Abstract
This editorial refers to ‘The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study’†, by M. D’Souza et al., on page 1621.
Immune checkpoint inhibitor (ICI) therapy induces an anticancer response by blockade of immune-evasive signalling. The first ICI used in patients was ipilimumab, which is an inhibitor of cytotoxic T-lymphocyte-associated protein 4 (CTLA4).1 In 2006, the first application of the programmed death 1 (PD1) inhibitor nivolumab followed. These initial studies revealed remarkable anticancer effects, and the first Food and Drug Association (FDA) approval was granted in 2011 for the treatment of stage IV melanoma. Since then, a total of seven ICIs have been approved, with >50 different indications including non-small cell lung cancer, hepatocellular cancer, cervical cancer, breast cancer, renal cell carcinoma, urothelial carcinoma, oesophageal cancer, gastric cancer, endometrial cancer, colorectal cancer (with high microsatellite instability), head and neck cancer, Merkel cell carcinoma, and Hodgkin’s lymphoma.1 Additionally, approvals for ICI therapy have expanded from late stage disease to the adjuvant and neoadjuvant setting, and it is estimated that 36.1% of all cancer patients are eligible for ICI treatment.2 The use of ICIs show an exponential growth rate that is higher than comparable approaches for targeted or immune-dependent cancer therapy regimens.2
ICI-related side effects are termed immune-related adverse events (irAEs). These are mainly triggered by the ICI’s T-cell cytotoxicity response that results in immune reaction affecting many organ systems, with the most common involving the skin, gut, lung, and liver. Myocarditis was the first described cardiac toxicity associated with ICIs3 and, using a pharmacovigilance database, was initially considered a rare event, with estimates ranging from 0.06% to 0.27%, with a fatal myocarditis event occurring from <0.01% to <0.17%.3 The true incidence of ICI-associated myocarditis was felt to be underestimated due to the wide range of clinical presentations, challenges in diagnosis, and a general lack of awareness of this condition.4
The study by D’Souza and colleagues which is presented in this issue of the European Heart Journal highlights some of these challenges and adds significantly to the discussion.5 From a nationwide Danish registry, they report a much higher frequency of cardiac events (a composite of arrhythmias, pericarditis, myocarditis heart failure, or cardiac death). The authors demonstrate a total risk of 6.6–9.7% for cardiac events in patients receiving ICI therapy compared with patients receiving non-ICI cancer therapy. The authors included data from 39, 199 consecutive cancer patients treated between 2011 and 2017, of whom 1100 were treated with an ICI. After stratification for the type of cancer, the authors noted a consistently increased hazard ratio of up to 4.93 for cardiac events in patients with ICI therapy. A significant effect was observed for PD1 inhibitors and CTLA4 inhibitors independently. When testing for the rates of individual events, arrhythmias, pericarditis, myocarditis, heart failure, and cardiovascular death were all increased in patients with lung cancer, while only the combined endpoint and arrhythmias were increased in melanoma patients. Additionally, they noted an increased risk for cardiac events that is sustained after the first 6 months of therapy, where prior data suggested that most cardiac events occurred within the first few months after initation of an ICI.6
How can we explain these higher risk ratios for cardiac irAEs as compared with previous reports? Misclassification may probably explain a significant component. Specifically, the standardized format for adverse events reporting for clinical trials, the ‘Common Terminology Criteria for Adverse Events’ (CTCAE), allows a wide variation in cardiovascular diagnoses ranging from complete heart block, to ventricular tachycardia, to left ventricular dysfunction, to heart failure and myocarditis. Is it reasonable to ask which of these in the setting of a new start on an ICI is not likely to be some manifestation of an ICI-related immune and inflammatory cardiac syndrome? Additionally, the diagnosis of some of these potential classifications is not straightforward. For example, the diagnosis of myocarditis, as illustrated in the current COVID-19 pandemic, can frequently be challenging. For ICI myocarditis, <50% of patients have a reduced left ventricular systolic ejection function,7 many are without some of the hallmark cardiac magnetic resonance findings,8 and case reports on subclinical forms and asymptomatic findings from laboratory or imaging testing are frequent. These observations suggest that myocarditis and other forms of myocardial involvement may have been missed in previous studies. This potential for underappreciation of concurrent or related cardiovascular disease, highlighted by recent publications in this journal,9 seems to have particularly affected patients with cancer and supports recent and ongoing research efforts and collaborations with oncologists applying comprehensive detection approaches to unselected cancer patients.10
