Supplemental digital content is available in the text.
Key Words: urinary microbiome, female bladder, interstitial cystitis, painful bladder
Abstract
Objective
Multiple studies show cultivatable bacteria in urine of most women. The existence of these bacteria challenges interstitial cystitis (IC)/painful bladder syndrome (PBS) diagnosis, which presumes a sterile bladder. The aims of this study were (1) to compare the female bladder microbiomes in women with IC/PBS and unaffected controls and (2) to correlate baseline bladder microbiome composition with symptoms.
Methods
This cross-sectional study enrolled 49 IC/PBS and 40 controls. All provided catheterized urine samples and completed validated questionnaires. A subset of the IC/PBS cohort provided voided and catheterized urine samples. All samples from both cohorts were assessed by the expanded quantitative urine culture (EQUC) protocol; a subset was assessed by 16S rRNA gene sequencing.
Results
Of the IC/PBS cohort, 49.0% (24/49) were EQUC positive; in these EQUC-positive samples, the most common urotypes were Lactobacillus (45.8%) and Streptococcus (33.3%). Of the controls, 40.0% were EQUC positive; of these EQUC-positive samples, the most common urotype was Lactobacillus (50.0%). The urotype distribution was significantly different (P < 0.05), as 16% of the IC/PBS cohort, but 0% of controls, were Streptococcus urotype (P < 0.01). Symptom-free IC/PBS participants were less likely to be EQUC positive (12.5%) than IC/PBS participants with moderate or severe symptoms (68.8% and 46.2%) and the control cohort (60%; P < 0.05).
Conclusion
Lactobacillus was the most common urotype. However, the presence of Lactobacillus did not differ between cohorts, and it did not impact IC/PBS symptom severity. Bacteria were not isolated from most participants with active IC/PBS symptoms. These findings suggest that bacteria may not be an etiology for IC/PBS.
Interstitial cystitis/painful bladder syndrome (IC/PBS) affects 2.7% to 6.5% of women in the United States1 and significantly impacts quality of life.2 Its economic impact in the United States exceeds $100 million per year.3 Despite its high incidence rate, impact on quality of life, and economic cost, IC/PBS pathophysiology is poorly understood. Key is the lack of information regarding IC/PBS etiology, with evidence suggesting that the cause may be multifactorial.4
The hallmark symptom of IC/PBS is chronic bladder pain without an associated underlying infection. Hence, IC/PBS diagnosis is one of the exclusion criteria, predicated on a negative standard urine culture. However, this diagnostic protocol is limited by the false assumption that the female bladder exists in a sterile state.5,6 The existence of the female urinary microbiota (FUM) has been revealed in nearly 80% of tested women through the combined use of high-throughput 16S rRNA gene sequencing and expanded quantitative urine culture (EQUC), which routinely detect bacteria in standard urine culture-negative samples.7–9
Our primary aim was to determine if the FUM differs between women with IC/PBS and women without bladder pain (controls). Our secondary aim was to assess if the FUM is associated with disease severity and/or particular symptoms. We hypothesized the FUM of participants with IC/PBS would differ from those without bladder pain and that FUM characteristics correlate with symptom severity.
