Abstract
DNA double-strand breaks (DSBs) are implicated in various physiological processes, such as class-switch recombination or crossing-over during meiosis, but also present a threat to genome stability. Extensive evidence shows that DSBs are a primary source of chromosome translocations or deletions, making them a major cause of genomic instability, a driving force of many civilization diseases, such as cancer. Therefore, there is a great need for a precise, sensitive and universal method for DSB detection, to enable both studying mechanisms of their formation and repair as well as exploring their therapeutic potential. Here, we provide a detailed protocol for our recently developed ultra-sensitive and genome-wide DSB detection method i-BLESS, which relies on encapsulation of cells in agarose beads and labeling breaks directly and specifically with biotinylated linkers. i-BLESS labels DSBs with single-nucleotide resolution, allows detection of ultra-rare breaks, takes five days to complete and can be applied to samples from any organism, as long as sufficient amount of starting material can be obtained. We also describe how to combine i-BLESS with our qDSB-Seq approach enabling measuring absolute DSB frequencies per cell and their precise genomic coordinates at the same time. Normalization with qDSB-Seq is especially useful for evaluation of spontaneous DSB levels and estimations of DNA damage induced rather uniformly in the genome (e.g. caused by irradiation or radiomimetic chemotherapeutics).
EDITORIAL SUMMARY
This protocol describes a genome-wide approach for ultrasensitive and quantitative detection of DNA double-strand breaks (DSBs) relying on encapsulating cells in agarose beads and labeling breaks with biotinylated adapters.
Introduction
Though stability of the genome is crucial for proper functioning of a cell, DNA is constantly subjected to damage resulting from normal cellular processes, such as replication, meiosis, transcription or antibody diversification, as well as caused by exogenous factors like chemical mutagens or radiation1. Among DNA lesions, the most dangerous are DNA double-strand breaks (DSBs), which form when both strands of the DNA double helix are broken simultaneously in close proximity. DSBs are highly cytotoxic and their failed or incorrect repair can lead to chromosomal translocations2. Such vast genome rearrangements can in turn cause cell death or result in tumorigenesis. Notably, translocations are present in virtually all types of cancers and are responsible for about 20% of cancer-related morbidity3. Impaired DSB repair is also linked to developmental, immunological and neurological disorders, as well as aging4,5. On the other hand, DSB induction can be used as a medical treatment. For instance, promising techniques relying on precise genome cleavage by CRISPR/Cas nucleases6, zinc-finger nucleases7 or transcription activator-like effector nucleases8 may enable gene editing in the near future9. Moreover, induction of DSBs is presently a main anti-cancer therapeutic strategy, since cancer cells typically have defective DNA damage repair pathways10 and are thus more vulnerable than healthy cells to irradiation or chemotherapeutic drugs. Better understanding of mechanisms of DSB formation, sensing and repair is therefore extremely important, yet it requires precise tools for quantitative and genome-wide detection of DSBs.
Major efforts were recently invested in developing high-resolution and specific DSB labeling methods. Commonly used ChIP-seq with antibody against phosphorylated histone H2AX (γ-H2AX) is no longer recognized as a satisfactory DSB detection tool, due to its low resolution and increasing evidence that γ-H2AX is not a specific marker of DSBs11,12,13. Another popular method, damaged DNA immunoprecipitation (dDIP) and the terminal deoxynucleotidyl transferase (TdT) mediated dUTP-biotin end-labeling to capture DNA at break sites, was described by Leduc et al.14. Unfortunately, dDIP involves DNA extraction before DSB labeling that results in high levels of signal from artificial DNA damage. Moreover, this and other techniques based on TdT, such as DSB-Seq15 and BrITL16, cannot distinguish between single-strand and double-stand DNA breaks. Finally, methods widely used for meiotic DSB mapping, such as CC-seq17 or ChIP coupled with single-stranded DNA (ssDNA) sequencing18,19, label only breaks with characteristic features (i.e. covalently bound proteins or 3’ssDNA overhangs coated with meiotic recombination proteins, respectively) and therefore cannot be considered as unbiased. Similarly, another DSB labeling technique, Break-Seq, which relies on incorporation of biotinylated dATPs during blunting of breaks, cannot detect all types of DSBs, but only those with 5-prime overhangs20,21.
