Abstract
Background
Allergic asthma causes morbidity in many individuals and novel precision-directed treatments would be valuable.
Objective
To examine the role of a novel innate molecule, repulsive guidance molecule b (RGMb), in murine models of allergic asthma.
Methods
In models of allergic asthma using OVA or cockroach allergen, mice were treated with anti-RGMb or control mAb and examined for airway inflammation and airway hyperreactivity (AHR), a cardinal feature of asthma. The mechanisms by which RGMb causes airways disease were also examined.
Results
We found that blockade of RGMb by treatment with anti-RGMb mAb effectively blocked the development of airway inflammation and AHR. Importantly, blockade of RGMb completely blocked the development of airway inflammation and AHR, even if treatment occurred only during the challenge (effector) phase. IL-25 played an important role in these models of asthma, since IL-25 receptor deficient mice failed to develop disease. RGMb was expressed primarily by innate cells in the lungs, including bronchial epithelial cells (known producers of IL-25), activated eosinophils and interstitial macrophages, which in the inflamed lung expressed the IL-25 receptor and produced IL-5 and IL-13. We also found that NEO1, the canonical receptor for RGMb, was expressed by interstitial macrophages and bronchial epithelial cells in the inflamed lung, suggesting that an innate RGMb-NEO1 axis might modulate allergic asthma.
Conclusions
These results demonstrate an important role for a novel innate pathway in regulating type 2 inflammation in allergic asthma, involving RGMb and RGMb-expressing cells such as interstitial macrophages and bronchial epithelial cells. Moreover, targeting this previously unappreciated innate pathway might provide an important treatment option for allergic asthma.
Keywords: Asthma, airway hyperreactivity, macrophages, Repulsive guidance molecule b, neogenin, IL-17RB, allergy
Capsule summary
We describe an important role for RGMb in asthma. Blockade of RGMb, expressed by bronchial epithelial cells, lung interstitial macrophages and eosinophils, abrogated allergic airways disease, even when treatment occurred late at the effector phase.
Graphical Abstract

Introduction
Asthma, which has increased dramatically in prevalence over the past 3 decades, is a major public health concern. Allergic asthma, the most common form of asthma, is mediated by type 2 inflammatory cells, including Th2 cells acting in concert with ILC2s and eosinophils, resulting in airway hyperreactivity (AHR), a cardinal feature of asthma. Airway epithelial cells, which are among the first to encounter inhaled allergens, initiate type 2 responses through the production of innate cytokines, including IL-25, IL-33 and TSLP. In particular, IL-25 plays a key role in type 2 inflammation in asthma (1–3) by binding to its receptor, IL-17RB, expressed on Th2 cells, ILC2s and Type 2 myeloid cells (4) and promoting the production of type 2 cytokines, thus orchestrating many of the classical features of asthma. However, little is known about innate pathways and molecules that affect and regulate IL-25-driven airway inflammation and AHR.
We examined a novel innate protein, repulsive guidance molecule b (RGMb), that appears to play a key role in IL-25 driven airway pathology. RGMb, a member of the RGM family, was discovered as a repulsive axon guidance cue, which directs embryonic brain development by linking the cell surface receptor neogenin (NEO1) (5–9) and bone morphogenetic proteins (BMPs) 2, 4 or 6 (10), providing a hub for multiple signaling pathways (7, 11) that play critical roles in bone (10, 12, 13) and lung (14–16) morphogenesis.
Several previous reports have suggested important postnatal roles for RGMb, NEO1 and BMPs 2, 4, and 6 in the lung. First, lung injury results in the induction of NEO1 expression, (17) and also activates the BMP pathway in bronchial epithelial cells (15), potentially promoting lung inflammation. Furthermore, in an allergic airway model, bronchial epithelial cells upregulated expression of BMPs 2, 4 and 6 and upregulated BMP/SMAD signaling (14), further indicating a postnatal role of BMPs in lung inflammation. Much less is known about the role of RGMb postnatally in the lung. However, the first report of a role for RGMb in the postnatal lung demonstrated that RGMb mRNA was highly expressed by lung interstitial macrophages, that RGMb bound to PD-L2, and that this RGMb-PD-L2 pathway played a key role in development of respiratory tolerance (18).
In the current report, using experimental mouse models of allergic asthma with OVA or cockroach allergen, we demonstrated a critical role for RGMb in coordinating type 2-lung inflammation. Thus, blockade of RGMb by treatment with an anti-RGMb mAb profoundly blocked the development of allergic asthma. Surprisingly, airway epithelial cells as well as interstitial macrophages in naive and inflamed lung expressed RGMb, as did activated eosinophils, which accumulated in the lungs of mice sensitized and challenged with OVA or CRA. Moreover, a large fraction of the RGMb+ interstitial macrophages in the inflamed lung expressed IL-17RB, the receptor for IL-25, which accounted for approximately 95% of the IL-17RB+ cells in the inflamed lung. This is important, since in this model, IL-25, but not IL-33, was required for the development of AHR. Furthermore, the RGMb+ macrophages in the inflamed lung produced IL-4, IL-5 and IL-13, and coexpressed NEO1, suggesting the importance of a type 2 innate RGMb-NEO1 signaling hub in these cells. Since treatment with anti-RGMb potently reduced airway inflammation and AHR, even when given late after sensitization, these findings suggest that anti-RGMb treatment may be effective as a treatment for ongoing, established asthma in humans.
Materials and Methods
Animals
BALB/cBy and C57Bl/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). PD-L2−/− mice on BALB/c background have been described (Keir et al, 2008). Mice were maintained and used according to institutional and National Institutes of Health guidelines in a pathogen-free facility. IL-17RB−/− mice were provided by Amgen (2). ST2−/− mice were provided by Andrew McKenzie (19). Animal protocols were approved by the Animal Care and Use Committees at Boston Children’s Hospital and the Dana-Farber Cancer Institute.
Antibodies
Monoclonal antibodies (mAbs) specific for IL-17RB and for NEO1 were generated by subcutaneous immunization of female Lewis strain rats (Harlan Sprague-Dawley) with mouse IL17-RB-Ig (Arg18-Gly286) or mouse neogenin-Ig (Ala42-Ile 1033, Asp442-Leu461 del) (R&D Systems). Hybridoma supernatants were screened and selected for use based on cell surface staining of mouse IL-17RB-transfected 300.19 cells or NEO-transfected 300.19 cells, respectively, and for lack of reactivity with untransfected cells (Fig E1). Anti-IL-17RB mAb 9B10, used in these studies, blocked binding of IL-17RB-huIgG Fc to ELISA plates coated with rIL-25 (Fig E1), further demonstrating its specificity for the IL-25 receptor. MAbs were purified using protein G-agarose (Pierce Biotechnology, Rockford, IL), and conjugated to fluorochromes by Biolegend, Inc or biotin-conjugated using standard techniques.
Generation of RGMb mAbs was previously described (18). Clone 9D1 was used for in vivo studies. RGMb mAb clone 9D1, used for in vivo studies, blocks the interaction of RGMb with BMP-2/4 and with PD-L2 (18) and partially blocks the interaction of RGMb with NEO1 (about 60%, data not shown). Since RGMb binds NEO1 at two distinct sites on the RGM molecule forming a complex of two RGMs with two NEO1 molecules (7), clone 9D1 may block interaction at one of these sites. RGMb mAb clone 9D3 was used for FACS, immunofluorescence and immunohistochemistry studies as its epitope is not covered by interactions with PD-L2, Neogenin or BMPs. PD-L2 mAb 2C9 blocks the interaction of PD-L2 with RGMb but does not block the interaction of PD-L2 with PD-1 (18).
Measurement of airway hyperreactivity
To induce AHR, mice were sensitized with 100μg OVA (ICN Biomedical) adsorbed in 2mg ALUM i.p. on day 0, and were given intranasal OVA (100 μg i.n.) or saline in 50 microlitre on day 7, 8 and 9. In some experiments, mice were treated with RGMb mAb 9D1, PD-L2 mAb 2C9 or control mAb i.p (500 μg/ treatment) as indicated. On day 10, mice were anesthetized with 50mg/kg body weight pentobarbital, tracheostomized and ventilated. Direct measurement of airway resistance and dynamic compliance was performed by invasive plethysmography (BUXCO systems) (20). Lung resistance was measured using invasive BUXCO (BUXCO electronics) in response to aerosolized saline (0.9% NaCl), followed by increasing doses of aerosolized acetyl-β-methylcholine chloride (methacholine) (0.125–40 mg/ml; Sigma-Aldrich).
