker RA, 2012. Neuropeptide Y and its role in CNS disease and repair. Exp. Neurol 238, 265–272. 10.1016/j.expneurol.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Diaz-delCastillo M, Christiansen SH, Appel CK, Falk S, Woldbye DPD, Heegaard AM, 2018. Neuropeptide Y is Up-regulated and Induces Antinociception in Cancer-induced Bone Pain. Neuroscience 384, 111–119. 10.1016/j.neuroscience.2018.05.025 [DOI] [PubMed] [Google Scholar]
- Dickie AC, Bell AM, Iwagaki N, Polgár E, Gutierrez-Mecinas M, Kelly R, Lyon H, Turnbull K, West SJ, Etlin A, Braz J, Watanabe M, Bennett DLH, Basbaum AI, Riddell JS, Todd AJ, 2019. Morphological and functional properties distinguish the substance P and gastrin-releasing peptide subsets of excitatory interneuron in the spinal cord dorsal horn. Pain 160, 442–462. 10.1097/j.pain.0000000000001406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross SE, Lowell BB, Wang Y, Goulding M, Ma Q, 2014. Identification of Spinal Circuits Transmitting and Gating Mechanical Pain. Cell 159, 1417–1432. 10.1016/j.cell.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A, Hope PJ, Lang CW, 1991. Microinjection of neuropeptide y into the superficial dorsal horn reduces stimulus-evoked release of immunoreactive substance p in the anaesthetized cat. Neuroscience 44, 733–740. 10.1016/0306-4522(91)90092-3 [DOI] [PubMed] [Google Scholar]
- Fu W, Nelson TS, Santos DF, Doolen S, Gutierrez JJP, Ye N, Zhou J, Taylor K, B., 2019. An NPY Y1 receptor antagonist unmasks latent sensitization and reveals the contribution of protein kinase A and Epac to chronic inflammatory pain. Pain 160, 1754–1765. 10.1097/j.pain.0000000000001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Wessel CR, Taylor BK, 2020. Neuropeptide Y tonically inhibits an NMDAR➔AC1➔TRPA1/TRPV1 mechanism of the affective dimension of chronic neuropathic pain. Neuropeptides 80, 102024. 10.1016/J.NPEP.2020.102024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T, Noguchi K, 2015. A potential anti-allodynic mechanism of GDNF following L5 spinal nerve ligation; Mitigation of NPY up-regulation in the touch sense pathway. Neuroscience 304, 240–249. 10.1016/j.neuroscience.2015.07.059 [DOI] [PubMed] [Google Scholar]
- Gao T, Ma H, Xu B, Bergman J, Larhammar D, Lagerström MC, 2018. The Neuropeptide Y System Regulates Both Mechanical and Histaminergic Itch. J. Invest. Dermatol 138, 2405–2411 10.1016/j.jid.2018.05.008 [DOI] [PubMed] [Google Scholar]
- Gibbs J, Flores CM, Hargreaves KM, 2004. Neuropeptide Y inhibits capsaicin-sensitive nociceptors via a Y1-receptor-mediated mechanism. Neuroscience 125, 703–709. 10.1016/j.neuroscience.2004.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Allen JM, Adrian TE, Kelly JS, Bloom SR, 1984. The distribution and origin of a novel brain peptide, neuropeptide Y, in the spinal cord of several mammals. J. Comp. Neurol 227, 78–91. 10.1002/cne.902270109 [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER, 2002. Correlations between neuronal morphology and electro-physiological features in the rodent superficial dorsal horn. J. Physiol 540, 189–207. 10.1113/jphysiol.2001.012890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Zhao C, Huang M, Huang T, Fan M, Xie Z, Chen Y, Zhao X, Xia G, Geng J, Cheng L, 2012. Tlx1/3 and Ptf1a Control the Expression of Distinct Sets of Transmitter and Peptide Receptor Genes in the Developing Dorsal Spinal Cord. J. Neurosci 32, 8509–8520. 10.1523/JNEUROSCI.6301-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Gautam M, Prasoon P, Kumar R, Ray SB, Kaler Jhajhria S, 2018. Involvement of Neuropeptide y in Post-Incisional Nociception in Rats. Ann. Neurosci 25, 268–276. 10.1159/000495130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Bell A, Polgár E, Watanabe M, Todd AJ, 2019. Expression of Neuropeptide FF Defines a Population of Excitatory Interneurons in the Superficial Dorsal Horn of the Mouse Spinal Cord that Respond to Noxious and Pruritic Stimuli. Neuroscience 416, 281–293. 10.1016/j.neuroscience.2019.