Abstract
Objectives
This study aims to evaluate the effectiveness of upper Michigan occlusal splint (OS) compared to mandibular OS in terms of pain, range of motion (ROM), and muscle activity as assessed by surface electromyography (sEMG) in patients affected by muscle-related temporomandibular disorders (TMD).
Patients and methods
In this randomized-controlled trial, a total of 40 adult patients (13 males, 27 females; mean age: 47.2±12.8 years; range, 22 to 56 years) with a diagnosis of myofascial pain, lasting from at least three months on at least one masseter muscle. The patients were randomly allocated into two groups: Group 1 (n=20) using upper Michigan OS and Group 2 (n=20) using mandibular OS. At baseline (T0), at one (T1), three (T2), and six months (T3), the following outcomes were assessed: myofascial pain by Visual Analog Scale (VAS) and ROM of mandible movements, activity of the main masticatory muscles through sEMG.
Results
There were no significant intra-group differences in the outcome measures assessed in both groups. However, Group 2 had a significantly higher right lateral mandibular ROM at T2 (7.1±3.1 vs. 9.8±2.3, respectively; p<0.05) and a significantly higher left lateral mandibular ROM at T3 (7.6±3.5 vs. 10.5±2.1, respectively; p<0.05). We found no significant difference in none of the sEMG parameters.
Conclusion
Our study results suggest that OS, independently from being built on the upper or lower arch, seems to not have significant effects in reducing pain over a six-month period in TMD patients.
Keywords: Electromyography, myofascial pain syndrome, occlusal splint, pain management, rehabilitation, temporomandibular joint disorder
Introduction
Temporomandibular disorders (TMDs) include several musculoskeletal and neuromuscular diseases involving the temporomandibular joint (TMJ), masticatory muscles and/or other associated structures.[1] Muscle-related TMD could be classified according to the Diagnostic Criteria for TMDs (DC/TMD)[1] as myalgia, tendonitis, myositis, and spasm. Myalgia can be further categorized into the following groups: local myalgia, myofascial pain, and myofascial pain with referral.
Myofascial pain is one of the most common causes of TMD,[2] affecting 85% of general population during their lifetime with an overall prevalence of approximately 46%.[3] Patients affected by myofascial pain have commonly a depressive state, lower independence in activities of daily living (ADLs), and an overall lower health-related quality of life (HRQoL).[4,5] Although the exact influence of myofascial pain is still unclear, several factors have been proposed to involve in the pathogenesis including overuse of a normally perfused muscle, ischemia of a normally working muscle, sympathetic reflexes that lead to changes in vascular supply, and altered psychological and emotional states, shared with fibromyalgia syndrome.[6,7]
Myofascial pain is commonly treated by analgesic and/or anti-inflammatory drugs, occlusal splints (OS), injections of botulinum type A toxin, instrumental physical therapies, and dry needling and trigger points injections.[8-12] In this scenario, OS may provide centric relation occlusion, eliminate posterior interferences, reduce neuromuscular activity, and obtain stable occlusal relationships with uniform tooth contacts throughout the dental arch.[13] Mandibular OS[14-16] and upper Michigan OS[17-19] are two of the OS approaches most commonly used in the treatment of TMDs. Although these treatments have already been extensively described in literature,[8,9,14-19] with a moderate-to-very low quality evidence supporting their effectiveness in the treatment of TMDs,[8] there are no data available comparing between the effects of these two approaches in terms of reduction of pain in muscle-related TMD patients. In the present study, therefore, we aimed to evaluate the effects of the upper Michigan OS compared to a mandibular flat OS in terms of reducing pain, improving range of motion (ROM), and changing muscle activity in patients affected by myofascial TMD.