MATERIALS AND METHODS
The institutional review board of Women & Infants Hospital approved this cross-sectional study. Two cohorts of women were recruited: women with IC/PBS who presented to the urogynecology clinic and unaffected women presenting for routine gynecologic care. Women ≥18 years old were eligible for the IC/PBS cohort if clinicians believed they fit the following description: “An unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than six weeks duration, in the absence of infection or other identifiable causes.” Participants who did not have bladder pain were eligible for the control cohort. Participants were excluded if they were currently pregnant, were experiencing vaginal itching/burning/discharge or gross hematuria, had anatomic anomalies (eg, urethral stricture and bladder surgery), had diagnosis of urinary retention, had recurrent urinary tract infection, had bladder cancer or pelvic radiation, or had a history of neurological disease/spinal cord injury. They were excluded if they had received/performed any of the following in the previous 4 weeks: antibiotics, vaginal douching, vaginal probiotics, or bladder instrumentation or intravesicular IC/PBS treatment; no other IC/PBS treatments were considered as part of the exclusion criteria, nor was this information explicitly gathered. Information was gathered on hormone use (both oral and vaginal, as well as probiotics), but these factors were not exclusion criteria. We attempted to recruit 40 to 50 participants per cohort, as previous studies demonstrated that approximately 40 urine samples detect most unique bacterial species.10,11
All participants completed a demographics questionnaire along with validated IC/PBS questionnaires: Genitourinary Pain Index (GUPI), Interstitial Cystitis Symptom Index (ICSI), Interstitial Cystitis Problem Index (ICPI), and the UTI Symptom Assessment (UTISA). The ICSI and ICPI measures presence and bother over the preceding month with a maximum score of 20 for the ICSI and 16 for the ICPI, with a minimal importance difference (MID) of 1.2 for both12; the GUPI measures bladder symptoms over the last week with maximum score of 45 (MID, 4)13 signifying most distress; and the UTISA measures presence (UTISA-P) and bother (UTISA-B) of bladder pain over the last 24 hours with a maximum score of 21 for each index (MID, 0.5–1.75)14; the final question on the UTISA (UTISA8) asks participants to rate their bladder symptoms during the last 24 hours.
All participants provided a single catheterized urine sample. The IC/PBS cohort was catheterized during their clinical care. A subset of the IC/PBS cohort also provided a midstream voided urine before the catheterized urine sample. Control participants were catheterized before undergoing benign gynecology procedures that did not require antibiotics. All urine samples were tested by EQUC; a subset was also subjected to 16S rRNA gene sequencing.
Samples for EQUC were prepared with urine C&S preservative (Becton Dickenson, Franklin Lakes, New Jersey) and shipped overnight with freezer packs for subsequent processing at Loyola University Chicago. The EQUC procedure has been described.15,16 The level of detection for EQUC is 10 colony-forming units per milliliter, represented by 1 colony of growth on any plate. Each morphologically distinct colony type was counted and isolated to purity for identification with matrix-assisted laser desorption/ionization time-of flight mass spectroscopy.
A portion of the urine samples was prepared with 10% AssayAssure universal urine collection media (Thermo Scientific, Waltham, Massachusetts) to preserve them for 16S rRNA sequencing analysis. They were stored at −80°C until processing as a single batch. The 16S rRNA gene was amplified using the polymerase chain reaction from extracted genomic DNA using primers targeting the V4 region as described17 with index sequences18 and sequenced in pools on an Illumina MiSeq at Loyola Genomics Facility.18,19 To control for potential DNA contamination, an extraction (no urine) negative control was processed with the samples and sequenced in every run. To ensure reproducibility, each sample was independently extracted and sequenced at least twice.
MiSeq postprocessing software was performed for initial quality control. Then, the mothur pipeline, formatted specifically for use with MiSeq-generated data, combined paired end reads, and removed sequences of incorrect length or those containing ambiguous bases or chimeric sequences. Within mothur, sequences were taxonomically assigned to operational taxonomic units and classified using a naïve Bayesian classifier and the mothur-customized Ribosomal Database Project training set v9. Bray-Curtis index is a measure of the degree of dissimilarity between 2 populations, with scores ranging from 0, meaning they share all of the same species, to 1, meaning they are wholly distinct and species do not overlap. To be considered positive, both replicates had to be dissimilar from the extraction control calculated via the Bray-Curtis dissimilarity index and have more reads than the extraction control. The remaining replicates were similar to each other according to the Bray-Curtis index, and the relative abundance and reads were averaged.