CRA-induced allergic airway model
German cockroach extract (CRA) purchased from Greer laboratories (XBP46D3A4, Lenoir, NC) was suspended in PBS to a protein concentration of 2mg/ml. For short stimulation protocol, mice were sensitized with 10μg of CRA in ALUM on day 0 and then challenged with 10μg of CRA in PBS intranasally on day 7, 8, and 9. Lung histology, FACS analysis of total lung cells, and BAL fluid were done at day 10. For chronic model, mice were immunized with 10μg of CRA emulsified in incomplete Freund’s adjuvant (IFA) on day 0, followed by challenge with 5μg of CRA i.n. on day 14, 18, 22, and 26. Anti-RGMb mAb was administered on day 13, 17, 21, and 25. Lung histology, FACS analysis of total lung cells, and BAL fluid analysis were performed on day 27.
BAL fluid, histopathology and FACS staining of lung cells
Following euthanasia, the lungs were lavaged three times with 1 ml of PBS containing 2% fetal bovine serum and the fluid pooled. The relative number of different types of leukocytes was determined from slide preparations of BAL fluid stained with H&E. For histopathology, lungs were flushed with PBS to remove blood, infused with 10% (vol/vol) formalin and embedded in paraffin. Lung sections 5μm in thickness were cut and stained with hematoxylin and eosin. H&E sections on slides were scored based on degree of inflammation as follows: 0, no significant inflammation; 1, small clusters (10–30 cells) of inflammatory cells adjacent to vessels and airways; 2, moderately sized clusters (30–100 cells) of inflammatory cells adjacent to vessels and airways; 3, large aggregates (>100 cells) of inflammatory cells adjacent to vessels and airways.
FACS Antibodies and Analysis
For FACS analysis of lung cells, the lung was perfused with PBS, cut into small pieces, digested in RPMI 1640 with 5% FBS, 1.6 mg/ml collagenase IV (Worthington Biochemical Corporation, MA) and 200u/ml DNase I (Roche) and then treated with red blood cell lysing buffer (Sigma). Antibodies used for staining can be found in Table E1. For FACS staining of RGMb expression, cells were first stained for surface marker expression, fixed and permeabilized using eBioscience Intracellular fixation and permeabilzation buffer (ThermoFisher Scientific), then stained with Alexafluor 647-conjugated RGMb mAb 9D3. For detection of intracellular cytokines, total lung cells were cultured in the presence of Golgi stop for 6h prior to staining for surface marker expression. Cells were then fixed, permeabilized, stained with anti-cytokine or control mAb and analyzed by FACS.
Immunofluorescence microscopy staining
Frozen sections (7 μm) of lung were prepared on day 10 from mice sensitized and challenged with OVA. Slides were then stained with antibodies in 2% rat serum, 0.5% BSA. See Table E1 for list of antibodies used.
Fluorescent images were visualized using Zeiss AxioImager upright microscope and Plan-NEOFLUAR 40X /1,3 lens (Carl Zeiss, New York, NY, USA). Pictures were acquired with the AxioCam Hrc and analyzed on the software AxioVision Rel. 4.8. Z-stack images were acquired using a Carl Zeiss LSM 700 Laser Scanning Microscope with ZEN 2009 imaging software (Carl Zeiss, Germany). Image analysis was performed using ImageJ (NIH). Resultant stacks were rendered in three dimensions using Bitplane Imaris 7.1 software (Bitplane, Concord, MA, USA)
Immunohistochemistry
IHC staining for RGMb or NEO1 was performed on sections from tissue frozen in OCT. Tissue was blocked with BLOXALL, and stained using the VECTASTAIN Elite ABC kit. Biotin-conjugated RGMb mAb 9D3, biotin-conjugated NEO1 mAb 3C9, or control antibody was used for detection of RGMb or NEO1. Following development with peroxidase substrate (DAB Chromogen DAKO K3468), slides were counterstained with hematoxylin.
Cell isolation and stimulation
Spleen cells were isolated by mechanical tissue disruption, depleted of B cells and restimulated in vitro with OVA. Culture supernatants were collected after 4 days for cytokine analysis by ELISA.
qPCR
Total RNA was isolated from lung tissues using RNeasy mini kit (QIAGEN), and cDNA was synthesized using iScript™ cDNA synthesis kit (Invitrogen, CA). qPCR using TaqMan universal master mix (Applied Biosystems) and gene-specific TaqMan probe was carried out in a 7500 Sequence Detection System (Applied Biosystems). The levels of target gene expression were normalized to GAPDH expression using the 2−ΔΔCT method. The following primers and probes were purchased from Applied Biosystems: mouse GAPDH (4352339E), IL-4 (Mm00445258_g1), IL-5 (Mm00439645_m1), IL-13 (Mm00434204_m1). IL-25 (Mm00499822_m1), IL-17RB (Mm00444709_m1).
Microarray
Total RNA was extracted using the RNeasy Kit (Qiagen). To obtain genome-wide expression profiles, RNA samples were sent to the Microarray Core Facility at the Dana-Farber Cancer Institute (Boston, MA) for amplification and hybridization on the Mouse Gene 1.0 ST array (Affymetrix; Santa Clara, CA). Raw data for sample populations were preprocessed and normalized using the RMA algorithm in the ExpressionFileCreator module in GenePattern (21). The resulting data was subsequently analyzed in the Multiplot Suite in GenePattern by first filtering out genes with low expression value (<100 in all subsets) and noisy genes (covariance greater than 0.8), and then selecting for genes upregulated or downregulated (fold change less than or equal to 0.6 or fold change greater than or equal to 1.6, respectively; one-way ANOVA Tukey HSD p-value<0.05) in the inflamed lung (isotype) relative to the lung from the saline treated group. These genes of interest were subsequently divided into further subgroups based on the degree of upregulation or downregulation resulting from treatment with RGMb mAb. Heatmaps were generated with the GENE-E module (http://www.broadinstitute.org/cancer/software/GENE-E/) in GenePattern (21) (and compiled for publication using Adobe Illustrator). GEO submission number: GSE79156.
Results
Treatment with anti-RGMb mAb inhibits the development of AHR
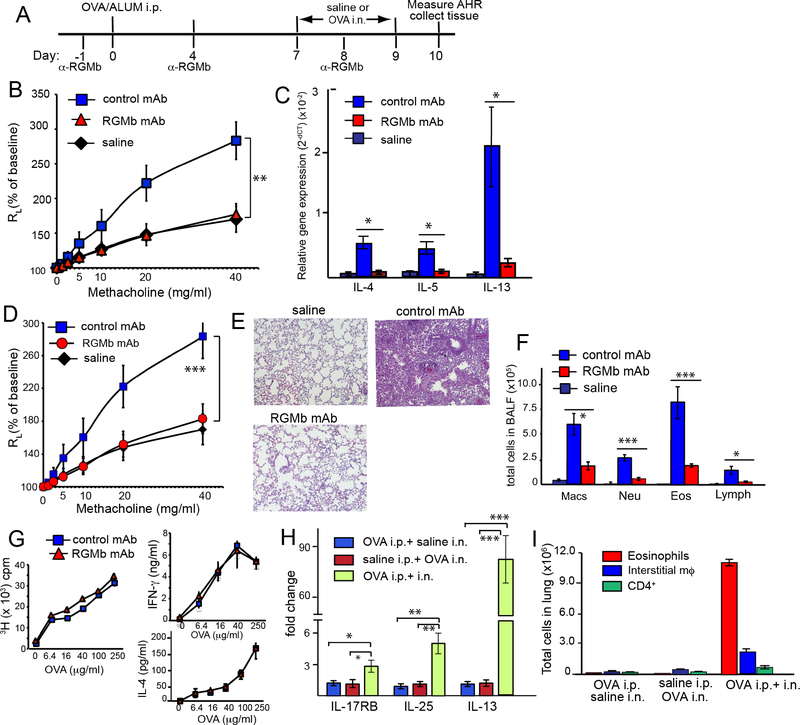
In an experimental mouse model of allergic asthma, treatment of OVA-sensitized and challenged mice with anti-RGMb mAb both at the time of sensitization and in the effector phase (days -1, 4 and 8) (Fig 1A), blocked the development of AHR (Fig 1B) and cytokine production (Fig 1C), as assessed on day 10. The reduction in airway inflammation was confirmed by gene expression studies, showing that Il4, Il13, Il33, as well as Ctla4, Cxcl5, Icos were increased in the inflamed airway but significantly reduced by treatment with anti-RGMb (Fig E2A). Expression of ifng, tslp, IL17a, IL22, IL23 was unchanged across the saline, RGMb mAb-treated and isotype control mAb-treated groups (Fig E2B). Furthermore, treatment with anti-RGMb mAb after sensitization had occurred, i.e., administration of anti-RGMb only on days 6 and 8, also greatly reduced AHR (Fig 1D) and airway inflammation (Fig 1E, F), indicating that anti-RGMb mAb can function at the effector stage to inhibit AHR and inflammation. Importantly, treatment with anti-RGMb mAb prior and during sensitization (days -1 and 4) did not block sensitization, as splenic T cells from OVA sensitized, anti-RGMb mAb-treated mice proliferated vigorously and produced IL-4 and IFN-γ on in vitro restimulation with OVA (Fig 1G). Therefore, the blockade of AHR and inflammation by anti-RGMb was not mediated through effects on T cell sensitization, which was required in our model, as T cell sensitization to OVA was required for expression of IL-25 and IL-13 and for OVA-induced airway inflammation (Fig 1H, I).