A major break-through in DSB labeling was achieved with the development of the BLESS (direct in situ Breaks Labeling, Enrichment on Streptavidin and next-generation Sequencing) method22. In BLESS, DSBs are labeled in situ by ligation of biotinylated and barcoded adapters, which enables direct, specific and genome-wide detection of breaks with single-nucleotide resolution. Application of the BLESS method has allowed detection of aphidicolin sensitive fragile sites22 and identification of off-target cutting sites of Cas9 endonuclease23. We also used it for studying DSB repair in the context of breaks mobility (i.e. increased motion of damaged sites within nucleus volume)24 and characterization of genome instability caused by loss of Ssb1 and Ssb2 proteins in mice25. However, BLESS data analysis was complicated due to high noise level26, which motivated the development of two improved techniques based on the BLESS protocol: DSBCapture27 and END-seq28. Independently, a very similar method called S1-seq29 was published. While these three methods use modified adapters that contain Illumina P5 and P7 sequences, therefore enabling sequencing without additional library preparation, DSBCapture requires multiple low speed centrifugations and thus, like BLESS, is only suitable for cells with relatively big nuclei (larger than ca. 4 μm in diameter). END-seq and S1-seq, on the other hand, use agarose plugs for cell encapsulation, which makes them in principle applicable to cells with nuclei of any size. While END-seq was only optimized for human and mouse cells28, S1-seq has been applied to both mouse and yeast cells29,30. However, usage of agarose plugs makes diffusion of reagents difficult, increasing reaction times and amount of reagents used. Moreover, though the S1 nuclease used in S1-Seq is capable of blunting very long overhangs, facilitating detection of considerably resected DNA ends, it can also transform the nicked DNA into DSBs31, resulting in unspecific labeling. Finally, another modification of BLESS called BLISS was recently proposed, in which DSBs are labeled with adapters containing a T7 promoter sequence, enabling linear amplification of tagged DNA by T7-mediated in vitro transcription32. This approach is especially useful for DSB detection in low-input samples, since it is less biased than exponential amplification by PCR, employed by other methods. This valuable technique, however, lacks proteinase K treatment before DSB labeling, which, as we previously established, is crucial for reducing the background signal observed in the original BLESS method20. Additionally, BLISS has been applied only to human and mouse cells.
To overcome these limitations, we have recently developed a new, improved version of BLESS method called immobilized BLESS (i-BLESS)20 (Fig. 1). In this approach, cells are immobilized in agarose beads, which are only about 100–300 μm in diameter, facilitating efficient diffusion of reagents. Use of agarose protects DNA from mechanical shearing, which, along with optimization of several protocol steps, allowed us to remarkably reduce noise and increase the sensitivity of the method20. Currently, i-BLESS is the most sensitive DSB labeling technique, enabling detection of one break at a given position in 100,000 cells20. i-BLESS was optimized for budding yeast cells and remains the only genome-wide and unbiased DSB labeling method for this model organism. Moreover, i-BLESS can be used for cells from any organism, regardless of their size, and has already been successfully applied to human cells33. We have also developed an approach called qDSB-Seq34, which enables quantification of absolute DSB numbers per cell and thus normalization between samples, when combined with i-BLESS or with another DSB labeling method. In this universal technique that can be combined with any ligation-based DSB detection method, spike-in DSBs are introduced and labeled together with studied DSBs. Next, their cutting frequency is established by qPCR or, more commonly, genomic DNA (gDNA) sequencing, in order to establish the ratio between numbers of i-BLESS reads and breaks frequency at enzyme cutting sites. This ratio can then be used to infer average frequency of studied DSBs per cell. Using i-BLESS and qDSB-Seq, we identified I-SceI off-target cleavage sites20, extending the repertoire of uncanonical I-SceI sites and redefining the common sequence motif recognized by this endonuclease35. We have also studied ultra-rare DSBs forming at G-quadruplex (G4) sites in untreated wild type yeast cells and discovered that G4s with shorter loops are more fragile and that, contrary to previous assumptions, G4s with loops longer than 4 nt are prone to breakage. Moreover, we quantified DSBs introduced by a radiomimetic drug Zeocin and those forming at replication fork barriers, discovering a camptothecin-dependent replication fork barrier34.
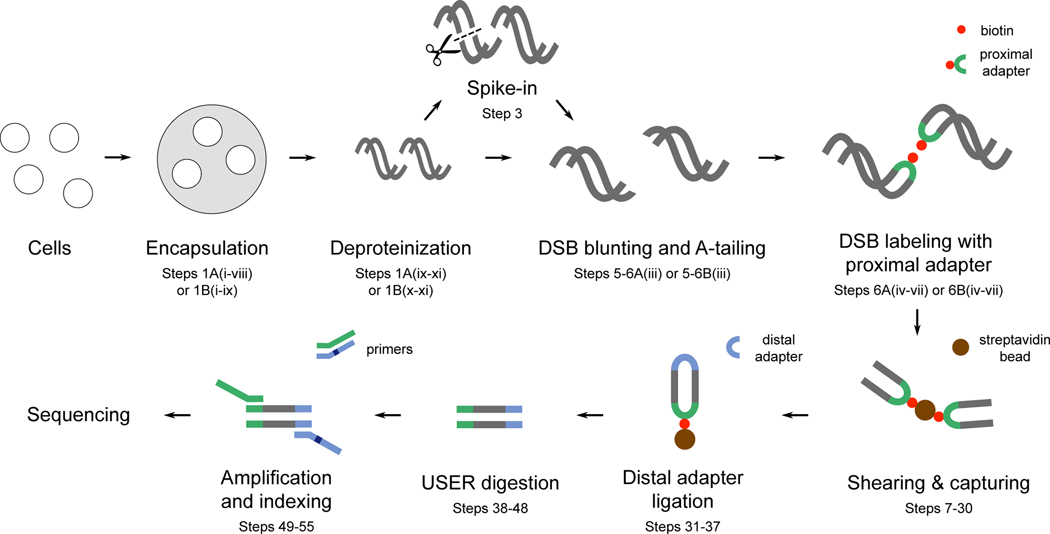
Fig. 1 |. Overview of the i-BLESS method.
i-BLESS workflow. Briefly, cells are encapsulated in agarose beads, lysed and deproteinized. Optionally, spike-in DSBs can be introduced by a restriction enzyme to quantify DSBs using the qDSB-Seq method. Next DSBs are blunted, A-tailed, labeled with a biotinylated adapter (proximal) and captured on streptavidin. The free ends of DNA fragments are ligated to the second adapter (distal), and the resulting fragments are linearized by USER digestion, amplified, and sequenced. This figure is based on a figure published previously 20.