Why is there an up to 9.7% rate of cardiac events among patients treated with an ICI? The pathophysiological mechanisms involved in ICI-related inflammatory and immune cardiac diseases are incompletely understood. In line with the general knowledge on irAEs, myocardial inflammation with involvement of the adaptive immune system and lymphocytic infiltration induces a cascade of reversible or irreversible damage to affected tissues.11 Infiltrating lymphocytes have been found within the myocardium of patients with ICI-related myocarditis in post-mortem samples and endomyocardial biopsies.8 However, fundamental questions remain unanswered. For example, a target structure for infiltrating immune cells has not yet been clearly identified. While Pd1 deficiency in a pre-clinical model led to the development of anticardiac troponin autoantibodies, no such effect was observed in patients.12 Recently, the immunoproteasome of immune cells was identified to predict the immune response in a mouse model of myocarditis after immunization with a cardiac troponin I peptide.13 Immunoproteasome deficiency led to mitigated cardiac inflammation, and reduced myocardial fibrosis, leading to improved cardiac function. Changes within the balance between effector and regulatory T cells, including enhanced expression of inhibitory PD1 receptors on T-cell subsets, were identified. The proposed mechanism also indicates a potential therapeutic benefit of pharmacological inhibition of the immune proteasome during myocarditis.13 Data also support the potential for antigenic mimicry between tumour cells and heart tissue,3 where, in a patient with melanoma and myocarditis and myositis, similar T-cell receptor clones were found within myocardium and skeletal muscle and were also greatly expanded within the tumour after treatment. This raises the possibility of shared epitopes in tumour and myocardial tissue.
These findings highlight that we are just beginning to understand the full nature of potential ICI-related cardiac complications and the increased relevance for cardio-oncology care. Given the wide variety for both presentations and the associated morbidity and mortality, treatment approaches represent a challenge. For example, fulminant myocarditis requires discontinuation of an ICI and high-dose corticosteroid therapy. If these approaches fail, then the next steps are unclear but are the subject of active research. The CTLA4 agonist abatacept facilitates a direct interaction with ICI signalling, and abatacept was successfully applied for the treatment of a patient with severe, steroid-refractory myocarditis after ICI therapy.14 Interestingly, the treatment was effective despite the patient receiving an ICI therapy targeting PD1, indicating the synergistic activity of both immune checkpoints. However, all forms of immunosuppressive therapy applied for ICI-related complications probably suppress anticancer efficacy. Recent evidence suggests that it may be possible to produce irAEs with preserved, or even enhanced, anticancer efficacy.15 Specifically, in an experimental model, blockade of tumour necrosis factor-α (TNF-α) signalling which was successfully applied for immune-related colitis, sustained anticancer effects.15 However, the caveat here is that TNF-α blockade is contraindicated in the presence of advanced heart failure (NYHA III and IV) and data are urgently needed testing the effect of TNF-α blockade for cardiovascular toxicities related to ICIs. Beyond the fulminant manifestations of ICI cardiac toxicity, fundamental treatment questions also remain regarding other clinical presentations. How should we approach a patient with an arrhythmia on an ICI or an isolated troponin elevation, or heart failure without imaging evidence of active cardiac inflammation? With recent data suggesting an association between ICI use and accelerated atherosclerosis and atherosclerotic-related cardiovascular events,16 what do we do with a patient with a vascular event such as an ischaemic stroke or myocardial infarction in the setting of an ICI? These treatment approaches are further complicated by the painful lessons learned from serial monitoring of the left ventricular ejection fraction among women with breast cancer on HER-2 antagonists such as herceptin, where lifesaving cancer therapies may be stopped due an asymptomatic decline in cardiac function, thus adversely impacting cancer outcomes.
This study also acts as the basis for some additional questions. What was the nature of the arrhythmias? Were they atrial or ventricular, malignant or more benign? What is the mechanism for the increase in some of these cardiac events? Can we dissociate cardiac toxicity from cancer efficacy among those on an ICI? How will we separate the cardiac injury or dysfunction seen with targeted therapy or traditional cytotoxic chemotherapy from that of an ICI when they are used in combination?