Figure 1:
Anti-RGMb mAb treatment blocks development of AHR and allergic airway inflammation
A. Protocol for OVA sensitization and challenge. B. OVA sensitized and challenged mice were treated with RGMb mAb or control mAb on days -1, 4, and 8. Results show the changes in lung resistance (RL). C. Level of target gene expression in mRNA from lung tissue was expressed relative to GAPDH. D. OVA sensitized and challenged mice were treated with RGMb or control mAb on days 6 and 8. Results show changes in lung resistance (RL). E. Lung sections from mice in (D) stained with H&E. F. Inflammatory cells in BAL fluid (cells/lung) of mice from Fig 1D. (B, D-F) n=4. G. OVA-sensitized mice were treated with RGMb mAb or control mAb on day -1 and day 4 and splenic T cell responses assayed on day 7. H. Level of IL-17RB, IL-25 and IL-13 gene expression in mRNA from lung tissue of mice sensitized and challenged with OVA and/or saline. I. The total number of eosinophils (CD11b+ Siglec-F+), interstitial macrophages (CD11b+ F4/80+CD11c− Siglec-F−) and CD4+ T cells in lung tissue from mice in H. Data are mean ± SEM and representative of three experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.005. B, D, control mAb compared with RGMb mAb group. Two-way ANOVA with Bonferroni post-hoc test. (C, F, H), control mAb compared with RGMb mAb group (unpaired t-test).
RGMb mAb inhibits established airways disease
Anti-RGMb treatment was also effective in a model of established airway inflammation, in which mice were exposed to several rounds of OVA i.n. and treated with anti-RGMb late on day 15 (Fig 2A). Following the first round of challenge with i.n. OVA (day 7–8), airway inflammation was shown to be present on day 9, with the presence of eosinophils, interstitial macrophages (F4/80+CD11b+CD11c−) and CD4+ lymphocytes (Fig 2B), and peri-bronchiolar cellular infiltration (Fig 2C). Importantly, just one dose of anti-RGMb on day 15 blocked airway inflammation measured on day 18 following a second round of i.n. OVA demonstrating that anti-RGMb inhibits established airways disease (Fig 2B, D). Lung inflammation is quantitated in Table E2A.
Figure 2:
RGMb mAb inhibits established airways disease and cockroach allergen-induced inflammation
A. Protocol for experiment shown in B-D. Mice were treated with RGMb mAb or control mAb on day 15. B. Lungs were collagenase digested and stained. The total number of eosinophils, interstitial macrophages, and CD4+ T cells in the lung on day 9 and day 18 are shown. C, D. Lung sections from mice on day 9 (C) and day 18 (D) stained with H&E (200X magnification). E. Protocol for experiment shown in F-H. Mice sensitized and challenged with cockroach allergen (CRA) were treated with RGMb or control mAb on days 6 and 8. F. Lungs were collagenase digested and stained as in B. G. Differential cell counts in BAL fluid (cells/lung) of mice shown in F. H. Lung sections from mice in (F, G), stained with H&E. Left panels: Lung tissue from negative control mouse that received intranasal saline but no cockroach antigen shows no significant inflammation. High power magnification of alveolar and bronchiolar epithelium is shown. Center panels: Lung tissue from positive control mice that received cockroach antigen. Dense perivascular and peribronchiolar inflammation is noted. High power magnification shows the inflammatory infiltrate consists of eosinophils, lymphocytes and interstitial macrophages. Bronchiolar epithelium shows abundant cytoplasmic mucin. Right panels: Lung tissue from mice that received cockroach antigen and anti-RGMb treatment. Mild peribronchiolar inflammation is noted. High power magnification shows minimal peribronchiolar inflammation and normal bronchiolar epithelium.
RGMb mAb inhibits cockroach allergen-induced airway inflammation
We also examined the effects of anti-RGMb in a model of cockroach antigen (CRA)-induced allergic asthma (Fig 2E), and showed that treatment with anti-RGMb significantly reduced the number of eosinophils, neutrophils, interstitial macrophages and lymphocytes in the lung and BAL fluid (Fig 2F, G). Examination of lung sections stained with H&E showed that exposure to CRA induced peribronchiolar and perivascular infiltrates consisting of eosinophils, lymphocytes and interstitial macrophages and significant bronchiolar mucus production (Fig 2H, middle panels). Anti-RGMb-treated mice showed significantly reduced peribronchial inflammation and normal bronchiolar epithelium (Fig 2H, right panels; inflammation quantitated in Table E2B). These results indicated that treatment with anti-RGMb effectively reduces lung inflammation in CRA-as well as OVA-induced allergen models, and in a model of established airway inflammation.
RGMb is expressed by innate rather than adaptive cells in the lung
Using immunohistochemistry (IHC), we found that RGMb was highly expressed by bronchial epithelial cells (Fig 3A) and by interstitial macrophages (Fig 3B) in both OVA sensitized and challenged mice and in saline control mice. Additionally, in the areas of inflammation in the lungs, other myeloid, but not lymphoid cells, expressed RGMb. This was confirmed using immunofluorescence microscopy, showing that CD11b+ and F4/80+ cells in the lung (markers of interstitial macrophages) highly express RGMb (Fig 3C, indicated with white arrows), whereas CD11c+ cells (DCs) and CD4+ T cells did not (Fig E3). In the F4/80+CD11b+ cells, most, though not all, of the RGMb expression was intracellular (Fig 3C, center panel), which was confirmed with confocal microscopy of CD11b+RGMb+ cells (Supplementary Video 1). These findings are consistent with our IHC studies (Fig 3B), and with other studies identifying interstitial macrophages as F4/80+CD11cneg in naïve mice (22), and also with our previous studies showing RGMb mRNA expression in naïve lung, with the highest RGMb mRNA expression in F4/80+CD11c− cells (18).
Figure 3:
RGMb is expressed by innate rather than adaptive cells in the lung.
A, B. IHC staining for RGMb on lung tissue from mice sensitized and challenged with OVA or saline shows strong staining in bronchiolar epithelium and interstitial macrophages. Far right panels of A and B show staining with control mAb. C-D. Immunofluorescent staining for RGMb of lung sections from mice sensitized and challenged with OVA. C. Left panel shows RGMb (red) and CD11b (blue). Center panel shows higher magnification of left panel. Right panel: White arrows indicate colocalization of RGMb (red), CD11b (blue), and F4/80 (green). Colocalization appears as white. D. Coexpression of RGMb (red) by cells that express Siglec-F (green), a marker of eosinophils, and CD11b (blue) is indicated with white arrows, right panel. Images representative of at least 5 animals and 3 experiments. E. Flow cytometric analysis of RGMb expression by eosinophils from lung digests of mice challenged with saline (left panel) or OVA (right panel). Analyses of one representative mouse per group are shown. Numbers on the plots reflect the percentage of cells in the plot that are present within the gated area. Data are representative of three experiments. F. Bar graph summary of data from experiment shown in E, 3 mice/group.
Eosinophils in the inflamed lung also expressed RGMb, since CD11b+ Siglec-F+ cells (eosinophils) expressed RGMb (Fig 3D, white arrows in the overlay). Moreover, flow cytometry showed that RGMb expression by eosinophils was highly upregulated in OVA-sensitized and challenged mice (>99% of activated eosinophils expressed RGMb compared with only 13–18% of the few eosinophils present in saline challenged mice) (Fig 3E, F).
