Abstract
Objective:
To compare the cognitive evolution of older adults with severe or profound hearing impairment after cochlear implantation with that of a matched group of older adults with severe hearing impairment who do not receive a cochlear implant (CI).
Design:
In this prospective, longitudinal, controlled, and multicenter study, 24 older CI users were included in the intervention group and 24 adults without a CI in the control group. The control group matched the intervention group in terms of gender, age, formal education, cognitive functioning, and residual hearing. Assessments were made at baseline and 14 months later. Primary outcome measurements included the change in the total score on the Repeatable Battery for the Assessment of Neuropsychological Status for Hearing impaired individuals score and on its subdomain score to assess cognitive evolution in both groups. Secondary outcome measurements included self-reported changes in sound quality (Hearing Implant Sound Quality Index), self-perceived hearing disability (Speech, Spatial, and Qualities of Hearing Scale), states of anxiety and depression (Hospital Anxiety and Depression Scale), and level of negative affectivity and social inhibition (Type D questionnaire).
Results:
Improvements of the overall cognitive functioning (p = 0.05) and the subdomain “Attention” (p = 0.02) were observed after cochlear implantation in the intervention group; their scores were compared to the corresponding scores in the control group. Significant positive effects of cochlear implantation on sound quality and self-perceived hearing outcomes were found in the intervention group. Notably, 20% fewer traits of Type D personalities were measured in the intervention group after cochlear implantation. In the control group, traits of Type D personalities increased by 13%.
Conclusion:
Intervention with a CI improved cognitive functioning (domain Attention in particular) in older adults with severe hearing impairment compared to that of the matched controls with hearing impairment without a CI. However, older CI users did not, in terms of cognition, bridge the performance gap with adults with normal hearing after 1 year of CI use. The fact that experienced, older CI users still present subnormal cognitive functioning may highlight the need for additional cognitive rehabilitation in the long term after implantation.
Keywords: Cochlear implant, Cognition, Cognitive decline, Older adults, RBANS-H
INTRODUCTION
An increasing number of studies have established that older adults with hearing impairment present an accelerated cognitive decline compared with their peers with normal hearing (Valentijn et al. 2005b; Lin et al. 2011, 2013; Gallacher et al. 2012; Mosnier et al. 2018). Although these studies point out the relationship between hearing loss and cognitive decline, the underlying mechanism of this relationship remains an open question.
Four hypotheses on the causal relationship between peripheral hearing loss and cognitive decline have been proposed and studied (Lindenberger & Baltes 1994; Baltes & Lindenberger 1997; Dennis & Cabeza 2008; Arlinger et al. 2009). In the first hypothesis, both hearing impairment and cognitive decline may result from one common genetic or environmental mechanism (e.g., widespread neural degeneration). This is called the common cause hypothesis. Another hypothesis is the cognitive load on perception hypothesis that states that reduced cognitive capacity places a heavier load on perception and, therefore, affects sensory processing. The other two hypotheses concern a causal relationship in the opposite direction. According to the sensory-deprivation hypothesis, peripheral hearing loss causes a permanent cognitive decline. Deafferentation and atrophy of the auditory system, and subsequent functional reorganization of cortical areas after auditory deprivation, may mediate the association between hearing loss and permanent cognitive decline. The impact of hearing loss on cognition may also be reversible, as is the case in the information-degradation hypothesis. This hypothesis suggests that perceptual difficulties cascade upwards, compromising higher-level cognitive processing. As more cognitive resources are diverted to perceptual processing, the available resources for the cognitive task are reduced, manifesting as a decline in cognitive performance.
The last hypothesis is of particular interest, as this implies that compensating for the perceptual difficulties through auditory rehabilitation may reduce the compromising effect of those perceptual difficulties on cognitive processing and, therefore, may improve cognitive performance. Indeed, a study by Amieva and colleagues (2015), which observed 3670 individuals of ages 65 years and older over a 25-year time period, indicated that the cognitive decline in adults with hearing impairment wearing hearing aids did not differ from that observed in the normal hearing controls. In contrast, the cognitive decline in older adults with hearing impairment without hearing aids was accelerated. This suggests that hearing aids may have a protective effect against accelerated cognitive decline. However, in general, studies investigating the impact of hearing aid use on cognition among older adults with moderate hearing loss yield mixed results, with some studies observing a positive effect of hearing aids on cognition (e.g., Acar et al. 2011; Dawes et al. 2015), while others find no effect (e.g., Valentijn et al. 2005a; van Hooren et al. 2005).
According to Lin et al. (2013), individuals with a greater degree of hearing loss are prone to higher risks of accelerated cognitive decline and incident cognitive impairment. Therefore, possible positive effects of hearing rehabilitation on cognition are postulated to be more pronounced with cochlear implant (CI) use in people with severe to profound hearing impairment than with hearing aid use in people with moderate to severe hearing impairment.
Although a lot of literature is available on the positive effects of hearing aids on cognition, a critical review by Claes et al. (2018a) showed the limited number of studies on the effects of cochlear implantation in adults with severe hearing impairment. Six pioneering studies were found eligible, corresponding to a total of 166 older CI users. Of the six articles selected for review, five reported improvements in cognition after cochlear implantation across all cognitive domains: learning and memory, language, perceptual-motor function, executive function, and complex attention (Mosnier et al. 2015; Castiglione et al. 2016; Cosetti et al. 2016; Ambert-Dahan et al. 2017; Jayakody et al. 2017). Only one study, the one by Sonnet et al. (2017), observed no significant change in cognitive performance after implantation.
However, after critically reviewing these studies, a high risk of bias was evidenced. Detailed information on the adaptation of cognitive tests for people with hearing impairment is frequently lacking; practice effects are not taken into account in most studies, and statistical information is missing or analyses are sub-optimal. One study appears to stand out because it made use of a clearly defined nonverbal cognitive test battery, and it included a control group (Jayakody et al. 2017). Therefore, more well-designed studies are required to verify whether cochlear implantation influences cognitive functioning in older adults.
