Abstract
Purpose of Review
Immune checkpoint inhibitors (ICIs) have improved the survival of several cancers. However, they may cause a wide range of immune-related adverse events (irAEs). While most irAEs are manageable with temporary cessation of ICI and immunosuppression, cardiovascular toxicity can be associated with high rates of morbidity and mortality. As ICIs evolve to include high-risk patients with preexisting cardiovascular risk factors and disease, the risk and relevance of ICI-associated cardiotoxicity may be even higher.
Recent Findings
Several cardiovascular toxicities such as myocarditis, stress cardiomyopathy, and pericardial disease have been reported in association with ICIs. Recent findings also suggest an increased risk of atherosclerosis with ICI use. ICI-associated myocarditis usually occurs early after initiation and can be fulminant. A high index of suspicion is required for timely diagnosis. Prompt treatment with high-dose corticosteroids is shown to improve outcomes.
Summary
Although the overall incidence is rare, ICI cardiotoxicity, particularly myocarditis, is associated with significant morbidity and mortality, making it a major therapy-limiting adverse event. Early recognition and prompt treatment with the cessation of ICI therapy and initiation of high-dose corticosteroids are crucial to improve outcomes. Cardio-oncologists will need to play an important role not just in the management of acute cardiotoxicity but also to reduce the risk of long-term sequelae.
Keywords: Immune checkpoint inhibitors, Immunotherapy, Cancer, Cardiotoxicity, Myocarditis, Pericarditis, Stress cardiomyopathy, COVID-19
Introduction
The introduction of immune checkpoint inhibitor (ICI) therapy can be considered one of the cardinal achievements in the field of oncology. ICIs enhance the host immune response against tumor cells by inhibiting intrinsic downregulators of the immune response, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) or its ligand, programmed cell death ligand 1 (PD-L1). To date, the US Food and Drug Administration (FDA) has approved seven agents (one CTLA-4-blocking antibody [ipilimumab]; three PD-1-blocking antibodies [nivolumab, pembrolizumab, and cemiplimab]; and three PD-L1-blocking antibodies [atezolizumab, avelumab, and durvalumab]) for 12 different cancers [1, 2]. While these agents have revolutionized the outcomes of a wide variety of cancers, activation of the immune system by these drugs can damage other organs, termed as immune-related adverse events (irAEs), particularly when used as combination immunotherapy [3••]. Although any organ system can be affected, irAEs most commonly involve the gastrointestinal tract, endocrine glands, skin, and liver [4]. Less often, the central nervous system and cardiovascular, pulmonary, musculoskeletal, and hematologic systems may also be involved. While many irAEs resolve successfully with temporary cessation of ICI therapy and immunosuppression, cardiotoxicity, particularly myocarditis, can be associated with significant morbidity and mortality [3••]. Here we review ICI-associated cardiotoxicity and its management.
Cardiotoxicity
Among the various forms of ICI cardiotoxicities, myocarditis is the most frequently reported due to its high morbidity and mortality. However, other manifestations such as cardiomyopathy, arrhythmias, acute coronary syndrome, and vasculitis have also been reported [5••, 6].
Although the exact mechanism of cardiotoxicity is incompletely understood, presence of common high-frequency T-cell receptor sequences in tumor and cardiac muscle cells raises the possibility of a shared antigen target [7, 8]. Animal models have shown that CTLA-4, PD-1, and PD-L1 have cardioprotective effects against immune-mediated damage after stress. Hence, inhibition by ICIs may make cardiac cells more susceptible to injury [9–11].
Incidence and Risk Factors
In an international multicenter registry, the reported overall prevalence of ICI-associated myocarditis was 1.14% and as high as 2.4% for combination (two or more ICIs) therapy [3••]. For patients undergoing treatment with nivolumab, troponin levels were elevated in 10% of patients without a clear reason, suggesting that the true incidence of subclinical or smoldering myocarditis might be even higher [12]. The true incidence of ICI-associated myocarditis remains unclear and is likely underestimated due to a combination of factors including the lack of conventional clinical symptoms, the potential for overlap with other cardiovascular illnesses, challenges in making the diagnosis, and a general lack of awareness about this condition.
