Abstract
Purpose:
Present clinical updates, current research findings, and consensus statements relevant to the care of the acute kidney injury (AKI) patient.
Principal findings:
AKI is one of the most frequent and debilitating complications of surgery and critical illness. Consensus criteria use serum creatinine and urine output measurements to diagnose AKI and allow for objective diagnosis and more accurate comparisons across populations. New serum and urine biomarkers may provide earlier evidence of AKI, but their clinical utility, while increasing, remains limited. Avoidance of nephrotoxins, intravascular fluid management, and maintenance of renal perfusion are the mainstays of preventative management and treatment of AKI. Optimal timing for the initiation of renal replacement therapy (RRT) is controversial and remains under investigation.
Conclusions:
Acute kidney injury continues to affect large numbers of patients receiving surgery or in the intensive care unit (ICU), but specific advances in resuscitation techniques, endpoint refinements, epidemiology, biomarkers, and pathology are providing the necessary framework to reduce AKI and associated morbidity.
In the past decade, preclinical research has provided insight into the pathobiology of acute kidney injury (AKI) and into the associated cellular mechanisms of renal damage and repair. In addition, results from clinical trials and observational cohort studies have improved our capacity to accurately diagnose AKI, manage patients with AKI, and predict future events in patients with AKI. While effective preventative and therapeutic treatments for AKI largely remain lacking, the clinical research framework to effectively test therapies has improved. In this report, we outline recent advances in the study of perioperative AKI. Among clinical updates we present current research findings and consensus statements relevant to the care of the AKI patient.
Incidence and risk factors for AKI
AKI is one of the most frequent and debilitating complications of surgery and critical illness. In the 21st century, AKI affects 13% of patients receiving open abdominal surgery, 25% of patients receiving cardiac surgery, and 57% of patients admitted to an ICU.[1]
The three most common precipitating events that lead to AKI are sepsis, surgery, and exposure to intravenous radiocontrast. The incidence of AKI is increased in patients with chronic kidney disease (CKD), diabetes, advanced age, African ancestry, chronic lung, liver, or cerebrovascular disease, and conditions that limit oxygen delivery to the kidneys including congestive heart failure, peripheral vascular disease, anemia, hypotension, venous congestion, and intravascular hypovolemia. It follows that anesthesiologists, intensivists, and surgeons must implement prevention strategies for AKI in patients with these risk factors who are exposed to sepsis, surgery, or radiocontrast.
Clinical presentation of AKI – Diagnosis
Current consensus criteria for AKI diagnosis are based on measured changes in urine output and serum creatinine. The most current iteration – the Kidney Disease Improving Global Outcomes (KDIGO) criteria – defines stage 1 AKI as a 0.3 mg/dl (≥26.5 μmol/l) serum creatinine increase from baseline within 48 hours of surgery, a 50% serum creatinine increase from baseline within 7 days of surgery, or urine output below 0.5 ml·kg−1·hr−1 for 6 hours. KDIGO criteria define stage 2 AKI as a 100% increase within 7 days of surgery or urine output below 0.5 ml·kg−1·hr−1 for 12 hours and stage 3 AKI as a 200% increase within 7 days of surgery or urine output below 0.3 ml·kg−1·hr−1 for 24 hours or anuria for 12 hours (Table 1).[2] The use of consensus criteria enables clinicians to objectively identify AKI, to compare AKI rates across providers, institutions, and patient populations, and to establish endpoints for therapeutic and prophylactic treatment trials. Unfortunately, changes in serum creatinine concentration are often insensitive and nonspecific for renal injury, because the association between renal injury and serum creatinine is often confounded by changes in creatinine production, myocyte injury, intravenous fluid administration, intravascular fluid extravasation, diuretic use, tubular creatinine secretion, and renal functional reserve. In fact, following cardiac surgery, many patients who do not develop AKI have a reduction in serum creatinine from baseline on postoperative day 1, whereas even a modest increase of serum creatinine on postoperative day 1 may be prognostic for subsequent diagnosis of AKI. These early changes reflect the impact of hemorrhage, resuscitation, and hemodilution as much as they do renal function and injury. Urine output is also non-specific for AKI and may lead to an over-diagnosis of AKI. Nonetheless, patients that meet AKI criteria by both an increase in serum creatinine and a decrease in urine output have significantly worse long-term renal outcomes and mortality than patients that only meet serum creatinine or urine output criteria.[3]
Table 1.
Kidney disease improving global outcomes (KDIGO) consensus criteria for AKI
| AKI Stage | Serum Creatinine Concentration | Urine Output |
|---|---|---|
| 1 | 50–100% increase within 7 days or 0.3 mg/dl 26.5 (μmol/l) increase within 48 hr |
<0.5 ml·kg−1·hr−1 or 6–12 hr |
| 2 | 100–200% increase within 7 days | <0.5 ml·kg−1·hr−1 for >12 hr |
| 3 | ≥200 increase, ≥4.0 mg/dl (354 μmol/l) with a ≥0.5 mg/dl (44 μmol/l) increase, or dialysis within 7 days | <0.3 ml·kg−1·hr−1 for ≥24 hr or anuria for ≥12 hr |
Biomarkers of renal injury that are expressed in the urine or plasma within hours of renal injury have been identified, and these markers may be more specific and sensitive for renal stress and injury than markers of impaired glomerular filtration (Table 2).[4, 5] Their clinical use for diagnosis and management of AKI, however, remains limited. In fact, the majority of these markers remain confined to research purposes. The FDA has approved the measurement of urinary tissue inhibitor of metalloproteinases (TIMP) 2 and insulin-like growth factor–binding protein (IGFBP) 7, markers of growth phase cell-cycle arrest, marketed as the Nephrocheck® test, to aid in the risk assessment for moderate or severe AKI within 12 hours of cardiac surgery, thus providing a commercially available test to measure kidney stress. The validation of Nephrocheck® for acute and persistent renal injury in different clinical settings is ongoing.
