Abstract
Patient: Female, 71-year-old
Final Diagnosis: Dermatomyositis
Symptoms: Fever • walking difficulties
Medication: —
Clinical Procedure: Corticosteroids
Specialty: Immunology • Oncology • Rheumatology
Objective:
Rare disease
Background:
Pembrolizumab is a humanized monoclonal antibody against programmed cell death-1 protein. Pembrolizumab sometimes causes immune-related adverse events (irAEs). Dermatomyositis is a rare irAE of immune checkpoint inhibitors. The presentation is usually acute, and symptoms include edema with erythema of the eyelids, erythema of the forehead, and muscle weakness in both thighs.
Case Report:
Here we report a case of pembrolizumab-induced dermatomyositis in a 71-year-old Japanese woman with cancer of unknown primary origin, who experienced a high fever and had difficulty walking after her sixth course of pembrolizumab. General physical examination revealed edema with a heliotrope rash, V-neck signs, and non-specific erythema of the forehead. Laboratory evaluation revealed that myogenic enzymes were within normal ranges. Autoantibody tests revealed that antinuclear antibodies were negative, and autoantibodies related to myositis and anti-acetylcholine receptor antibodies were also negative. A magnetic resonance imaging scan of the thighs revealed signal abnormalities in the left lateral and distal vastus medialis muscle. The patient was treated with corticosteroids, subsequently followed by intravenous immunoglobulin therapy, which led to an improvement of the symptoms.
Conclusions:
Pembrolizumab-induced dermatomyositis is rare. Corticosteroids have been administered in many cases, and this case also suggests the efficacy of intravenous immunoglobulin therapy in treating immune checkpoint inhibitor-related dermatomyositis. This case highlights practical management of pembrolizumab-induced dermatomyositis.
Keywords: Dermatomyositis; Drug-Related Side Effects and Adverse Reactions; Immunoglobulins, Intravenous; Programmed Cell Death 1 Receptor
Background
Pembrolizumab is a humanized monoclonal antibody against programmed cell death-1 (PD-1) protein and is an immune checkpoint inhibitor (ICI) first approved by the Food and Drug Administration in 2014. ICIs, including pembrolizumab, sometimes cause immune-related adverse events (irAEs) [1]. These irAEs differ greatly from adverse events caused by conventional cytotoxic anticancer agents and molecular-targeted drugs in that irAEs involve various organs, including the skin, the digestive system, lungs, thyroid gland, and pituitary gland [1]. irAEs are thought to result from excessive autoimmune reactions by activated T cells. The frequency of irAEs is relatively low, and if they are mild, treatment with ICIs can be continued with careful management. However, in moderate to severe cases, discontinuation of ICIs and treatment with corticosteroids is required. Dermatomyositis can occur as an irAE, but only a few cases have been reported. Here, we report a case of dermatomyositis induced by pembrolizumab treatment.
Case Report
A 71-year-old woman was referred to Hamanomachi Hospital because of high fever and difficulty walking after her sixth course of pembrolizumab. Her medical history included an appendectomy at age 20, kidney biopsy at age 60 for chronic glomerulonephritis, and ovarian cyst surgery at age 65.
She was diagnosed with a cancer of unknown primary origin 16 months earlier by a biopsy of the cervical lymph nodes. Immunostaining of the biopsy revealed CK7(+), CK19(+), TTF-1(+), p40(–), SPA(+), 34βE12(+), and Napsin A(+), suggesting lung adenocarcinoma. PD-L1 had a tumor proportion score of 25–49%. However, imaging studies did not show any lung lesions, so we initially diagnosed a cancer of unknown primary origin in the patient. She was then treated with 8 courses of carboplatin and paclitaxel. She had progressive disease with enlarged lymph nodes in the left-neck, supraclavicular, mediastinum, and left axillary lymph nodes. Since the initial pathological immunostaining suggested primary lung adeno-carcinoma as the origin, pembrolizumab was selected as the second line of treatment. The first course was administered 5 months before presentation. She developed pembrolizumab-induced colitis (grade 2) after the second course, which improved with prednisolone (PSL) administration, and the dose of PSL was tapered to 15 mg/d. After the fourth course, a contrast-enhanced computed tomography (CT) scan showed shrinkage of the lymph node metastasis. We then decided to continue with pembrolizumab treatment and performed the sixth course. Three days after administration of the sixth course of pembrolizumab, she developed a high fever and difficulty walking and was admitted to Hamanomachi Hospital.