In addition to methods relying on adapter ligation to DSBs, several approaches based on the ability of DSBs to translocate have been developed, such as GUIDE-seq36, LAM-HTGTS26 or IDLV37. These techniques use so called “bait DSBs” which are joined with studied DSBs by DNA repair mechanisms. Integration takes place in living cells, which requires cell transfection with appropriate “bait” – double stranded oligodeoxynucleotides in GUIDE-seq, integrase-defective lentiviral vector in IDLV, and a cassette with an I-SceI recognition sequence in LAM-HTGTS. This requirement limits the application of these methods to cells in which transfection can be conducted, therefore precluding studying DSBs in clinical samples, apart from the fact that cell transfection is itself a challenging process. Moreover, bait-based techniques can only detect DSBs capable of joining “bait DSBs” via non-homologous end joining. However, we have recently reported that the choice of DNA repair pathway, and thus the ability to translocate, depends on the chromatin environment around a given DSB24. Lastly, these methods detect all DSBs that were translocated over an extended period of time, rather than labeling those present in cells at a given moment, which, depending on the studied problem, may be an advantage or a disadvantage. For instance, such an approach would be unsuitable for a time-course experiment, making it impossible to e.g. study dynamics of DSB repair, as done by us previously using BLESS24.
Applications of i-BLESS
i-BLESS is currently the only unbiased method enabling specific and genome-wide DSB detection with single-nucleotide resolution and ultra-high sensitivity in budding yeast cells. Usage of Saccharomyces cerevisiae allows obtaining insights relevant to human biology, as in spite of different cell and genome size, many fundamental cellular processes in this model organism are analogous to those occurring in human cells. Notably, one third of yeast genes have homologs in human36, and 20% of human disease-related genes have functional equivalents in yeast38,39. Moreover, due to availability of extensive annotations for the S. cerevisiae genome, inexpensive and fast culture, and easy genetic manipulations, budding yeast is a powerful system for studying mechanisms of genomic instability. Importantly, i-BLESS is a universal DSB-labeling method, in principle applicable to all types of cells, regardless of their size (assuming they can be dissociated into single cells) and already successfully applied to human33. Below, we provide some recommendations on how to adapt the protocol to various model organisms. Thanks to usage of agarose immobilization, DSBs are labeled in all the DNA present in the cell, including mitochondrial DNA (mtDNA) (unlike in methods based on nuclear extraction, such as BLESS or DSBCapture), providing a unique opportunity to study breaks occurring in mitochondria.
i-BLESS can be used both to investigate the role of various proteins in maintenance of genomic integrity and to determine the influence of chemical and physical mutagens on genome stability. The single-nucleotide resolution of our method enables not only precise determination of break sites, but also discerning between different mechanisms of breaks formation based on DSB patterns40. By combining i-BLESS with qDSB-Seq, it is possible to quantify DSBs induced by different doses of drugs, including chemotherapeutics, in healthy or affected cells, which could be potentially used in development of new therapies.
The extraordinary sensitivity of i-BLESS enables detection of ultra-rare breaks, e.g. we were able to identify DSBs occurring naturally at G4 structures in yeast. Using qDSB-Seq, we estimated that these breaks occur with an average frequency of 4 DSBs per 1,000 cells for all G4 sites and 2 DSBs in 100,000 cells at individual loci, making them undetectable by any other DSB labeling method. Such high sensitivity makes i-BLESS particularly useful for identification of endonuclease off-target cleavage sites. In case of clinical application of genome-editing nucleases such as CRISPR/Cas9, given the large size of cell population that would be subjected to modifications, it is extremely important to exclude any possibility of even very rare off-target genome alterations, to prevent oncogenic transformation36. Our method could therefore serve for development and validation of engineered nucleases.
Our protocol can be divided into three parts: cell immobilization (Steps 1–2), qDSB-Seq (Step 3), and i-BLESS labeling (Steps 4–55). With certain modifications, each part can be combined with other methods to improve their performance, enable samples quantification or label different types of DNA damage in parallel, e.g. single-strand breaks (SSBs). Steps describing embedding of cells in agarose beads can be used with both SSB detection (e.g. GLOE-seq41) and DSB detection (e.g. END-seq, S1-seq, BLISS) techniques. Such application would require modification of these methods to be compatible with usage of agarose beads (i.e. increased reaction volumes, centrifugation conditions, addition of washing steps). The i-BLESS protocol could serve as a guidance for such adaptations. qDSB-Seq steps can be introduced into DSB labeling protocols that do not employ agarose beads, however, intensive proteinase K treatment prior to spike-in induction is required in order to make the DNA accessible to restriction enzymes. Such deproteinization is possible only when some immobilization technique is applied, as otherwise unprotected DNA is prone to mechanical damage, resulting in artifactual DSBs. If an approach different from agarose beads encapsulation is used, the exact conditions of spike-in induction must be established de novo.