Patients and Methods
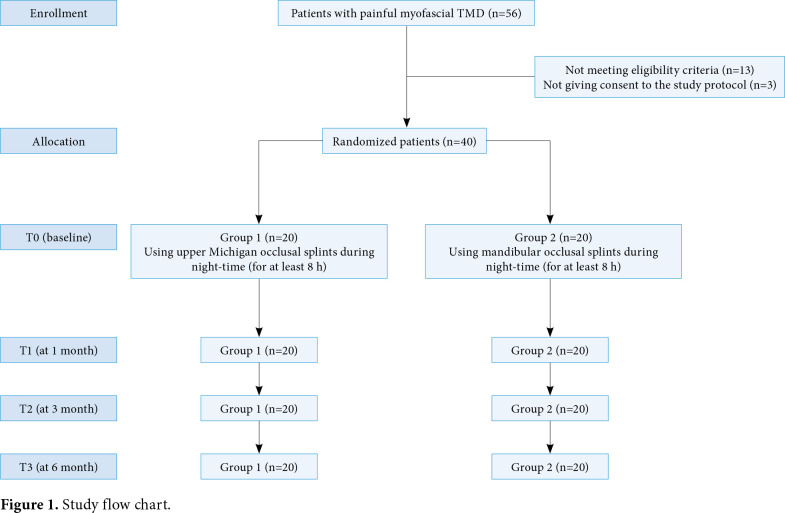
In this randomized-controlled trial (RCT), we recruited adult patients consecutively referring to a Gnathology Unit of a tertiary care hospital between January 2018 and June 2019. Inclusion criteria were as follows: having a diagnosis of myofascial pain according to the DC/TMD;[1] a Visual Analog Scale (VAS) score of ≥4; and pain lasting from at least three months on at least one masseter muscle. Exclusion criteria were as follows: having a history of TMJ disc displacement or arthralgia or osteoarthritis; having a history of head trauma; previous or concomitant treatment with oral splint; presence of oral removable prosthesis; drug addiction; allergy to acrylic resin; ongoing anti-inflammatory or rehabilitative treatments; high risk of obstructive sleep apnea syndrome as assessed by Stop-Bang questionnaire;[20] having a history of mental problems; and having a history of therapy for bruxism. Finally, out of 56 patients with painful myofascial TMD, a total of 40 adult patients (13 males, 27 females; mean age: 47.2±12.8 years; range, 22 to 56 years) who met the inclusion criteria were included in the study. The study flow chart is shown in Figure 1. A written informed consent was obtained from each patient. The study protocol was approved by the AOU Città della Salute e della Scienza di Torino Ethics Committee (Date: 12/09/2016, No: 0089207). The study complied with conducted in accordance with the principles of the Declaration of Helsinki and pertinent national and international regulatory requirements.
Figure 1. Study flow chart.
Intervention
The patients were randomly allocated in a 1:1 ratio into two groups by an independent investigator using an online software (www.random.org, Dublin, Ireland): upper Michigan OS (Group 1, n=20) and mandibular OS (Group 2, n=20).
In Group 1, the OS was in contact with the mandibular supporting cusps and had cuspid guidance discluding the supporting cusp contact almost as soon as lateral or protrusive mandibular movements were made.[17] In Group 2, the OS was constructed to allow only posterior contacts (from the second premolar to the second/first permanent molar), without static and dynamic anterior contacts,[16] following the biomechanical models proposed by Ferrario et al.[15] All patients underwent the treatment with OS during night time, for at least 8 h per night for a total of six months.
Outcomes
The primary outcome of this study was the reduction of myofascial pain as assessed by the VAS. The secondary outcomes were the ROM of mandible movements and the activity of the main masticatory muscles (superficial masseter and anterior temporalis) as evaluated by surface electromyography (sEMG). All outcome measures were assessed at baseline (T0), one month (T1), three months (T2), and six months (T3) by a single observer who was unblinded to the problems of the patients and experienced in the diagnosis and treatment of TMD according to the DC/TMD.[1]
Instruments
The VAS is used to evaluate the intensity of myofascial pain perceived by the patient at the clinical evaluation, scoring from 0 (no pain) to 10 (worst pain ever).
The ROM was assessed for the following mandible movements:
Active opening (AO): measured from the right lower central incisor margin to the right upper central incisor margin, when the patients carried out a maximum mouth opening without feeling pain;
Passive opening (PO): measured from the right lower central incisor margin to the right upper central incisor margin, when the patients carried out a maximum mouth opening, while the clinician was forcing the AO;
Protrusion (PR): measured from the upper incisor and the lower incisor, when the patients carried out the PR movement;
Lateral excursion mandibular movement (LAT): measured from the superior interincisal line to the inferior interincisal line, when the patients carried out left and right lateral excursion mandibular movements. The sEMG was also used to assess the main masticatory muscles (superficial masseter and anterior temporalis) activity using the Foremg® device (4T QuattroTi S.r.l. Cislago, Varese, Italy), and the OTBioLab® software (OT Bioelettronica, Torino, Italy). The concentric bipolar electrodes (CoDe®: Concentric Detection, OT Bioelettronica, Torino, Italy) were used to detect the myoelectric activity.[21,22] To reduce skin impedance, the skin was carefully cleaned with antiseptic gel prior to electrodes placement, and recordings were performed 5 to 6 min later, allowing the conductive paste to adequately moisten the skin surface.[23] Electromyographic recordings were performed before and after splint treatment pacing electrodes by palpation of the muscle in the main direction of the muscle fibers,[24] and accordingly to anatomical references described.[25] The EMG activity of jaw elevator muscles was detected with disposable bipolar silver chloride (AgCl) concentric electrodes using a 16-mm radius applied on the muscles’ bellies, while a reference electrode was applied on the forehead.[26] The EMG activity was recorded using four of the eight channels of the instrument. The analogic EMG signal was amplified, digitized, and digitally filtered. The signals were sampled at 800 Hz with 8-bit resolution. The EMG channels were filtered between 10 and 400 Hz with a gain of 4,300. The instrument was directly interfaced with a computer that presented the data graphically and recorded them for further quantitative and qualitative analyses.