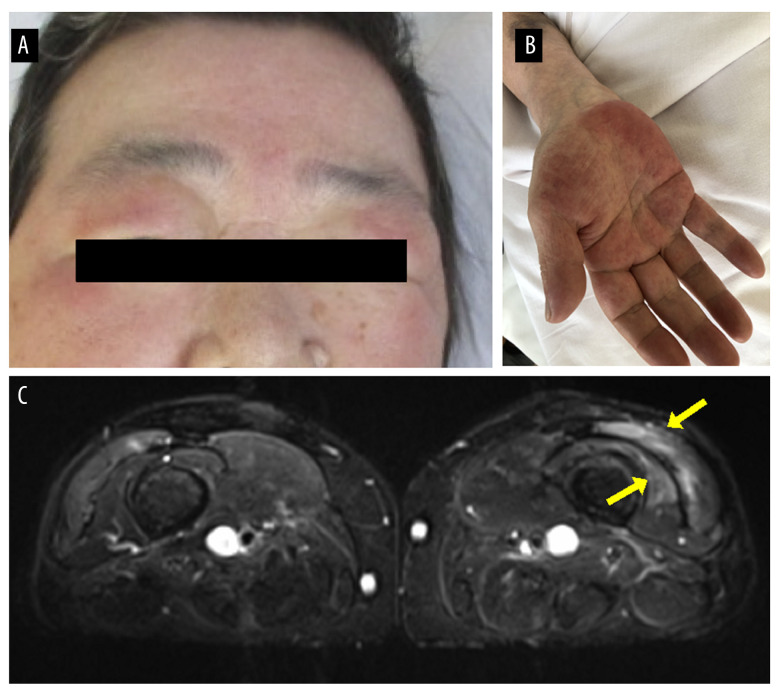
The patient had a temperature of 39.2°C, blood pressure of 94/66 mmHg, pulse of 92 beats/min, and SpO2 of 92% (room air). The low SpO2 level on admission could have been due to hyperthermia; the patient showed no hypoxia during the admission. General physical examination revealed edema with erythema of the eyelids, indicating a heliotrope rash (Figure 1A), erythema of the chest (V-neck signs), and non-specific erythema of the forehead. Edema was present in all 4 limbs (Figure 1B). There was muscle weakness in both thighs, but no diurnal variation was noted. There was no swelling or tenderness of the joints.
Figure 1.
Clinical features of the patient. (A) Edema with erythema of the eyelids indicating heliotrope rash and (B) edema of the right palm. (C) A magnetic resonance imaging scan of both thighs showed signal abnormalities in the left lateral and distal vastus medialis muscles.
Laboratory evaluation revealed that C-reactive protein was elevated (9.3 mg/dL). Myogenic enzymes were within normal ranges, and there were no other abnormal findings. Two sets of blood cultures were negative. Urinalysis showed no abnormalities, and a polymerase chain reaction test for SARS-CoV-2 was negative. Autoantibody tests revealed that antinuclear antibodies were negative, and autoantibodies related to myositis were also negative (anti-aminoacyl tRNA synthetase, anticardiolipin, and anti-transcriptional intermediary factor 1-γ antibodies). There have been reports of myasthenia gravis associated with ICIs [2], but anti-acetylcholine receptor antibodies were negative. The patient’s electrocardiogram was normal in sinus rhythm, and cardiac/abdominal echography showed no significant abnormal findings. A chest CT showed no complications of interstitial pneumonia or thymoma. A magnetic resonance imaging (MRI) scan of the thighs showed signal abnormalities in the left lateral and distal vastus medialis muscle (Figure 1C). An MRI scan of the head and spine showed no evidence of metastatic tumors or other abnormalities.