Demographical data and clinical characteristics were compared between cohorts using χ2 tests. Continuous variables were analyzed with the 2-sample t test or the Kruskal-Wallis test; categorical variables were analyzed with Pearson χ2 or Fisher exact tests. For microbiome statistical analysis, after taxonomic assignment via mothur, Bray-Curtis dissimilarity was used to cluster bacterial community profiles into urotypes. All statistical analyses were conducted using SAS v9.4 (SAS Software, Cary, North Carolina) or RStudio v1.2.1335 (RStudio, Boston, Massachusetts), and statistical significance were assessed at the α = 0.05 level.
RESULTS
The mean (SD) ages (in years) of the participants were 51 (15) for the IC/PBS cohort and 45 (13) for the controls (P < 0.05; Table 1). Most identified as white/non-Hispanic: 67.3% for the IC/PBS cohort and 72.5% for controls (P > 0.05). About a quarter identified as Hispanic: 26.5% for the IC/PBS cohort and 22.5% of the controls (P > 0.05). More participants were postmenopausal in the IC/PBS cohort than the controls: 51.0% versus 25.0% (P = 0.023).
TABLE 1.
Demographics
| Patient and Clinical Characteristics | IC/PBS Cohort (n = 49) | Control Cohort (n = 40) | P* |
|---|---|---|---|
| Age (y), mean (SD) | 51 (16) | 45 (13) | 0.047 |
| Race, n (%) | |||
| White/Caucasian | 33 (67.3) | 29 (72.5) | 0.77 |
| Hispanic† | 13 (26.5) | 9 (22.5) | 0.85 |
| Black/African American | 1 (2.0) | 1 (2.5) | 1.0 |
| Native American | 2 (4.1) | 0 (0) | 0.50 |
| Unknown/not reported | 0 (0) | 1 (2.5) | 0.45 |
| Menopausal status, n (%) | |||
| Premenopausal | 11 (22.4) | 22 (55.0) | 0.0033 |
| Postmenopausal | 25 (51.0) | 10 (25.0) | 0.023 |
| Not sure | 8 (16.3) | 3 (7.5) | 0.33 |
| Unknown/not reported | 5 (10.2) | 5 (12.5) | 0.75 |
| Parous, n (%) | |||
| Yes | 40 (81.6) | 28 (70.0) | 0.30 |
| No | 7 (14.3) | 5 (12.5) | 1.0 |
| Unknown/not reported | 2 (4.1) | 7 (17.5) | 0.072 |
| Smoking, n (%) | |||
| Never | 33 (67.3) | 24 (60.0) | 0.62 |
| Current | 7 (14.3) | 3 (7.5) | 0.50 |
| Previous | 9 (18.4) | 12 (30.0) | 0.3 |
| Unknown/not reported | 0 (0) | 1 (2.5) | 0.45 |
| Urine pH, mean | 5.51 | 6.28 | <0.0005 |
*Categorical variables calculated via χ2 or Fisher test. Continuous variables via Student t test.
†Includes white and black Hispanic.
The EQUC detected bacteria in 44.9% (40/89) of the catheterized urine samples. Of the IC/PBS cohort, 49.0% (24/49) were positive by EQUC, which detected 19 unique species from members of this cohort (Fig. 1). In the EQUC-positive IC/PBS samples, the most common urotype (defined as >50% relative abundance) at the genus level was Lactobacillus (45.8%), followed by Streptococcus (33.3%; Fig. 2A). Of the controls, 40.0% (16/40) were positive by EQUC, which detected 14 unique species from members of this cohort (Fig. 1). In the EQUC-positive control samples, the most common urotype was Lactobacillus (50%; Fig. 2B). At the genus level, the overall urotype distribution differed significantly (P = 0.016), mostly because 33.3% of the EQUC-positive IC/PBS cohort, but none of the controls had the Streptococcus urotype (P = 0.013; Figs. 2A, B). The rarefaction curve for this study can be found in Supplemental Digital Content Figure 1, http://links.lww.com/FPMRS/A127, which demonstrates sufficient sampling size to detect unique species.
FIGURE 1.