Since there exists a high between-individual variability in rate of change in cognitive abilities over time, confounding factors like education, cognitive training, and gender should be taken into account in cognitive hearing research. Moreover, there is increasing evidence that personality traits may influence older adults’ cognitive profile too (Curtis et al. 2015). The review of Curtis et al. identified some relatively consistent relationships, including positive associations between openness and cognitive ability and associations of conscientiousness with slower rates of cognitive decline. They therefore suggest to assess personality traits when investigating cognition in older adults.
The present prospective, longitudinal, controlled, multicenter study tries to meet the recommendations of the critical review by Claes et al. (i.e., to control for practice effects by introducing a group of control participants, to use appropriate statistical tests, and an appropriate cognitive assessment tool for people with hearing impairment) to answer the research question: “What is the effect of cochlear implantation on the cognitive evolution in older adults with severe or profound hearing impairment?”
MATERIALS AND METHODS
Design
To investigate the effect of cochlear implantation on the cognitive evolution in older adults, a prospective, longitudinal, controlled, multicenter study was conducted in five investigational sites over a 4-year period (April 2015 to August 2019): Antwerp University Hospital (Antwerp, Belgium), La Paz University Hospital (Madrid, Spain), World Hearing Center (Warsaw, Poland), Yorkshire Auditory Implant Service (Bradford, United Kingdom), and Fiona Stanley Hospital (Perth, Australia). During this period, all consecutive older adults with severe or profound hearing impairment, who met the inclusion criteria for either the intervention or the control group, were invited to participate in the study. Before the start of the study, at least one investigator from each participating center attended a training course covering the content of the multicenter protocol and administration of all the included assessments. All participating centers are members of the HEARRING group, a multidisciplinary group of 30 expert clinics that aim to identify evidenced-based standards that can provide each potential implant recipient with the best possible hearing implant solution for the individual hearing loss. Data were collected at baseline T0 and 14 months later at the follow-up test interval T14. In case of the intervention group, T0 was scheduled 1 month prior to cochlear implantation. The study was approved by the local ethics committees and competent authorities (Antwerp 15/17/181; Madrid PI-2504; Warsaw KB/16/2016; Bradford 16/EM/0437; Perth RGS0000000335). All participants gave their written informed consent in accordance with the Declaration of Helsinki prior to participation (Fig. 1).
Fig. 1.
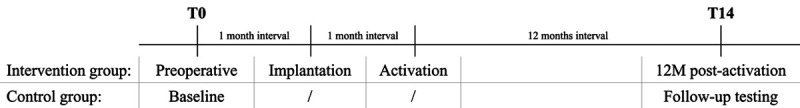
Overview test intervals. T0 corresponds to baseline testing. In case of the intervention group, T0 was scheduled 1 month prior to cochlear implantation. In both the intervention and control group, follow-up testing was performed at T14, which was scheduled 14 months after baseline testing.
Subjects
Although there are commonly used definitions of old age, there is no general agreement on the age at which a person becomes “old.” Therefore, the authors decided to include patients aged 55 years or older because this age was the youngest mean age in which presence of hearing loss was shown to increase dementia (Gallacher et al. 2012). The sample size calculation is based on the primary outcome variable, the Repeatable Battery for the Assessment of Neuropsychological Status for Hearing impaired individuals (RBANS-H) total score, and was performed at design stage. The approach with the minimum/optimum number of subjects to answer the research question was chosen; that is the minimum accepted level of 0.75, which means there is a 7.5 in 10 chance of detecting a difference between both groups. The proposed sample size of 24 intervention subjects and 24 controls holds 75% power to detect a difference of 14 standard deviations (=Cohen’s d; Claes et al. 2018b). Therefore, data analysis was scheduled when 24 subjects and 24 matching controls completed the study (T0–T14). Of the 30 patients who were included in the intervention group, four left the study before completion because they were not willing to do the extra tests at T14. For the control group, 24 out of the 36 designated adults were included after the matching procedure.
Intervention Group
Inclusion criteria for the intervention group were as follows: (1) aged 55 years or older, (2) postlingually, bilaterally, and severely to profoundly hearing impaired, (3) receiver of a unilateral CI (MED-EL, Innsbruck, Austria) in accordance to the respective local national reimbursement criteria. Prospective participants were excluded from the study if they were unable to complete the test protocol due to additional impairments, like uncorrected vision or if their self-reported CI usage was less than 10 hours a day. In total, 24 older adults (14 male and 10 female) were found to be eligible for inclusion to the intervention group with a mean age of 72 years (SD, 7 years) at baseline. Their audio processor was activated approximately 4 weeks after cochlear implantation, and the processor settings were optimized during regular local programming sessions.
Control Group
Using a matched study design, the control group consisted of older adults (≥55 years old) with postlingual, bilateral, severe or profound hearing loss, who matched the gender, mean age, duration of formal education, cognitive functioning, and residual hearing at baseline of the intervention group, but who were not scheduled for cochlear implantation. Contraindications for cochlear implantation in this group included the following: the participant had health conditions that did not allow anesthesia to be administered (8%), the participant’s ear anatomy did not allow cochlear implantation (8%), the participant was still on a CI waiting list at the time of the study (8%), the participant did not meet the local criteria for reimbursement (46%), or the participant did not want to undergo surgery (29%). In total, 24 (10 male and 14 female) out of the 36 designated adults were included to the control group. At baseline, their mean age was 73 years (SD, 9 years; Table 1).
TABLE 1.