During the recordings, the patients sat with their heads unsupported and were asked to maintain a natural erect posture with open eyes, with feet positioned on the floor, hands were resting on their legs. Four recordings were performed during this study. All measurements were done using the same electrodes positioned in the same cutaneous area, using same cables and oral appliance, to reduce biological and technical noise. The patients were asked to relax for 3 min between each recording to avoid fatigue.
A series of sEMG indices[27-29] were used to perform the evaluation of the muscular activity. The indices were computed as follows:
Percentage overlapping coefficient (POC):[27] an index of the symmetric distribution of muscular activity as determined by occlusion, calculated for each couple of homologous muscles, anterior temporal and superficial masseter; its ranges from 0% (no symmetry) to 100% (perfect symmetry), where a POC of >85% is considered normal.
Barycenter (BAR):[27] an index providing data on the principal occlusal center of pressure, calculating to compare the muscle activity of masseter and temporalis muscles; the occlusal center of pressure (clench on the occlusal surfaces compared to clench on the cotton rolls) may be displaced onwards (temporalis prevalence) or backwards (masseter prevalence); it ranges from -100% (temporalis muscle prevalence) to +100% (masseter muscle prevalence).
Impact coefficient (IMPACT):[27] a measure of the total electrical activity, by calculating the area under the muscular waveforms of all four analyzed muscles; it assesses the muscle work performed during the selected task.
Torque coefficient (TC):[28] an index computed in case of an unbalanced contractile activity of contralateral masseter and temporalis muscles, ranging from 0% (unbalanced standardized masseter and temporalis potentials) to 100% (well comparable standardized masseter and temporalis potentials).
Asymmetry index (ASIM):[29] an index that quantifies the asymmetrical masticatory muscle activity to identify the dominant side; it ranges from -100% to +100%, where a negative number indicates a left-side muscle dominance and a positive number a right-side one.
Statistical analysis
Considering the VAS as the primary outcome and taking into account an effect size of 1.3 as described by Keskinruzgar et al.,[30] the minimum sample size was calculated as 11 patients per group. Considering a 10% dropout rate, 24 patients (12 in each group) were needed. The null hypothesis was defined as: H0: μ1= μ2, indicating that media variations of all analyzed variables must be the same in the two different study groups. The null hypothesis can be rejected, if some of the variables reached statistical significance. The power of the study was 90% with a type I error level of 0.05.
Statistical analysis was performed using the IBM SPSS version 23.0 software (IBM Corp., Armonk, NY, USA). Continuous variables were presented in mean ± standard deviation or median (min-max), while categorical variables were expressed in number and frequency. The Shapiro-Wilk test was performed to assess the distribution of all continuous data, as the data did not show a normal distribution. The differences between single variables at different timepoints were assessed by the two-way Friedman Analysis of Variance (ANOVA) for repeated measures and Dunn’s post-hoc test. The Wilcoxon rank-sum test was used to compare continuous variables between the two groups at different timepoints. A p value of <0.05 was considered statistically significant.
Results
All patients completed the treatment without any withdrawal. The mean age was 46.0±14.8 years in Group 1 and 48.5±10.6 years in Group 2. There was no statistically significant difference in the baseline demographic characteristics between the groups.
We observed a trend in decreasing pain as assessed by VAS in both groups, particularly after six months (T3-T0) of the Michigan OS treatment (mean VAS: 5.1±2.5 vs. 3.9±1.6, respectively; p=0.061); however, there were no statistically significant intra- or inter-group differences in the VAS scores. As depicted in Table 1, there were no significant intra-group differences in all the other outcome measures. However, Group 2 had a significantly higher mean right lateral mandibular ROM at T2 (7.1±3.1 vs. 9.8±2.3, respectively; p<0.05) and a significant higher left lateral mandibular ROM at T3 (7.6±3.5 vs. 10.5±2.1, respectively; p<0.05). In addition, no significant inter- and intra-group differences were observed in any sEMG parameters (Table 2).