Limitations of the method
The major limitation of i-BLESS is the requirement for a relatively high number of cells, e.g. 2.5×109 yeast cells or 1.0×107 human cells. While obtaining such number of cells from cell culture is relatively easy, in case of some tissues, clinical samples or time-course experiments it can be challenging or even impossible. To adapt the protocol to a lower amount of starting cells, proportionally smaller amounts of each reagent would need to be used during the agarose bead encapsulation step. Our standard protocol was tested successfully for encapsulation of 0.5 mL cell suspension (containing 2.5×108 yeast cells) mixed with 0.5 mL agarose and 2 mL of liquid paraffin. For smaller volumes, ensuring proper stirring can be challenging and would therefore require optimization. Additionally, the number of encapsulated cells needs to be sufficient to obtain at least 1 μg of DNA after isolation from agarose, to provide enough material for generation of a good quality library in the subsequent steps. To establish the minimal number of cells needed for recovery of 1 μg of labeled DNA, the efficiency of isolation for a given experimental setup can be determined by performing Steps 1–26 of the protocol using TE buffer for washes and incubations in Steps 3–6. I-BLESS also shares the requirements of all direct DSB labeling methods for fresh or fixed cells stored for a short time, as longer storage or freezing of cells could result in artifactual fragmentation of DNA.
Encapsulation of DNA in agarose substantially increases the volume of a sample, resulting in two disadvantages. First, costs of the procedure are raised as larger volumes of buffers are used. Nevertheless, thanks to efficient diffusion in agarose beads, the amounts of enzymes used are equal or slightly higher than those recommended by the manufacturer thus the increase of overall costs is moderate. Secondly, the increased volume of reactions requires dividing samples into multiple tubes, which hinders preparation of many samples at the same time. We recommend handling no more than 8 yeast or 16 human samples simultaneously (both corresponding to 48 tubes; due to lower volume, more human samples can be processed at the same time).
It should be also noted that the obtained DSB data is population-averaged and might not represent the pattern of DSBs in individual cells, both in terms of their locations and numbers. For example, presence of apoptotic cells in the analyzed cell population can result in considerable data distortion. A partial solution to this challenge is complementing DSB sequencing with methods that provide a distribution of DSBs in a cell population, as described elsewhere40.
Lastly, DSBs occurring in living cells are subjected to various processes involved in their repair, including end resection, that can create long (up to 10 kb42) 3’ overhangs. Such overhangs are shortened during the ends blunting step of the procedure, resulting in detection of break signal some distance from the actual damage site. This problem can be solved by employment of mutants lacking proteins essential for end resection or usage of chemicals hindering resection (e.g. mirin43). However, as there are multiple independent pathways involved in breaks resection, it might be impossible to fully inhibit this process by either of the proposed methods. Alternatively, resection can be modelled using available data28,29,44, but ideally should be based on a time-course labeling of DSBs induced in vivo at defined regions of the genome (e.g. using DIvA system45) in cycling cells. It should be also noted that the maximum size of a resected DNA end that can be blunted using T4 DNA polymerase under conditions proposed in our protocol has not been determined yet, while S1-seq and END-seq are capable of labeling 3’overhangs that are up to 2 kb and 5 kb long, respectively28,29.
Experimental design
Organism-specific modifications
The i-BLESS protocol is composed of ten main parts (two of which are optional) and takes approximately 31 working hours over 5 days to complete. Below we provide detailed protocols for human and yeast cells, but i-BLESS can be applied to cells and tissues from any organism, provided they can be dissociated into single cells and that a sufficient number of cells is obtained. For multicellular organisms researchers should use verified protocols for the isolation of a given cell type (for tissue dissociation protocols see e.g. 46–48); if sufficient number of cells cannot be obtained, appropriate cell lines should be used (e.g. A6 or XLK-WG for Xenopus laevis, At7 for Arabidopsis thaliana and a variety of cell lines for Drosophila melanogaster). In case of organisms with genome and cell size comparable to human, such as Mus musculus or Xenopus laevis, the protocol variant for human cells should be used, while in case of organisms with genome and cell size comparable to or smaller than yeast, we recommend using the workflow for yeast cells. Additionally, in case of cells surrounded by a cell wall, its digestion needs to be performed as in the yeast version of the protocol. More detailed recommendations on protocol modifications for selected model organisms can be found in Table 1.
Table 1.