Overview of the characteristics of the intervention and the control group at baseline T0
| Intervention Group | Control Group | |
|---|---|---|
| Number | 24 | 24 |
| Mean age T0 (y) (SD)* | 72 (7) | 73 (9) |
| Mean formal education (y) (SD)* | 10 (3) | 10 (4) |
| RBANS-H Total Score (SD)* | 82 (14) | 72 (4) |
| Immediate memory | 86 (13) | 73 (17) |
| Visuospatial constructional | 81 (18) | 76 (19) |
| Language | 96 (11) | 91 (11) |
| Attention | 76 (20) | 73 (17) |
| Delayed memory | 91 (13) | 77 (18) |
| RH best ear (SD)* | 20.25 (13.51)% | 27.10 (14.21)% |
| Gender* | ||
| Male | 58% | 42% |
| Female | 42% | 58% |
| HADS | ||
| Anxiety score | 7.7 (3.1) | 7.1 (4.3) |
| Depression score | 6.4 (4.2) | 6.1 (3.7) |
| HISQUI score | 33 (13) | 74 (23) |
| SSQ12 score | 1.25 (1.10) | 4.13 (1.89) |
| DS14 score | 28 (15) | 19 (13) |
| Aetiology | ||
| Unknown | 75% | 67% |
| Ototoxicity | 8% | 8% |
| Hereditary | 8% | 4% |
| Otosclerosis | 4% | 0% |
| Trauma | 4% | 0% |
| Menière | 0% | 13% |
| Mastoiditis | 0% | 4% |
| Meningitis | 0% | 4% |
| Implanted ear | ||
| Right | 16 | NA |
| Left | 4 | NA |
| HA use T0 | ||
| Yes | 71% | 100% |
| No | 29% | 0% |
| Tinnitus presence T0 | ||
| Yes | 42% | 50% |
| No | 58% | 50% |
| Retired | ||
| Yes | 92% | 96% |
| No | 8% | 4% |
Variables indicated with an asterisk (*) are used for matching. DS14, Type D questionnaire; HA, hearing aid; HADS, Hospital Anxiety and Depression Scale; HISQUI19, Hearing Implant Sound Quality Index–19; N/A Not Applicable; RBANS-H Repeatable Battery for the Assessment of Neuropsychological Status for Hearing impaired individuals; RH percentage of Residual Hearing; SSQ12, Speech, Spatial, and Qualities of hearing Scale–12; T0 indicates baseline.
Statistics
IBM SPSS Statistics version 24 (IBM Corp., New York, NY) was used for the statistical analyses. The primary objective of the study was to compare the cognitive evolution over time (delta T = T14–T0) between the intervention and the control group. To look for changes over time within one group, pairwise comparisons were performed. Subsequently, linear mixed models, including group (intervention and control group), time (T0 and T14) and the interaction, Group × Time, were performed on the RBANS-H total score and the subdomain scores. The significance of interaction indicates if there is a difference in effect between the two groups. The same tests were applied to investigate the secondary objective: to compare the evolution of self-reported outcomes over time (delta T = T14–T0) between the intervention and the control group. Significance levels of p < 0.05 are indicated with one asterisk; levels of p < 0.01 with two asterisks.
Since random assignment to the intervention or to the control group was not possible, a group-matched study design was chosen to create balanced groups. In a first step, the following cofounders were identified based on previous literature (Lin et al. 2013; Lipnicki et al. 2017): (1) gender; (2) mean age at baseline; (3) mean duration of formal education, which was defined as the number of years following formal education starting from the age of six; (4) mean cognitive functioning at baseline, which was assessed with the RBANS-H and which was included as a matching variable to avoid bias of regression to the mean; and (5) percentage of residual hearing in the best ear at T0, which was based upon the Hearing Preservation Classification System introduced by Skarzynski et al. in 2013 (Skarzynski et al. 2013). In both groups, the mean duration of hearing loss was 33 years. Since the chance to be implanted depends on some cofounders, a multivariate logistic regression analysis was performed to calculate the propensity. Group matching aimed the lowest difference in propensity score of the intervention and the control group. A detailed overview of the participants’ characteristics can be found in Table 1.
Primary Outcome Measurements
Repeatable Battery for the Assessment of Neuropsychological Status for Hearing Impaired Individuals
The primary outcome measurements are the changes in cognitive functioning between the two test moments (T0 and T14), assessed by means of the RBANS-H (Claes et al. 2016). The RBANS-H is a modification of the RBANS and was especially developed to examine cognition in individuals with hearing impairment (Randolph et al. 1998). All participating centers were trained to administer the RBANS-H (in their native languages: Dutch, Spanish, Polish and English) to minimize assessor bias. This cognitive test battery consists of 12 subtests and assesses five cognitive subdomains (i.e., “Immediate memory,” “Visuospatial/constructional,” “Language,” “Attention,” and “Delayed memory”).
In contrast to the original RBANS, the RBANS-H provides audiovisual presentation. It includes a PowerPoint presentation with the written instructions shown to the participant on an external screen, along with the standard oral instructions. In addition, simultaneous auditory and visual stimulation is provided in four of the 12 subtests (“List learning,” “Story memory,” “Digit span,” and “List recognition”). In the original RBANS format, the items of these subtests were only presented orally.
The score for each of the 12 subtests contributes to one of the five cognitive subdomains. The raw total scores of two subtests are needed for the conversion to an index score for the domains of “Immediate memory,” “Visuospatial/constructional,” “Language,” and “Attention.” For the index score of the subdomain “Delayed memory,” the raw scores of the subtests “List recall,” “Story recall,” and “Figure recall” are summed. This sum, in combination with the total raw score for the “List recognition” subtest, is used to derive the “Delayed memory” index score. The RBANS-H total score is computed by combining the five subdomain scores. The subdomain and total scores are age-corrected standard scores, scaled to a normal distribution with a mean of 100 and a SD of 15. A detailed description of the modified RBANS-H can be found in Claes et al. (2016).
Secondary Outcome Measurements
Together with the primary outcome measurements, the secondary outcome measurements were administered at baseline (T0) and at a follow-up visit (T14) for both the intervention and control group.
Audiometric Assessment
The audiometric test battery included unaided pure-tone thresholds for air conduction, measured using insert earphones at 125, 250, 500, 1000, 2000, 3000, 4000, 6000, and 8000 Hz (ISO 8253-1, 2010). Speech perception in quiet and in noise were also part of the local clinical routine test batteries. However, due to language differences, these outcomes were not part of the multicenter analysis.
Hearing Implant Sound Quality Index
The Hearing Implant Sound Quality Index (HISQUI) was introduced by Amann and Anderson (Amann & Anderson 2014) and was used to quantify the self-perceived level of auditory benefit experienced by hearing implant users in everyday listening situations study (Mertens et al. 2015; Calvino et al. 2016). The HISQUI consists of 19 items scored on a 7-point Likert scale, ranging from “always” (7) to “never” (1). The total score is calculated by adding the scores on the 19 items, with a maximal score of 133. Based on the total score achieved, the self-reported sound quality is classified as very poor (<30), poor (30–59), moderate (60–89), good (90–109), or very good (110–133).