The risk factors for ICI cardiotoxicity are poorly understood. An international registry identified combination therapy, diabetes, obesity, and anti-CTLA-4 therapy as independent risk factors for cardiotoxicity [6]. Pre-existing autoimmune disease may also be an independent risk factor [5••]. Furthermore, the risk of myocarditis may differ between various classes of ICIs. Some have reported that the prevalence of myocarditis is highest with anti-CTLA-4 monotherapy (3.3%), followed by anti-PD-L1 (2.4%) and anti-PD-1 agents (0.5%) [3••]. However, others have noted similar toxicity profiles for both anti-PD-1 and anti-PD-L1 agents [13].
Pre-existing cardiovascular risk factors may also be associated with the development of ICI-associated myocarditis. In a multicenter international registry, patients who developed myocarditis had a greater prevalence of hypertension (60% vs 48%, p < 0.009) and tobacco use (48% vs 17%, p < 0.001) and were more likely to be on a statin (39% vs 29%, p = 0.04) and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (32% vs 23%, p = 0.04). Conversely, pre-existing coronary artery disease, atrial fibrillation, and chronic kidney disease were not identified as risk factors for myocarditis [14]. However, these risk factors were identified by univariate rather than multivariable regression analysis, and hence, their true implications cannot yet be confirmed.
Clinical Presentation
ICI-associated myocarditis can present with a spectrum of symptoms ranging from fatigue and myalgias to chest pain and shortness of breath and can sometimes even present as syncope and sudden cardiac death [3, 7, 15, 16]. In an international multicenter registry, nearly 50% of patients with ICI-associated myocarditis developed major adverse cardiovascular events (MACE) including atrial and ventricular arrhythmias, complete heart block, heart failure, cardiogenic shock, or death [3••]. Although fulminant myocarditis with heart failure and arrhythmias has been more commonly reported, subclinical or smoldering myocarditis with minimal signs and symptoms may also occur.
Timing of Cardiotoxicity
Myocarditis typically occurs early after initiation of ICI therapy with a median time of 2 months, with majority of cases occurring within 3 months [3••]. However, cardiotoxicity can occur at any time during ICI therapy and can occasionally occur even after cessation of therapy due to persistent effects of the drug [17].
Diagnosis
As ICI-associated myocarditis is an emerging entity, there are no specific guidelines for its diagnosis. Because its presentation can be nonspecific, a high degree of suspicion is required to make the diagnosis, especially since it can have a rapidly progressive and fatal course. While it is reasonable to consider myocarditis in any patient on ICIs presenting with either nonspecific or specific cardiovascular symptoms, other potential causes such as acute coronary syndrome, stress-induced cardiomyopathy, type II myocardial infarction, viral myocarditis, and pneumonitis should be considered. The clinical presentation alone may be unable to distinguish ICI-associated myocarditis from other cardiac disorders. Hence, additional diagnostic tests, such as coronary computed tomography angiography or invasive coronary angiography, play a pivotal role in eliminating acute coronary syndrome. Many patients may have taken other potentially cardiotoxic drugs (such as anthracyclines) in the past, and the adverse cardiovascular effects of these drugs should also be considered in the differential. If other irAEs are present in a patient with cardiac symptoms, the probability of ICI-associated myocarditis increases. In particular, myocarditis is more commonly seen in association with ICI-related myasthenia gravis and myositis compared with other irAEs [16]. The proposed mechanism for this association is the presence of shared antigens on cardiac and skeletal muscles.