Recommendations on protocol modifications for selected model organisms
| Organism name | Amount of starting material | Genome size | Method variant | Needed modifications |
|---|---|---|---|---|
| Homo sapiens | 107 cells | 3.1 Gb | human | None |
| Mus musculus | 107 cells | 2.6 Gb | human | None |
| Xenopus laevis | 107 cells | 3.1 Gb | human | None |
| Arabidopsis thaliana | 2.5×108 cells | 136 Mb | human | After Step 1A(viii) use cellulase-macerozyme solution53 to create protoplasts, then proceed to Step 1A(x) |
| Drosophila melanogaster | 2.5×108 cells | 139.5 Mb | human | None |
| Saccharomyces cerevisiae | 2.5×109 cells | 12 Mb | yeast | None |
| Schizosaccharomyces pombe | 2.5×109 cells | 14 Mb | yeast | None |
| Escherichia coli | 7.5×109 cells | 4.6 Mb | yeast | In Step 1A(ix) use lysozyme instead of lyticase (the exact reaction conditions need to be established, start with amount of enzyme and time of treatment used in your laboratory for standard applications) |
Cell harvesting
The starting material for i-BLESS is freshly collected cells. Yeast cells can be fixed and stored up to a week, if DSB labeling cannot be conducted immediately after their induction. For human cells we do not recommend cell fixation, as it makes encapsulation in agarose less efficient. The number of cells depends on the genome size of a studied organism, as the starting amount of DNA in cells should be around 50 μg (recommendations on amount of starting material for selected model organisms can be found in Table 1). Cells should be collected in the gentlest possible way to avoid mechanical damage of DNA; in case of adherent cell lines enzymatic treatment should be used instead of scraping. After collection, the fraction of apoptotic cells needs to be determined (e.g. using Trypan Blue), and if it is higher than 5%, apoptotic cells should be removed (e.g. by low speed centrifugation), unless the intention is to study apoptosis, as signal from apoptotic cells obscures the data40. Alternatively, it can be computationally separated from the signal from cells of interest, as we discuss elsewhere40. Collected cells should be placed on ice and immediately subjected to encapsulation in agarose. However, cells cannot be frozen at any time, as ice crystals forming during freezing could lead to mechanical shearing and fragmentation of DNA. For each studied condition, a proper control needs to be prepared (i.e. negative and/or vehicle controls), along with at least two biological replicates, to ensure meaningful statistical analysis.
Cell encapsulation and lysis
Immediately after harvest, cells should be subjected to encapsulation in agarose (Supplementary Movie 1). Low melting point agarose should be melted and equilibrated at 40°C together with liquid paraffin in advance, to allow efficient processing of the samples. Once the agarose beads are prepared, they should be examined under a microscope to evaluate proper immobilization. Encapsulation can be considered successful if most of the beads contain multiple cells (cells should occupy the majority of a given bead volume) and only a few cells are present outside of beads (see Fig. 2a,c and Fig. 2b, d for examples of proper and improper encapsulation, respectively). After encapsulation, cells with a cell wall require its lysis by an appropriate enzyme, e.g. lyticase in case of yeast. Progress of digestion should be monitored under a microscope, to ensure that most cells have turned into spheroplasts or protoplasts (Fig. 3a). Lastly, cells are lysed and proteins are removed by an intensive proteinase K treatment, which drastically reduces noise level in DSB sequencing data20. The efficiency of lysis and protein removal needs to be checked by examining beads mixed with a DNA staining dye (e.g. DAPI); proper lysis should lead to even dispersion of DNA within beads (see Fig. 3b-c and Fig. 3d-e for examples of proper and improper lysis, respectively).
Fig. 2 |. Encapsulation of cells in agarose beads.
(a-b) Examples of (a) proper and (b) improper encapsulation of yeast cells in agarose beads. (c-d) Examples of (c) proper and (d) improper encapsulation of LN18 human cells in agarose beads (upper row: phase contrast, lower row: DAPI staining). While proper encapsulation results in the presence of multiple cells in a bead with cells occupying most of the bead volume, improperly prepared beads contain only few cells. Scale bars denote 10 μm.
Fig. 3 |. Spheroplasting and lysis.
(a) Examples of proper cell wall digestion of encapsulated yeast cells. (b-c) Examples of DAPI-stained (b) yeast and (c) human chromatin after proper cell lysis and deproteinization. Proper lysis leads to even dispersion of DNA within beads. (d-e) Examples of DAPI-stained (d) yeast and (e) human chromatin after improper cell lysis and deproteinization. Scale bars denote 10 μm.
Spike-in DSB induction (qDSB-Seq DSB quantification)
After lysis and protein removal, samples can be subjected to induction of spike-in DSBs by a restriction enzyme, which allows normalization and quantification of DSBs in the studied samples using our qDSB-Seq approach34. We highly recommend including this step, as otherwise one can only determine DSB hotspots in the genome, but gains no information about differences in DSB level between studied conditions and control. To avoid an excessive number of sequencing reads generated by spike-in DSBs, we advise to use rare-cutting enzymes (i.e. those with at least 8 bp recognition sequences), such as NotI and SgrDI for yeast and human cells, respectively. It is also important to ensure that the conditions of the digestion reaction are such that the cutting efficiency is within the recommended range of 12–62 %, since in our experience this efficiency leads to a nearly constant ratio of the number of labeled reads per DSB, which is required for accurate quantification34. For NotI and SgrDI enzymes, we provide digestion conditions (time of incubation and enzyme concentration) that lead to optimal cutting efficiency, however, if other enzymes are used, these conditions must be determined empirically. This can be done by performing Steps 1–3 and 7–25, and sequencing thus obtained gDNA or using it for qPCR reaction (for more information on how to calculate cutting efficiency using these methods, see Box 2 and 34).