Speech, Spatial, and Qualities of Hearing Scale–12
The Speech, Spatial, and Qualities of Hearing Scale–12 is a 12-item questionnaire and was used in the present study to assess self-reported hearing disabilities in both groups. It is a short form of the Speech, Spatial, and Qualities of Hearing Scale (Gatehouse & Noble 2004). The total score ranges from zero to ten, with a lower score indicating a higher degree of hearing disability.
Hospital Anxiety and Depression Scale
To identify symptoms of depression and anxiety in both groups, the Hospital Anxiety and Depression Scale was administered. This is a reliable instrument for detecting states of depression and anxiety (Zigmond & Snaith 1983). This self-assessment questionnaire consists of seven items in the subscale “Depression” (e.g., “I still enjoy the things I used to enjoy.”) and seven items in the subscale “Anxiety” (e.g., “I get a sort of frightened feeling as if something awful is about to happen.”).
Type D questionnaire
The Type D questionnaire was used to identify participants with a type D personality (Denollet 2005). This type of personality is characterized by two global traits, negative affectivity, and social inhibition and is relatively independent from changes in mood status. People with high negative affectivity tend to experience more negative emotions over time and across situations. Social inhibition refers to the tendency to inhibit the expression of emotions and behaviors in social interactions to avoid others’ disapproval. Both traits are assessed by means of seven items. Each item is rated on a five-point Likert scale from zero (false) to four (true). A person is classified as type D if both the score on negative affectivity and the score on social inhibition are greater than or equal to ten.
RESULTS
Primary Outcome Measurements
RBANS-H Improvement
Figure 2 shows the mean differences between the RBANS-H scores at T0 and T14 for both the intervention and the control group (delta T = T14–T0). Therefore, an improvement of cognitive functioning is represented by a positive mean value; a mean value of zero indicates that no change was measured over time. For the intervention group, Wilcoxon Signed-Rank tests revealed significant improvements (p < 0.01) for “Immediate memory” (mean delta T = 11.9), “Attention” (mean delta T = 10.4), “Delayed memory” (mean delta T = 7.5), and the total RBANS-H score over time (mean delta T = 9.1). For the control group, “Immediate memory” (mean delta T = 8.2, p < 0.05), “Language” (mean delta T = 8.0, p < 0.01), and the total RBANS-H score (mean delta T = 4.9, p < 0.01) improved significantly. To correct for the practice effect, the bias of being tested at least twice, mixed models were fitted. A marginal trend toward significance (p = 0.05) was found for the interaction between time and either of the groups with regards to the total RBANS-H score. In other words, after correcting for the improvement found in the control group, the improvement of the total RBANS-H score in the intervention group after cochlear implantation remained significant. In addition, in comparison to the control group, a significant improvement in the “Attention” subdomain was found (p = 0.02) for the intervention group. The significant interaction between time and the “Language” domain was found for the control group. One could hypothesize that the control group were able to rely on the practice effect more than the intervention group. In other words, both the “RBANS-H total score” (marginal p value of 0.05) and the score of the “Attention” subdomain improved significantly after cochlear implantation compared to the control group. The improvement found for the other subdomains in the intervention group was nullified after correction for a possible practice effect by applying mixed models.
Fig. 2.
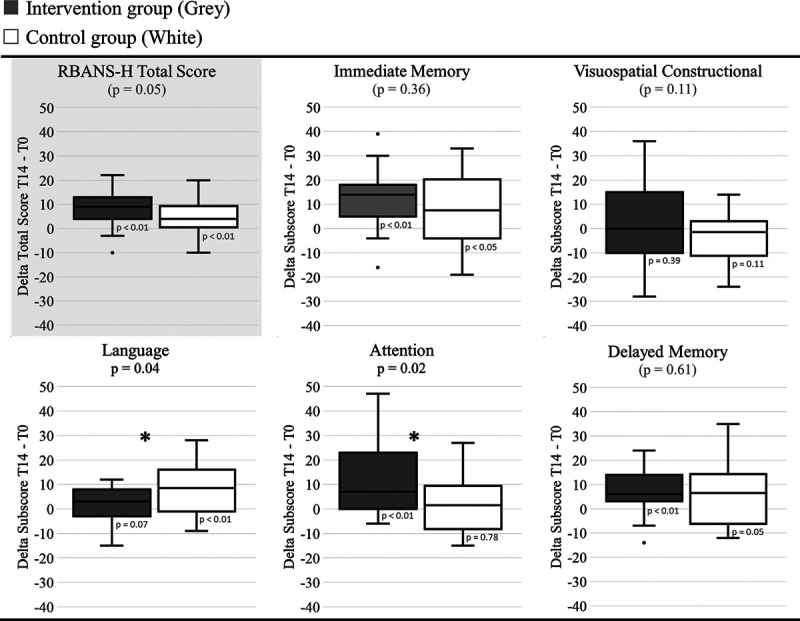
Cognitive outcome measurements. Boxplots of the differences between the outcomes on T14 and T0 for the subscores and total scores of the RBANS-H for the intervention group (gray) and the control group (white) are shown. A score of zero indicates no difference between T0 and T14. Boxplots represent the minimum, 1st quartile, median, 3rd quartile, and maximum of the RBANS-H scores. Levels of significance for the interaction between time and both groups with regard to the RBANS scores are presented in the title (mixed model). The gray plot on top represents the primary outcome and total score of RBANS-H. Significant improvements of RBANS-H scores over time for the intervention group compared to the control group are indicated with an asterisk (p < 0.05). p Values for the improvements over time within one group are presented below the boxplots (Wilcoxon Signed-Rank Test).
Secondary Outcome Measurements
Audiometric Assessment
The rate of residual hearing in the best ear at baseline, quantified by using the hearing preservation classification scale (Skarzynski et al. 2013), was used for matching purposes. The median residual hearing in the best ear was 20% in the intervention group and 27% in the control group.