Because there were no obvious signs of infection and because symptoms started immediately after pembrolizumab administration, irAE was the most likely cause. The diagnosis of pembrolizumab-induced dermatomyositis (grade 3) was made based on the presence of a heliotrope rash, as well as weakness of the quadriceps muscles and abnormal signals in the left lateral and distal vastus medialis muscle in MRI of the thighs. Muscle biopsies and electromyography were not performed due to their invasiveness. The possibility of infection was considered as low. Kinetics of the blood C-reactive protein level seemed to reflect the disease status with a delay of about half a day, which is often observed in many other clinical settings, such as infectious diseases.
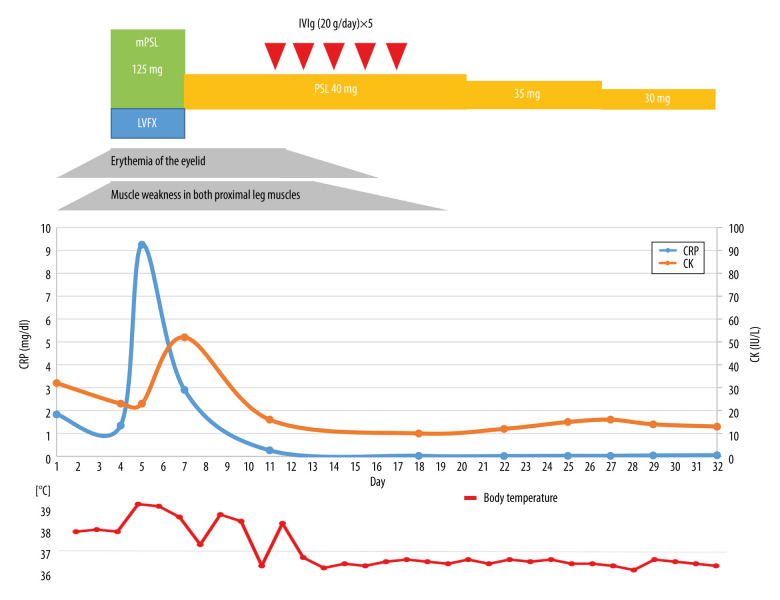
Methylprednisolone (mPSL; 125 mg/d) was started after admission and continued for 3 days. Levofloxacin was temporarily administered after blood culture collection, considering the possibility of sepsis.
After the mPSL dosage was reduced to 40 mg/d, but the patient’s fever flared to 38°C or more, and inflammatory findings worsened (Figure 2). It was difficult to increase the dose of corticosteroids in this case because of severe steroid-induced cataracts, as well as oral candidiasis. We considered administering immunosuppressive agents; however, because of renal dysfunction and the risk of opportunistic infections due to immunosuppression, we proceeded with intravenous immunoglobulin (IVIg) therapy instead of immunosuppressants [3]. If that did not work, we planned to use a combination of immunosuppressive agents. High-dose IVIg was then administered for 5 days. Afterward, the weakness of the quadriceps improved, and the patient was able to walk on her own. The inflammatory response also became negative. PSL was gradually reduced to 30 mg/d with no relapse of symptoms. Finally, the patient was considered to be in remission and was discharged. After discharge from the hospital, PSL was further tapered to 5 mg every 2 weeks. Her dermatomyositis symptoms have not flared up, and remission has continued. No apparent progression of cancer was observed over 6 months after pembrolizumab discontinuation.
Figure 2.
The clinical course of the case. CK – creatine kinase; CRP – C-reactive protein; IVIg – intravenous immunoglobulin; LVFX – levofloxacin; mPSL – methylprednisolone; PSL – prednisolone.