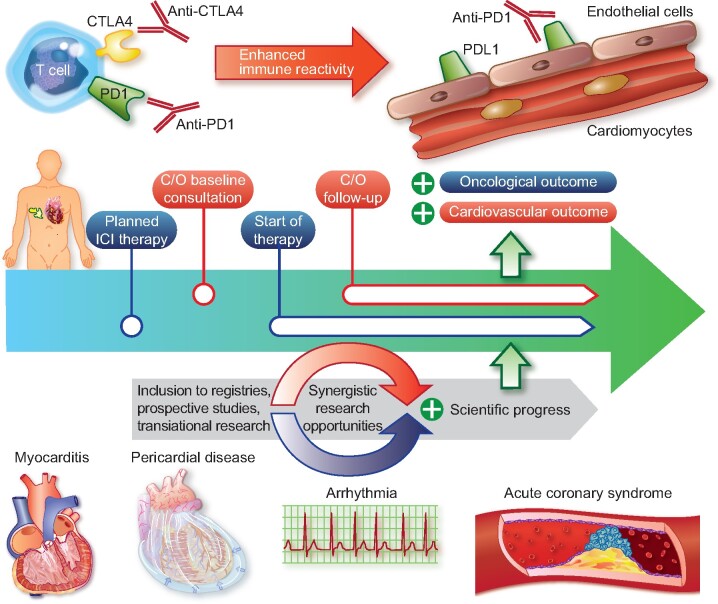
In conclusion, perhaps is it time for a broader description of ICI-induced cardiovascular complications to include the term ‘ICI-related cardiovascular disease’ and this is supported by the important insights presented by D’Souza and colleagues. Immediate steps include increasing our awareness for a broader range of potential cardiac toxicities related to ICI treatment (Take home figure). Longer term steps include broadening collaborations with our oncology and pharmaceutical partners, and expanded clinical research efforts in parallel and based on innovative basic experimental insights. These and other steps are needed to move this forward so we can improve cardiovascular outcomes among our cancer patients treated with an ICI.
Take home figure .
Systematic assessment for a better and precise characterization of ICI-related cardiovascular disease is required. This requires baseline and follow-up assessment and inclusion of patients in large systematic registries to identify potential adverse events including myocarditis, pericarditis, arrhythmia (e.g. atrioventricular block), and acute coronary syndromes. C/O, cardio-oncology.
Funding
This work was supported by the National Institutes of Health/National Heart, Lung, Blood Institute [T32HL076136; R01HL137562-, R01HL130539, and K24HL150238 to T.G.N.]. T.G.N.was also supported, in part, through a kind gift from A. Curtis Greer and Pamela Kohlberg. The figure was produced using Servier Medical Art.
Conflict of interest: M.T. has received personal funding and advisory fees from Bayer Vital, Astra Zeneca, Daiichi Sankyo, and Bristol Myers Squibb. T.G.N. has received advisory fees from Parexel, BMS, H3 Biomedicine, AbbVie, and Intrinsic Imaging, and grant funding from Astra Zeneca. E.L. has no conflicts to declare.
Footnotes
† doi: 10.1093/eurheartj/ehaa884.
Contributor Information
Matthias Totzeck, Department of Cardiology and Vascular Medicine, West German Heart and Vascular Center, Medical Faculty, University Hospital Essen, Essen, Germany.
Esther Lutgens, Experimental Vascular Biology Division, Department of Medical Biochemistry, University of Amsterdam, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, Amsterdam, The Netherlands; Institute for Cardiovascular Prevention (IPEK), Ludwig-Maximilians Universität, München, Germany and German Center for Cardiovascular Research (DZHK), partner site Munich Heart Alliance, Munich, Germany.