Table 2.
Features and utility of markers of acute kidney injury (AKI). Data represent approximations since these features of AKI depend on severity and persistence of injury
| Feature | Functional marker | Damage marker | Time of onset | Time of peak expression | AUROC vs. KDIGO | Marker of prognosis | Clinical availability | Favorable Cost |
|---|---|---|---|---|---|---|---|---|
| Urine output | ★★ | ★ | 1 hr | 24 hr | 1.00 | ★ | ★★★★ | ★★★★★ |
| Serum creatinine | ★★★ | ★★ | 24 hr | 72 hr | 1.00 | ★ | ★★★★★ | ★★★★ |
| Serum cystatin C | ★★★★ | ★★ | 24 hr | 72 hr | 0.63 | ★ | ★ | ★ |
| Urine NGAL | ★ | ★★★★ | 2 hr | 6 hr | 0.70 | ★★ | ★ | ★ |
| Urine KIM-1 | ★ | ★★★★ | 6 hr | 24 hr | 0.68 | ★★ | ★ | ★ |
| Urine IGFBP7• TIMP-2 | ★ | ★★★★ | 1 hr | 6hr | 0.75 | ★★ | ★★★ | ★★ |
| Dialysis | ★★★★ | ★★ | 36 hr | NA | 1.00 | ★★★★ | ★★★★★ | ★★★★★ |
| MAKE 30 | ★★★★ | ★★ | 30 days | NA | NA | ★★★ | ★★★★ | ★★★★ |
| MAKE 90 | ★★★★ | ★★ | 90 days | NA | NA | ★★★★ | ★★★★ | ★★★★ |
★ = Least relevant or favorable; ★★★★★ = Most relevant or favorable; NA = not applicable.
AUROC, area under the receiver operating characteristic curve – range 0.00–1.00 where a higher value indicates superior association with KDIGO criteria AKI; KDIGO, kidney disease improving global outcomes; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1; IGFBP7, insulin-like growth factor binding protein 7; TIMP-2, tissue inhibitor of metalloproteinase 2; MAKE, major adverse kidney events, a dichotomous endpoint at 30 or 90 days defined as a 25% eGFR decline, new dialysis, or death
Prevention of AKI
Intravascular fluid management remains a mainstay of AKI prevention. The best composition of isotonic fluid has until recently remained unclear. In critically ill patients, a balanced salt solution that approximates plasma concentrations of sodium, chloride, and potassium, such as Plasma-Lyte©, Hartman’s solution, or lactated Ringer’s solution has been associated with less major adverse kidney events (MAKE) at 30 days compared to hyperchloremic solutions such as ‘normal’ (0.9%) saline,[6] and recent evidence suggests that balanced isotonic salt solutions may protect the kidneys compared to isotonic sodium chloride. The SMART trial, a large randomized trial of balanced salt solution or saline treatment immediately upon presentation to the intensive care unit found that a balanced salt solution reduces MAKE30 compared to saline.[7] Therefore, aside from select patient populations such as those with increased intracranial pressure, a balanced salt solution may be preferable treatment in acutely ill patients. Recovery pathways that promote restrictive administration of intravenous fluids with a goal of advancing early recovery in patients undergoing major surgery have become popular despite limited evidence. The RELIEF trial, however, randomized 3000 major abdominal surgery patients to restrictive (approximately 1.5L of balanced crystalloid solution during a 4 hour surgery followed by 0.8ml/kg/hr for at least 24 hours postoperatively) or liberal (approximately 3L of balanced crystalloid during a 4 hour surgery followed by 1.5 ml/kg/hr for at least 24 hours postoperatively) intravenous fluid administration during surgery and in the early postoperative period and found that restrictive fluid administration was not associated with increased disability-free survival.[8] In addition, pre-specified secondary analyses noted increased acute kidney injury in the restrictive administration group compared to the liberal administration group. Additional large pragmatic RCTs, including PLUS (NCT02721654), BaSICS (NCT02875873) and SOLAR (NCT02565420) will provide more evidence on the relative renal benefits and harms of commonly administered crystalloid intravenous fluids.
Radiocontrast-associated AKI may also be affected by choice of resuscitative crystalloid fluid. For example, administration of intravenous isotonic fluid decreases AKI when compared with hypotonic fluid in patients receiving intravenous radiocontrast. Based on the rationale that sodium bicarbonate may reduce oxidative stress in the nephron or in the blood by scavenging reactive oxygen species and by decreasing reactive oxygen species generated via iron-dependent mechanisms, clinician scientists have hypothesized that administration of sodium bicarbonate decreases radiocontrast induced AKI. Recent trials in patients exposed to radiocontrast, however, found no difference in rates or severity of contrast-induced AKI or persistent renal injury between patients administered isotonic sodium bicarbonate and patients administered isotonic sodium chloride.[9]
Colloid solutions have been the subject of many investigations examining their effect on kidney injury and renal recovery. Using the rationale that increased colloid oncotic pressure might limit intravascular fluid extravasation, starch solutions were historically administered as part of intravascular volume resuscitation. Some hydroxylethyl starch solutions have been noted to increase the risk of AKI and RRT and lead to increased mortality in septic patients.[10] Data regarding the use of albumin as a resuscitative solution are inconclusive. In septic patients, no effect of albumin resuscitation on AKI or need for RRT has been noted in large meta-analyses.[11] In a study of hypoalbuminemic patients undergoing off pump coronary artery bypass surgery, administration of 20% albumin preoperatively increased urine output and decreased the risk of AKI.[12] The administration of albumin is more costly than administration of crystalloid solutions, and results of trials examining the effects of albumin on AKI are mixed. There may be some benefit to its administration in hypoalbuminemic patients, but there is insufficient evidence to support widespread use of albumin as a resuscitative fluid to prevent AKI.