Table 1. Inter-group and intra-group differences in terms of pain and range of motion.
| VAS | AO ROM (mm) | PO ROM (mm) | PR ROM (mm) | LAT to right ROM (mm) | LAT to left ROM (mm) | |||||||
| Group 1 (n=20) | Group 2 (n=20) | Group 1 (n=20) | Group 2 (n=20) | Group 1 (n=20) | Group 2 (n=20) | Group 1 (n=20) | Group 2 (n=20) | Group 1 (n=20) | Group 2 (n=20) | Group 1 (n=20) | Group 2 (n=20) | |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | |
| T0 | 5.1±2.5 | 4.1±2.1 | 41.5±5.7 | 43.6±5.4 | 43.7±5.8 | 46.3±4.7 | 6.8±3.2 | 8.2±3.1 | 8.9±2.4 | 8.5±2.6 | 7.5±2.8 | 9.7±3.4 |
| T1 | 4.8±3.1 | 3.7±1.9 | 43.0±6.9 | 41.5±8.3 | 45.0±7.2 | 45.3±8.3 | 6.5±4.0 | 9.1±2.2 | 8.5±2.3 | 8.7±3.2 | 8.9±4.3 | 9.2±3.8 |
| T2 | 3.5±2.9 | 4.2±2.1 | 41.7±7.8 | 43.2±9.1 | 46.9±7.5 | 46.8±7.1 | 6.9±3.5 | 8.7±2.2 | 8.1±2.2 | 9.5±1.9 | 7.6±3.5* | 10.5±2.1* |
| T3 | 3.9±1.6 | 3.8±1.9 | 44.2±4.0 | 45.1±7.1 | 46.5±5.0 | 48.2±6.6 | 6.9±3.8 | 8.8±2.8 | 7.1±3.1* | 9.8±2.3* | 8.5±3.1 | 10.1±1.7 |
| VAS: Visual Analog Scale; AO: Active opening; ROM: Range of motion; PO: Passive opening; PR: Protrusion; LAT: Lateral excursion mandibular movement; SD: Standard deviation; * p<0.05 as inter-group difference; Group 1: Michigan occlusal splint; Group 2: Mandibular occlusal splint; Wilcoxon rank-sum test was used to evaluate inter-group differences and the two-way Friedman Analysis of Variance (ANOVA) for repeated measures and Dunn’s post-hoc test were used to evaluate intra-group differences. | ||||||||||||
Table 2. Between-group and intra-group differences in terms of surface electromyography (sEMG) parameters using cotton rolls and occlusal splints.
| Cotton rolls | ||||||||||||
| AT POC (%) | SM POC (%) | BAR (%) | IMPACT (pV/pV x 100 x s) | TORS (%) | ASIM (%) | |||||||
| Group 1 (n=20) |
Group 2 (n=20) |
Group 1 (n=20) |
Group 2 (n=20) |
Group 1 (n=20) |
Group 2 (n=20) |
Group 1 (n=20) |
Group 2 (n=20) |
Group 1 (n=20) |
Group 2 (n=20) |
Group 1 (n=20) |
Group 2 (n=20) |
|
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | |
| TO | 79.2413.1 | 70.8430.8 | 81.9421 | 80.5412.2 | 84.5414.4 | 70.94 30.9 | 86.3431.5 | 98.1421.8 | 87.545.8 | 74.54 31.5 | 3.3412.8 | 4.5414.7 |
| T1 | 83.049.6 | 83.0412.1 | 85.549.7 | 83.4412.3 | 83.049.6 | 89.144.9 | 85.549.7 | 93.8422.3 | 88.847.9 | 88.244.8 | -1.049.4 | -2.5414.6 |
| T2 | 77421.1 | 84.349.2 | 79.2414.1 | 84.148.1 | 81.0±21 | 89.345.1 | 115.7420.9 | 92.7412.6 | 80.1420.9 | 89.045.3 | 1.947.6 | 1.7249.3 |
| T3 | 80.2422.3 | 76.7422.5 | 82.7413.7 | 88.842.5 | 82.0422.9 | 76.246.2 | 120.2485.8 | 99.6416.7 | 82.9422.9 | 85.8411.7 | -1.8412.9 | -0.87416.5 |
| Stabilization splints | ||||||||||||
| AT POC (%) | SM POC (%) | BAR (%) | IMPACT (pV/pV x 100 x s) | TORS (%) | ASIM (%) | |||||||
| Group 1 (n=20) |
Group 2 (n=20) |
Group 1 (n=20) |
Group 2 (n=20) |
Group 1 (n=20) |
Group 2 (n=20) |
Group 1 (n=20) |
Group 2 (n=20) |
Group 1 (n=20) |
Group 2 (n=20) |
Group 1 (n=20) |
Group 2 (n=20) |
|
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | |
| TO | 73.2420.0 | 66.7431.5 | 81.9411.8 | 77.4414.3 | 80.5413.3 | 68.2431 | 89.2424.3 | 130.9418.6 | 81.7413.5 | 72.0431.8 | 0.3420.4 | -0.2421.9 |
| T1 | 73.7420.6 | 81.9410.8 | 81.5415.0 | 79.9412.5 | 79.8416 | 85.9410.7 | 84.3430.0 | 87.8422.3 | 82.2414.6 | 88.447.0 | 0.3418.5 | -6.84415.4 |
| T2 | 71.8425.5 | 83.148.7 | 68.9427.1 | 82.0415.7 | 76.1421.5 | 83.1±15.7 | 98.9462.3 | 79.1429.4 | 75.6424.7 | 88.846.5 | 5.0421.7 | 1.7249.3 |
| T3 | 77.7423.8 | 72.3425.9 | 79418.1 | 85.945.0 | 76.2422.9 | 85.447.1 | 116.9482.8 | 91.2431.0 | 80.1425.6 | 84.0412.0 | 0.2413.3 | -0.87416.5 |