Tomas G Neilan, Cardiovascular Imaging Research Center, Division of Cardiology and Department of Radiology, Massachusetts General Hospital, Boston, MA, USA; Cardio-Oncology Program, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 2018;29:84–91. [DOI] [PubMed] [Google Scholar]
- 2. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2019;2:e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr, Anders RA, Sosman JA, Moslehi JJ. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. D’Souza M, Nielsen D, Svane IM, Rasmussen PV, Madelaire C, Fosbøl E, Køber L, Gustafsson F, Andersson C, Gislason G, Torp-Pedersen C, Schou M. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J 2021;42:1621–1631. [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, Zubiri L, Chen CL, Sullivan RJ, Alvi RM, Rokicki A, Murphy SP, Jones-O’Connor M, Heinzerling LM, Barac A, Forrestal BJ, Yang EH, Gupta D, Kirchberger MC, Shah SP, Rizvi MA, Sahni G, Mandawat A, Mahmoudi M, Ganatra S, Ederhy S, Zatarain-Nicolas E, Groarke JD, Tocchetti CG, Lyon AR, Thavendiranathan P, Cohen JV, Reynolds KL, Fradley MG, Neilan TG. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation 2020;141:2031–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Awadalla M, Mahmood SS, Groarke JD, Hassan MZO, Nohria A, Rokicki A, Murphy SP, Mercaldo ND, Zhang L, Zlotoff DA, Reynolds KL, Alvi RM, Banerji D, Liu S, Heinzerling LM, Jones-O’Connor M, Bakar RB, Cohen JV, Kirchberger MC, Sullivan RJ, Gupta D, Mulligan CP, Shah SP, Ganatra S, Rizvi MA, Sahni G, Tocchetti CG, Lawrence DP, Mahmoudi M, Devereux RB, Forrestal BJ, Mandawat A, Lyon AR, Chen CL, Barac A, Hung J, Thavendiranathan P, Picard MH, Thuny F, Ederhy S, Fradley MG, Neilan TG. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol 2020;75:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, Zlotoff DA, Murphy SP, Stone JR, Golden DLA, Alvi RM, Rokicki A, Jones-O’Connor M, Cohen JV, Heinzerling LM, Mulligan C, Armanious M, Barac A, Forrestal BJ, Sullivan RJ, Kwong RY, Yang EH, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Moslehi JJ, Coelho-Filho OR, Ganatra S, Rizvi MA, Sahni G, Tocchetti CG, Mercurio V, Mahmoudi M, Lawrence DP, Reynolds KL, Weinsaft JW, Baksi AJ, Ederhy S, Groarke JD, Lyon AR, Fradley MG, Thavendiranathan P, Neilan TG. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 2020;41:1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez-Sendon J, Alvarez-Ortega C, Zamora Aunon P, Buno Soto A, Lyon AR, Farmakis D, Cardinale D, Canales Albendea M, Feliu Batlle J, Rodriguez Rodriguez I, Rodriguez Fraga O, Albaladejo A, Mediavilla G, Gonzalez-Juanatey JR, Martinez Monzonis A, Gomez Prieto P, Gonzalez-Costello J, Serrano Antolin JM, Cadenas Chamorro R, Lopez Fernandez T. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J 2020;41:1720–1729. [DOI] [PubMed] [Google Scholar]
- 10. Rassaf T, Totzeck M, Backs J, Bokemeyer C, Hallek M, Hilfiker-Kleiner D, Hochhaus A, Lüftner D, Müller OJ, Neudorf U, Pfister R, von Haehling S, Lehmann LH, Bauersachs J. Onco-Cardiology: Consensus Paper of the German Cardiac Society, the German Society for Pediatric Cardiology and Congenital Heart Defects and the German Society for Hematology and Medical Oncology. Clin Res Cardiol 2020;109:1197–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology—strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol 2019;280:163–175. [DOI] [PubMed] [Google Scholar]
- 12. Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 2003;9:1477–1483. [DOI] [PubMed] [Google Scholar]
- 13. Bockstahler M, Fischer A, Goetzke CC, Neumaier HL, Sauter M, Kespohl M, Muller AM, Meckes C, Salbach C, Schenk M, Heuser A, Landmesser U, Weiner J, Meder B, Lehmann L, Kratzer A, Klingel K, Katus HA, Kaya Z, Beling A. Heart-specific immune responses in an animal model of autoimmune-related myocarditis mitigated by an immunoproteasome inhibitor and genetic ablation. Circulation 2020;141:1885–1902. [DOI] [PubMed] [Google Scholar]
- 14. Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, Kerneis M. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med 2019;380:2377–2379. [DOI] [PubMed] [Google Scholar]
- 15. Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, de Andrea C, Rodriguez-Ruiz ME, Perez-Gracia JL, Marquez-Rodas I, Llacer C, Alvarez M, de Luque V, Molina C, Teijeira A, Berraondo P, Melero I. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019;569:428–432. [DOI] [PubMed] [Google Scholar]
- 16. Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, Mosarla RC, Lee C, Zlotoff DA, Raghu VK, Hartmann SE, Gilman HK, Gong J, Zubiri L, Sullivan RJ, Reynolds KL, Mayrhofer T, Zhang L, Hoffmann U, Neilan TG. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 2020;doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]