Repair of “dirty” ends
Some DSB inductors, i.e. ionizing radiation or radiomimetic compounds (e.g. bleomycin or neocarzinostatin) generate so called “dirty” DSB ends, which contain chemically modified DNA ends, for example 5’-hydroxyl, 3’-phosphate or 3’-phosphoglycolate termini49. While 5’-hydroxyl ends are phosphorylated by T4 Polynucleotide Kinase in the next step (DNA ends blunting), DSBs with modified 3’ ends cannot be blunted or ligated, precluding their labeling by i-BLESS. When a substantial level of “dirty” ends is expected, we highly recommend performing “dirty” end repair by NEBNext® FFPE DNA Repair Mix, which repairs blocked 3´ ends. It should be noted, however, that some mutations or treatments might result in the formation of unusual ends, such as 2′,3′-cyclic phosphate ends occurring in RNase H2-deficient cells50, which cannot be repaired by this approach. Such breaks would therefore not be labeled by i-BLESS unless an alternative solution for removal of end modifications is found.
DNA ends blunting and labeling
Next, DNA ends are blunted and dA-tailed. Thorough washing between these two steps is necessary to prevent dA-tailed termini from blunting by residual T4 DNA polymerase, as usage of agarose beads precludes enzyme thermal inactivation (it requires incubation at 65°C, which would result in melting of agarose beads). After that, DSBs are ligated to a biotinylated proximal adapter (P) by T4 DNA ligase, which can only ligate dsDNA, ensuring specificity of labeling. Adapter P has a hairpin structure, which prevents concatemer formation, and contains the Illumina P5 sequence. For first-time users, or in case of application of the protocol to an organism other than yeast or cultured human cells, it is recommended to include ligation controls (without adapter P and T4 DNA ligase) to confirm the proper working of the protocol.
DNA isolation
After the DSBs are labeled, agarose beads are subjected to proteinase K treatment in order to remove residual proteins and T4 DNA ligase (to prevent its activity during DNA fragmentation). Next, the encapsulated DNA is subjected to initial sonication. Though sonication of DNA embedded in agarose is less effective and precise than sonicating purified DNA, it generates DNA fragments below 10 kb, allowing for their more efficient recovery from agarose. Subsequently, DNA is purified from agarose using Zymoclean Large Fragment DNA Recovery Kit and is again sonicated to create fragments with an average length of 300–400 bp, which is within the optimal range for next generation sequencing on Illumina platforms. It is critical to ensure a proper size distribution of fragments at this step, since there is no more shearing or size-selection during the further steps of the protocol. We highly recommend random DNA fragmentation (for instance by sonication) to avoid bias caused by nonrandom enzymatic fragmentation.
Distal adapter ligation
After gDNA sonication, the labeled fragments are captured on streptavidin, increasing the specificity of i-BLESS. Distal ends of captured fragments are blunted, A-tailed and ligated to the distal adapter (D) containing the Illumina P7 sequence. As for ligation with adapter P, for first-time users or in case of application of the protocol to another organism, we recommend including ligation controls (without adapter D and T4 DNA ligase).
USER digestion and amplification of labeled fragments
Next, the resulting fragments are released from streptavidin beads and linearized by digestion with USER enzyme. The library is indexed and amplified with 15–20 cycles of PCR; the optimal number of cycles should be determined empirically for a given set of samples. We recommend preparing a negative PCR control with water instead of template DNA to ensure that reagents are not contaminated and to exclude the possibility of unspecific product formation. Lastly, the quality of the library is checked using Agilent Bioanalyzer or Fragment Analyzer and its quantity is determined using Qubit 2.0 or later. An example of a high-quality library is presented on Fig. 4a. For next generation sequencing on Illumina platforms, one should prepare at least 10 μl of 4 nM library of fragments within the range of 200–800 bp in length. The library that passes these requirements can be sequenced on an appropriate Illumina sequencer.
Fig. 4 |. Anticipated results.
(a) Examples of DNA fragments size distribution in good quality libraries prepared from yeast (left) and human (right) cells. (b-d) Examples of DSB mapping with i-BLESS. (b) i-BLESS signal normalized to 1×106 total reads for mec1–1 cells treated with hydroxyurea (200 mM HU for 1 h), and untreated and Zeocin-treated (100 μg mL−1 for 1 h) wt yeast cells, shown for a representative fragment of chromosome XI. Reads were processed using our computational pipeline and mapped to the yeast reference genome (sacCer3). Dotted vertical lines mark replication origins. (c) The qDSB-Seq method allows establishing correspondence between the frequency of DSBs at a given position in the genome (here enzyme cutting efficiency), and the number of i-BLESS reads mapped to this location. Even though the ratio between DSB frequency and number of reads is experiment-specific (note the difference between left and right chart), as long as the qDSB-Seq protocol recommendations are followed, the proportion remains remarkably stable (correlation coefficient R>0.9) between different locations in the genome and different break frequencies, thus allowing for unbiased DSB quantification based on read counts. (d) The same results as in panel b, for untreated and Zeocin-treated wt yeast cells, but normalized using the qDSB-Seq approach with NotI spike-in. Data from (b-d) have been published previously20,34.