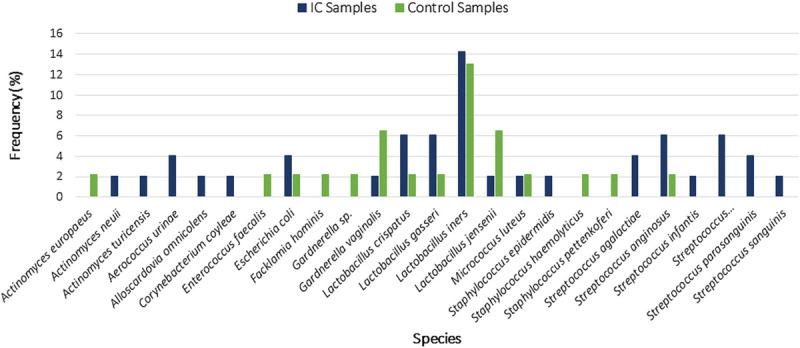
Comparison of species in the IC/PBS and control cohorts as determined by EQUC. The columns represent the percent frequency of detection of a species between to 2 cohorts.
FIGURE 2.
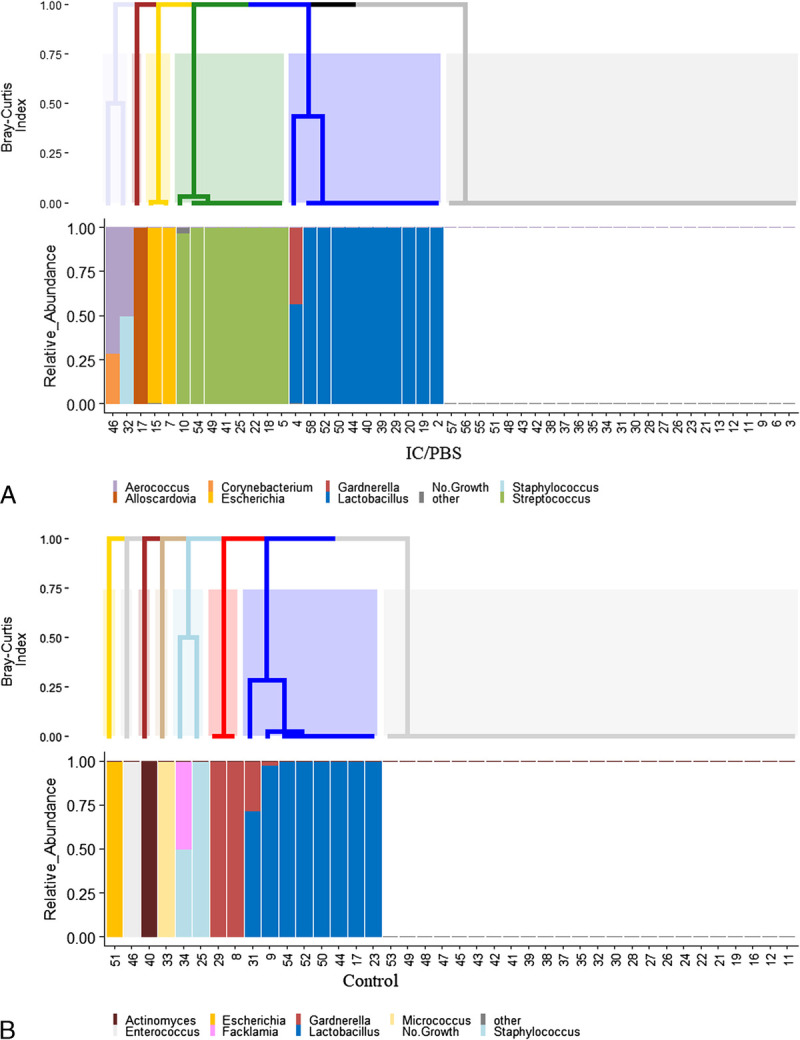
Urotypes in the IC/PBS (A) and control (B) cohorts as determined by EQUC. Dendrogram produced using the Bray-Curtis index (top). Histogram showing relative abundance for each specimen (bottom).