Self-Reported Improvement
As shown in Figure 3, a significantly larger self-reported hearing improvement over time for the intervention group was found, measured with the HISQUI and the Speech, Spatial, and Qualities of Hearing Scale–12, compared to that of the control group (p < 0.01). The reported level of anxiety and depression remained stable in both groups. In other words, cochlear implantation did not influence the level of anxiety measured with the Hospital Anxiety and Depression Scale in older CI users compared to the control group. The negative affectivity (p < 0.01) and social inhibition (p < 0.05) decreased significantly after cochlear implantation compared to the control group. That means that cochlear implantation in older adults has a positive impact on their experiences of negative emotions, their tendency to inhibit the expression of their emotions, and their behavior in social interactions. Notably, 20% fewer Type D personalities (both the score on negative affectivity and on social inhibition ≥10) were measured after cochlear implantation. In the control group, participants categorized with Type D personality increased by 13% over time.
Fig. 3.
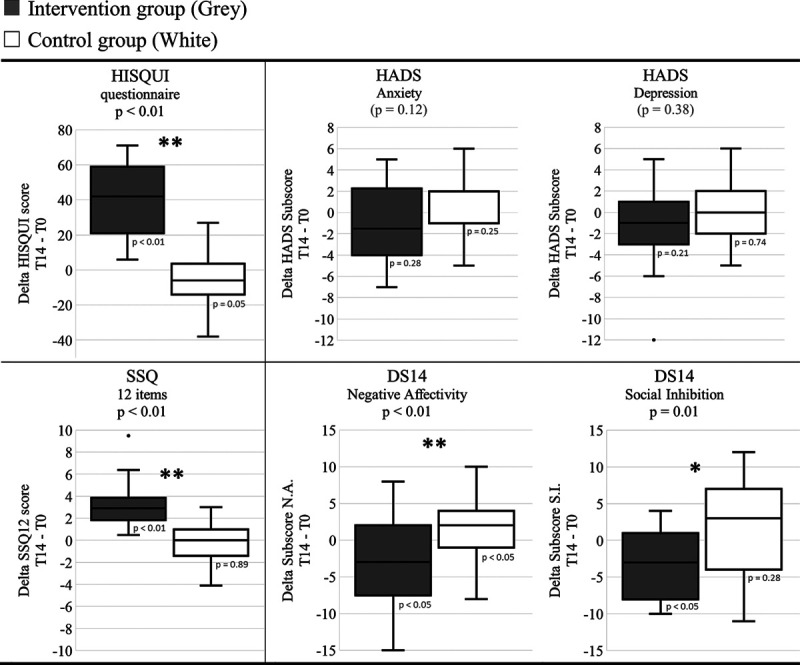
Self-reported outcome measurements. Boxplots of the differences between the self-reported outcomes at T14 and T0 for the questionnaires for the intervention group (gray) and the control group (white) are shown. A score of zero indicates no difference between T0 and T14. Boxplots represent the minimum, 1st quartile, median, 3rd quartile, and maximum of the subjective delta scores. Levels of significance for the interaction between time and both groups with regard to the scores are presented in the title (mixed model). Significant differences of the scores over time for the intervention group compared to the control group are indicated with one (p < 0.05) or two asterisks (p < 0.01). p Values for the change over time within one group are presented below the boxplots (Wilcoxon Signed-Rank Test). Hearing Implant Sound Quality Index–19 [HISQUI19], Speech, Spatial, and Qualities of hearing Scale–12 [SSQ12], Hospital Anxiety and Depression Scale [HADS], and Type D questionnaire [DS14].
DISCUSSION
The present study found significant improvements in “Immediate Memory,” “Attention,” and “Delayed Memory” in the intervention group. However, as mentioned in the critical review by Claes et al. (2018a), the use of two versions of the RBANS-H (or other cognitive assessment tools) is not enough to completely eliminate practice effects. Therefore, a controlled design is indispensable to correct for practice effects. Consequently, the addition of a control group that was missing in previous studies is the most important strength of the present study. By the implementation of our matched control group into the mixed model, the significant improvement in “Immediate Memory” and “Delayed Memory” disappeared. The observed improvements in cognitive functioning after CI use is, therefore, mainly attributable to improvements in the “Attention” domain. Similar findings were reported by Völter et al. (2018) and Mosnier et al. (2015) who investigated “Attention” using a reaction time paradigm. Again, these results should be interpreted with caution because no control group was included in these studies.
It is important to note, however, that the inclusion of a comparable control group is not at all straightforward. A randomized controlled design, in which eligible CI candidates are randomly assigned to either the intervention group or the control group, would be ideal to investigate the effect of cochlear implantation on cognition. Yet, not providing a CI to CI candidates who do meet the criteria for cochlear implantation is far from ethical. Another way of implementing a control group is making use of the existing waiting time for CI subsidy, as was the case in some of our control participants. Candidates who were waiting for the subsidy took part in the control group and are tested at the same interval as the CI users. Some control participants in our study did not undergo cochlear implantation due to health reasons. Therefore, one could speculate that more patients in the control group are suffering from various diseases, including perhaps cardiovascular diseases, which are known to be independently associated with the development of dementia (Justin et al. 2013). Due to the small sample size of this population, we were not able to correct for this potential bias. Other control participants did not meet the local inclusion criteria (e.g., CI criteria in Belgium at the time of inclusion: mean preoperative hearing loss at 0.5, 1, and 2 kHz of at least 85 dB HL at the better ear) and, therefore, were included in the control group. They were the ideal matches for the intervention group to, for example, the control group in Warsaw, where there were less strict rules for reimbursement (De Raeve & Wouters 2013). Due to the small sample size, we were not able to investigate the effect of preoperative hearing aid use, which was more present in the control group. Although the effect of vestibular function on the cognitive profile was not part of the present study, one could expect that associated vestibular areflexia was present in some of our subjects. As we know from the study of Dobbels et al. (2019) that vestibular loss is linked to decreased performance on the attention subscale of the RBANS-H, this could have influenced the cognitive outcomes in subjects with associated vestibular deficits (e.g., Menière disease of meningitis).