If spike-in DSBs were induced, gDNA from a given sample (obtained after second sonication) should be sequenced along with the i-BLESS library, to determine enzyme cutting efficiency at its individual recognition sites. This enables calculating the ratio between read number and break frequency that is used for DSB quantification. As gDNA is isolated after DSB labeling and some of the fragments already contain the proximal adapter with Illumina P5 sequence, it needs to be removed by SapI treatment prior to library construction. Then the library can be generated using standard commercial kits (e.g. ThruPLEX DNA-seq Kit, Rubicon Genomics). Since induction of spike-in DSBs by a restriction enzyme can result in an unbalanced i-BLESS library (i.e. with low initial sequence diversity), for next generation sequencing on Illumina platforms we highly recommend pooling both i-BLESS and gDNA libraries on the same lane, according to Illumina guidance for index pooling and following our modified protocols for generation of high-quality sequencing data from low-diversity samples51.
Materials
Biological materials for yeast i-BLESS
Approximately 2.5×109 yeast cells in logarithmic phase.
CRITICAL Single cell suspension is needed. Cells need to be collected without disrupting their structure, i.e. using small centrifugation forces, up to 700 g. After collection, cells can be fixed with 2% formaldehyde for 5 min, followed by quenching with 125 mM glycine for 5 min, and stored at 4°C for up to one week, or immediately subjected to i-BLESS procedure. At no time cells can be frozen.
Biological materials for mammalian/higher eukaryote i-BLESS
1.2×107 cells from human or mouse cell lines or tissues. The basic protocol has been tested with HEK293 (CLS Cat# 300192/p777_HEK293, https://scicrunch.org/resolver/CVCL_0045), HeLa (CLS Cat# 300194/p772_HeLa, https://scicrunch.org/resolver/CVCL_0030), LN18 (ATCC Cat# CRL-2610, https://scicrunch.org/resolver/CVCL_0392) and GM19239 (Coriell Cat# GM19239, https://scicrunch.org/resolver/CVCL_9634) cell lines.
CRITICAL Single cell suspension is needed. Cells need to be collected without disrupting their structure, e.g. by trypsinization, not by cell scraping. After collection cells should be immediately subjected to the i-BLESS procedure. At no time cells can be frozen.
!CAUTION All cell lines must be regularly checked for mycoplasma contamination.
Common reagents
!CAUTION Wear protective clothing and gloves when handling any reagent listed in this protocol.
Sodium chloride (NaCl, reagent grade, BioShop, cat. no. SOD001.5)
EDTA solution (0.5 M, pH 8.0, Invitrogen, cat. no. AM9261)
TRIS Base (BioShop, cat. no. TRS001.5)
Magnesium chloride solution (MgCl2, 1 M, molecular biology grade, Sigma-Aldrich, cat. no. M1028–100ML)
Dithiothreitol (DTT, 1 M, molecular biology grade, Sigma-Aldrich, cat. no. 43816–10ML)
Tris (1 M, pH 8.5, BioShop, cat. no. TRS333.500)
-
N-Lauroylsarcosine sodium salt (BioShop, cat. no. SLS002.100)
!CAUTION This reagent is flammable, harmful if swallowed, causes skin irritation and serious eye irritation, and may cause respiratory irritation. Keep away from heat and open flames, use eye and face protection, use in a well-ventilated area.
Agarose (low melting point, analytical grade, Prona, cat. no. RN100)
Liquid paraffin (Merck, cat. no. 107160)
Proteinase K (20 mg/mL, RNA grade, Invitrogen, cat. no. 25530049)
-
PMSF (BioShop, cat. no. PMSF123.25)
!CAUTION This reagent is toxic if swallowed and causes severe skin burns and eye damage. Avoid breathing dust, use eye and face protection.
-
Isopropanol (BioShop, cat. no. ISO920.1)
!CAUTION This reagent is highly flammable, causes serious eye irritation, and may cause drowsiness or dizziness. Keep away from heat and open flames. Keep container tightly closed. Avoid inhalation, use only outdoors or in a well-ventilated area, wear eye and face protection.
NEBNext® FFPE DNA Repair Mix (New England Biolabs, cat.no. M6630L)
Quick Blunting Kit (New England Biolabs, cat. no. E1201L)
BSA (20 mg/mL, New England Biolabs, cat. no. B9000S)
NEBNext® dA-Tailing Module (New England Biolabs, cat.no. E6053L)
NEBNext® dA-Tailing Reaction Buffer (New England Biolabs, cat. no. B6059S)
T4 DNA Ligase (New England Biolabs, cat. no. M0202L)
T4 DNA Ligase Reaction Buffer (New England Biolabs, cat. no. B0202S)
Zymoclean™ Large Fragment DNA Recovery Kit (Zymo Research, cat. no. D4046)
-
Agencourt AMPure XP beads (Beckman Coulter, cat. no. A63881)
CRITICAL AMPure XP beads are stored at 4°C and must be brought to room temperature (RT: 20–25°C) before use.
-
High Sensitivity DNA Kit (Agilent, cat. no. 5067–4626)
CRITICAL High Sensitivity DNA reagents are stored at 4°C and must be brought to RT before use.