Anemia can decrease renal oxygen delivery, and a hemoglobin concentration less than 8 g/dL (5 mmol/l) during noncardiac surgery is associated with a 400% increase in AKI.[13] Transfusion with packed red blood cells, however, has also been associated with AKI.[14] The TRICS III trial randomized 4860 cardiac surgery patients to a transfusion threshold of 9.5 g/dl or 7.5 g/dl. Transfusion threshold did not affect new renal failure requiring dialysis, despite increased transfusion in the higher threshold group, and a kidney substudy of this trial noted no differences in mild, moderate, or severe AKI between randomized treatment groups. [15] Transfusion of packed red blood cells stored longer prior to transfusion also increase the risk of AKI. Putative mechanisms that have been proposed include increased inflammation, oxidative stress, or impaired oxygen delivery. Current recommendations emphasize prevention of anemia and judicious blood product transfusion in surgical patients at risk for AKI to balance the risks of anemia and transfusion.
Preventative management for AKI also includes maintenance of renal perfusion and the avoidance of medications that decrease glomerular filtration in patients that are hypovolemic, such as NSAIDS and ACE inhibitors, and avoidance of nephrotoxins, including intravenous radiocontrast and aminoglycosides. Identifying patients who are hypovolemic and quantifying the degree of intravascular depletion remains a significant clinical problem, particularly since excess resuscitation and hypervolemia lead to venous congestion, a condition associated with AKI in cohorts of patients receiving surgery, with sepsis, admitted to the ICU, and with heart failure.[16] No longer can clinicians feel they are protecting the kidneys by administering large amounts of intravenous (IV) fluids. Clinicians should maintain renal perfusion by preserving or augmenting cardiac output, treating hypotension with agents that also increase cardiac output such as norepinephrine or IV fluids while avoiding hypervolemia, and avoiding severe anemia. Vasoactive agents that exclusively increase systemic vascular resistance, such as phenylephrine or vasopressin, may not best preserve renal perfusion while treating hypotension. In an RCT of patients with vasoplegic shock after cardiac surgery receiving norepinephrine, however, patients treated with vasopressin were less likely to develop a composite endopoint of mortality or organ dysfunction compared to patients treated with additional norepinephrine. Vasopressin-treated patients had a hazard ratio of 0.26 for renal failure compared to norepinephrine-treated patients, and additional trials examining vasopressin should be performed.[17]
Even though renal ischemia is responsible for a large portion of perioperative AKI, treatments to augment renal perfusion or decrease renal oxygen consumption, including the administration of dopamine receptor agonists or diuretics, have largely failed. Dopamine agonists increase renal perfusion, glomerular filtration, sodium delivery to the tubule, solute reabsorption requirements, and renal tubule oxygen consumption. Diuretics decrease renal oxygen consumption but can lead to hypovolemia and do not decrease AKI. Other preventative therapies, including perioperative administration of statins in patients having cardiac surgery, sodium bicarbonate,[18] corticosteroids,[19] and alkaline phosphatase have yielded mixed results but generally failed to reduce AKI. Remote ischemic preconditioning is performed by inflating a cuff on a limb (most commonly the arm) to suprasystolic pressure for 3 cycles of 5 minutes. In cardiac surgery patients, two large RCTs have found that RIPC does not decrease risk of AKI while another has observed a protective effect against AKI.
AKI progression, management, and prognosis
Until specific therapeutics are available, AKI treatment must focus on supportive care and avoiding further insults. While numerous modifiable risk factors such as anemia, hypotension, impaired cardiac output, hemolysis, rhabdomyolysis, exposure to nephrotoxic drugs, and blood transfusion have been identified, most pharmacologic prevention (and salvage) treatment strategies have failed to reverse surgical AKI or limit its progression.[20] Goal-directed hemodynamic management is a reasonable approach as it uses protocolized monitoring and interventions with prespecified hemodynamic targets. Broadly speaking this patient management strategy has also failed to consistently limit AKI, although a study in cardiac surgery patients demonstrated that stroke volume optimization – achieved by monitoring the effects of 250cc fluid challenges on left ventricle stroke volume – reduced the incidence of AKI from 19.9% to 6.5% (P=0.002).[21]
Acidosis, electrolyte derangements, intravascular volume overload, and severe uremia typically herald the onset of AKI that requires RRT. It is unclear if early or late initiation of RRT reverses critical illness, improves survival, or improves long-term kidney function. Three recent randomized clinical trials have addressed this question. Zarbock and colleagues found evidence that initiation of RRT within 8-hours of stage 2 AKI reduces 90-day mortality, duration of RRT, duration of mechanical ventilation, duration of hospitalization, and increases recovery of renal function compared to initiation of RRT within 12-hours of stage 3 AKI in 231 critically ill patients (ELAIN trial).[22] At one year follow up, decreased MAKE and mortality and enhanced renal recovery persisted in ELAIN trial patients who received early RRT. Gaudry and colleagues, however, found that patients with stage 3 AKI assigned immediate RRT had similar 60-day mortality to patients assigned RRT after development of significant renal failure sequelae and experienced increased catheter-related blood stream infections and delayed return of renal function (AKIKI trial).[23] Barbar and colleagues randomized 488 patients with early-stage septic shock and renal failure, defined by RIFLE criteria, to RRT at 12 hours after documented renal failure or at 48 hours if renal recovery had not occurred (IDEAL-ICU trial).[24] This trial was stopped early for futility, with no significant difference in the primary outcome of death at 90 days between groups. Importantly, the ELAIN trial included mostly surgical patients with a high prevalence of fluid overload, while the AKIKI and IDEAL-ICU trials included mostly medical patients with a high prevalence of sepsis. Perhaps the initiation of RRT while patients were still in moderate AKI led to an early RRT treatment benefit in the ELAIN trial. The STARRT-AKI trial (NCT02568722) aims to enroll and randomize 2866 patients with stage 2 AKI or specific clinical criteria to early or late RRT and will provide more evidence. Thus, at present it remains unclear if initiation of RRT in patients with moderate AKI salvages the kidneys compared to waiting until patients recover or develop more severe AKI.