Besides the bias of the practice effect, the review by Claes et al. (2018a) also mentioned the suitability of cognitive tests to examine cognition among older adults with hearing impairment as having a possible risk of bias. If, for instance, a verbal memory test is administered to a person with hearing loss, this person may not perceive the words that should be committed to memory and may, as a consequence, not perform at their best (Dupuis et al. 2015). Moreover, even if the person with a hearing impairment can hear the words, it may have required more effort to correctly perceive them, which leaves less cognitive resources available for the process of remembering them (Pichora-Fuller et al. 2016). This, in turn, may also lead to an underestimation of the cognitive abilities of a person with hearing impairment. Since CI use can improve hearing and speech perception, the negative effect of the hearing loss is likely to be greater before than after implantation (Clark et al. 2012; Cosetti & Lalwani 2015). Therefore, by selecting an inadequate cognitive assessment tool or by not adequately modifying an existing test, an improvement in postimplantation test scores may be generated by improved hearing rather than by improved cognition.
The present study successfully employed the RBANS-H, which provides audiovisual presentation of the instructions and the test items to avoid this risk of bias (Claes et al. 2016). Another assessment tool to assess cognition in adults with hearing impairment is the hearing impaired Montreal Cognitive Assessment, which was recently introduced (Lin et al. 2017). This is a cognitive screening test, which is solely visual. Since it was developed as a screening tool, the hearing impaired Montreal Cognitive Assessment is less sensitive and can only differentiate between normal and abnormal cognitive function.
In the present study, at baseline, both the intervention group and the control group of older adults with hearing impairment had notably worse overall cognitive functioning (RBANS-H total score at T0 for the interventional group: 81.83 [13.66]) compared to their peers with normal hearing (RBANS-H total score is scaled to a normal distribution with a mean of 100 and a SD of 15). These findings correspond to the evidence from several studies mentioned in the introduction that conclude that hearing loss is associated with accelerated cognitive decline. The study by Lin et al., for example, showed that individuals with hearing loss (i.e., pure-tone average of hearing thresholds at 0.5 to 4 kHz above 25 dB HL in the better ear) had a 30% to 40% accelerated rate of cognitive decline and a 24% increased risk of incident cognitive impairment over a 6-year period compared to individuals with normal hearing (Lin et al. 2013). Therefore, one should advocate for people with hearing loss to be advised to follow the WHO recommendations for reducing risk of cognitive impairment that apply to all people, including physical activity as the recommendation backed up by the strongest evidence (Chen et al. 2009).
Moreover, at baseline, 41% of all included subjects with severe hearing loss were identified with a Type D personality. Since Type D has a negative influence on health, rehabilitation should check and focus on the personality traits of older adults with severe hearing loss. The study found that the observed negative affectivity and social inhibition in a population with severe sensorineural hearing loss significantly decreased after cochlear implantation. However, we did not find an association between the personality traits of our subjects and their (change in) cognitive abilities like they did in patients with cardiac diseases (Unterrainer et al. 2016).
The review from Claes et al. (2018a) introduced three hypotheses with regard to the effect of cochlear implantation on cognitive decline in older adults. According to the first, there is no effect of the CI on the cognitive decline in older adults. The accelerated cognitive decline continues in the same way as it did prior to the implantation, and the distinction in cognitive performance between individuals with normal hearing and impaired hearing continues to increase. Based on the results presented in this study, the second hypothesis seems the most plausible. Namely, that receiving a CI stops or slows down the acceleration of cognitive decline and induces an age-expected rate of decline. Although cofounders were taken into account in the propensity analysis, we cannot claim causality due to hidden cofounders.
A limitation of the study is the variable auditory rehabilitation. Auditory rehabilitation is recommended in the intervention group, whereas it is not part of the follow-up program of the control group. Therefore, future research is necessary to disentangle the possible confounding effect of practice from the effects of the auditory rehabilitation. Moreover, even if the auditory rehabilitation is responsible for the improvements in cognition, it is not clear to what extent this effect is generated by the CI or by the additional care and auditory rehabilitation program. Although this program is exclusively geared towards improving auditory perception with the CI, it may indeed indirectly influence cognition as well. Therefore, future research should include the same rehabilitation programs in intervention and control groups. Alternatively, it may also be interesting to investigate the effect of specific cognitive training among poor-performing CI recipients on their speech perception capabilities with the CI (Claes et al. 2018b).
Older adults with severe hearing impairment did not bridge the performance gap with adults with normal hearing in terms of cognition. Therefore, we can say that it is very unlikely that one year of CI use induces a catch-up (as stated in the third hypothesis) with older adults with normal hearing, resulting in an age-expected cognitive trajectory (Claes et al. 2018a). The fact that experienced, unilateral CI users still present subnormal cognitive functioning one year after implantation has implications for auditory rehabilitation after cochlear implantation and may highlight the need for additional cognitive rehabilitation in the long-term after implantation. Moreover, it is crucial to further monitor cognitive aging in older adults who received a CI many years ago. By doing so, it may become clear whether compensating for severe or profound hearing loss may delay the onset and/or progression of dementia, or whether there is a cut-off point from which influencing cognitive functioning is no longer possible.
ACKNOWLEDGMENTS
The authors thank Kristien Wouters (Antwerp University Hospital) for her contributions to the statistical analyses and Wilnelia Adams (MED-EL) for language editing a version of this article. The study was supported by the HEARRING group.
Footnotes
At the time of submission, A. J. C. was employed at Cochlear Benelux. However, data collection was performed during her employment at the Antwerp University Hospital and the University of Antwerp.
This manuscript does not necessarily reflect the opinions of Cochlear Benelux, and Cochlear Benelux did not have any impact on the results or the content of the manuscript.
G. M. coordinated the multicenter study, reviewed data from all sites, provided interpretive analysis, and drafted the paper. E. A., A. J. C., M. C., I. S. C., E. M., K. B., W. S., C. K., L. T., J. R., J. M. C., D. T.-V., R. M., and A. A. performed the experiments, collected data at the respective sites, and provided critical revision. V. T., P. V. d. H., V. V. R., J. G., L. L., P. S., H. S., and C. R. provided critical revision. All authors read and approved the final article.
The Antwerp University Hospital (UZA) currently receives a research grant from MED-EL, Innsbruck, Austria.
published online ahead of print October 9, 2020.