Discussion
Tumor cells express PD-L1 and PD-L2, which are ligands for PD-1, and the presence of PD-L1 or PD-L2 on tumor cells inhibits tumor elimination by T cells and natural killer cells [4]. Pembrolizumab is a humanized IgG4 monoclonal antibody that shows affinity for PD-1. It binds to the PD-1 expressed on activated T cells and prevents it from binding to PD-L1, allowing T-cell activation to eliminate cancer cells. Because of this immune system-related mechanism of action, it may sometimes cause irAEs.
Nivolumab, which preceded pembrolizumab on the market, caused myositis in 0.04% of cases (4/9869) [5]. In addition, nivolumab-induced myositis was associated with no presence of autoantibodies [5]. In clinical trials (KEYNOTE-024 and KEYNOTE-010) for pembrolizumab, on the other hand, myositis occurred in 0.3-0.6% of cases [6,7], and there were several reports of dermatomyositis [8–10], specifically 2 cases of autoantibody-negative dermatomyositis and 1 case of anti-TIF1-γ antibody-positive dermatomyositis [11]. This case is, thus, valuable, because dermatomyositis was induced by pembrolizumab treatment.
Restarting ICIs for patients after they develop irAEs can cause recurrence of the irAEs or presentation of new irAEs. Among patients with non-small-cell lung cancers who were treated with PD-L1 inhibitors, 26% developed recurrent irAEs and another 26% developed new irAEs, when re-treated with PD-L1 inhibitors [12]. In addition, treatment with PD-1 inhibitors in cancer patients with pre-existing autoimmune diseases reportedly exacerbated the autoimmune diseases in 38% of patients [13]. Given these findings, the fact that this patient had first developed pembrolizumab-induced colitis may have increased the risk of developing dermatomyositis as a second irAE.
A weakness of this case is that we did not perform a muscle biopsy or electromyography due to the invasiveness of these tests. The skin manifestations of heliotrope rash, weakness in both thighs, MRI findings, and an elevated inflammatory response on blood tests suggest that this patient would have yielded findings consistent with dermatomyositis. However, skin biopsy might have provided stronger evidence of dermatomyositis with minimal invasiveness.
Corticosteroids are often administered to treat ICI-associated dermatomyositis, but there is no established treatment or recourse for steroid-refractory patients. However, in a review by Solimando et al [3], IVIg proved beneficial in treatment of steroid-refractory ICI-related dermatomyositis. The patient had grade 3–4 dermatomyositis that was resistant to treatment with 125 mg/d of mPSL, followed by 40 mg/d of PSL. Her symptoms improved after administration of IVIg, and thereafter, symptoms did not flare up during corticosteroid reduction. In a review of IVIg for autoimmune myositis, it was reported that marked improvement in symptoms was obtained 4–6 months after the start of IVIg [14]. In contrast, a case report of a patient treated with IVIg for steroid-resistant dermatomyositis in Japan showed improvement of symptoms immediately after IVIg treatment, suggesting that IVIg can be rapidly effective in some patients [15]. Further studies are needed to determine the efficacy of IVIg in treating ICI-related dermatomyositis.
Many patients with polymyositis/dermatomyositis generally have antibodies to some kind of autoantigen, and biopsies show infiltration of inflammatory cells, mainly macrophages and lymphocytes, which respond to corticosteroids and immunosuppressive drugs [16]. In the present case and that reported by Berger et al [17], autoantibodies were negative on commercially available tests, although this does not necessarily mean that autoantibodies were not present. We believe unknown autoantibodies could be involved in the pathogenesis of the disease. As subtypes of polymyositis/dermatomyositis can be classified according to autoantibodies, and autoanti-body tests are useful in clinical practice in cases such as clinically amyopathic dermatomyositis, which is associated with interstitial pneumonia with poor prognosis [18], autoantibodies in ICI-related myositis could predict disease behavior and prognosis. Since ICIs will continue to be used worldwide, it is necessary to document more cases and to conduct research leading to identification of autoantibodies.