A “furosemide stress test” is the administration of furosemide to a patient who has oliguria or AKI in order to better predict the progression of AKI and the need for subsequent RRT. It is based off a study that demonstrated that the volume of urine output following a 1–1.5 mg/kg dose of intravenous furosemide produced a C-statistic of 0.82 and 0.87 to predict development of stage 3 AKI in two cohorts of patients with stage 1 or 2 AKI.[25] A urine output less than 200 ml in the two hours following furosemide administration had 87.1% sensitivity and 84.1% specificity to predict progression to stage 3 AKI. The authors of this study caution that urine output should be replaced with intravenous fluids during a furosemide stress test unless the patient is frankly intravascularly overloaded, as numerous studies have demonstrated that the administration of furosemide to reverse oliguria does not reduce but increases AKI. Outside of a furosemide stress test, furosemide should be reserved to treat intravascular hypervolemia.
Hospital mortality for AKI patients who require dialysis remains a staggering 30%.[1] Renal failure is frequently accompanied by respiratory failure, arrhythmias, cardiovascular failure, severe infections, polyneuropathy, weakness, and delirium.[26] There is increasing evidence that these organ injuries do not just occur simultaneously in response to a common insult, but that impaired fluid and electrolyte homeostasis, reduced waste (fixed acids and urea) elimination, renal cytokine production, decreased cytokine clearance, neurohormonal signaling pathways, or impaired neutrophil activity cause deleterious effects to extra-renal organs. For example, experimental renal ischemia-reperfusion precipitates neutrophil margination in the contralateral kidney, spleen, and lungs,[27] macrophage infiltration in lung and myocardial interstitium,[28] and causes lung and brain edema. These extra-renal effects of experimental AKI may explain the clinical observations that even stage 1 AKI increases the risk of extra-renal organ failure and is associated with a 7-fold increase in 30-day mortality.
Pathophysiology of AKI development and resolution – short- and long-term effects
Perioperative AKI is primarily a result of renal malperfusion, oxidative damage, and inflammation.[29] The cortico-medullary junction is relatively hypoxic (PO2 10–20 mmHg) in normal conditions, and during surgery and critical illness, systemic changes in cardiac output, systemic vascular resistance, and renal vein pressure alter cortical and medullary perfusion. At the same time, activation of the sympathetic nervous system, stimulation of the renin angiotensin aldosterone system, secretion of vasopressin, and release of endothelin-1 further alter the renal microcirculation. Ischemia and reperfusion cycles induce reactive oxygen species (ROS) production from renal mitochondria. ROS impair endothelial function and perfusion homeostasis by eliminating nitric oxide, inciting pro-inflammatory transcription factors including nuclear factor-kappa B, and damaging surrounding tissues by oxidizing lipids, DNA, and additional proteins. Neutrophils and macrophages are recruited into the renal interstitium to regulate inflammation. The tubular epithelium loses polarity and its brush border. Necrotic and apoptotic cells are lost into the lumen, and myofibroblasts deposit collagen in the interstitium as the renal parenchyma becomes fibrotic.[30] Septic AKI and radiocontrast-induced AKI, although unique in their presentation, are also products of altered microcirculation, oxidative damage, and renal inflammation.
The kidneys can recover from AKI. After tubular cell loss, surviving cells, stimulated by paracrine signaling from bone marrow-derived progenitor cells, dedifferentiate, proliferate, migrate along the basement membrane, and differentiate into mature tubule cells, replenishing the tubular epithelium.[30] This process, however, is frequently maladaptive. The severity of AKI and repetitive AKI events increase risk of CKD progression (Figure). Even if renal function recovers following AKI, patients that suffer AKI are at increased risk for developing subsequent episodes of AKI or worsening CKD.
Figure.