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Furuta T, Watanabe M, Todd AJ, 2016. A quantitative study of neurochemically defined excitatory interneuron populations in laminae I–III of the mouse spinal cord. Mol. Pain 12, 1–18. 10.1177/1744806916629065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenraets K, Albisetti GW, Foster E, Wildner H, 2018. Adeno-associated Virus-mediated Transgene Expression in Genetically Defined Neurons of the Spinal Cord. J. Vis. Exp 1–11. 10.3791/57382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenraets K, Foster E, Johannssen H, Kandra V, Frezel N, Steffen T, Jaramillo V, Paterna JC, Zeilhofer HU, Wildner H, 2017. Spinal nociceptive circuit analysis with recombinant adeno-associated viruses: the impact of serotypes and promoters. J. Neurochem 142, 721–733. 10.1111/jnc.14124 [DOI] [PubMed] [Google Scholar]
- Häring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lönnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O, Lagerström MC, Linnarsson S, Ernfors P, 2018. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci 21, 869–880. 10.1038/s41593-018-0141-1 [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Brumovsky P, Shi T, Pedrazzini T, Villar M, 2007. NPY and pain as seen from the histochemical side. Peptides 28, 365–372. 10.1016/j.peptides.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Hu J, Huang T, Li T, Guo Z, Cheng L, 2012. C-Maf is required for the development of dorsal horn laminae III/IV neurons and mechanoreceptive DRG axon projections. J. Neurosci 32, 5362–5373. 10.1523/JNEUROSCI.6239-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XY, Boublik JH, Spicer MA, Rivier JE, Brown MR, Yaksh TL, 1991. The antinociceptive effects of spinally administered neuropeptide Y in the rat: systematic studies on structure-activity relationship. J. Pharmacol. Exp. Ther 258, 243–8. [PubMed] [Google Scholar]
- Huang J, Polgár E, Solinski HJ, Mishra SK, Tseng PY, Iwagaki N, Boyle KA, Dickie AC, Kriegbaum MC, Wildner H, Zeilhofer HU, Watanabe M, Riddell JS, Todd AJ, Hoon MA, 2018. Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci 21, 707–716. 10.1038/s41593-018-0119-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Huang T, Xiang Y, Xie Z, Chen Y, Yan R, Xu J, Cheng L, 2008. Ptf1a, Lbx1 and Pax2 coordinate glycinergic and peptidergic transmitter phenotypes in dorsal spinal inhibitory neurons. Dev. Biol 322, 394–405. 10.1016/j.ydbio.2008.06.031 [DOI] [PubMed] [Google Scholar]
- Intondi AB, Dahlgren MN, Eilers MA, Taylor BK, 2008. Intrathecal neuropeptide Y reduces behavioral and molecular markers of inflammatory or neuropathic pain. Pain 137, 352–365. 10.1016/j.pain.2007.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson JET, Ma H, Lagerström MC, 2019. Neuropeptide Y in itch regulation. Neuropeptides 78, 101976. 10.1016/j.npep.2019.101976 [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ, 2003. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci. 26, 696–705. 10.1016/j.tins.2003.09.017 [DOI] [PubMed] [Google Scholar]
- Jiang N, Furue H, Katafuchi T, Yoshimura M, 2003. Somatostatin directly inhibits substantia gelatinosa neurons in adult rat spinal dorsal horn in vitro. Neurosci. Res 47, 97–107. 10.1016/S0168-0102(03)00183-4 [DOI] [PubMed] [Google Scholar]
- Jung SJ, Chang JW, Won R, Cha MH, Nam TS, Lee HJ, Lee BH, 2009. Modulation of neuropathic pain by galanin and neuropeptide y at the level of the medulla in rats. Int. J. Neurosci 119, 1941–1955. 10.1080/00207450903263661 [DOI] [PubMed] [Google Scholar]
- Kar S, Quirion R, 1992. Quantitative autoradiographic localization of [125I] neuropeptide Y receptor binding sites in rat spinal cord and the effects of neonatal capsaicin, dorsal rhizotomy and peripheral axotomy. Brain Res. 574, 333–337. 10.1016/0006-8993(92)90836-X [DOI] [PubMed] [Google Scholar]
- Kim SJ, Chung WH, Rhim H, Eun SY, Jung SJ, Kim J, 2002. Postsynaptic action mechanism of somatostatin on the membrane excitability in spinal substantia gelatinosa neurons of juvenile rats. Neuroscience 114, 1139–1148. 10.1016/S0306-4522(02)00245-2 [DOI] [PubMed] [Google Scholar]