RGMb+ interstitial macrophages express IL-17RB and produce IL-5 and IL-13
Since the RGMb+ interstitial macrophages appeared to be similar to recently described “type 2 myeloid (T2M)” cells (F4/80+CD11b+Gr-1mid, IL-17RB+ cells that produce IL-13 and IL-4) (4), we examined the RGMb+ cells for expression of IL-17RB and type 2 cytokines. Flow cytometry showed that most (80–90%) of the CD11b+F4/80+ macrophages expressed RGMb, as expected (Fig 4A, C), and surprisingly, many of these CD11b+RGMb+ cells from OVA challenged mice also expressed IL-17RB (Fig 4D). They did not express ST2, the receptor for IL-33 (data not shown). Histologically, the interstitial macrophages purified by FACS from lungs of OVA sensitized and challenged mice contained numerous cytoplasmic vacuoles and often displayed indented, kidney-shaped nuclei (Fig 4F, left panel), similar to that described for T2M cells (4) and lung interstitial macrophages (22), which were also shown to express F4/80 and CD11b. In contrast, eosinophils displayed the typical eosinophil morphology with red cytoplasmic granules (Fig 4F, right panel).
Figure 4.
Interstitial macrophages from OVA sensitized and challenged mice express RGMb and produce type 2 cytokines
A-D. Flow cytometric analysis of cells from lung digests of mice challenged with saline (A, B) or OVA (C, D). Interstitial macrophages (F4/80+CD11b+CD11c−Siglec-Fneg cells) were stained for IL-17RB, intracellular and surface RGMb, and intracellular cytokines. A, C. RGMb expression and B,D, IL-17RB expression and intracellular cytokine expression by interstitial macrophages. Numbers on the plots reflect the percentage of cells in the plot that are present within the gated area. Analyses of one representative mouse per group are shown. Data are representative of three experiments. E. Coexpression of IL-17RB (green) with CD11b (blue) (left panel, white arrows) and RGMb (red), (right panel, white arrows). (F) Morphology of eosinophils and interstitial macrophages FACS-purified from the lungs of OVA-sensitized and challenged mice. Cells were sorted as CD45+Siglec-F+CD11bmid (eosinophils) and CD45+Siglec-FnegCD11b+ F4/80+CD11cneg (interstitial macrophages) and stained with HEMA-3 stain. (G) Bar graph representation of experiment shown in Figure 4B, D, summary of 3 mice/group.
We also found that the F4/80+ CD11b+ macrophages from sensitized and challenged mice that expressed IL-17RB (40 to 60% in various experiments) produced IL-5, IL-13 and some IL-4 (Fig 4D), shown as a bar graph summary in Fig 4G. Moreover, while many FACS-sorted interstitial macrophages expressed IL-17RB (~67%), upon culture (48 hours) with recombinant IL-25, they responded by increasing their expression of IL-17RB (>98%) (Fig E4). Together, these findings suggest that in our model, RGMb+ macrophages express IL-17RB+ and can respond to IL-25, which likely leads to the production of IL-5 and IL-13 and a role as effector cells.
Most IL-17RB+ cells in inflamed lungs are RGMb+ interstitial macrophages
The number of inflammatory cells in the lung increased following intranasal challenge of sensitized mice with OVA (Fig 5A). 24 hours following the first intranasal challenge, the number of CD4+ T cells in the lung was significantly increased compared with control mice. The numbers of eosinophils and interstitial macrophages increased more slowly but were markedly different from control mice on days 9 and 10. Most of the inflammatory cells in the lung on day 10 were eosinophils, followed by the RGMb+ interstitial macrophages and CD4+ T cells (ILC2s and iNKT cells were present in the lungs in much lower numbers) (Fig 5B and Table E3). Importantly, most (about 95%) of the IL-17RB+ cells present in the inflamed lung on day 10 were interstitial macrophages (that expressed RGMb) (Fig 5C), suggesting that interstitial macrophages were the primary cells responding to IL-25. Moreover, IL-17RB+ interstitial macrophages were present at much higher frequencies in the lungs of OVA-challenged mice than in the lungs of saline challenged mice (Fig 5C, E, Table E3). Treatment with anti-RGMb mAb, even with only one dose during the effector phase of the protocol, greatly reduced the number of IL-17RB+ cells in the lungs (Fig 5E) as well as the total numbers of inflammatory cells (Fig 5D), suggesting an important role for IL-17RB+ RGMb+ interstitial macrophages.
Figure 5.
Numbers of interstitial macrophages and eosinophils in the lung increase following allergen exposure, and this is inhibited by treatment with RGMb mAb.
A-C. Lungs from mice sensitized and challenged with OVA or saline as in Fig 1A were harvested on the indicated day, collagenase digested and analyzed by flow cytometry. A. The total number of eosinophils, interstitial macrophages, and CD4+ T cells in the lung on days 8, 9 and 10 are shown. B. Lungs from mice sensitized and challenged with OVA or saline were collagenase digested and analyzed by flow cytometry. Total numbers of eosinophils, interstitial macrophages, CD4+ T, ILC2 and iNKT, and (C) numbers of IL-17RB+ cells of these cell types in the lung on day 10 are shown. D, E. Mice sensitized and challenged with OVA were treated with RGMb mAb or isotype control on days 0, 4 and 8 (α-RGMb), or on day 6 and day 8 (α-RGMb effector phase). D. Total numbers of eosinophils, interstitial macrophages and CD4+ T cells in the lung on day 10 and (E) IL-17RB+ numbers of these cell types are shown. n=4. Data are mean ± SEM. * P<0.05; ** P<0.01; *** P<0.005. Unpaired t test. (F) Protocol for experiment shown in G-J. Mice sensitized and challenged with cockroach allergen (CRA) were treated with RGMb mAb or isotype control on days 13, 17, 21 and 25. G-H. Numbers of IL-17RB+ eosinophils, interstitial macrophages, and CD4+ T cells (G) and (H) total numbers of cells of these cell types in the lung on day 27 are shown. (I) Differential cell counts in BAL fluid (cells/lung) of mice shown in G-H. J. Lung sections from mice in (G, H) stained with H&E (200X magnification).
Exposure of mice to CRA in a chronic exposure model over 27 days (Fig 5F), resulted in greater lung inflammation with significant accumulations of eosinophils, interstitial macrophages and CD4+ T cells in the lung (Fig5G–I, 5J (middle panel). Histological inflammation is quantitated in Table E2C. As with OVA, the most numerous IL-17RB+ cells were interstitial macrophages (Fig 5G). Treatment with anti-RGMb mAb on days 13, 17, 21 and 25 significantly reduced the number of IL17RB+ cells, as well as the total number of inflammatory cells in the lung (Fig 5G–H) and BAL fluid (Fig 5I). Moreover, IL-17RB expression by interstitial macrophages present in the lungs following anti-RGMb treatment was substantially reduced, nearly to the level of that observed in saline control mice (Fig E5). Examination of lung sections stained with H&E showed that exposure to CRA induced peribronchiolar and perivascular infiltrates consisting of eosinophils, lymphocytes and interstitial macrophages (Fig 5J, middle panel), while anti-RGMb-treated mice showed minimal peribronchial inflammation and normal bronchiolar epithelium (Fig 5J, right panel, inflammation quantitated in Table E2C), indicating that treatment with anti-RGMb blocks the accumulation/expansion of inflammatory cells, particularly of RGMb+ interstitial macrophages, that occurs in the lungs in response to allergen.
IL-17RB but not ST2 is required for allergen-induced AHR
IL-25 (23, 24) but not IL-33 (25, 26) played a critical role in our model of asthma, since IL-33 receptor deficient ST2−/− mice sensitized and challenged with OVA developed similar levels of AHR and inflammation as did WT mice (Fig 6A, B). Furthermore, treatment with anti-RGMb mAb blocked the development of AHR and lung inflammation in both the ST2−/− and WT mice. In contrast, IL-17RB−/− mice failed to develop AHR (Fig 6C) and airway inflammation (Fig 6D) following OVA sensitization and challenge. Moreover, mRNA for IL-17RB, IL-25 and IL-13 was elevated in lungs of OVA sensitized mice at 3 and 24 hr after challenge with OVA in the lungs (Fig 6E). Together, these studies indicate that IL-25 and IL-17RB were critical for the development of allergen-induced AHR.
Figure 6.