REFERENCES
- Acar B., Yurekli M. F., Babademez M. A., Karabulut H., Karasen R. M. Effects of hearing aids on cognitive functions and depressive signs in elderly people. Arch Gerontol Geriatr, (2011). 52, 250–252. [DOI] [PubMed] [Google Scholar]
- Amann E., Anderson I. Development and validation of a questionnaire for hearing implant users to self-assess their auditory abilities in everyday communication situations: the Hearing Implant Sound Quality Index (HISQUI19). Acta Otolaryngol, (2014). 134, 915–923. [DOI] [PubMed] [Google Scholar]
- Ambert-Dahan E., Routier S., Marot L., Bouccara D., Sterkers O., Ferrary E., Mosnier I. Cognitive evaluation of cochlear implanted adults using CODEX and MoCA screening tests. Otol Neurotol, (2017). 38, e282–e284. [DOI] [PubMed] [Google Scholar]
- Amieva H., Ouvrard C., Giulioli C., Meillon C., Rullier L., Dartigues J. F. Self-reported hearing loss, hearing aids, and cognitive decline in elderly adults: A 25-year study. J Am Geriatr Soc, (2015). 63, 2099–2104. [DOI] [PubMed] [Google Scholar]
- Arlinger S., Lunner T., Lyxell B., Pichora-Fuller M. K. The emergence of cognitive hearing science. Scand J Psychol, (2009). 50, 371–384. [DOI] [PubMed] [Google Scholar]
- Baltes P. B., Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging, (1997). 12, 12–21. [DOI] [PubMed] [Google Scholar]
- Calvino M., Gavilán J., Sánchez-Cuadrado I., Pérez-Mora R. M., Muñoz E., Lassaletta L. Validation of the Hearing Implant Sound Quality Index (HISQUI19) to assess Spanish-speaking cochlear implant users’ auditory abilities in everyday communication situations. Acta Otolaryngol, (2016). 136, 48–55. [DOI] [PubMed] [Google Scholar]
- Castiglione A., Benatti A., Velardita C., Favaro D., Padoan E., Severi D., Pagliaro M., Bovo R., Vallesi A., Gabelli C., Martini A. Aging, cognitive decline and hearing loss: Effects of auditory rehabilitation and training with hearing aids and cochlear implants on cognitive function and depression among older adults. Audiol Neurootol, (2016). 21Suppl 121–28. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Lin K. P., Chen Y. C. Risk factors for dementia. J Formos Med Assoc, (2009). 108, 754–764. [DOI] [PubMed] [Google Scholar]
- Claes A. J., Mertens G., Gilles A., Hofkens-Van den Brandt A., Fransen E., Van Rompaey V., Van de Heyning P. The Repeatable Battery for the Assessment of Neuropsychological Status for Hearing Impaired Individuals (RBANS-H) before and after cochlear implantation: A protocol for a Prospective, Longitudinal Cohort Study. Front Neurosci, (2016). 10, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes A. J., Van de Heyning P., Gilles A., Van Rompaey V., Mertens G. Cognitive outcomes after cochlear implantation in older adults: A systematic review. Cochlear Implants Int, (2018a). 19, 239–254. [DOI] [PubMed] [Google Scholar]
- Claes A. J., Van de Heyning P., Gilles A., Van Rompaey V., Mertens G. Cognitive performance of severely hearing-impaired older adults before and after cochlear implantation: Preliminary results of a prospective, longitudinal cohort study using the RBANS-H. Otol Neurotol, (2018b). 39, e765–e773. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Yeagle J., Arbaje A. I., Lin F. R., Niparko J. K., Francis H. W. Cochlear implant rehabilitation in older adults: Literature review and proposal of a conceptual framework. J Am Geriatr Soc, (2012). 60, 1936–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosetti M. K., Lalwani A. K. Is cochlear implantation safe and effective in the elderly? Laryngoscope, (2015). 125, 1279–1281. [DOI] [PubMed] [Google Scholar]
- Cosetti M. K., Pinkston J. B., Flores J. M., Friedmann D. R., Jones C. B., Roland J. T., Jr, Waltzman S. B. Neurocognitive testing and cochlear implantation: insights into performance in older adults. Clin Interv Aging, (2016). 11, 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R. G., Windsor T. D., Soubelet A. The relationship between Big-5 personality traits and cognitive ability in older adults—a review. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, (2015). 22, 42–71. [DOI] [PubMed] [Google Scholar]
- Dawes P., Emsley R., Cruickshanks K. J., Moore D. R., Fortnum H., Edmondson-Jones M., McCormack A., Munro K. J. Hearing loss and cognition: the role of hearing aids, social isolation and depression. PLoS One, (2015). 103e0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raeve L., Wouters A. Accessibility to cochlear implants in Belgium: state of the art on selection, reimbursement, habilitation, and outcomes in children and adults. Cochlear Implants Int, (2013). 14Suppl 1S18–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis N. A., Cabeza R. Craik F. I. M., Salthouse T. A. (Eds.), Neuroimaging of healthy cognitive aging. In The Handbook of Aging and Cognition, (2008). 3rd ed. (pp. Psychology Press.1–54. [Google Scholar]
- Denollet J. DS14: standard assessment of negative affectivity, social inhibition, and Type D personality. Psychosom Med, (2005). 67, 89–97. [DOI] [PubMed] [Google Scholar]
- Dobbels B., Mertens G., Gilles A., Claes A., Moyaert J., van de Berg R., Van de Heyning P., Vanderveken O., Van Rompaey V. Cognitive Function in Acquired Bilateral Vestibulopathy: A Cross-Sectional Study on Cognition, Hearing, and Vestibular Loss. Front Neurosci, (2019). 13, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis K., Pichora-Fuller M. K., Chasteen A. L., Marchuk V., Singh G., Smith S. L. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, (2015). 22, 413–437. [DOI] [PubMed] [Google Scholar]