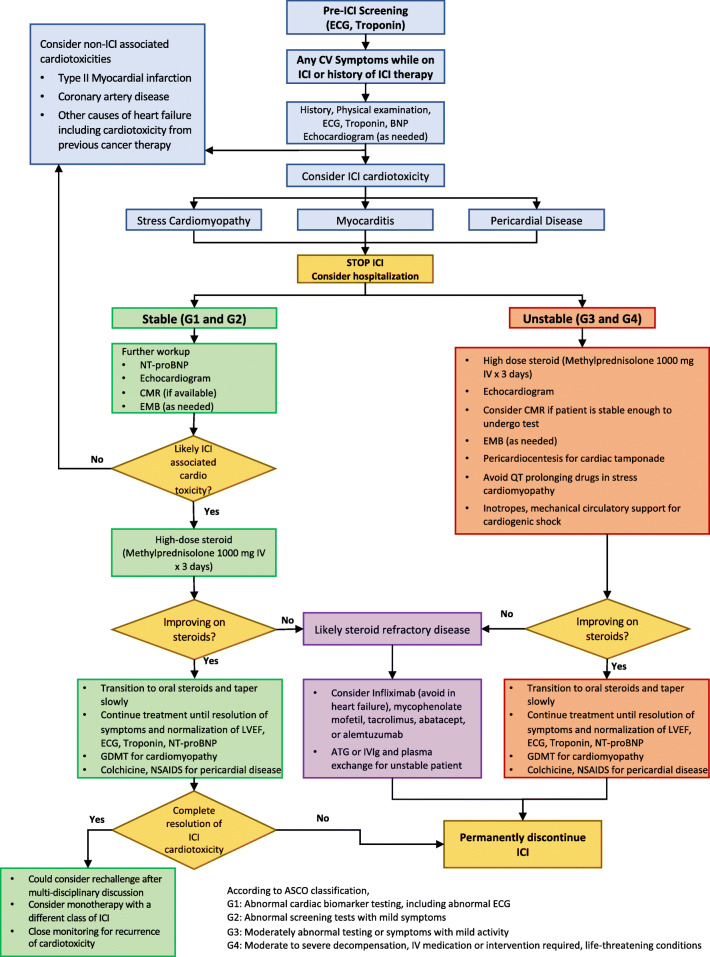
A combination of several imaging and non-imaging tests is often required to make the diagnosis of ICI-associated myocarditis (Fig. 1). In the following sections, we describe various diagnostic tests and discuss their utility and limitations.
Fig. 1.
Proposed algorithm for management of ICI-associated myocarditis. Abbreviations: ATG, anti-thymocyte globulin; CMR, cardiac magnetic resonance imaging; ECG, electrocardiogram; EMB, endomyocardial biopsy; GDMT, guideline-directed medical therapy; ICI, immune checkpoint inhibitor; IVIg, intravenous immunoglobulin; LVEF, left ventricular ejection fraction; NSAID, nonsteroidal anti-inflammatory drug; NT-proBNP, N-terminal pro-brain natriuretic peptide
Electrocardiogram
An electrocardiogram (ECG) is usually the first test performed in patients presenting with cardiovascular symptoms. While ECG abnormalities are reported in most cases, a normal ECG does not rule out ICI-associated myocarditis [3••]. Most often, the findings are nonspecific and may include sinus tachycardia, QRS/QT prolongation, conduction abnormalities, diffuse T-wave inversion, abnormal Q waves, atrial or ventricular arrhythmias, or local/diffuse ST elevation. In a retrospective study, QRS prolongation was noted more frequently in patients with myocarditis compared to controls, and it was also associated with a higher occurrence of MACE [3••]. This finding implies that any subtle new conduction abnormality should not be overlooked even in relatively asymptomatic or minimally symptomatic patients.
Cardiac Troponin
While cardiac troponin is one of the most sensitive tests, with increased levels noted in 94% of patients with ICI-associated myocarditis [3••], it is not specific for myocarditis. However, troponin may have prognostic implications since higher troponin levels are associated with worse cardiovascular outcomes in patients with ICI-associated myocarditis [3••].
Brain Natriuretic Peptide (BNP)/N-Terminal pro-BNP
Natriuretic peptides can be elevated, especially in patients with volume overload/heart failure. However, they lack sensitivity and specificity for ICI-associated myocarditis and hence are not particularly helpful in making the diagnosis [3••, 5••].