IL-25 but not IL-33 signaling is required for development of OVA-induced AHR ST2−/− (A, B), IL-17RB−/− (C,D), or WT mice sensitized and challenged with OVA or saline as in Fig 1A were assessed for AHR on day 10. Mice in (A) were treated with control mAb or RGMb mAb on day -1, 4 and 8 as indicated. B,D. Inflammatory cells in BAL fluid (cells/lung) of the mice in A and C. E. Expression of il-17rb, il-25, and il-13 mRNA in lung of WT mice at 3h and 24h after last allergen challenge, measured by qPCR. GAPDH was used as an internal control. Values are normalized to saline mice at 3h. A, C, n=4. Data shown are means ± SD. * P<0.05; ** P<0.01; *** P<0.005. Two-way ANOVA with the Bonferroni post-hoc test. B, D, E, unpaired t test.
The RGMb pathway in allergic asthma is independent of PD-L2
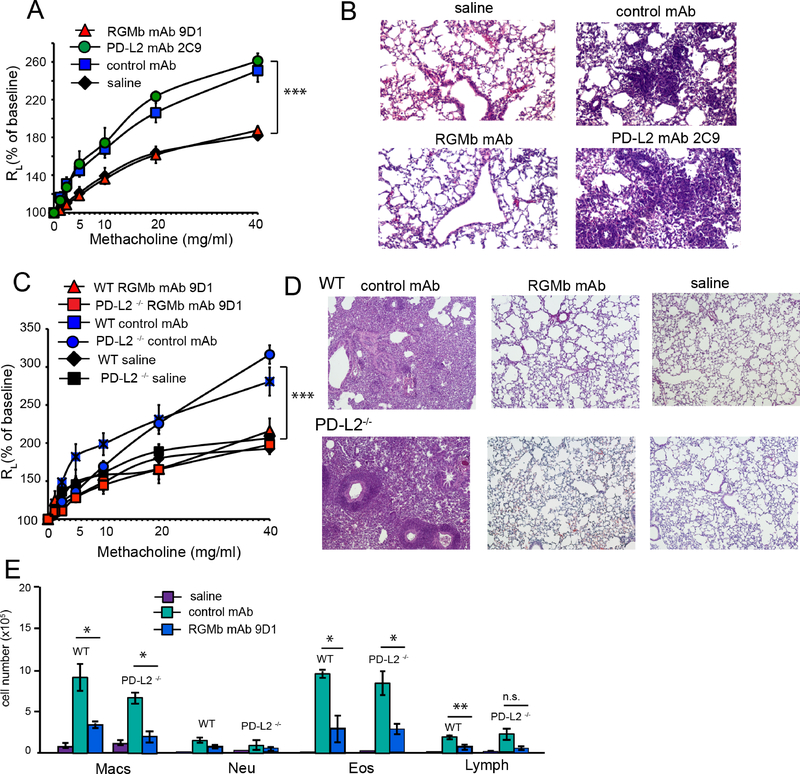
Since we recently demonstrated that RGMb can promote respiratory tolerance by interacting with one of its ligands, PD-L2 (18), we asked if this pathway had a role in the development of AHR. Treatment with an anti-PD-L2 mAb (2C9) that blocks the interaction of PD-L2 with RGMb but does not inhibit the interaction of RGMb with NEO1 or BMPs 2,4 or 6 (18), had no effect on AHR or airway inflammation (Fig 7A, B), whereas treatment with anti-RGMb abolished AHR (Fig 7C, D). These findings were confirmed with PD-L2−/− mice, which developed robust AHR and inflammation when sensitized and challenged with OVA (27), which was abolished by treatment with anti-RGMb mAb (Fig 7C, D, E). These results demonstrated that the interaction of RGMb with PD-L2 did not affect the development of AHR.
Figure 7:
RGMb blockade of AHR and lung inflammation does not involve RGMb:PD-L2 interaction
A. OVA sensitized and challenged mice were treated with RGMb mAb 9D1, PD-L2 mAb 2C9 or isotype control on days -1, 4, and 8. B. Lung tissue from the mice in (A) was stained with H&E. C. PD-L2 deficient or WT mice sensitized and challenged with OVA were treated with RGMb mAb or isotype control (500 μg) on days -1, 4, and 8. ‘WT saline’ and ‘PD-L2−/− saline’ denote WT and PD-L2−/− mice sensitized with OVA and challenged with saline as negative controls. D. Lung sections from mice in (C) were stained with H&E. E. Inflammatory cells in BAL fluid (cells/lung) of the mice in (C). (A-D) n=4. Data are mean ± SEM and representative of three experiments. * P<0.05; ** P<0.01; *** P<0.005. A, C, control mAb group compared to RGMb mAb group. Two-way ANOVA with Bonferroni post-hoc test. E, (unpaired t-test).
Neogenin is expressed by F4/80+ CD11b+ cells following OVA sensitization and challenge
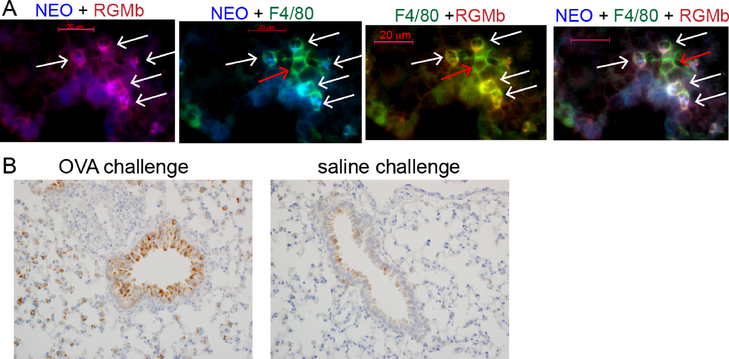
Since the canonical pathway for RGMb signaling is via binding the receptor NEO1 (7, 9), providing a hub for interactions with bone morphogenetic proteins (BMPs) (11, 28), we examined NEO1 expression in lung. Using a novel anti-NEO mAb, we found that NEO1 colocalized with expression of RGMb in the lungs of OVA sensitized and challenged mice (Fig 8A, left panel). Numerous cells were observed that coexpressed NEO1, F4/80 and RGMb (Fig 8A, right panel, white arrows), although not all RGMb+ cells expressed NEO1, and a few F4/80+ cells expressed neither NEO1 nor RGMb (red arrows). Confocal microscopy showed that NEO1 was predominantly expressed intracellularly although some surface expression was detectable (Figure E6, Video 2). Using immunohistochemistry (IHC) to detect NEO1, we found that bronchial epithelium from OVA challenged mice showed diffuse strong cytoplasmic staining for NEO1. NEO1 was also expressed by interstitial macrophages in lungs of the OVA challenged mice. In contrast, bronchial epithelium from saline challenged mice showed patchy weak cytoplasmic staining for NEO1, and NEO1 expression was not detected on interstitial macrophages (Fig 8B).
Figure 8.
NEO1 is expressed by RGMb+ interstitial macrophages and bronchiolar epithelium in lung tissue from OVA sensitized and challenged mice.
A. Left: Colocalization of NEO1 (blue) and RGMb (red) appears magenta (white arrows). 2nd panel: Colocalization of F4/80 (green) and NEO1 (blue) (white arrows). 3rd panel: Colocalization of F4/80 (green) and RGMb (red) appears yellow (white arrows). Right: Triple colocalization of NEO1, RGMb and F4/80 appears white (white arrows). Images representative of at least 4 animals. B. IHC staining of lung tissue for Neogenin. Bronchiolar epithelium from the saline challenged group shows patchy weak cytoplasmic staining. In the OVA challenged group, the bronchiolar epithelium shows diffuse strong cytoplasmic staining. NEO1-positive interstitial macrophages are also present in the OVA challenged lungs as compared to the saline treated mice.
Discussion
Using several murine models of asthma we discovered that RGMb contributes greatly to the development of allergen-induced AHR and type 2 lung inflammation through novel innate mechanisms. The importance of RGMb in the lung is supported by our findings that RGMb was expressed in the lung by 1) bronchial epithelial cells, which are known to respond to environmental stimuli by secreting IL-25, IL-33, TSLP, and GMCSF, thus initiating type 2 responses (3, 29, 30), and also by 2) interstitial macrophages, which expressed the IL-25 receptor and responded to IL-25 by secreting IL-5 and IL-13, a critical cytokine causing AHR (31, 32). Following exposure to either OVA or CRA, RGMb+ interstitial macrophages greatly increased in numbers in the lung. Moreover, treatment with anti-RGMb mAb blocked the expansion of RGMb+ interstitial macrophages and eosinophils in the lungs, and inhibited development of AHR, even when given late after sensitization. Thus our studies identify RGMb as a novel innate molecular entity critically involved in the development of AHR, and suggest that targeting RGMb could be an effective therapy for established asthma.