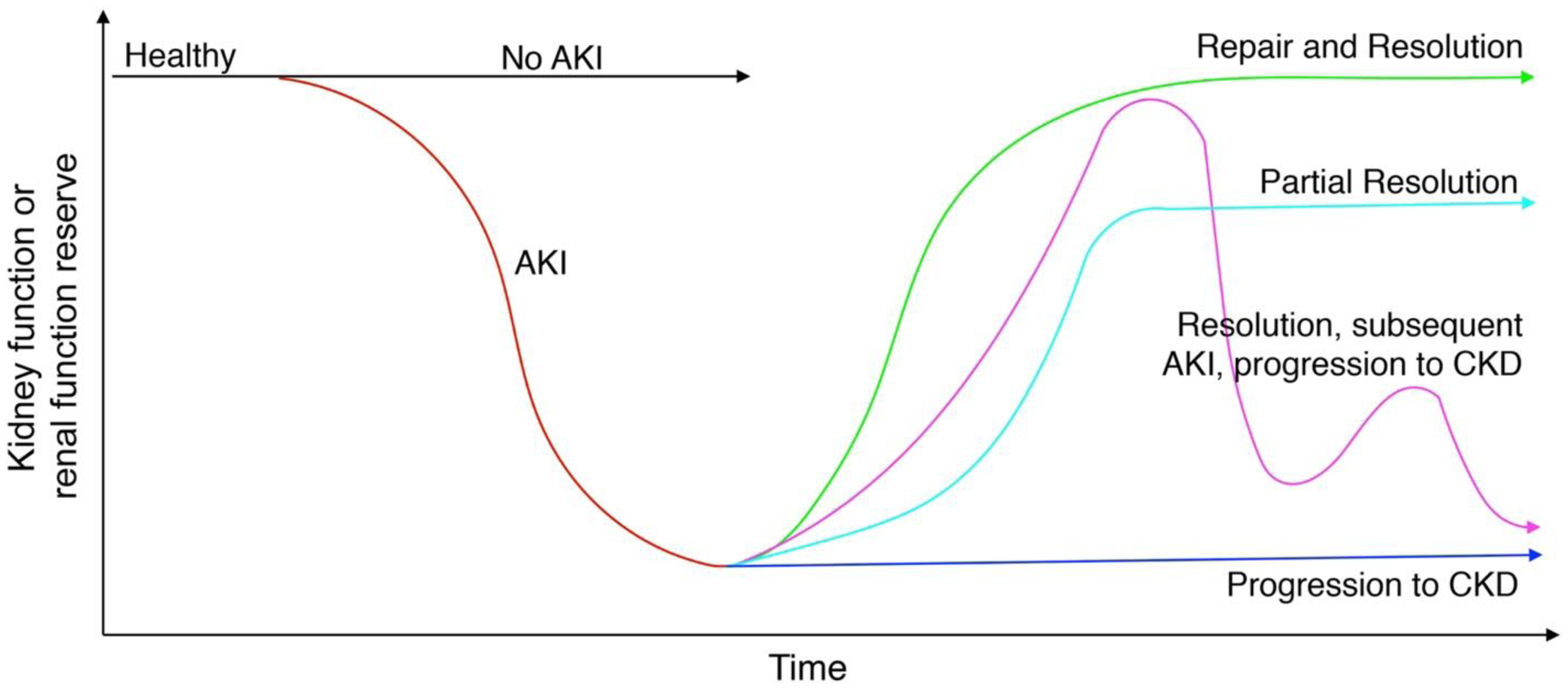
Renal function trajectories in patients who do and do not suffer acute kidney injury (AKI) and do and do not progress to chronic kidney disease (CKD).
AKI is a common, complicated, and dreadful complication of acute illness. Patients often recover from AKI but suffer subsequent morbidity. Our understanding of the pathophysiology of AKI development, however, has improved, and recent empiric treatments have emerged. Future work will focus on prophylactic therapy for AKI and extending patient monitoring and treatment after AKI resolution.
Implications statement:
Acute kidney injury continues to affect large numbers of patients receiving surgery or in the ICU, but advances in resuscitation techniques, endpoint refinements, epidemiology, biomarkers, and pathology are providing the necessary framework to reduce AKI.
Objectives of this Continuing Professional Development (CPD) module:
After reading this module, the reader should be able to:
List 4–6 objectives
Describe risk factors for the development of perioperative AKI
Identify patients who meet consensus criteria for the diagnosis of AKI
Understand treatment goals for reducing the risk of AKI
Learn to manage patients with AKI to improve outcomes
CLINICAL CASE.
Introduction:
Ms. Henle, a 68-year old woman, presented to the emergency department with a fever and a cold swollen left foot. She has a history of hyperlipidemia, congestive heart failure, and stage III (defined as an estimated glomerular filtration rate [eGFR] between 30 and 60 mL/min/1.73 m2) chronic kidney disease (CKD). Her heart rate is 93 bpm and blood pressure 95/50 mmHg. Her left foot is mottled, and distal pulses are diminished. Her serum creatinine measures 1.4 mg/dl (124 μmol/l). She is urgently taken for angiography and angioplasty under monitored anesthesia care. She receives a Foley catheter to monitor urine output. She receives 1.5 L of intravenous Plasma-Lyte©, several phenylephrine boluses to treat hypotension, and 125 ml of radiocontrast during left popliteal artery dilation and stenting. In the post-anesthesia care unit, her heart rate is 115 bpm and blood pressure 82/42 mmHg. Serum potassium measures 5.4 mEq/L. She has ST segment depression on lead V5. She is somnolent and anuric. Her resident physician orders a 500 mL bolus of IV 0.9% saline, concerned that administration of a balanced fluid containing potassium could worsen her hyperkalemia, and a phenylephrine infusion. She is transferred to the intensive care unit for further treatment.
Question 1
Which of the following is NOT recommended for preventative management of AKI for this patient:
Administration of balanced crystalloid IV fluids
Administration of a dopamine receptor agonist
Prompt recognition and treatment of intraoperative hypotension
Optimizing myocardial oxygen delivery and consumption
Avoidance of intravenous radiocontrast
Question 2
Mechanisms of perioperative AKI, and consequently viable targets for development of therapies, include:
Intraoperative renal ischemia and reperfusion injury
Intraoperative oxidative stress
Cholesterol emboli during vascular surgery
Acute inflammation
All of the above
Question 3
In the ICU Ms. Henle’s left leg pulses are restored, but all her extremities are cool. A surface echocardiogram reveals an undilated but hypocontractile left ventricle, a dilated and hypocontractile right ventricle, moderate tricuspid valve regurgitation, and an inferior vena cava measuring 2cm in diameter with limited respiratory variability. She remains anuric and is diagnosed with AKI.
Which of the following regarding the diagnosis of AKI is FALSE?