Echocardiogram
This is usually the initial imaging test for the assessment of patients with suspected ICI-associated myocarditis. Although patients with ICI-associated myocarditis can develop cardiomyopathy, even severe forms like fulminant myocarditis may present with a normal left ventricular ejection fraction (LVEF) [5••]. Importantly, a normal LVEF does not preclude MACE. In an international retrospective registry, 51% of patients with myocarditis had a normal LVEF, and among those who experienced MACE, 38% occurred in patients with a normal LVEF [3••, 18]. Emerging data suggests that global longitudinal strain (GLS) obtained with 2-D echocardiography may help with both the diagnosis and prognosis of ICI-associated myocarditis. A retrospective study of 101 patients with ICI-associated myocarditis showed that GLS was lower in patients with myocarditis compared with controls, for patients with both a preserved and a reduced LVEF. A lower GLS value was strongly associated with subsequent MACE among patients with myocarditis regardless of whether they had preserved or reduced LVEF [18].
Cardiac Magnetic Resonance Imaging
Among all the imaging tests, cardiac magnetic resonance imaging (CMR) is the preferred test for diagnosing ICI-associated myocarditis. The diagnosis of myocarditis is made using the updated Lake Louise criteria [19]. Myocardial inflammation and edema can be visualized by CMR techniques like late gadolinium enhancement (LGE) and T2-weighted imaging, which provide excellent spatial resolution and tissue characterization [19]. However, the utility of CMR is limited as it may not be readily available, and severely ill patients may not tolerate the test. Additionally, recent data from an international registry showed that most patients with ICI-associated myocarditis had a normal LVEF on CMR and only 46% had LGE, making CMR an insensitive tool for the diagnosis of ICI-associated myocarditis. LGE on CMR has been shown to be an effective tool for risk stratification and prognostication in myocarditis due to other causes. However, in patients with ICI-associated myocarditis, LGE was not associated with MACE [20]. This suggests that LGE on CMR cannot be used exclusively to diagnose ICI-associated myocarditis and additional parameters of tissue characterization (such as T1 and T2 mapping) may need to be routinely utilized in the evaluation of these patients.
Endomyocardial Biopsy
Endomyocardial biopsy is the most specific diagnostic test for ICI myocarditis. The biopsy from a typically affected area shows inflammatory infiltrates (usually T cell–predominant lymphocytic infiltrate) in the myocardium and may also demonstrate the presence of cell-specific markers such as T lymphocytes (CD3), macrophages (CD68), or human leukocyte antigens [5••, 21]. Because endomyocardial biopsy poses a risk of cardiac perforation, it is not performed on a routine basis. However, it should be considered in patients with a high clinical suspicion for ICI-associated myocarditis and negative or ambiguous non-invasive imaging. While ICI-associated myocarditis can be a patchy process, lowering the sensitivity of endomyocardial biopsy in some patients, a negative biopsy in a hemodynamically stable patient, without other corroborating evidence, may allow careful re-introduction of ICI therapy in patients lacking effective alternative cancer treatments.
Management
The management of ICI-associated myocarditis is primarily based on knowledge gained through clinical experience and from retrospective registry data, rather than prospective clinical trials. When ICI-associated myocarditis is suspected, ICI therapy should be withheld, and the necessary workup promptly initiated. Further management is dictated by (1) the degree of suspicion for ICI-associated myocarditis and (2) the intensity of the clinical presentation. Intensity is categorized into four grades according to ASCO guidelines—G1 and G2 are considered stable and minimally symptomatic whereas G3 and G4 are very symptomatic or unstable patients [22]. Depending on these factors, the patient may need to be hospitalized, especially if the patient is unstable or has significant abnormalities on diagnostic testing. Prompt initiation of immunosuppressive therapy is critical to managing and improving the prognosis of ICI-associated myocarditis (Fig. 1).
Corticosteroids
These are the first agents used to manage ICI-associated myocarditis.
Dose
High-dose corticosteroids are recommended, typically starting with intravenous methylprednisolone 1000 mg daily for 3 days, followed by oral prednisone 1 mg/kg/day with a slow taper over several weeks [5••]. Data from an international registry of 126 patients showed that a higher initial dose of corticosteroids was associated with improved outcomes [23••].
Timing
Along with dosage, the timing of initiation of corticosteroid therapy also plays a significant role. Prompt initiation of corticosteroids, within 24 h of presentation, was associated with improved outcomes [23••]. Furthermore, low-dose corticosteroids administered early after presentation were associated with better outcomes than high-dose corticosteroids administered at a later time [23••]. This data supports the need for early initiation of therapy, and in some cases, even before confirmation of the diagnosis.