The critical role of RGMb-expressing interstitial macrophages in asthma was suggested by a previous study in a chronic cockroach allergen model describing macrophages, called Type 2 myeloid (T2M) cells, that produced IL-13 (4). T2M cells, expressing the surface markers IL-17RB+F4/80+CD11b+Gr-1mid, accumulated in the lungs of mice following repeated allergen exposure and were critical in asthma pathogenesis (4). In another setting, F4/80+CD11b+ macrophages were also shown to play an essential role in IL-13-dependent lung inflammation in which depletion of these cells at the time of airway challenge prevented recruitment of eosinophils to the airways (33). We greatly extended these studies and demonstrated that lung interstitial macrophages that produce IL-5 and IL-13 expressed RGMb, and treatment with anti-RGMb inhibited airway inflammation and AHR. A recent report which showed that targeting RGMb decreased lung inflammation, is consistent with our findings (34).
Moreover, we showed that the RGMb+ interstitial macrophages also played an important role because they expressed IL-17RB, the receptor for IL-25, which was required in our mouse model of allergen-driven AHR. An important role for IL-25 in human asthma has been demonstrated in clinical studies (35, 36) and confirmed by human genetic studies (37). IL-25 is produced by inflamed airway epithelial cells (3, 35), eosinophils (38), tuft cells in the intestinal and respiratory tracts (39–41) and by solitary chemosensory cells (SCC) in the airways (42). IL-25 then, in combination with neuropeptides such as neuromedin U released from sensory neurons in the lungs and calcitonin gene-related peptide released from pulmonary neuroendocrine cells, can promote type 2 immunity in allergen-driven AHR (1, 2) (43–45) by enhancing the production of IL-4, IL-5 and IL-13 from ILC2s, Th2 cells and RGMb+ interstitial macrophages (2, 23, 44–46). While RGMb+ interstitial macrophages in saline control mice express only low levels of IL-17RB, we found that following either OVA or CRA sensitization and challenge, RGMb+ interstitial macrophages were the most numerous IL-17RB-expressing cells, accounting for about 98% of IL-17RB+ cells in the lung. Both IL-25 and IL-17RB mRNA and protein increase in the lungs following infection with RSV or rhinovirus (35, 47), or following allergen exposure as shown in the present study and in a previous study (4). IL-25 was shown to induce IL-17RB surface expression by human CD4+ T cells (48), and IL-17RB expression by human monocytic cells was induced and amplified by IL-4 and IL-25 (49). We found that while about 50% of the RGMb+ interstitial macrophages taken directly from inflamed lungs expressed IL-17RB, upon incubation with rIL-25, expression increased to >98%.
In OVA- or CRA-sensitized and challenged mice treated with anti-RGMb mAb, IL-17RB expression by interstitial macrophages was reduced almost to the level of saline control mice. These findings suggest that production of IL-25 by cells such as bronchial epithelial cells in the inflamed lung results in enhanced expression of IL-17RB on interstitial macrophages and subsequent production of type 2 inflammatory cytokines, and suggests that treatment with anti-RGMb mAb may inhibit production of IL-25.
The important role of RGMb-expressing cells (epithelial cells, interstitial macrophages and eosinophils) was shown by treating mice with anti-RGMb, which blocked the accumulation of eosinophils and RGMb+ interstitial macrophages. Blocking IL-25 in the challenge phase with anti-IL-25 mAb was shown to prevent AHR but did not reduce cell infiltration or eosinophil number in BAL fluid (1), indicating that RGMb plays a more dominant role than IL-25 at this time point. In contrast, IL-33 was not required in our model since development of AHR occurred normally in mice deficient in ST2, the receptor for IL-33. IL-33 has been shown to be required in other models of asthma, such as that induced by influenza infection, in which infected alveolar macrophages produce IL-33, which then activates ILC2s (50, 51).
The number of RGMb+ macrophages in the lung increased rapidly following allergen challenge. This increase may be due to local proliferation of lung resident macrophages driven by the cytokine IL-4 (52, 53), or recruitment from the blood or bone marrow (38). The number of eosinophils, which are recruited into the airways by local production of IL-5 and by eotaxin produced by IL-13-exposed epithelial cells (54), also increased following allergen challenge.
Allergen-primed T cells, particularly pathogenic effector Th2 cells (55, 56), are required in our model, and we believe they are involved in initiating the anamnestic allergic inflammatory response in the lung, perhaps in conjunction with iNKT cells and ILC2s (57). Although ILC2s have been shown to have critical roles in many models of asthma, particularly with protease containing allergens that damage airway epithelium (58) or in the response to viral infection (47, 50, 59) ILC2s may not be required in all conditions leading to asthma. For example, ILC2s were found to be dispensable in inducing type 2 lung inflammation when T cells were first sensitized with allergen and adjuvant (60). Moreover, in our models ILC2s were present at low numbers in the lung and did not increase after allergen challenge.
RGMb signals via linking the NEO1 and BMP pathways, i.e., by binding BMP2, BMP4 or BMP6 (11, 28, 61), which are key signaling molecules, and NEO1 (5–8), thus forming a BMP-RGM-NEO1 super-receptor complex. We speculate that RGMb may upregulate airway inflammation and AHR by forming a super-receptor complex with NEO1 and BMPs 2, 4 or 6, shown to be upregulated in bronchial epithelial cells in acute allergic airway inflammation (14, 15), and interstitial macrophages in the inflamed lung, thus activating BMP signaling pathways. This is consistent with the observation that when both NEO1 and RGM are expressed on the same cell, as we observed for interstitial macrophages and bronchial epithelial cells in inflamed lung, cis (same-cell) interactions occur in a BMP-dependent manner (11). BMPs mediate clustering of RGM-NEO at the cell surface (7, 11) and engagement of BMPs triggers intracellular phosphorylation (62), leading to downstream signaling by the SMAD cascade or alternative pathways (63). The RGMb mAb used in our in vivo studies blocks the interaction of RGMb with BMP2 and BMP4 (18), and partially blocks the interaction of RGMb with NEO1 (data not shown), likely disrupting the formation of the signaling hub.
Although both RGMb and NEO proteins are cell surface molecules, they are primarily localized intracellularly (Fig 3, Video 1 and 2), which is in agreement with recent data from crystal structure analysis suggesting that the RGM-NEO1 complex transports BMP dimers from the cell surface to endosomes which are rich in BMP type I receptors (11). Although the site of action of BMP receptors is under debate, endocytosis has been shown to be important for BMP signaling (64).
Anti-RGMb mAb treatment may inhibit airway inflammation at several stages of the allergic response, by blocking expansion of the RGMb-expressing macrophages, which, through production of IL-13, contribute to lung smooth muscle contraction and eosinophil recruitment (54). RGMb mAb treatment may also inhibit production of type 2 mediators by bronchial epithelial cells, which we have shown express RGMb. Bronchial epithelial cells have been termed “the gatekeepers of allergic sensitization” (29) since upon detection of inhaled allergens through pattern recognition receptors they release cytokines which initiate type 2 responses. Further studies will be required to fully elucidate these mechanisms.
It should be noted that RGMb appears to have two distinct functions in the lung, involving two distinct immunologic pathways. We previously described a pathway in which RGMb, through interaction with PD-L2, promotes respiratory tolerance in the lung (18). Respiratory tolerance, induced by exposure to OVA in the respiratory tract without prior T cell priming, was inhibited by treatment with an anti-PD-L2 antibody, which blocked the interaction of PD-L2 with RGMb, but not the interaction of RGMb with NEO1 or BMPs (18). In the inflammatory pathway described herein, which involves allergen sensitization prior to allergen challenge in the respiratory tract, administration of anti-PD-L2 mAb which blocks the RGMb:PD-L2 interaction had no effect on AHR. In contrast, in lung inflammation an RGMb:NEO1:BMPs interaction may be involved (without PD-L2), based on our finding that a) NEO1 is expressed in the inflamed lung on bronchial epithelial cells and interstitial macrophages, b) the anti-RGMb mAb we used inhibits interaction of RGMb with BMPs 2, 4, and NEO1, c) previous work demonstrated elevation of BMPs 2 and 4 and NEO in inflamed lung (14, 17) and d) crystal structure analyses demonstrated that RGMb forms a ternary complex with NEO1 and BMP 2, 4 or 6, creating a potent signaling hub (11). Upstream events such as the activation of epithelial cells by allergen or sensitized T cells likely promotes the RGMb:NEO1:BMPs pathway. Alternatively, it is possible that an RGMb interaction with BMPs 2, 4, 6 or with an unidentified ligand is involved. In any case, in the development of AHR, the RGMb pathway is distinct from that in respiratory tolerance.