| AT: Anterior temporal muscle; POC: Percentage overlapping coefficient; SM: Superficial masseter muscle; BAR: Barycenter; IMPACT: Impact coefficient; TORS: Torque coefficient; ASIM: Asymmetry index; SD: Standard deviation; * p<0.05 as inter-group difference; Group 1: Michigan occlusal splint; Group 2: Mandibular occlusal splint; Wilcoxon rank-sum test was used to evaluate inter-group differences and the two-way Friedman Analysis of Variance (ANOVA) for repeated measures and Dunn’s post-hoc test were used to evaluate intra-group differences. | ||||||||||||
No side effect or compliance problem were reported with the use of splints which were also prescribed at the end of the study.
Discussion
Myofascial pain adversely affects ADLs and HRQoL of TMD patients. Therefore, several treatments can be applied to reduce the invaliding pain, including pharmacological therapy, rehabilitative treatments, instrumental physical therapies, and OS.[8-12]
In this study, we compared the effects of both upper Michigan OS and mandibular splint in terms of myofascial pain, ROM, and muscle activity in TMD patients. Our study results showed that OS, independently from being built on the upper or lower arch, had no significant effects in reducing pain over a six-month period in TMD patients. Nonetheless, it was interesting to notice that no side effects were reported by the study participants and their compliance to OS prescription was successful as testified by no dropouts throughout the study period.
Although there were no significant inter- and intra-group differences for all the considered outcomes, we noticed a trend in decreasing pain in patients of both groups. It should be considered that pain-related TMDs embracing clinical problems involving the masticatory system, the TMJ, may also impact HRQoL in these subjects.[31,32] In a recent systematic review, Dahlström and Carlsson[33] reported that subjective TMD symptoms, including myofascial pain, had a greater impact than clinical findings on the oral health-related quality of life (OHRQoL). Indeed, TMDs have a greater impact compared to other diseases which may affect the oral health status, thus leading to a poor OHRQoL.[33-36]
To date, although the widely recognized application of OS for treating TMDs is in use in patients with muscle-related disorders,[8,9,37-39] there are no head-to-head studies comparing the effects of upper and lower OS. In a recent study, Al-Moraissi et al.[8] performed a network meta-analysis of 48 RCTs to assess the effectiveness of various types of OS in the management of TMD and found that there was moderate-to-very low-quality evidence supporting the effectiveness of OS combined to counselling therapy to achieve the maximum improvement for TMD patients. On the contrary, findings of our RCT did not confirm a reduction in terms of pain after treatment in both upper Michigan and mandibular OS groups after six months of treatment. The splint- induced modification in the relative activity between the masseter and temporal muscles has been also reported by other authors,[40,41] and even if it cannot be presented statistically and clinically related to the reduction in TMD pain, it is believed to play an important role in this effect.[15] However, Zhang et al.[42] performed a double-blind, RCT in 36 patients with myofascial pain and confirmed that OS could have a significant positive effect on clinical outcomes. Furthermore, the authors showed, by sEMG analysis, that the wearing of OS might reduce fatigue in the masticatory muscles with a correlation between the OS treatment and electromyographic changes in the masticatory muscles; however, their findings were effective only in the short-term, considering that the follow-up visit was at one month from the beginning of OS treatment. Other studies also confirmed an improvement in sEMG parameters in both the masseter and the temporal muscles after treatment with OS.[43,44] On the other hand, in our study, as far as electromyography is concerned, we found only a trend, albeit not significant, in changing of sEMG parameters after OS treatment, compared to baseline.