- Kline RH, Wiley RG, 2008. Spinal -Opioid Receptor-Expressing Dorsal Horn Neurons: Role in Nociception and Morphine Antinociception. J. Neurosci 28, 904–913. 10.1523/JNEUROSCI.4452-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight KH, Brand FM, Mchaourab AS, Veneziano G, 2007. Implantable intrathecal pumps for chronic pain: Highlights and updates. Croat. Med. J 48, 22–34. [PMC free article] [PubMed] [Google Scholar]
- Kuphal KE, Solway B, Pedrazzini T, Taylor BK, 2008. Y1 receptor knockout increases nociception and prevents the anti-allodynic actions of NPY. Nutrition 24, 885–891. 10.1016/j.nut.2008.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ, 2009. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 10, 895–926. 10.1016/j.jpain.2009.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons LL, Wiley RG, 2012. Neuropeptide Y receptor-expressing dorsal horn neurons: Role in nocifensive reflex and operant responses to aversive cold after CFA inflammation. Neuroscience 216, 158–166. 10.1016/j.neuroscience.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD, 2011. The Functional Organization of Cutaneous Low-Threshold Mechanosensory Neurons. Cell 147, 1615–1627 10.1016/j.cell.2011.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ji R, Xiong L, Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, 2013. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J. Clin. Invest 123, 4050–4062. 10.1172/JCI70026.4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Gao T, Jakobsson JET, Weman HM, Xu B, Larhammar D, Lagerström MC, 2020. The neuropeptide Y Y2 receptor is coexpressed with NPPB in primary afferent neurons and Y2 activation reduces histaminergic and IL-31-induced itch. J. Pharmacol. Exp. Ther 372, 73–82. 10.1124/jpet.119.262584 [DOI] [PubMed] [Google Scholar]
- Magnussen C, Hung S-P, Ribeiro-da-Silva A, 2015. Novel Expression Pattern of Neuropeptide Y Immunoreactivity in the Peripheral Nervous System in a Rat Model of Neuropathic Pain. Mol. Pain 11, 1–12. 10.1186/s12990-015-0029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahinda TB, Taylor BK, 2004. Intrathecal neuropeptide Y inhibits behavioral and cardiovascular responses to noxious inflammatory stimuli in awake rats. Physiol. Behav 80, 703–711. 10.1016/j.physbeh.2003.12.007 [DOI] [PubMed] [Google Scholar]
- Malet M, Leiguarda C, Gastón G, McCarthy C, Brumovsky P, 2017. Spinal activation of the NPY Y1 receptor reduces mechanical and cold allodynia in rats with chronic constriction injury. Peptides 92, 38–45. 10.1016/j.peptides.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Mark MA, Colvin LA, Duggan AW, 1998. Spontaneous release of immunoreactive neuropeptide y from the central terminals of large diameter primary afferents of rats with peripheral nerve injury. Neuroscience 83, 581–589. 10.1016/S0306-4522(97)00402-8 [DOI] [PubMed] [Google Scholar]
- Mark MA, Jarrott B, Colvin LA, MacMillan SJA, Duggan AW, 1997. The release of immunoreactive neuropeptide Y in the spinal cord of the anaesthetized rat and cat. Brain Res. 754, 195–203. 10.1016/S0006-8993(97)00061-9 [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Chen W, Fu W, Taylor BK, 2019. Neuropeptide Y release in the rat spinal cord measured with Y1 receptor internalization is increased after nerve injury. Neuropharmacology 158, 107732. 10.1016/j.neuropharm.2019.107732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado ML, Gibert-Rahola J, Chover AJ, Micó JA, 1996. Effect on nociception of intracerebroventricular administration of low doses of neuropeptide Y in mice. Life Sci. 58, 2409–2414. 10.1016/0024-3205(96)00244-5 [DOI] [PubMed] [Google Scholar]