In summary, we identified a novel innate immune pathway in the lungs in which blockade of RGMb inhibited the development of AHR and lung inflammation. We identified lung cells that expressed RGMb and NEO1, and that modulated the development of AHR. RGMb mAb treatment was effective when given during the challenge phase of the allergic lung response and even following several rounds of allergen exposure. RGMb treatment may be effective in reducing IL-25-driven disease, including allergen-induced AHR, RSV-induced pulmonary disease (47) and rhinovirus-induced allergic airway inflammation (35), which have both been shown to be driven by IL-25. Agents that target RGMb could provide an effective therapy for asthma.
Supplementary Material
Supplementary Video 1.RGMb is expressed intracellularly and on the cell surface. A Z stack movie of a CD11b+ cell (blue) shows that a substantial amount of RGMb (red) is expressed intracellularly, localized in the cytoplasm of the cell. Overlap of RGMb with CD11b on the cell surface appears as magenta. One video representative of 5 is shown.
Supplementary Video 2. Neogenin and RGMb proteins are colocalized and expressed intracellularly and on the cell surface. A Z stack movie of two CD11b+ cells stained with CD11b (blue), RGMb (red) and NEO1 (green) mAbs shows that that RGMb and NEO1 expression colocalize, both internally and on the cell surface. Overlap of RGMb with NEO appears as yellow-green and yellow. One video representative of 4 is shown.
Key Messages.
RGMb regulates type 2 inflammation in allergic asthma
RGMb is expressed primarily by innate cells in the lungs, including activated eosinophils and interstitial macrophages, and by bronchial epithelial cells.
Treatment with anti-RGMb mAb following allergen sensitization inhibits allergen-induced asthma (AHR) and lung inflammation
Acknowledgements
This work was supported by National Institutes of Health Grants: PO1 AI-054456 (Freeman, DeKruyff, Umetsu), R01 AI089955 (Freeman, DeKruyff) and by the Sean N. Parker Center for Allergy and Asthma Research, Stanford University School of Medicine (DeKruyff).
We thank Amgen for providing the IL-17RB deficient mice and Andrew N.J. McKenzie, MRC Laboratory of Molecular Biology, Cambridge, UK, for providing the ST2 deficient mice.
Abbreviations
- AHR
airway hyperreactivity
- BAL
bronchoalveolar lavage
- BMP
bone morphogenetic protein
- NEO1
neogenin
- PD-L2
programmed death ligand 2
- qPCR
quantitative real-time reverse transcription polymerase chain reaction
- RGMb
repulsive guidance molecule b
- RGMb+ macrophage
F4/80+CD11b+CD11cneg macrophage
- RGMs
repulsive guidance molecules
Footnotes
Conflict of interest disclosure: RDK has no financial or commercial conflict of interest. RDK, GF, YX, SY have patents pending on the use of RGMb reagents in asthma and cancer. GF has patents/pending royalties on the PD-1 pathway from Bristol-Myers-Squibb, Roche, Merck, EMD-Serono, Boehringer-Ingelheim, AstraZeneca, and Novartis. GF has served on advisory boards for CoStim, Novartis, Roche, Eli Lilly, Bristol-Myers-Squibb, Seattle Genetics, and Bethyl Laboratories.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol 2007. December;120(6):1324–31. PubMed PMID: 17889290. [DOI] [PubMed] [Google Scholar]
- 2.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008. September 15;181(6):4299–310. PubMed PMID: 18768888. [DOI] [PubMed] [Google Scholar]
- 3.Suzukawa M, Morita H, Nambu A, Arae K, Shimura E, Shibui A, et al. Epithelial cell-derived IL-25, but not Th17 cell-derived IL-17 or IL-17F, is crucial for murine asthma. J Immunol. 2012. October 1;189(7):3641–52. PubMed PMID: 22942422. Pubmed Central PMCID: 3812057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen BC, Budelsky AL, Baptist AP, Schaller MA, Lukacs NW. Interleukin-25 induces type 2 cytokine production in a steroid-resistant interleukin-17RB+ myeloid population that exacerbates asthmatic pathology. Nat Med. 2012. May;18(5):751–8. PubMed PMID: 22543263. Pubmed Central PMCID: 3378776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samad TA, Srinivasan A, Karchewski LA, Jeong SJ, Campagna JA, Ji RR, et al. DRAGON: a member of the repulsive guidance molecule-related family of neuronal- and muscle-expressed membrane proteins is regulated by DRG11 and has neuronal adhesive properties. J Neurosci. 2004. February 25;24(8):2027–36. PubMed PMID: 14985445. Epub 2004/02/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsunaga E, Tauszig-Delamasure S, Monnier PP, Mueller BK, Strittmatter SM, Mehlen P, et al. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol. 2004. August;6(8):749–55. PubMed PMID: 15258591. Epub 2004/07/20. eng. [DOI] [PubMed] [Google Scholar]
- 7.Bell CH, Healey E, van Erp S, Bishop B, Tang C, Gilbert RJ, et al. Structure of the repulsive guidance molecule (RGM)-neogenin signaling hub. Science. 2013. July 5;341(6141):77–80. PubMed PMID: 23744777. Epub 2013/06/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad S, Stimpfle F, Montazeri S, Oldekamp J, Seid K, Alvarez-Bolado G, et al. RGMb controls aggregation and migration of Neogenin-positive cells in vitro and in vivo. Mol Cell Neurosci. 2009. February;43(2):222–31. PubMed PMID: 19944164. Epub 2009/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 9.Rajagopalan S, Deitinghoff L, Davis D, Conrad S, Skutella T, Chedotal A, et al. Neogenin mediates the action of repulsive guidance molecule. Nat Cell Biol. 2004. August;6(8):756–62. PubMed PMID: 15258590. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Xie J, Lee D, Liu Y, Jung J, Zhou L, et al. Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation. Dev Cell. 2010. July 20;19(1):90–102. PubMed PMID: 20643353. Epub 2010/07/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healey EG, Bishop B, Elegheert J, Bell CH, Padilla-Parra S, Siebold C. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nature structural & molecular biology. 2015. June;22(6):458–65. PubMed PMID: 25938661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. J Cell Biochem. 2004. September 1;93(1):93–103. PubMed PMID: 15352166. Epub 2004/09/08. eng. [DOI] [PubMed] [Google Scholar]
- 13.Yoon BS, Pogue R, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, et al. BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development. 2006. December;133(23):4667–78. PubMed PMID: 17065231. Epub 2006/10/27. eng. [DOI] [PubMed] [Google Scholar]
- 14.Rosendahl A, Pardali E, Speletas M, Ten Dijke P, Heldin CH, Sideras P. Activation of bone morphogenetic protein/Smad signaling in bronchial epithelial cells during airway inflammation. Am J Respir Cell Mol Biol. 2002. August;27(2):160–9. PubMed PMID: 12151307. Epub 2002/08/02. eng. [DOI] [PubMed] [Google Scholar]
- 15.Sountoulidis A, Stavropoulos A, Giaglis S, Apostolou E, Monteiro R, Chuva de Sousa Lopes SM, et al. Activation of the canonical bone morphogenetic protein (BMP) pathway during lung morphogenesis and adult lung tissue repair. PLoS One. 2012;7(8):e41460. PubMed PMID: 22916109. Epub 2012/08/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996. June;122(6):1693–702. PubMed PMID: 8674409. [DOI] [PubMed] [Google Scholar]
- 17.Mirakaj V, Jennewein C, Konig K, Granja T, Rosenberger P. The guidance receptor neogenin promotes pulmonary inflammation during lung injury. FASEB J. 2012. April;26(4):1549–58. PubMed PMID: 22198383. Epub 2011/12/27. eng. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014. May 5;211(5):943–59. PubMed PMID: 24752301. Pubmed Central PMCID: 4010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013. October;132(4):933–41. PubMed PMID: 23810766. [DOI] [PubMed] [Google Scholar]
- 20.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature Medicine. 2003;9:582–88. [DOI] [PubMed] [Google Scholar]
- 21.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006. May;38(5):500–1. PubMed PMID: 16642009. [DOI] [PubMed] [Google Scholar]
- 22.Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest. 2009. December;119(12):3723–38. PubMed PMID: 19907079. Epub 2009/11/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001. December;15(6):985–95. PubMed PMID: 11754819. [DOI] [PubMed] [Google Scholar]
- 24.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002. July 1;169(1):443–53. PubMed PMID: 12077275. [DOI] [PubMed] [Google Scholar]