Lactobacillus iners was the most common species in both cohorts and was detected at similar relatively low frequencies: 14% and 13% for the IC/PBS and control cohorts, respectively (Fig. 1). No other species was detected in more than 6% of either cohort. The most outstanding difference between the 2 cohorts was the frequency of Streptococcus species in the IC/PBS cohort, with the most common being S. anginosus (6%) and members of the S. mitis/oralis/pneumonia group (6%).
Symptom severity during the last 24 hours, as measured by UTISA8, showed that IC/PBS participants who were symptom-free were likely to be EQUC positive (87.5%). In contrast, IC/PBS participants with “moderate” or “severe” pain during the last 24 hours were less likely to be EQUC positive (31.2% and 53.8%, respectively), as were members of the control cohort (40%; P < 0.05; Table 2). The most common urotype for EQUC-positive IC/PBS cohort members with moderate or severe pain was Lactobacillus (18.8% and 38.5%, respectively), but this proportion did not differ significantly from IC/PBS participants without pain during the last 24 hours (25.0%) or the control cohort (20.0%; P = 0.51; Table 2), and we observed no difference in Lactobacillus urotypes between our cohorts on any of the pain scales (Table 3). Half of the IC/PBS cohort who reported no symptoms over the last 24 hours had a Streptococcus urotype, whereas no member of the IC/PBS cohort who reported severe symptoms had a Streptococcus urotype (P < 0.05). Finally, biomass was not statistically significant between the IC/PBS and control cohorts (Table 2). Urine pH of the IC/PBS cohort was significantly lower than that of the control group (5.51 vs 6.28, P < 0.0005; Table 1), but did not differ by IC/PBS symptom severity (Table 2). As expected, there was a statistically significant difference between IC/PBS cohort and control cohort on all validated questionnaires (Supplemental Table 1, http://links.lww.com/FPMRS/A125).
TABLE 2.
IC/PBS Severity and Associated Urotypes by EQUC
| Control Cohort (n = 40) | IC No Symptoms (n = 8) |
IC Mild Symptoms (n = 10) |
IC Moderate Symptoms (n = 16) |
IC Severe Symptoms (n = 13) |
P* | P† | P Value‡ | |
|---|---|---|---|---|---|---|---|---|
| Negative, n (%) | 24 (60.0) | 1 (12.5) | 6 (60%) | 11 (68.8) | 6 (46.2) | 0.09 | 0.02 | 0.06 |
| Positive, n (%) | 16 (40) | 7 (87.5) | 4 (40) | 5 (31.2) | 7 (53.8) | |||
| Urotype, n (%) | ||||||||
| Lactobacillus | 8 (20.0) | 2 (25.0) | 1 (10) | 3 (18.8) | 5 (38.5) | 0.57 | 0.67 | 0.47 |
| Escherichia | 1 (2.5) | 0 (0) | 1 (10) | 0 (0) | 1 (7.7) | 0.42 | 1 | 0.66 |
| Streptococcus | 0 (0) | 4 (50.0) | 1 (10) | 2 (12.5) | 0 (0) | 0.00022 | 0.00036 | 0.016 |
| Aerococcus | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 0.36 | 1 | 0.66 |
| Alloscardovia | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0.092 | 0.17 | 0.17 |
| Mixed | 1 (2.5) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0.48 | 1 | 0.38 |
| Urine pH (SD) | 6.28 ± 1.02 | 5.6 ± 0.52 | 5.5 ± 0.53 | 5.5 ± 0.72 | 5.5 ± 0.58 | 0.0001 | nd | nd |
| Biomass of positive samples, CFU/mL | 35 (20–100,000) |
310 (10–2375) |
135 (35–25173) |
50 (30–1250) |
400 (40–2905) |
0.63 | 0.48 | 0.46 |
*Between all IC groups and control cohort via Fisher exact test and Kruskal-Wallis test.