Duration
There is limited data regarding the optimal duration of corticosteroid therapy and the duration should be decided on a case-by-case basis. In general, corticosteroids should be tapered slowly (over at least 4–6 weeks) and only after resolution of symptoms, normalization of LVEF, or stabilization of arrhythmias [5••, 14].
Other Immunosuppressive Therapies
Besides corticosteroid therapy, the utility of other immunosuppressive agents is not well established. Due to limited evidence, these therapies should only be considered in patients with corticosteroid-refractory ICI-associated myocarditis. The need for additional immunosuppressive agents can be further classified based on the patient’s clinical status.
Tacrolimus and mycophenolate mofetil can be considered if there is evidence of high-grade myocarditis on endomyocardial biopsy [5••, 6, 14] or in cases with persistent symptoms/biomarker elevation despite high-dose corticosteroids. Infliximab has been associated with worsening heart failure [24] and more recently reports of increased CV deaths [25]. Therefore, it is not a suitable primary agent.
Anti-thymocyte globulin, intravenous gamma globulin, and plasmapheresis should be considered in patients with ICI-associated myocarditis who are hemodynamically unstable. The evidence regarding their use is limited and is derived mostly from patients with cardiac allograft rejection. Abatacept, a CTLA-4 agonist, and alemtuzumab, a CD52 monoclonal antibody, have also been shown to be effective in the treatment of corticosteroid-refractory ICI-associated myocarditis but the evidence is based on case reports, and hence, their use is limited [26, 27].
Supportive Therapy
Given the paucity of data regarding the efficacy and safety of different agents used for the management of hemodynamically unstable patients with ICI-associated myocarditis, a multidisciplinary and individualized approach to decision-making is critical to achieve the best outcomes. Mechanical circulatory support may be needed in some patients and should be considered after weighing the prognosis from a cancer standpoint. In patients with cardiomyopathy, guideline-directed medical therapy should be started after stabilization. Similarly, in patients with arrhythmias, appropriate antiarrhythmics and anticoagulation therapy should be considered.
Prognosis
ICI-associated myocarditis is associated with a high mortality with estimated rates of 38% to 46%. Non-fatal MACE including heart failure, cardiogenic shock, cardiac arrest, ventricular arrhythmias, and complete heart block have also been reported in approximately 50% of cases [3••, 12]. The lack of systematic screening strategies may contribute to overestimation of these complications and underreporting of subclinical or smoldering myocarditis that presents with minimal signs and symptoms.
Screening and Surveillance
The American Society of Clinical Oncology Clinical Practice Guideline recommends a screening ECG and consideration of troponin measurement prior to initiating ICI, particularly in patients receiving dual ICI therapy [22]. Retrospective registry data show that 70% of patients who developed myocarditis on ICI therapy had a normal baseline LVEF [18]. Similarly, baseline GLS did not differ between patients who developed ICI myocarditis and those who did not [18]. Therefore, screening echocardiography is not recommended at baseline. However, in our opinion, baseline ECG, troponin, and echocardiography should be considered in patients with pre-existing CV disease and autoimmune disease and in those with a prior history of exposure to other cardiotoxic therapies.
Stress Cardiomyopathy
There have been several reports of takotsubo (stress induced) cardiomyopathy in patients receiving ICI therapy [13, 28]. In a small retrospective study of 30 patients with unselected ICI-related cardiotoxicity, 14% had a clinical presentation consistent with takotsubo cardiomyopathy [13]. The incidence of takotsubo cardiomyopathy is increased in patients treated with a combination of ICI and cytotoxic chemotherapy [28]. In contrast to ICI-associated myocarditis, the underlying pathophysiology of ICI-related takotsubo cardiomyopathy is believed to be non-inflammatory. While the diagnosis of takotsubo cardiomyopathy is usually based on the clinical presentation, ECG, cardiac biomarkers (troponin and NT-proBNP), and typical apical ballooning pattern on echocardiography, CMR can be helpful to exclude myocarditis [28]. Acute coronary syndrome and underlying obstructive coronary artery disease should also be ruled out as an underlying etiology.