- 25.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999. October 4;190(7):895–902. PubMed PMID: 10510079. Pubmed Central PMCID: 2195643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005. November;23(5):479–90. PubMed PMID: 16286016. [DOI] [PubMed] [Google Scholar]
- 27.Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010. January;3(1):81–91. PubMed PMID: 19741598. Pubmed Central PMCID: 2845714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia Y, Babitt JL, Bouley R, Zhang Y, Da Silva N, Chen S, et al. Dragon enhances BMP signaling and increases transepithelial resistance in kidney epithelial cells. J Am Soc Nephrol. 2010. April;21(4):666–77. PubMed PMID: 20167703. Epub 2010/02/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014. September;134(3):499–507. PubMed PMID: 25171864. [DOI] [PubMed] [Google Scholar]
- 30.Sheih A, Parks WC, Ziegler SF. GM-CSF produced by the airway epithelium is required for sensitization to cockroach allergen. Mucosal Immunol. 2017. May;10(3):705–15. PubMed PMID: 27731325. Pubmed Central PMCID: 5389932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wills-Karp M Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004. December;202:175–90. PubMed PMID: 15546393. [DOI] [PubMed] [Google Scholar]
- 32.Walter D, McIntire J, Berry G, McKenzie A, Donaldson D, DeKruyff R, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–75. [DOI] [PubMed] [Google Scholar]
- 33.Borthwick LA, Barron L, Hart KM, Vannella KM, Thompson RW, Oland S, et al. Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis. Mucosal Immunol. 2016. January;9(1):38–55. PubMed PMID: 25921340. Pubmed Central PMCID: 4626445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie X, Chen W, Zhu Y, Huang B, Yu W, Wu Z, et al. B7-DC (PD-L2) costimulation of CD4(+) T-helper 1 response via RGMb. Cellular & molecular immunology. 2017. May 8;14:1–10. PubMed PMID: 28479601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beale J, Jayaraman A, Jackson DJ, Macintyre JD, Edwards MR, Walton RP, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014. October 1;6(256):256ra134. PubMed PMID: 25273095. Pubmed Central PMCID: 4246061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol. 2011. July;128(1):116–24. PubMed PMID: 21570719. [DOI] [PubMed] [Google Scholar]
- 37.Jung JS, Park BL, Cheong HS, Bae JS, Kim JH, Chang HS, et al. Association of IL-17RB gene polymorphism with asthma. Chest. 2009. May;135(5):1173–80. PubMed PMID: 19118269. [DOI] [PubMed] [Google Scholar]
- 38.Dolgachev V, Petersen BC, Budelsky AL, Berlin AA, Lukacs NW. Pulmonary IL-17E (IL-25) production and IL-17RB+ myeloid cell-derived Th2 cytokine production are dependent upon stem cell factor-induced responses during chronic allergic pulmonary disease. J Immunol. 2009. November 01;183(9):5705–15. PubMed PMID: 19828636. [DOI] [PubMed] [Google Scholar]
- 39.Harris N IMMUNOLOGY. The enigmatic tuft cell in immunity. Science. 2016. March 18;351(6279):1264–5. PubMed PMID: 26989236. [DOI] [PubMed] [Google Scholar]
- 40.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018. August;560(7718):319–24. PubMed PMID: 30069044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bankova LG, Dwyer DF, Yoshimoto E, Ualiyeva S, McGinty JW, Raff H, et al. The cysteinyl leukotriene 3 receptor regulates expansion of IL-25-producing airway brush cells leading to type 2 inflammation. Science immunology. 2018. October 5;3(28). PubMed PMID: 30291131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohanski MA, Workman AD, Patel NN, Hung LY, Shtraks JP, Chen B, et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018. August;142(2):460–9 e7. PubMed PMID: 29778504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017. September 14;549(7671):277–81. PubMed PMID: 28869974. Pubmed Central PMCID: 5714273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klose CSN, Mahlakoiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature. 2017. September 14;549(7671):282–6. PubMed PMID: 28869965. Pubmed Central PMCID: 6066372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018. June 8;360(6393). PubMed PMID: 29599193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chesne J, Cardoso V, Veiga-Fernandes H. Neuro-immune regulation of mucosal physiology. Mucosal Immunol. 2018. August 8. PubMed PMID: 30089849. [DOI] [PubMed] [Google Scholar]
- 47.Petersen BC, Dolgachev V, Rasky A, Lukacs NW. IL-17E (IL-25) and IL-17RB promote respiratory syncytial virus-induced pulmonary disease. J Leukoc Biol. 2014. January 9. PubMed PMID: 24407884. Pubmed Central PMCID: 3984969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bredo G, Storie J, Shrestha Palikhe N, Davidson C, Adams A, Vliagoftis H, et al. Interleukin-25 initiates Th2 differentiation of human CD4(+) T cells and influences expression of its own receptor. Immunity, inflammation and disease. 2015. December;3(4):455–68. PubMed PMID: 26734466. Pubmed Central PMCID: 4693727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weathington NM, Kanth SM, Gong Q, Londino J, Hoji A, Rojas M, et al. IL-4 Induces IL17Rb Gene Transcription in Monocytic Cells with Coordinate Autocrine IL-25 Signaling. Am J Respir Cell Mol Biol. 2017. September;57(3):346–54. PubMed PMID: 28421819. Pubmed Central PMCID: 5625225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011. July;12(7):631–8. PubMed PMID: 21623379. Pubmed Central PMCID: 3417123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012. January;129(1):216–27 e1–6. PubMed PMID: 22119406. Epub 2011/1½9. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011. June 10;332(6035):1284–8. PubMed PMID: 21566158. Pubmed Central PMCID: 3128495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, et al. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med. 2013. October 21;210(11):2477–91. PubMed PMID: 24101381. Pubmed Central PMCID: 3804948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001. October;108(4):594–601. PubMed PMID: 11590387. [DOI] [PubMed] [Google Scholar]
- 55.Muehling LM, Lawrence MG, Woodfolk JA. Pathogenic CD4+ T cells in patients with asthma. J Allergy Clin Immunol. 2017. April 22. PubMed PMID: 28442213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang HE, Pollack JL, et al. A tissue checkpoint regulates type 2 immunity. Nat Immunol. 2016. December;17(12):1381–7. PubMed PMID: 27749840. Pubmed Central PMCID: 5275767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010. July;11(7):577–84. PubMed PMID: 20562844. Pubmed Central PMCID: 3114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012. March 23;36(3):451–63. PubMed PMID: 22425247. [DOI] [PubMed] [Google Scholar]
- 59.Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol. 2016. September;138(3):814–24 e11. PubMed PMID: 27156176. Pubmed Central PMCID: 5014571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014. April;133(4):1142–8. PubMed PMID: 24679471. [DOI] [PubMed] [Google Scholar]
- 61.Samad TA, Rebbapragada A, Bell E, Zhang Y, Sidis Y, Jeong SJ, et al. DRAGON, a bone morphogenetic protein co-receptor. J Biol Chem. 2005. April 8;280(14):14122–9. PubMed PMID: 15671031. Epub 2005/0½7. eng. [DOI] [PubMed] [Google Scholar]
- 62.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003. June 13;113(6):685–700. PubMed PMID: 12809600. [DOI] [PubMed] [Google Scholar]
- 63.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003. October 9;425(6958):577–84. PubMed PMID: 14534577. [DOI] [PubMed] [Google Scholar]
- 64.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003. May;5(5):410–21. PubMed PMID: 12717440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1.RGMb is expressed intracellularly and on the cell surface. A Z stack movie of a CD11b+ cell (blue) shows that a substantial amount of RGMb (red) is expressed intracellularly, localized in the cytoplasm of the cell. Overlap of RGMb with CD11b on the cell surface appears as magenta. One video representative of 5 is shown.
Supplementary Video 2. Neogenin and RGMb proteins are colocalized and expressed intracellularly and on the cell surface. A Z stack movie of two CD11b+ cells stained with CD11b (blue), RGMb (red) and NEO1 (green) mAbs shows that that RGMb and NEO1 expression colocalize, both internally and on the cell surface. Overlap of RGMb with NEO appears as yellow-green and yellow. One video representative of 4 is shown.