Conclusions
Pembrolizumab-induced dermatomyositis is rare, and autoantibodies were negative on commercially available tests in the current report. Occurrence of other autoimmune diseases enhances the risk of pembrolizumab-induced dermatomyositis. IVIg might be a treatment option in cases for which corticosteroids are ineffective.
References:
- 1.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–68. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 2.Hibino M, Maeda K, Horiuchi S, et al. Pembrolizumab-induced myasthenia gravis with myositis in a patient with lung cancer. Respirol Case Rep. 2018;6(7):e00355. doi: 10.1002/rcr2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solimando AG, Crudele L, Leone P, et al. Immune checkpoint inhibitor-related myositis: From biology to bedside. Int J Mol Sci. 2020;21(9):3054. doi: 10.3390/ijms21093054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott J, Jimeno A. Pembrolizumab: PD-1 inhibition as a therapeutic strategy in cancer. Drugs Today (Barc) 2015;51(1):7–20. doi: 10.1358/dot.2015.51.1.2250387. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki S, Ishikawa N, Konoeda F, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017;89(11):1127–34. doi: 10.1212/WNL.0000000000004359. [DOI] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 7.Kwok G, Yau TCC, Chiu JW, et al. Pembrolizumab (keytruda) Hum Vaccin Immunother. 2016;12(11):2777–89. doi: 10.1080/21645515.2016.1199310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah M, Tayar JH, Abdel-Wahab N, et al. Myositis as an adverse event of immune checkpoint blockade for cancer therapy. Semin Arthritis Rheum. 2019;48(4):736–40. doi: 10.1016/j.semarthrit.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Seki M, Uruha A, Ohnuki Y, et al. Inflammatory myopathy associated with PD-1 inhibitors. J Autoimmun. 2019;100:105–13. doi: 10.1016/j.jaut.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Touat M, Maisonobe T, Knauss S, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91(10):e985–94. doi: 10.1212/WNL.0000000000006124. [DOI] [PubMed] [Google Scholar]
- 11.Kadota H, Gono T, Shirai Y, et al. Immune checkpoint inhibitor-induced myositis: A case report and literature review. Curr Rheumatol Rep. 2019;21(4):10. doi: 10.1007/s11926-019-0811-3. [DOI] [PubMed] [Google Scholar]
- 12.Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6(9):1093–99. doi: 10.1158/2326-6066.CIR-17-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–76. doi: 10.1093/annonc/mdw443. [DOI] [PubMed] [Google Scholar]
- 14.Mulhearn B, Bruce IN. Indications for IVIG in rheumatic diseases. Rheumatology (Oxford) 2015;54(3):383–91. doi: 10.1093/rheumatology/keu429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi N, Muramitsu M, Kanamoto K, et al. [A case of steroid-resistant dermatomyositis effectively controlled by intravenous immunoglobulin therapy] Shizuoka Red Cross Hospital Research Report. 2009;29(1):54–58. [in Japanese] [Google Scholar]
- 16.Findlay AR, Goyal NA, Mozaffar T. An overview of polymyositis and dermatomyositis. Muscle Nerve. 2015;51(5):638–56. doi: 10.1002/mus.24566. [DOI] [PubMed] [Google Scholar]
- 17.Berger M, Legeay AL, Souci S, et al. Pembrolizumab-induced dermatomyositis in a patient with metastatic melanoma. Eur J Cancer. 2018;104:227–30. doi: 10.1016/j.ejca.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Gil B, Merav L, Pnina L, et al. Diagnosis and treatment of clinically amyopathic dermatomyositis (CADM): A case series and literature review. Clin Rheumatol. 2016;35(8):2125–30. doi: 10.1007/s10067-015-2928-8. [DOI] [PubMed] [Google Scholar]