Cessation of ICI therapy is the first step in the management of ICI-related takotsubo cardiomyopathy [13]. While there are case reports of improvement [28], the data is limited to recommend routine high-dose corticosteroid therapy. Guideline-directed heart failure therapy should be used in cases of cardiomyopathy [29]. QT-prolonging drugs should be avoided.
Pericardial Disease
Pericardial disease is the second most common ICI-associated cardiotoxicity. In an analysis of adverse drug reactions reported to the World Health Organization’s VigiBase database, ICI-related pericardial disease had a prevalence of 13.6% [30], and in a second retrospective study of ICI-related cardiotoxicity, 7% of cases had a pericardial effusion [13]. ICI-related pericardial disease has a reported mortality of 21% [31]. Pericarditis can occur in isolation or together with ICI-associated myocarditis (myopericarditis). Shortness of breath is the most common symptom of ICI-related pericardial disease [30]. Additional symptoms can range from precordial pain in the absence of pericardial effusion or jugular venous congestion and cardiogenic shock in cases of cardiac tamponade due to pericardial effusion [32]. Diagnostic evaluation of ICI-associated pericardial disease includes a detailed physical examination, ECG, and echocardiogram [30, 33]. Concomitant presence of ICI-elated myocarditis should be evaluated and CMR can aid in the assessment of myocardial involvement. Tissue pathology reveals lymphocytic infiltration and a fibrinous exudate [34]. Cancer-associated pericardial effusion represents an important alternative diagnosis and examination of pericardial fluid cytology may help differentiate the underlying pathology [32].
ICI therapy should be discontinued in all cases of pericarditis. Immunosuppressive therapy with high-dose corticosteroids can be given as initial therapy. Colchicine and nonsteroidal anti-inflammatory drugs may be used as adjunctive treatment [33]. In cases of steroid-refractory pericarditis, mycophenolate mofetil, infliximab, or anti-thymocyte globulin may be considered but lack systematic evidence [30, 33]. Complications like cardiac tamponade should be managed with pericardiocentesis, as per the guidelines [32].
Re-challenge with ICI
ICI-associated myocarditis is considered a high-grade adverse event and current guidelines recommend permanent discontinuation of ICI therapy [22]. However, patients with a milder course and complete cardiac recovery may benefit from continuing or restarting ICI therapy, especially if their cancer is responsive to ICI treatment. The decision to re-challenge a patient who has suffered ICI-associated myocarditis must be individualized and considered carefully by a multidisciplinary team. The various factors to consider include cancer status and alternative treatment options, severity of cardiotoxicity, regression of toxicity with immunosuppressive therapy, and patient preference. If the decision is made to re-challenge a patient, monotherapy with an alternative agent should be used. Systemic monitoring for cardiovascular symptoms, coupled with surveillance for asymptomatic disease with serial troponins and periodic cardiac imaging, is recommended [6]. Data regarding re-challenge with ICIs after the development ICI-associated myocarditis is very limited but retrospective studies suggest that an anti-PD-1 agent can be safely resumed after initially experiencing an irAE with an anti-CTLA-4 ICI [35].
As with ICI-associated myocarditis, there is limited data regarding the safety of re-challenging patients with ICI therapy in cases with ICI-related takotsubo cardiomyopathy or pericardial disease. Therefore, ICI re-challenge should be evaluated on a case-by-case basis after recovery of LV function or pericarditis and with intensified cardiac monitoring [28, 30, 33].
Long-term Sequelae of ICI Therapy
A recent multivariate analysis demonstrated a 3-fold higher risk of cardiovascular events after starting an ICI (HR, 3.3 [95% CI, 2.0–5.5]; P < 0.001) [36]. In this same study, 2.32% of patients had a cardiovascular event prior to starting an ICI, and 4.2% had a cardiovascular event after initiation of ICI. Imaging showed that the rate of progression of total aortic plaque volume was > 3-fold higher with ICIs (from 2.1%/year pre to 6.7%/year post) [36]. This data suggests that ICIs are associated with a higher rate of atherosclerosis and aortic plaque progression. Animal models have shown that the PD-1/PD-L1 pathway downregulates the proatherogenic T-cell response [37, 38]. Additionally, PD-1-deficient myeloid progenitors upregulate genes involved in cholesterol synthesis and uptake, and downregulate genes promoting cholesterol metabolism. This cumulatively leads to markedly increased cellular cholesterol levels [36]. Although these risks may not outweigh the cancer-related benefits, candidates for ICI therapy should undergo screening and modification of traditional CV risk factors and scrutiny for cardiac events during ICI treatment.