Changes in serum creatinine are often insensitive and nonspecific for renal injury.
Using only urine output to diagnose AKI is nonspecific and may lead to overdiagnosis.
Patients who meet AKI criteria by both serum creatinine and decrease in urine output have similar long-term renal outcomes to patients who meet only one criterion.
The use of the majority of biomarkers of renal injury remains limited to research.
The Kidney Disease Improving Global Outcomes (KDIGO) criteria define stage 1 AKI as a 0.3 mg/dl (26.5 μmol/l) serum creatinine increase from baseline with 2 days of surgery, a 50% increase of serum creatinine within 1 week of surgery, or urine output below 0.5 ml·kg−1·hr−1 for 6 hours.
Question 4
A right internal jugular vein central line is placed and a norepinephrine infusion initiated to maintain a mean arterial blood pressure of 60 mmHg. The phenylephrine infusion is discontinued. Ms. Henle’s central venous pressure is 24 mmHg supine, heart rate 90 bpm, respiratory rate 24 bpm, peripheral arterial hemoglobin oxygen saturation 97% on 4 liters/minute oxygen via nasal cannula, venous hemoglobin oxygen saturation 58%, and she complains of dyspnea. Auscultation reveals bilateral rales in her dependent lung fields. Her intensivist administers her 100 mg of furosemide, keeps her NPO, and monitors her respiratory status.
The furosemide “stress test” administered to this patient:
Decreases renal tubule oxygen consumption and decreases AKI.
Can help predict the patient’s progression to more severe AKI.
Should have been avoided because of the patient’s intravascular hypovolemia.
Will require that additional IV fluid administration to replace urine output in response to the diuretic.
Question 5
Six hours after the furosemide bolus she has made 45 ml of urine, her jugular veins are distended to the base of her ears, and she continues to complain of dyspnea. An intravenous dialysis catheter is placed in her left internal jugular vein, and she is initiated on hemodialysis to remove intravascular fluid.
During the progression of AKI, renal replacement therapy (dialysis) should be initiated:
Early following the development of stage II AKI to prevent long-term CKD.
Late because patients might not need dialysis, and dialysis increases the need for long-term renal replacement therapy.
At present it remains unclear if initiation of renal replacement therapy in patients with moderate AKI salvages the kidneys compared to waiting for other established indications for dialysis.
There is no evidence that timing of renal replacement therapy impacts patient outcomes.
Explanations for answers
Question 1
Which of the following is NOT recommended for preventative management of AKI for this patient:
-
A. Administration of balanced crystalloid IV fluids
Recommended. The SMART trial demonstrated a reduction in major adverse kidney events at 30 days in patients treated with balanced crystalloid solutions compared to high chloride solutions such as 0.9% saline. [7]
-
B. Administration of a dopamine receptor agonist
Not recommended. Dopamine agonists increase renal perfusion, glomerular filtration, sodium delivery to the tubule, solute reabsorption requirements, and renal tubule oxygen supply but fail to decrease the progression of AKI. Furthermore, low dose dopamine agonist infusions may increase the incidence of hypotension. [20]
-
C. Prompt recognition and treatment of intraoperative hypotension
Recommended. Intraoperative hypotension is strongly associated with AKI in the perioperative period. [1, 20]
-
D. Optimizing myocardial oxygen delivery and consumption
Recommended. Optimizing cardiac performance increases renal perfusion and renal oxygenation, both of which may be important in preventing AKI. [1]
-
E. Avoidance of intravenous radiocontrast
Recommended. Avoidance of nephrotoxins is a mainstay for the prevention of AKI.
Question 2
Mechanisms of perioperative AKI, and consequently viable targets for development of therapies, include:
-
Intraoperative renal ischemia and reperfusion injury
True statement but not recommended answer. Intraoperative renal ischemia and reperfusion is relatively common during surgery due to reductions in cardiac output, blood pressure, and hematocrit. Venous congestion also decreases renal perfusion. Reperfusion elicits a surge in the production of reactive oxygen species which in turn induce inflammation. Markers of these processes are independently associated with AKI.[21]
-
Intraoperative oxidative stress
True statement but not recommended answer. Increased intraoperative oxidative stress may be the first molecular signal associated with perioperative AKI, and markers of oxidative damage, for example, F2-isoprostanes in plasma and urine, reliably predict development of AKI. [29]
-
Cholesterol emboli during vascular surgery
True statement but not recommended answer. Manipulation of the aorta during aorta cross clamp for cardiac surgery or during vascular procedures including transcutaneous procedures leads to cholesterol emboli which have been noted in renal biopsies and are associated with increased. AKI.
-
Acute inflammation
True statement but not recommended answer. Increased markers of inflammation and leukocytes, both locally in the renal parenchyma and systemically in the blood are associated with increased AKI. Due to the later expression relative to surgery, however, it is difficult to know if inflammation is a mechanism of AKI or a response to AKI.[3, 27]
-
All of the above
Recommended answer
Question 3
In the ICU Ms. Henle’s left leg pulses are restored, but all her extremities are cool. A surface echocardiogram reveals an undilated but hypocontractile left ventricle, a dilated and hypocontractile right ventricle, moderate tricuspid valve regurgitation, and an inferior vena cava measuring 2cm in diameter with limited respiratory variability. She remains anuric and is diagnosed with AKI.
Which of the following regarding the diagnosis of AKI is FALSE?
-
Changes in serum creatinine are often insensitive and nonspecific for renal injury.
True. Creatinine is the most common laboratory test used to diagnose AKI. Serum creatinine concentrations, however, can be confounded by changes in creatinine production, myocyte injury, intravenous fluid administration, intravascular fluid extravasation, diuretic use, tubular creatinine secretion, and renal functional reserve. [1]
-
Using only urine output to diagnose AKI is nonspecific and may lead to overdiagnosis.