†Between IC no-symptom and control cohort via Fisher exact test and Kruskal-Wallis test.
‡Between the IC no, mild, moderate, and severe symptom groups via Fisher exact test and Kruskal-Wallis test.
CFU, colony-forming units; nd, not determined.
TABLE 3.
IC/PBS Pain Questionnaires and the Presence of Lactobacillus
| Lactobacillus | No Lactobacillus | P* | |
|---|---|---|---|
| Female GUPI, median (IQR) | 24 (3.5–32) | 9 (1–29) | 0.25 |
| IC symptom index score, median (IQR) | 7 (2–10) | 5 (1.25–10.75) | 0.62 |
| IC problem index score, median (IQR) | 7 (0–11.5) | 2.5 (0–11) | 0.43 |
| UTISA8, median (IQR) | 1 (0–2.5) | 0 (0–2.5) | 0.35 |
*Calculated via Kruskal-Wallis test.
IQR, interquartile range.
To serve as a control for our collection technique, a subset of catheterized urine samples underwent 16S rRNA gene sequencing, with a lower detection rate of 40.9% (27/66). Of the 16 IC/PBS samples that were positive by both methods, 13 matched well, 1 matched somewhat, and 1 did not match (Supplemental Figure 2A, http://links.lww.com/FPMRS/A128). Of the 5 control samples that were positive by both methods, 3 matched well, 1 matched somewhat, and 2 did not (Supplemental Figure 2B, http://links.lww.com/FPMRS/A129). Of the subset of 46 IC/PBS participants and 23 controls that were sequenced, there was a significant association between the presence of Streptococcus and the IC/PBS cohort without active symptoms, as observed with EQUC data (P = 0.017; Supplemental Table 2, http://links.lww.com/FPMRS/A126).
A subset of the IC/PBS cohort also provided voided samples. The EQUC detected bacteria in all but 1 voided sample (9/10); the EQUC also detected Candida in 1. In contrast, EQUC detected bacteria in only 5 of the matched catheterized samples and did not detect Candida (Supplemental Figure 3, http://links.lww.com/FPMRS/A130). The voided samples contained 30- to 10,000-fold more biomass than did their paired catheterized samples.
DISCUSSION
We assessed urine from women with IC/PBS using catheterized samples and EQUC. Bacteria were not detected in most samples. When bacteria were detected, the Lactobacillus urotype was most common. Symptom severity for the IC/PBS cohort was affected by urotype, but not in the hypothesized manner; we found no association with pain scores and the Lactobacillus urotype. Interestingly, Streptococcus was only detected in our IC/PBS cohort but was associated with less severe symptoms. In previous studies, S. anginosus was associated with urge urinary incontinence.10 It is possible that Streptococcus species are more common in “irritative voiding” groups, such as urge urinary incontinence and IC/PBS. Because EQUC can detect yeast, our negative findings suggest that yeast is not a major inciting factor in IC/PBS, in contrast to a previous report.20 Lastly, we observed no association between IC/PBS symptoms in the last 24 hours and higher bacterial abundance. Thus, our results do not support an association between the bladder microbiota and either presence of IC/PBS or severity of IC/PBS symptoms.
Results from our paired catheterized versus voided samples reaffirm that voided urine does not accurately represent bladder microbiota. They demonstrate that using voided samples to describe the bladder microbiome would lead to a significantly elevated false-positive rate. Certainly, there is value in describing the genitourinary microbiome (voided urine) of women with IC/PBS, but our results suggest that true sampling of the bladder cannot be accomplished with voided urine. Our results can most directly be compared with those of Abernethy et al,21 who assessed catheterized urine by 16S rRNA gene sequencing. They found Lactobacillus to be the urotype in 38% of their IC/PBS cohort and 78% of their control cohort. Women who lacked Lactobacillus exhibited higher pain scores as reported by the ICSI and ICPI. In contrast, we observed no difference in Lactobacillus urotypes between cohorts on any pain scale.