- Melnick IV, 2012. Cell type-specific postsynaptic effects of neuropeptide Y in substantia gelatinosa neurons of the rat spinal cord. Synapse 66, 640–649. 10.1002/syn.21550 [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA, 2013. The cells and circuitry for itch responses in mice. Science. 340, 968–971. 10.1126/science.1233765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa A, Furue H, Katafuchi T, Jiang N, Yasaka T, Kato G, Yoshimura M, 2005. Action of neuropeptide Y on nociceptive transmission in substantia gelatinosa of the adult rat spinal dorsal horn. Neuroscience 134, 595–604. 10.1016/j.neuroscience.2005.04.045 [DOI] [PubMed] [Google Scholar]
- Moehring F, Halder P, Seal RP, Stucky CL, 2018. Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron 100, 349–360. 10.1016/j.neuron.2018.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TD, Colmers WF, Smith P. a, 2004. Opioid-like actions of neuropeptide Y in rat substantia gelatinosa: Y1 suppression of inhibition and Y2 suppression of excitation. J. Neurophysiol 92, 3266–3275. 10.1152/jn.00096.2004 [DOI] [PubMed] [Google Scholar]
- Murase K, Nedeljkov V, Randić M, 1982. The actions of neuropeptides on dorsal horn neurons in the rat spinal cord slice preparation: an intracellular study. Brain Res. 234, 170–176. 10.1016/0006-8993(82)90483-8 [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Lucas G, Blakeman KH, Hao JX, Xu XJ, Wiesenfeld-Hallin Z, Thorén P, Ernfors P, 2001. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature 409, 513–517. 10.1038/35054063 [DOI] [PubMed] [Google Scholar]
- Nazli M, Morris R, 2000. Expression of neuropeptide Y and neuropeptide Y Y1 receptors and neuronal markers following axotomy in the rat spinal cord and gracile nucleus. Anat. Histol. Embryol 29, 97–101. 10.1046/j.1439-0264.2000.00242.x [DOI] [PubMed] [Google Scholar]
- Nelson TS, 2019. Dorsal Horn PKC␥ Interneurons Mediate Mechanical Allodynia through 5-HT 2A R-Dependent Structural Reorganization. J. Neurosci 39, 6221–6223. 10.1523/JNEUROSCI.0291-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TS, Fu W, Donahue RR, Corder GF, Hökfelt T, Wiley RG, Taylor BK, 2019. Facilitation of neuropathic pain by the NPY Y1 receptor-expressing subpopulation of excitatory interneurons in the dorsal horn. Sci. Rep 9, 1–14. 10.1038/s41598-019-43493-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Zhang ET, Carvajal C, Gardell L, Quirion R, Dumont Y, Lai J, Porreca F, 2002. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J. Neurosci 22, 9858–9867. 10.1523/jneurosci.22-22-09858.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Albisetti GW, Sivakumar N, Wildner H, Santello M, Johannssen HC, Zeilhofer HU, 2019. How Gastrin-Releasing Peptide Opens the Spinal Gate for Itch. Neuron 103, 1–16. 10.1016/j.neuron.2019.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Fatima M, Li A, Lee H, Cai W, Horwitz L, Hor CC, Zaher N, Cin M, Slade H, Huang T, Xu XZS, Duan B, 2019. Identification of a Spinal Circuit for Mechanical and Persistent Spontaneous Itch. Neuron 103, 1135–1149. 10.1016/j.neuron.2019.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Dallel R, Todd AJ, 2020. Recent advances in our understanding of the organization of dorsal horn neuron populations and their contribution to cutaneous mechanical allodynia. J. Neural Transm. 127, 505–525. 10.1007/s00702-020-02159-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Seal RP, 2016. Neural circuits for pain: Recent advances and current views. Science. 354, 578–584. 10.7554/eLife.22866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Williams SPG, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, Seal RP, 2015. Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron 87, 797–812. 10.1016/j.neuron.2015.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean HB, Bourojeni F, Tsao D, Davidova A, Sotocinal SG, Mogil JS, Kania A, Sharif-Naeini R, 2019. Recruitment of Spinoparabrachial Neurons by Dorsal Horn Calretinin Neurons. Cell Rep. 28, 1429–1438. 10.1016/j.celrep.2019.07.048 [DOI] [PubMed] [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan LY, Ribeiro-da-Silva A, Braz JM, Basbaum AI, Sharif-Naeini R, 2015. Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Rep. 13, 1246–1257. 10.1016/j.celrep.2015.09.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapunar D, Vukojević K, Kostić S, Puljak L, 2011. Attenuation of pain-related behavior evoked by injury through blockade of neuropeptide y Y2 receptor. Pain 152, 1173–1181. 10.1016/j.pain.2011.01.045 [DOI] [PubMed] [Google Scholar]
- Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC, Kelley MW, Levine AJ, 2018. Massively Parallel Single Nucleus Transcriptional Profiling Defines Spinal Cord Neurons and Their Activity during Behavior. Cell Rep. 22, 2094–2106. 10.1016/j.celrep.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi TJS, Li J, Dahlström A, Theodorsson E, Ceccatelli S, Decosterd I, Pedrazzini T, Hökfelt T, 2006. Deletion of the neuropeptide Y Y1 receptor affects pain sensitivity, neuropeptide transport and expression, and dorsal root ganglion neuron numbers. Neuroscience 140, 293–304. 10.1016/j.neuroscience.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Smith K, Browne T, Davis O, Coyle A, Boyle K, Watanabe M, Dickinson S, Iredale J, Gradwell M, Jobling P, Callister R, Dayas C, Hughes D, Graham B, 2019. Calretinin positive neurons form an excitatory amplifier network in the spinal cord dorsal horn. Elife 8, e49190. 10.7554/eLife.49190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Boyle KA, Madden JF, Dickinson SA, Jobling P, Callister RJ, Hughes DI, Graham BA, 2015. Functional heterogeneity of calretinin-expressing neurons in the mouse superficial dorsal horn: Implications for spinal pain processing. J. Physiol 593, 4319–4339. 10.1113/JP270855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK, 2007. Spinal mechanisms of NPY analgesia. Peptides 28, 464–474. 10.1016/j.peptides.2006.09.029 [DOI] [PubMed] [Google Scholar]
- Solinski HJ, Kriegbaum MC, Tseng PY, Earnest TW, Gu X, Barik A, Chesler AT, Hoon MA, 2019. Nppb Neurons Are Sensors of Mast Cell-Induced Itch. Cell Rep. 26, 3561–3573. 10.1016/j.celrep.2019.02.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solway B, Bose SC, Corder G, Donahue RR, Taylor BK, 2011. Tonic inhibition of chronic pain by neuropeptide Y. PNAS 108, 7224–7229. 10.1073/pnas.1017719108/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1017719108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Silva E, Stein T, Mascarin LZ, Dornelles FN, Bicca MA, Tonussi CR, 2020. Intra-articular injection of 2-pyridylethylamine produces spinal NPY-mediated antinociception in the formalin-induced rat knee-joint pain model. Brain Res. 1735, 146757. 10.1016/j.brainres.2020.146757 [DOI] [PubMed] [Google Scholar]
- Taiwo OB, Taylor BK, 2002. Antihyperalgesic effects of intrathecal neuropeptide Y during inflammation are mediated by Y1 receptors. Pain 96, 353–363. 10.1016/S0304-3959(01)00481-X [DOI] [PubMed] [Google Scholar]
- Tan CMJ, Green P, Tapoulal N, Lewandowski AJ, Leeson P, Herring N, 2018. The role of neuropeptide Y in cardiovascular health and disease. Front. Physiol 9, 1–13. 10.3389/fphys.2018.01281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V, 1982. Neuropeptide Y—a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296, 659–660. 10.1038/296659a0 [DOI] [PubMed] [Google Scholar]
- Taylor BK, Abhyankar SS, Vo NTT, Kriedt CL, Churi SB, Urban JH, 2007. Neuropeptide Y acts at Y1 receptors in the rostral ventral medulla to inhibit neuropathic pain. Pain 131, 83–95. 10.1016/j.pain.2006.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Corder G, 2014. Endogenous Analgesia, Dependence, and Latent Pain Sensitization. Curr. Top. Behav. Neurosci 20, 283–325. 10.1007/7854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Fu W, Kuphal KE, Stiller CO, Winter MK, Chen W, Corder GF, Urban JH, McCarson KE, Marvizon JC, 2014. Inflammation enhances Y1 receptor signaling, neuropeptide Y-mediated inhibition of hyperalgesia, and substance P release from primary afferent neurons. Neuroscience 256, 178–194. 10.1016/j.neuroscience.2013.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, 2017. Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Mol. Pain 13, 1–19. 10.1177/1744806917693003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R, Croll SD, Vercollone J, Reinhardt J, Griffiths J, Zabski S, Anderson KD, Adams NC, Gowen L, Sleeman MW, Valenzuela DM, Wiegand SJ, Yancopoulos GD, Murphy AJ, 2007. Mice genetically deficient in neuromedin U receptor 2, but not neuromedin U receptor 1, have impaired nociceptive responses. Pain 130, 267–278. 10.1016/j.pain.2007.01.036 [DOI] [PubMed] [Google Scholar]