True. Urine output alone is nonspecific to AKI and its use in isolation may lead to overdiagnosis, particularly in surgical patients who have been administered boluses of IV fluids, are experiencing intravascular and interstitial fluid shifts, or have received diuretics.
-
Patients who meet AKI criteria by both serum creatinine and decrease in urine output have similar long-term renal outcomes to patients who meet only one criterion.
False. Recommended answer. Patients who meet AKI criteria by creatinine concentration and oliguria have worse long-term renal outcomes compared to those who only meet one criterion. [3]
-
The use of the majority of biomarkers of renal injury remains limited to research.
True. While biomarkers of renal injury may be able to detect AKI earlier than urine output and serum creatinine values, their use remains largely limited to research. [4]
-
The Kidney Disease Improving Global Outcomes (KDIGO) criteria define stage 1 AKI as a 0.3 mg/dl (26.5 μmol/l) serum creatinine increase from baseline with 2 days of surgery, a 50% increase of serum creatinine within 1 week of surgery, or urine output below 0.5 ml·kg−1·hr−1 for 6 hours.
True. KDIGO criteria define stage 1 AKI as a 0.3 mg/dl (26.5 μmol/l) serum creatinine increase from baseline with 2 days of surgery, a 50% increase of serum creatinine within 1 week of surgery, or urine output below 0.5 ml·kg−1·hr−1 for 6 hours. [2]
Question 4
A right internal jugular vein central line is placed and a norepinephrine infusion initiated to maintain a mean arterial blood pressure of 60 mmHg. The phenylephrine infusion is discontinued. Ms. Henle’s central venous pressure is 24 mmHg supine, heart rate 90 bpm, respiratory rate 24 bpm, peripheral arterial hemoglobin oxygen saturation 97% on 4 liters/minute oxygen via nasal cannula, venous hemoglobin oxygen saturation 58%, and she complains of dyspnea. Auscultation reveals bilateral rales in her dependent lung fields. Her intensivist administers her 100 mg of furosemide, keeps her NPO, and monitors her respiratory status.
The furosemide “stress test” administered to this patient:
-
Decreases renal tubule oxygen consumption and decreases AKI
Not recommended. There is not evidence that furosemide reduces the risk of AKI or renal replacement therapy, and in fact, the administration of furosemide may increase AKI. [20, 25]
-
Can help predict the patient’s progression to more severe AKI.
Recommended. Urine output following 1–1.5 mg/kg intravenous furosemide produced a C-statistic of 0.82 and 0.87 in two cohorts of patients with stage 1 or 2 AKI to predict development of stage 3 AKI.[25]
-
Should have been avoided because of the patient’s intravascular hypovolemia.
Not recommended. The patient does not have signs of intravascular hypovolemia. Rather, the patient has signs of intravascular volume overload.
-
Will require additional IV fluid administration to replace urine output in response to the diuretic.
Not recommended. This patient has hemodynamic indicators (CVP of 24 mmHg) and clinical signs and symptoms (rales, dyspnea) of intravascular volume overload. Additional IV fluid administration is contraindicated unless the patient becomes intravascularly hypovolemic. [26]
Question 5
Six hours after the furosemide bolus she has made 45 ml of urine, her jugular veins are distended to the base of her ears, and she continues to complain of dyspnea. An intravenous dialysis catheter is placed in her left internal jugular vein, and she is initiated on hemodialysis to remove intravascular fluid.
During the progression of AKI, renal replacement therapy (dialysis) should be initiated:
-
Early following the development of stage II AKI to prevent long-term CKD.
Not recommended. The initiation of renal replacement therapy in the early phase remains controversial. The AKIKI trial noted increased catheter related infections with early initiation of RRT and similar 60-day mortality to patients assigned late RRT. [23]
-
Late because patients might not need dialysis, and dialysis increases the need for long-term renal replacement therapy.
Not recommended. The appropriate timing for the initiation of renal replacement therapy remains controversial. The ELAIN trial noted improvement in mortality and long term renal function with early initiation. [22]
-
At present it remains unclear if initiation of renal replacement therapy in patients with moderate AKI improves kidney function in the long term compared to waiting for other established indications for dialysis.
Recommended. There remains conflicting evidence on whether early or late initiation of renal replacement therapy is superior for improved long term kidney function in patients. [1, 26]
-
There is no evidence that timing of renal replacement therapy impacts patient outcomes.
Not recommended. The ELAIN trial noted a reduction in mortality, long term kidney dysfunction, and other morbidities when renal replacement therapy was initiated early in patients with stage 3 AKI.[22]
Funding sources:
Support was provided by the United States National Institutes of Health (K23GM102676, K23GM129662, and R01GM112871) and the Vanderbilt University Department of Anesthesiology.
Footnotes
Conflicts of interest: The authors declare no competing interests.