Similar to our findings, other studies have also failed to detect a protective role of Lactobacillus. Siddiqui and colleagues22 collected voided samples, performed 16S rRNA gene sequencing, and found a decrease in microbial richness in IC/PBS urine, and 92% of their voided IC/PBS urine samples isolated Lactobacillus compared with 57% of controls. Furthermore, the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Network reported that IC/PBS participants (n = 181, voided urine, 16S rRNA sequencing) had an “overabundance” of Lactobacillus.
Additional negative data were reported by Meriwether et al,23 Bresler et al,24 and Nickel et al.25 These studies offer a wealth of findings that significantly advance our understanding of IC/PBS, with the overarching conclusion being that the FUM detected by EQUC and/or 16S rRNA gene sequencing from voided urine samples does not reveal a difference between IC/PBS participants and asymptomatic controls.
The strengths of our study are as follows: (1) it is one of a very few that uses catheterized urine samples; (2) it is the only study to analyze samples by an enhanced urine culture method (EQUC) and 16S rRNA gene sequencing; (3) we included proper controls to test our methods: voided versus catheterized pairs; (4) our exclusion criteria required an antibiotic wash-out period; and (5) it is the largest sample size to date of catheterized urine from women with IC/PBS.
Weaknesses of our study include a small but statistically significant difference in age and the lack of menopause matching, as hormonal status does impact the genitourinary microbiome.26 Furthermore, we did not exclude patients on oral or vaginal hormones nor recent intercourse, which could have impacted our results. Our samples were collected and prepared at Women & Infants Hospital in Providence, Rhode Island, and shipped overnight to Loyola University Education and Research Collaboration (LUEREC) in Illinois for EQUC processing. All LUEREC collaborative institutions throughout the country use these preparation and shipping techniques; however, we cannot ignore the possibility that the shipping impacted the culture-based data, as our detection rate is significantly less than other published studies from LUEREC that typically ranges from 70% to 80%.
We did not find an undetected/dominant uropathogen that could revolutionize the treatment of IC/PBS, but we did make interesting observations. The FUM of women with IC/PBS determined by catheterized urine and analyzed with EQUC and 16S rRNA gene sequencing does not differ from controls. The presence of Lactobacillus is unlikely associated with symptom severity, and Candida does not seem to be a major contributor to IC/PBS. Given that these results conflict with some that have been reported, it seems that we might be back at square one with IC/PBS—the disease process is generally independent of microbes. Also, we cannot overlook the observation that the urine pH from IC/PBS participants was significantly lower than that of controls, which is consistent with the common IC/PBS therapies that aim to deacidify urine. It is also possible that lower pH represents a host response to resident microbiota, thus normal microbes, but abnormal response. This study and others have not assessed viruses and nonyeast eukaryotic microbes, which also comprise the FUM; these are a natural next step for future investigation.
Supplementary Material
Footnotes
This article published online ahead of print on April 6, 2020.
Current addresses: Kristin M. Jacobs, Division of Female Pelvic Medicine and Reconstructive Surgery, Rush University, Chicago, IL.
Current addresses: Travis K. Price, Department of Pathology and Laboratory Medicine, University of California Los Angeles, Los Angeles, CA.
Current addresses: Krystal Thomas-White, Department of Microbiology, Stanford University, Palo Alto, CA.
The authors have declared they have no conflict of interest.
The authors received funding from PFD Research Grant.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.fpmrs.net).
Contributor Information
Travis K. Price, Email: tprice1@luc.edu.
Krystal Thomas-White, Email: krystalwhit@gmail.com.
Thomas Halverson, Email: thalverson@luc.edu.
Abigail Davies, Email: abigail_davies@brown.edu.
Deborah L. Myers, Email: dmyers@wihri.org.
Alan J. Wolfe, Email: awolfe@luc.edu.