- Tracey DJ, Romm MA, Yao NNL, 1995. Peripheral hyperalgesia in experimental neuropathy: exacerbation by neuropeptide Y. Brain Res. 669, 245–254. 10.1016/0006-8993(94)01265-J [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennett GJ, 1991. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci. Lett 124, 200–203. 10.1016/0304-3940(91)90093-9 [DOI] [PubMed] [Google Scholar]
- White DM, 1997. Intrathecal neuropeptide Y exacerbates nerve injury-induced mechanical hyperalgesia. Brain Res. 750, 141–146. 10.1016/S0006-8993(96)01340-6 [DOI] [PubMed] [Google Scholar]
- White DM, Mansfield K, 1996. Vasoactive intestinal polypeptide and neuropeptide Y act indirectly to increase neurite outgrowth of dissociated dorsal root ganglion cells. Neuroscience 73, 881–887. 10.1016/0306-4522(96)00055-3 [DOI] [PubMed] [Google Scholar]
- Wiley RG, Kline RH, 2000. Neuronal lesioning with axonally transported toxins. J. Neurosci. Methods 103, 73–82. 10.1016/S0165-0270(00)00297-1 [DOI] [PubMed] [Google Scholar]
- Wiley RG, Lemons LL, Kline RH, 2009. Neuropeptide Y receptor-expressing dorsal horn neurons: Role in nocifensive reflex responses to heat and formalin. Neuroscience 161, 139–147. 10.1016/j.neuroscience.2008.12.017 [DOI] [PubMed] [Google Scholar]
- Xu IS, Hao J-X, Xu X-J, Hökfelt T, Wiesenfeld-Hallin Z, 1999. The effect of intrathecal selective agonists of Y1 and Y2 neuropeptide Y receptors on the flexor reflex in normal and axotomized rats. Brain Res. 833, 251–257. 10.1016/S0006-8993(99)01551-6 [DOI] [PubMed] [Google Scholar]
- Xu IS, Luo L, Ji RR, Hökfelt T, Xu XJ, Wiesenfeld-Hallin Z, 1998. The effect of intrathecal neuropeptide Y on the flexor reflex in rats after carrageenan-induced inflammation. Neuropeptides 32, 447–452. 10.1016/S0143-4179(98)90070-1 [DOI] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Hökfelt T, Wiesenfeld-Hallin Z, 1994. The effects of intrathecal neuropeptide Y on the spinal nociceptive flexor reflex in rats with intact sciatic nerves and after peripheral axotomy. Neuroscience 63, 817–826. 10.1016/0306-4522(94)90526-6 [DOI] [PubMed] [Google Scholar]
- Xu Y, Lopes C, Qian Y, Liu Y, Cheng L, Goulding M, Turner EE, Lima D, Ma Q, 2008. Tlx1 and Tlx3 Coordinate Specification of Dorsal Horn Pain-Modulatory Peptidergic Neurons. J. Neurosci 28, 4037–4046. 10.1523/JNEUROSCI.4126-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji RR, 2015. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med 21, 1326–1331. 10.1038/nm.3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalamuri SM, Brennan TJ, Spofford CM, 2013. Neuropeptide y is analgesic in rats after plantar incision. Eur. J. Pharmacol 698, 206–212. 10.1016/j.ejphar.2012.10.036 [DOI] [PubMed] [Google Scholar]
- Yu XH, Cao CQ, Mennicken F, Puma C, Dray A, O’Donnell D, Ahmad S, Perkins M, 2003. Pro-nociceptive effects of neuromedin U in rat. Neuroscience 120, 467–474. 10.1016/S0306-4522(03)00300-2 [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Xu ZQ, Kopp J, Arvidsson U, Elde R, Hökfelt T, 1994. Localization of neuropeptide Y Y1 receptors in the rat nervous system with special reference to somatic receptors on small dorsal root ganglion neurons. Proc. Natl. Acad. Sci. U. S. A 91, 11738–11742. 10.1073/pnas.91.24.11738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tong YG, Bao L, Hokfelt T, 1999. The neuropeptide Y Y1 receptor is a somatic receptor on dorsal root ganglion neurons and a postsynaptic receptor on somatostatin dorsal horn neurons. Eur. J. Neurosci 11, 2211–2225. 10.1046/j.1460-9568.1999.00638.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.