References
- 1.Meersch M, Schmidt C, Zarbock A (2017) Perioperative Acute Kidney Injury: An Under-Recognized Problem. Anesth Analg 125:1223–1232. 10.1213/ANE.0000000000002369 [DOI] [PubMed] [Google Scholar]
- 2.KDIGO AKI Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138 [Google Scholar]
- 3.Kellum JA, Sileanu FE, Murugan R, et al. (2015) Classifying AKI by Urine Output versus Serum Creatinine Level. J Am Soc Nephrol JASN 26:2231–2238. 10.1681/ASN.2014070724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han WK, Waikar SS, Johnson A, et al. (2008) Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int 73:863–869. 10.1038/sj.ki.5002715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashani K, Al-Khafaji A, Ardiles T, et al. (2013) Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17:R25. 10.1186/cc12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yunos NM, Bellomo R, Hegarty C, et al. (2012) Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 308:1566–1572. 10.1001/jama.2012.13356 [DOI] [PubMed] [Google Scholar]
- 7.Semler MW, Self WH, Wanderer JP, et al. (2018) Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med 378:829–839. 10.1056/NEJMoa1711584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myles PS, Bellomo R, Corcoran T, et al. (2018) Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N Engl J Med 378:2263–2274. 10.1056/NEJMoa1801601 [DOI] [PubMed] [Google Scholar]
- 9.Weisbord SD, Gallagher M, Jneid H, et al. (2017) Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. N Engl J Med. 10.1056/NEJMoa1710933 [DOI] [PubMed] [Google Scholar]
- 10.Perner A, Haase N, Guttormsen AB, et al. (2012) Hydroxyethyl Starch 130/0.42 versus Ringer’s Acetate in Severe Sepsis. N Engl J Med 367:124–134. 10.1056/NEJMoa1204242 [DOI] [PubMed] [Google Scholar]
- 11.Wiedermann CJ, Dunzendorfer S, Gaioni LU, et al. (2010) Hyperoncotic colloids and acute kidney injury: a meta-analysis of randomized trials. Crit Care 14:R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee E-H, Kim W-J, Kim J-Y, et al. (2016) Effect of Exogenous Albumin on the Incidence of Postoperative Acute Kidney Injury in Patients Undergoing Off-pump Coronary Artery Bypass Surgery with a Preoperative Albumin Level of Less Than 4.0 g/dl: Anesthesiology 124:1001–1011. 10.1097/ALN.0000000000001051 [DOI] [PubMed] [Google Scholar]
- 13.Walsh M, Garg AX, Devereaux PJ, et al. (2013) The Association Between Perioperative Hemoglobin and Acute Kidney Injury in Patients Having Noncardiac Surgery: Anesth Analg 117:924–931. 10.1213/ANE.0b013e3182a1ec84 [DOI] [PubMed] [Google Scholar]
- 14.Marik PE, Corwin HL (2008) Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature*: Crit Care Med 36:2667–2674. 10.1097/CCM.0b013e3181844677 [DOI] [PubMed] [Google Scholar]
- 15.Mazer CD, Whitlock RP, Fergusson DA, et al. (2017) Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N Engl J Med 377:2133–2144. 10.1056/NEJMoa1711818 [DOI] [PubMed] [Google Scholar]
- 16.Chen KP, Cavender S, Lee J, et al. (2016) Peripheral Edema, Central Venous Pressure, and Risk of AKI in Critical Illness. Clin J Am Soc Nephrol CJASN 11:602–608. 10.2215/CJN.08080715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, et al. (2017) Vasopressin versus Norepinephrine in Patients with Vasoplegic Shock after Cardiac Surgery: The VANCS Randomized Controlled Trial. Anesthesiol J Am Soc Anesthesiol 126:85–93. 10.1097/ALN.0000000000001434 [DOI] [PubMed] [Google Scholar]
- 18.McGuinness SP, Parke RL, Bellomo R, et al. (2013) Sodium bicarbonate infusion to reduce cardiac surgery-associated acute kidney injury: a phase II multicenter double-blind randomized controlled trial. Crit Care Med 41:1599–1607. 10.1097/CCM.0b013e31828a3f99 [DOI] [PubMed] [Google Scholar]
- 19.Whitlock RP, Devereaux PJ, Teoh KH, et al. (2015) Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet Lond Engl 386:1243–1253. 10.1016/S0140-6736(15)00273-1 [DOI] [PubMed] [Google Scholar]
- 20.Joannidis M, Druml W, Forni LG, et al. (2017) Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017 : Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med 43:730–749. 10.1007/s00134-017-4832-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson R, Meeran H, Valencia O, Al-Subaie N (2014) Goal-directed therapy after cardiac surgery and the incidence of acute kidney injury. J Crit Care 29:997–1000. 10.1016/j.jcrc.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 22.Zarbock A, Kellum JA, Schmidt C, et al. (2016) Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 315:2190–2199. 10.1001/jama.2016.5828 [DOI] [PubMed] [Google Scholar]
- 23.Gaudry S, Hajage D, Schortgen F, et al. (2016) Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med 375:122–133. 10.1056/NEJMoa1603017 [DOI] [PubMed] [Google Scholar]
- 24.Barbar SD, Clere-Jehl R, Bourredjem A, et al. (2018) Timing of Renal-Replacement Therapy in Patients with Acute Kidney Injury and Sepsis. N Engl J Med 379:1431–1442. 10.1056/NEJMoa1803213 [DOI] [PubMed] [Google Scholar]
- 25.Chawla LS, Davison DL, Brasha-Mitchell E, et al. (2013) Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care Lond Engl 17:R207. 10.1186/cc13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle JF, Forni LG (2016) Acute kidney injury: short-term and long-term effects. Crit Care 20:188. 10.1186/s13054-016-1353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awad AS, Rouse M, Huang L, et al. (2009) Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int 75:689–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tokuyama H, Kelly DJ, Zhang Y, et al. (2007) Macrophage infiltration and cellular proliferation in the non-ischemic kidney and heart following prolonged unilateral renal ischemia. Nephron Physiol 106:p54–62 [DOI] [PubMed] [Google Scholar]
- 29.Billings FT, Pretorius M, Schildcrout JS, et al. (2012) Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol JASN 23:1221–1228. 10.1681/ASN.2011090940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonventre JV, Yang L (2011) Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121:4210–4221. 10.1172/JCI45161 [DOI] [PMC free article] [PubMed] [Google Scholar]