- Gallacher J., Ilubaera V., Ben-Shlomo Y., Bayer A., Fish M., Babisch W., Elwood P. Auditory threshold, phonologic demand, and incident dementia. Neurology, (2012). 79, 1583–1590. [DOI] [PubMed] [Google Scholar]
- Gatehouse S., Noble W. The speech, spatial and qualities of hearing scale (SSQ). Int J Audiol, (2004). 43, 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakody D. M. P., Friedland P. L., Nel E., Martins R. N., Atlas M. D., Sohrabi H. R. Impact of Cochlear Implantation on Cognitive Functions of Older Adults: Pilot Test Results. Otol Neurotol, (2017). 38, e289–e295. [DOI] [PubMed] [Google Scholar]
- Justin B. N., Turek M., Hakim A. M. Heart disease as a risk factor for dementia. Clin Epidemiol, (2013). 5, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Metter E. J., O’Brien R. J., Resnick S. M., Zonderman A. B., Ferrucci L. Hearing loss and incident dementia. Arch Neurol, (2011). 68, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Yaffe K., Xia J., Xue Q. L., Harris T. B., Purchase-Helzner E., Satterfield S., Ayonayon H. N., Ferrucci L., Simonsick E. MHealth ABC Study Group. Hearing loss and cognitive decline in older adults. JAMA Intern Med, (2013). 173, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin V. Y., Chung J., Callahan B. L., Smith L., Gritters N., Chen J. M., Black S. E., Masellis M. Development of cognitive screening test for the severely hearing impaired: Hearing-impaired MoCA. Laryngoscope, (2017). 127Suppl 1S4–S11. [DOI] [PubMed] [Google Scholar]
- Lindenberger U., Baltes P. B. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging, (1994). 9, 339–355. [DOI] [PubMed] [Google Scholar]
- Lipnicki D. M., Crawford J. D., Dutta R., Thalamuthu A., Kochan N. A., Andrews G., Lima-Costa M. F., Castro-Costa E., Brayne C., Matthews F. E., Stephan B. C., Lipton R. B., Katz M. J., Ritchie K., Scali J., Ancelin M. L., Scarmeas N., Yannakoulia M., Dardiotis E., Lam L. C., et al. Cohort Studies of Memory in an International Consortium (COSMIC). Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study. PLoS Med, (2017). 14, e1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens G., Kleine Punte A., De Bodt M., Van de Heyning P. Sound quality in adult cochlear implant recipients using the HISQUI19. Acta Otolaryngol, (2015). 135, 1138–1145. [DOI] [PubMed] [Google Scholar]
- Mosnier I., Bebear J. P., Marx M., Fraysse B., Truy E., Lina-Granade G., Mondain M., Sterkers-Artières F., Bordure P., Robier A., Godey B., Meyer B., Frachet B., Poncet-Wallet C., Bouccara D., Sterkers O. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg, (2015). 141, 442–450. [DOI] [PubMed] [Google Scholar]
- Mosnier I., Vanier A., Bonnard D., Lina-Granade G., Truy E., Bordure P., Godey B., Marx M., Lescanne E., Venail F., Poncet C., Sterkers O., Belmin J. Long-term cognitive prognosis of profoundly deaf older adults after hearing rehabilitation using cochlear implants. J Am Geriatr Soc, (2018). 66, 1553–1561. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller M. K., Kramer S. E., Eckert M. A., Edwards B., Hornsby B. W., Humes L. E., Lemke U., Lunner T., Matthen M., Mackersie C. L., Naylor G., Phillips N. A., Richter M., Rudner M., Sommers M. S., Tremblay K. L., Wingfield A. Hearing Impairment and Cognitive Energy: The Framework for Understanding Effortful Listening (FUEL) Ear Hear, (2016). 37Suppl 15S–27S. [DOI] [PubMed] [Google Scholar]
- Randolph C., Tierney M. C., Mohr E., Chase T. N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol, (1998). 20, 310–319. [DOI] [PubMed] [Google Scholar]
- Skarzynski H., van de Heyning P., Agrawal S., Arauz S. L., Atlas M., Baumgartner W., Caversaccio M., de Bodt M., Gavilan J., Godey B., Green K., Gstoettner W., Hagen R., Han D. M., Kameswaran M., Karltorp E., Kompis M., Kuzovkov V., Lassaletta L., Levevre F., et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol, (2013). 564), Suppl(3–13. [DOI] [PubMed] [Google Scholar]
- Sonnet M. H., Montaut-Verient B., Niemier J. Y., Hoen M., Ribeyre L., Parietti-Winkler C. Cognitive Abilities and Quality of Life After Cochlear Implantation in the Elderly. Otol Neurotol, (2017). 38, e296–e301. [DOI] [PubMed] [Google Scholar]
- Unterrainer J., Michal M., Rahm B., Hadzibegovic J., Wild P. S., Schulz A., Münzel T., Blettner M., Lackner K., Pfeiffer N., Blankenberg S., Denollet J., Beutel M. E. Association of Type D personality with cognitive functioning in individuals with and without cardiovascular disease - The Gutenberg Health Study. Int J Cardiol, (2016). 214, 256–261. [DOI] [PubMed] [Google Scholar]
- Valentijn S. A., van Boxtel M. P., van Hooren S. A., Bosma H., Beckers H. J., Ponds R. W., Jolles J. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. J Am Geriatr Soc, (2005a). 53, 374–380. [DOI] [PubMed] [Google Scholar]
- Valentijn S. A., van Boxtel M. P., van Hooren S. A., Bosma H., Beckers H. J., Ponds R. W., Jolles J. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. J Am Geriatr Soc, (2005b). 53, 374–380. [DOI] [PubMed] [Google Scholar]
- van Hooren S. A., Anteunis L. J., Valentijn S. A., Bosma H., Ponds R. W., Jolles J., van Boxtel M. P. Does cognitive function in older adults with hearing impairment improve by hearing aid use? Int J Audiol, (2005). 44, 265–271. [DOI] [PubMed] [Google Scholar]
- Völter C., Götze L., Dazert S., Falkenstein M., Thomas J. P. Can cochlear implantation improve neurocognition in the aging population? Clin Interv Aging, (2018). 13, 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A. S., Snaith R. P. The hospital anxiety and depression scale. Acta Psychiatr Scand, (1983). 67, 361–370. [DOI] [PubMed] [Google Scholar]