REFERENCES
- 1.Berry SH Elliott MN Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011;186(2):540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michael YL Kawachi I Stampfer MJ, et al. Quality of life among women with interstitial cystitis. J Urol 2000;164(2):423–427. [PubMed] [Google Scholar]
- 3.Anger JT Zabihi N Clemens JQ, et al. Treatment choice, duration, and cost in patients with interstitial cystitis and painful bladder syndrome. Int Urogynecol J 2011;22(4):395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grover S Srivastava A Lee R, et al. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 2011;3(1):19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levinson WJ, Jawetz E. Medical Microbiology and Immunology. Vol 6th ed. New York: Lange Medical Books/McGraw-Hill; 2000. [Google Scholar]
- 6.Madigan MTB, Thomas D. Brock Biology of Microorganisms. San Francisco, CA: Pearson Education; 2009. [Google Scholar]
- 7.Wolfe AJ, Brubaker L. “Sterile urine” and the presence of bacteria. Eur Urol 2015;68(2):173–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakeman MM, Roovers JP. Urinary tract infections in women with urogynaecological symptoms. Curr Opin Infect Dis 2016;29(1):92–97. [DOI] [PubMed] [Google Scholar]
- 9.Whiteside SA Razvi H Dave S, et al. The microbiome of the urinary tract—a role beyond infection. Nat Rev Urol 2015;12(2):81–90. [DOI] [PubMed] [Google Scholar]
- 10.Pearce MM Hilt EE Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio 2014;8(4):e01283–e01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas-White KJ Hilt EE Fok C, et al. Incontinence medication response relates to the female urinary microbiota. Int Urogynecol J 2016;27(5):723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Leary MP Sant GR Fowler FJ Jr., et al. The interstitial cystitis symptom index and problem index. Urology 1997;49(Suppl 5A):58–63. [DOI] [PubMed] [Google Scholar]
- 13.Clemens JQ Calhoun EA Litwin MS, et al. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology 2009;74(5):983–987, quiz 987 e981-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayson D Wild D Doll H, et al. Validation of a patient-administered questionnaire to measure the severity and bothersomeness of lower urinary tract symptoms in uncomplicated urinary tract infection (UTI): the UTI Symptom Assessment Questionnaire. BJU Int 2005;96(3):350–359. [DOI] [PubMed] [Google Scholar]
- 15.Hilt EE McKinley K Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol 2014;52(3):871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price TK Dune T Hilt EE, et al. The clinical urine culture: enhanced techniques improve detection of clinically relevant microorganisms. J Clin Microbiol 2016;54(5):1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane DJ Pace B Olsen GJ, et al. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A 1985;82(20):6955–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caporaso JG Lauber CL Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012;6(8):1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozich JJ Westcott SL Baxter NT, et al. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 2013;79(17):5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nickel JC Stephens A Landis JR, et al. Urinary fungi associated with urinary symptom severity among women with interstitial cystitis/bladder pain syndrome (IC/BPS). World J Urol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abernethy MG Rosenfeld A White JR, et al. Urinary microbiome and cytokine levels in women with interstitial cystitis. Obstet Gynecol 2017;129(3):500–506. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui H Lagesen K Nederbragt AJ, et al. Alterations of microbiota in urine from women with interstitial cystitis. BMC Microbiol 2012;12:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meriwether KV Lei Z Singh R, et al. The vaginal and urinary microbiomes in premenopausal women with interstitial cystitis/bladder pain syndrome as compared to unaffected controls: a pilot cross-sectional study. Front Cell Infect Microbiol 2019;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bresler L Price TK Hilt EE, et al. Female lower urinary tract microbiota do not associate with IC/PBS symptoms: a case-controlled study. Int Urogynecol J 2019. [DOI] [PubMed] [Google Scholar]
- 25.Nickel JC Stephens-Shields AJ Landis JR, et al. A culture-independent analysis of the microbiota of female interstitial cystitis/bladder pain syndrome participants in the MAPP research network. J Clin Med 2019;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas-White KJ Kliethermes S Rickey L, et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am J Obstet Gynecol 2017;216(1):55.e1–55.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