Impact of COVID-19
Current data suggests that patients with a history of cancer are at a higher risk of acquiring severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and patients with the novel coronavirus disease 2019 (COVID-19) are likely to experience a more severe course and higher mortality. This is especially true in patients with concomitant cancer and cardiovascular disease [39–41]. SARS-CoV-2 rarely infects cardiac myocytes and the predominant mechanism of myocardial injury is activation of the host inflammatory response with associated microvascular dysfunction and thrombosis. Myocardial autopsy specimens from COVID-19 patients demonstrate increased macrophage rather than T cell infiltration, without associated myocyte necrosis [42]. Thus, they do not meet the histopathologic criteria for myocarditis [43].
However, like ICI-associated myocarditis, the diagnosis of SARS-CoV-2 myocarditis is often made on the basis of elevated troponin and positive MRI findings. Hence, SARS-CoV-2 should be considered in the differential diagnosis of a patient with suspected ICI-associated myocarditis. During the COVID-19 pandemic, it is important to differentiate SARS-CoV-2 myocarditis from ICI-associated myocarditis to guide appropriate management and improve prognosis.
Conclusion
While ICI therapy has revolutionized outcomes for a wide variety of cancers, activation of the immune system by ICIs may lead to various irAEs. Although the overall incidence is rare, ICI cardiotoxicity, particularly myocarditis, is associated with significant morbidity and mortality, making it a major therapy-limiting adverse event. Early recognition and prompt treatment with cessation of ICI therapy and initiation of high-dose corticosteroids are crucial to improve outcomes in patients with ICI cardiotoxicity.
Declarations
Conflict of Interest
Rushin P. Patel, Rohan Parikh, Krishna S. Gunturu, Rana Zouveenoor Tariq, Sourbha S. Dani, and Sarju Ganatra declare no conflict of interest. Anju Nohria has received research funding from Amgen, and has received compensation for service as a consultant from Takeda Oncology, AstraZeneca, and Boehringer Ingelheim.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Footnotes
This article is part of the Topical Collection on Cardio-oncology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sarju Ganatra, Email: Sarju.ganatra@lahey.org.
Anju Nohria, Email: Anohria@bwh.harvard.edu.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 3.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 5.Ganatra S, Neilan TG. Immune checkpoint inhibitor-associated myocarditis. Oncologist. 2018;23(8):879–886. doi: 10.1634/theoncologist.2018-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganatra S, Parikh R, Neilan TG. Cardiotoxicity of immune therapy. Cardiol Clin. 2019;37(4):385–397. doi: 10.1016/j.ccl.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuben A, Petaccia de Macedo M, McQuade J, Joon A, Ren Z, Calderone T, et al. Comparative immunologic characterization of autoimmune giant cell myocarditis with ipilimumab. Oncoimmunology. 2017;6(12):e1361097. doi: 10.1080/2162402X.2017.1361097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Love VA, Grabie N, Duramad P, Stavrakis G, Sharpe A, Lichtman A. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res. 2007;101(3):248–257. doi: 10.1161/CIRCRESAHA.106.147124. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9(12):1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 12.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dal’bo N, Patel R, Parikh R, Shah SP, Guha A, Dani SS, et al. Cardiotoxicity of contemporary anticancer immunotherapy. Curr Treat Options Cardiovasc Med. 2020;22(12):62. doi: 10.1007/s11936-020-00867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136(21):2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 16.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg DD, Vaduganathan M, Nohria A, Davids MS, Alyea EP, Torre M, et al. Immune-related fulminant myocarditis in a patient receiving ipilimumab therapy for relapsed chronic myelomonocytic leukaemia. Eur J Heart Fail. 2017;19(5):682–685. doi: 10.1002/ejhf.806. [DOI] [PubMed] [Google Scholar]