Nonetheless, there are some limitations to this study. First, the study was conducted during a six-month period which could provide us the short- or mid-term results of OS use. Second, in our study, no counselling therapy was given to the patients, which was recently demonstrated by Al-Moraissi et al.[8] to produce, in combination with OS, the maximum improvement for patients affected by TMD. Third, there is a lack of control group not undergoing splint therapy, that could better point out the effects of upper Michigan and lower mandibular OS. However, we initially hypothesized that both methods would be effective in TMD patients, as shown in many previous studies.[14-19] Nevertheless, to the best of our knowledge, this is the first RCT comparing the effects of upper Michigan and lower mandibular OS in reducing myofascial pain and on muscle activity as assessed by sEMG.
In conclusion, the findings of the present study suggested that a 6-month treatment with OS, independently from being built on the upper or lower arch, seems to not have significant effects in reducing myofascial pain in patients affected by muscle-related TMD. Future large-scale, prospective studies with a longer follow-up should be performed to gain a better understanding of the effectiveness of OS in these patients. Based on our results, we suggest that the treatment of muscle-related TMDs should be multifactorial including OS, analgesic drugs, psychological support, and an adequate and patient-tailored rehabilitation.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldemir K, Üstüner E, Erdem E, Demiralp AS, Oztuna D. Ultrasound evaluation of masseter muscle changes in stabilization splint treatment of myofascial type painful temporomandibular diseases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:377–383. doi: 10.1016/j.oooo.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Fleckenstein J, Zaps D, Rüger LJ, Lehmeyer L, Freiberg F, Lang PM, et al. Discrepancy between prevalence and perceived effectiveness of treatment methods in myofascial pain syndrome: results of a cross-sectional, nationwide survey. BMC Musculoskelet Disord. 2010;11:32–32. doi: 10.1186/1471-2474-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber LH, Sikdar S, Armstrong K, Diao G, Heimur J, Kopecky J, et al. A systematic comparison between subjects with no pain and pain associated with active myofascial trigger points. PM R. 2013;5:931–938. doi: 10.1016/j.pmrj.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastore GP, Goulart DR, Pastore PR, Prati AJ, de Moraes M. Self-medication among myofascial pain patients: A preliminary study. Open Dent J. 2018;12:347–353. doi: 10.2174/1874210601812010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fricton J. Myofascial pain: Mechanisms to management. Oral Maxillofac Surg Clin North Am. 2016;28:289–311. doi: 10.1016/j.coms.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Gündüz N, Polat A, Erzincan E, Turan H, Sade I, Tural Ü. Psychiatric comorbidity and childhood trauma in fibromyalgia syndrome. Turk J Phys Med Rehabil. 2018;64:91–99. doi: 10.5606/tftrd.2018.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Moraissi EA, Farea R, Qasem KA, Al-Wadeai MS, Al-Sabahi ME, Al-Iryani GM. Effectiveness of occlusal splint therapy in the management of temporomandibular disorders: network meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg. 2020;49:1042–1056. doi: 10.1016/j.ijom.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Kuzmanovic Pficer J, Dodic S, Lazic V, Trajkovic G, Milic N, Milicic B. Occlusal stabilization splint for patients with temporomandibular disorders: Meta-analysis of short and long term effects. e0171296PLoS One. 2017;12 doi: 10.1371/journal.pone.0171296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado D, Martimbianco ALC, Bussadori SK, Pacheco RL, Riera R, Santos EM. Botulinum toxin type a for painful temporomandibular disorders: Systematic review and meta-analysis. J Pain. 2020;21:281–293. doi: 10.1016/j.jpain.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Keskin Tunç S, Ünalan Değirmenci B, Alpaslan Yaylı N, Aslan Ş, Akdeniz MŞ. Evaluation the effects of low-level laser therapy on disc displacement with reduction. Turk J Phys Med Rehabil. 2020;66:24–30. doi: 10.5606/tftrd.2020.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aksu Ö, Pekin Doğan Y, Sayıner Çağlar N, Şener BM. Comparison of the efficacy of dry needling and trigger point injections with exercise in temporomandibular myofascial pain treatment. Turk J Phys Med Rehabil. 2019;65:228–235. doi: 10.5606/tftrd.2019.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Arch Phys Med Rehabil. 2002;83:1406–1414. doi: 10.1053/apmr.2002.34834. [DOI] [PubMed] [Google Scholar]
- 14.Ferrario VF, Sforza C. Biomechanical model of the human mandible in unilateral clench: distribution of temporomandibular joint reaction forces between working and balancing sides. J Prosthet Dent. 1994;72:169–176. doi: 10.1016/0022-3913(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 15.Ferrario VF, Sforza C, Tartaglia GM, Dellavia C. Immediate effect of a stabilization splint on masticatory muscle activity in temporomandibular disorder patients. J Oral Rehabil. 2002;29:810–815. doi: 10.1046/j.1365-2842.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- 16.De Giorgi I, Castroflorio T, Cugliari G, Deregibus A, et al. Does occlusal splint affect posture. A randomized controlled trial. Cranio. 2020;38:264–272. doi: 10.1080/08869634.2018.1511265. [DOI] [PubMed] [Google Scholar]
- 17.Ramfjord SP, Ash MM. Reflections on the Michigan occlusal splint. J Oral Rehabil. 1994;21:491–500. doi: 10.1111/j.1365-2842.1994.tb01164.x. [DOI] [PubMed] [Google Scholar]
- 18.Capp NJ. Temporomandibular joint dysfunction--its relevance to restorative dentistry. Part 2: Splint therapy and restorative considerations. Restorative Dent. 1986;2:62:64–68. [PubMed] [Google Scholar]
- 19.Capp NJ. Occlusion and splint therapy. Br Dent J. 1999;186:217–222. doi: 10.1038/sj.bdj.4800069. [DOI] [PubMed] [Google Scholar]
- 20.Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK, Memtsoudis S, et al. Validation of the STOP-bang questionnaire as a screening tool for obstructive sleep apnea among different populations: A systematic review and meta-analysis. e0143697PLoS One. 2015;10 doi: 10.1371/journal.pone.0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farina D, Cescon C. Concentric-ring electrode systems for noninvasive detection of single motor unit activity. IEEE Trans Biomed Eng. 2001;48:1326–1334. doi: 10.1109/10.959328. [DOI] [PubMed] [Google Scholar]
- 22.Castroflorio T, Deregibus A, Bargellini A, Debernardi C, Manfredini D. Detection of sleep bruxism: comparison between an electromyographic and electrocardiographic portable holter and polysomnography. J Oral Rehabil. 2014;41:163–169. doi: 10.1111/joor.12131. [DOI] [PubMed] [Google Scholar]
- 23.Ferrario VF, Sforza C, Colombo A, Ciusa V. An electromyographic investigation of masticatory muscles symmetry in normo-occlusion subjects. J Oral Rehabil. 2000;27:33–40. doi: 10.1046/j.1365-2842.2000.00490.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferrario VF, Sforza C, D’Addona A, Miani A Jr. Reproducibility of electromyographic measures: a statistical analysis. J Oral Rehabil. 1991;18:513–521. doi: 10.1111/j.1365-2842.1991.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 25.Castroflorio T, Farina D, Bottin A, Piancino MG, Bracco P, Merletti R. Surface EMG of jaw elevator muscles: effect of electrode location and inter-electrode distance. J Oral Rehabil. 2005;32:411–417. doi: 10.1111/j.1365-2842.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- 26.Castroflorio T, Icardi K, Becchino B, Merlo E, Debernardi C, Bracco P, et al. Reproducibility of surface EMG variables in isometric sub-maximal contractions of jaw elevator muscles. J Electromyogr Kinesiol. 2006;16:498–505. doi: 10.1016/j.jelekin.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Ferrario VF, Sforza C, Tartaglia GM, Dellavia C. Immediate effect of a stabilization splint on masticatory muscle activity in temporomandibular disorder patients. J Oral Rehabil. 2002;29:810–815. doi: 10.1046/j.1365-2842.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferrario VF, Tartaglia GM, Galletta A, Grassi GP, Sforza C. The influence of occlusion on jaw and neck muscle activity: a surface EMG study in healthy young adults. J Oral Rehabil. 2006;33:341–348. doi: 10.1111/j.1365-2842.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- 29.Naeije M, McCarroll RS, Weijs WA. Electromyographic activity of the human masticatory muscles during submaximal clenching in the inter-cuspal position. J Oral Rehabil. 1989;16:63–70. doi: 10.1111/j.1365-2842.1989.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 30.Keskinruzgar A, Kucuk AO, Yavuz GY, Koparal M, Caliskan ZG, Utkun M. Comparison of kinesio taping and occlusal splint in the management of myofascial pain in patients with sleep bruxism. J Back Musculoskelet Rehabil. 2019;32:1–6. doi: 10.3233/BMR-181329. [DOI] [PubMed] [Google Scholar]