- 18.Awadalla M, Mahmood SS, Groarke JD, Hassan MZO, Nohria A, Rokicki A, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. 2020;75(5):467–478. doi: 10.1016/j.jacc.2019.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41(18):1733–1743. doi: 10.1093/eurheartj/ehaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maisch B, Portig I, Ristic A, Hufnagel G, Pankuweit S. Definition of inflammatory cardiomyopathy (myocarditis): on the way to consensus. A status report. Herz. 2000;25(3):200–209. doi: 10.1007/s000590050007. [DOI] [PubMed] [Google Scholar]
- 22.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology. 2018;36(17):1714–1768. doi: 10.1200/jco.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141(24):2031–2034. doi: 10.1161/CIRCULATIONAHA.119.044703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon HJ, Cote TR, Cuffe MS, Kramer JM, Braun MM. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003;138(10):807–811. doi: 10.7326/0003-4819-138-10-200305200-00008. [DOI] [PubMed] [Google Scholar]
- 25.Cautela J, Zeriouh S, Gaubert M, Bonello L, Laine M, Peyrol M, et al. Intensified immunosuppressive therapy in patients with immune checkpoint inhibitor-induced myocarditis. J Immunother Cancer. 2020;8(2). 10.1136/jitc-2020-001887. [DOI] [PMC free article] [PubMed]
- 26.Esfahani K, Buhlaiga N, Thebault P, Lapointe R, Johnson NA, Miller WH., Jr Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380(24):2375–2376. doi: 10.1056/NEJMc1903064. [DOI] [PubMed] [Google Scholar]
- 27.Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380(24):2377–2379. doi: 10.1056/NEJMc1901677. [DOI] [PubMed] [Google Scholar]
- 28.Ederhy S, Cautela J, Ancedy Y, Escudier M, Thuny F, Cohen A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc Imaging. 2018;11(8):1187–1190. doi: 10.1016/j.jcmg.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955–1971. doi: 10.1016/j.jacc.2018.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Upadhrasta S, Elias H, Patel K, Zheng L. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis Transl Med. 2019;5(1):6–14. doi: 10.1016/j.cdtm.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–ee58. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 34.Altan M, Toki MI, Gettinger SN, Carvajal-Hausdorf DE, Zugazagoitia J, Sinard JH, et al. Immune checkpoint inhibitor-associated pericarditis. J Thorac Oncol. 2019;14(6):1102–1108. doi: 10.1016/j.jtho.2019.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250–255. doi: 10.1093/annonc/mdx642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020. 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed]
- 37.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 Checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutgens E, Seijkens TTP. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. Journal for immunotherapy of cancer. 2020;8(1):e000300. doi: 10.1136/jitc-2019-000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganatra S, Dani SS, Redd R, Rieger-Christ K, Patel R, Parikh R, et al. Outcomes of COVID-19 in patients with a history of cancer and comorbid cardiovascular disease. J Natl Compr Canc Netw. 2020:1–10. 10.6004/jnccn.2020.7658. [DOI] [PubMed]
- 40.Ganatra S, Hammond SP, Nohria A. The novel coronavirus disease (COVID-19) threat for patients with cardiovascular disease and cancer. JACC CardioOncol. 2020;2(2):350–355. doi: 10.1016/j.jaccao.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenihan D, Carver J, Porter C, Liu JE, Dent S, Thavendiranathan P, et al. Cardio-oncology care in the era of the coronavirus disease 2019 (COVID-19) pandemic: an International Cardio-Oncology Society (ICOS) statement. CA Cancer J Clin. 2020;70(6):480–504. doi: 10.3322/caac.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawakami R, Sakamoto A, Kawai K, Gianatti A, Pellegrini D, Nasr A, et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77(3):314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganatra S, Dani SS, Shah S, Asnani A, Neilan TG, Lenihan D, et al. Management of cardiovascular disease during coronavirus disease (COVID-19) pandemic. Trends Cardiovasc Med. 2020;30(6):315–325. doi: 10.1016/j.tcm.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]