- 31.Barros Vde M, Seraidarian PI, Côrtes MI, de Paula LV. The impact of orofacial pain on the quality of life of patients with temporomandibular disorder. J Orofac Pain. 2009;23:28–37. [PubMed] [Google Scholar]
- 32.Oghli I, List T, Su N, Häggman-Henrikson B. The impact of oro-facial pain conditions on oral health-related quality of life: A systematic review. J Oral Rehabil. 2020;47:1052–1064. doi: 10.1111/joor.12994. [DOI] [PubMed] [Google Scholar]
- 33.Dahlström L, Carlsson GE. Temporomandibular disorders and oral health-related quality of life. A systematic review. Acta Odontol Scand. 2010;68:80–85. doi: 10.3109/00016350903431118. [DOI] [PubMed] [Google Scholar]
- 34.de Sire A, Baricich A, Ferrillo M, Migliario M, Cisari C, Invernizzi M. Buccal hemineglect: is it useful to evaluate the differences between the two halves of the oral cavity for the multidisciplinary rehabilitative management of right brain stroke survivors. A cross-sectional study. Top Stroke Rehabil. 2020;27:208–214. doi: 10.1080/10749357.2019.1673592. [DOI] [PubMed] [Google Scholar]
- 35.Machado V, Botelho J, Proença L, Alves R, Oliveira MJ, Amaro L, et al. Periodontal status, perceived stress, diabetes mellitus and oral hygiene care on quality of life: a structural equation modelling analysis. BMC Oral Health. 2020;20:229–229. doi: 10.1186/s12903-020-01219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kjeldsted E, Dalton SO, Frederiksen K, Andersen E, Nielsen AL, Stafström M, et al. Association between human papillomavirus status and health-related quality of life in oropharyngeal and oral cavity cancer survivors. Oral Oncol. 2020;109:104918–104918. doi: 10.1016/j.oraloncology.2020.104918. [DOI] [PubMed] [Google Scholar]
- 37.Ferrario VF, Sforza C, Miani A Jr, D'Addona A, Barbini E. Electromyographic activity of human masticatory muscles in normal young people. Statistical evaluation of reference values for clinical applications. J Oral Rehabil. 1993;20:271–280. doi: 10.1111/j.1365-2842.1993.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 38.Al-Ani MZ, Davies SJ, Gray RJ, Sloan P, Glenny AM. WITHDRAWN: Stabilisation splint therapy for temporomandibular pain dysfunction syndrome. CD002778Cochrane Database Syst Rev. 2016;1 doi: 10.1002/14651858.CD002778.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene CS, Menchel HF. The Use of Oral Appliances in the Management of Temporomandibular Disorders. Oral Maxillofac Surg Clin North Am. 2018;30:265–277. doi: 10.1016/j.coms.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Naeije M, Hansson TL. Short-term effect of the stabilization appliance on masticatory muscle activity in myogenous craniomandibular disorder patients. J Craniomandib Disord. 1991;5:245–250. [PubMed] [Google Scholar]
- 41.Visser A, McCarroll RS, Oosting J, Naeije M. Masticatory electromyographic activity in healthy young adults and myogenous craniomandibular disorder patients. J Oral Rehabil. 1994;21:67–76. doi: 10.1111/j.1365-2842.1994.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang FY, Wang XG, Dong J, Zhang JF, Lü YL. Effect of occlusal splints for the management of patients with myofascial pain: a randomized, controlled, double-blind study. Chin Med J (Engl) 2013;126:2270–2275. [PubMed] [Google Scholar]
- 43.Visser A, Naeije M, Hansson TL. The temporal/masseter co-contraction: an electromyographic and clinical evaluation of short-term stabilization splint therapy in myogenous CMD patients. J Oral Rehabil. 1995;22:387–389. doi: 10.1111/j.1365-2842.1995.tb00789.x. [DOI] [PubMed] [Google Scholar]
- 44.Sheikholeslam A, Holmgren K, Riise C. A clinical and electromyographic study of the long-term effects of an occlusal splint on the temporal and masseter muscles in patients with functional disorders and nocturnal bruxism. J Oral Rehabil. 1986;13:137–145. doi: 10.1111/j.1365-2842.1986.tb00646.x. [DOI] [PubMed] [Google Scholar]