Qubit dsDNA HS Assay Kit (Invitrogen, cat. no. Q32851)
Dynabeads™ MyOne™ Streptavidin C1 (Invitrogen, cat. no. 65001)
-
Triton X-100 (BioShop, cat. no. TRX506.500)
!CAUTION This reagent is highly flammable, causes serious eye irritation, and may cause drowsiness or dizziness. Keep away from heat and open flames. Keep container tightly closed. Avoid inhalation, use only outdoors or in a well-ventilated area, wear eye and face protection.
NEBNext® Ultra™ End Repair/dA-Tailing Module (New England Biolabs, cat. no. E7442L)
USER® Enzyme (New England Biolabs, cat. no. M5505L)
Phusion High-Fidelity DNA Polymerase (Thermo Fisher, cat. no. F530S)
dNTP mix (10 mM each, Invitrogen, cat. no. 18427089)
-
99.8% (vol/vol) Ethanol (POCH, cat. no. BA6480111)
!CAUTION This reagent is highly flammable. Keep away from heat and open flames, use in a well-ventilated area.
Nuclease free water (Applied Biosystems, cat.no. AM9937)
Table 2.
Adapter sequences
| Name | Sequence (5’→3’) |
|---|---|
| Adapter P | P-GATCGGAAGAGCGTCGTGTAGGGAAAGAGTGTUUU[btn T]UUUACACTCTTTCCC TACACGACGCTCTTCCGATC*T |
| Adapter D | P-GATCGGAAGAGCACACGTCTGAACTCCAGTCACUUUUUUUGTGACTGGAGTTCA GACGTGTGCTCTTCCGATC*T |
[btn T] - Biotin-dT
- phosphorothioate bond
Table 3.
Primer sequences
| Name | Sequence (5’→3’) | Index name | Index sequence |
|---|---|---|---|
| P5 | AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGAC | ||
| P7–1 | CAAGCAGAAGACGGCATACGAGATAACGTGATGTGACTGGAGT TCAGACG*T | 1 | AACGTGAT |
| P7–2 | CAAGCAGAAGACGGCATACGAGATAAACATCGGTGACTGGAGT TCAGACG*T | 2 | AAACATCG |
| P7–3 | CAAGCAGAAGACGGCATACGAGATATGCCTAAGTGACTGGAGT TCAGACG*T | 3 | ATGCCTAA |
| P7–4 | CAAGCAGAAGACGGCATACGAGATAGTGGTCAGTGACTGGAGT TCAGACG*T | 4 | AGTGGTCA |
| P7–5 | CAAGCAGAAGACGGCATACGAGATACCACTGTGTGACTGGAGT TCAGACG*T | 5 | ACCACTGT |
| P7–6 | CAAGCAGAAGACGGCATACGAGATACATTGGCGTGACTGGAGT TCAGACG*T | 6 | ACATTGGC |
| P7–7 | CAAGCAGAAGACGGCATACGAGATCAGATCTGGTGACTGGAGT TCAGACG*T | 7 | CAGATCTG |
| P7–8 | CAAGCAGAAGACGGCATACGAGATCATCAAGTGTGACTGGAGT TCAGACG*T | 8 | CATCAAGT |
| P7–9 | CAAGCAGAAGACGGCATACGAGATCGCTGATCGTGACTGGAGT TCAGACG*T | 9 | CGCTGATC |
| P7–10 | CAAGCAGAAGACGGCATACGAGATACAAGCTAGTGACTGGAGT TCAGACG*T | 10 | ACAAGCTA |
| P7–11 | CAAGCAGAAGACGGCATACGAGATCTGTAGCCGTGACTGGAGT TCAGACG*T | 11 | CTGTAGCC |
| P7–12 | CAAGCAGAAGACGGCATACGAGATAGTACAAGGTGACTGGAGT TCAGACG*T | 12 | AGTACAAG |
| P7–13 | CAAGCAGAAGACGGCATACGAGATAACAACCAGTGACTGGAGT TCAGACG*T | 13 | AACAACCA |
| P7–14 | CAAGCAGAAGACGGCATACGAGATAACCGAGAGTGACTGGAG TTCAGACG*T | 14 | AACCGAGA |
| P7–15 | CAAGCAGAAGACGGCATACGAGATAACGCTTAGTGACTGGAGT TCAGACG*T | 15 | AACGCTTA |
| P7–16 | CAAGCAGAAGACGGCATACGAGATAAGACGGAGTGACTGGAG TTCAGACG*T | 16 | AAGACGGA |
| P7–17 | CAAGCAGAAGACGGCATACGAGATAAGGTACAGTGACTGGAGT TCAGACG*T | 17 | AAGGTACA |
| P7–18 | CAAGCAGAAGACGGCATACGAGATACACAGAAGTGACTGGAG TTCAGACG*T | 18 | ACACAGAA |
| P7–19 | CAAGCAGAAGACGGCATACGAGATACAGCAGAGTGACTGGAG TTCAGACG*T | 19 | ACAGCAGA |
| P7–20 | CAAGCAGAAGACGGCATACGAGATACCTCCAAGTGACTGGAGT TCAGACG*T | 20 | ACCTCCAA |
| P7–21 | CAAGCAGAAGACGGCATACGAGATACGCTCGAGTGACTGGAGT TCAGACG*T | 21 | ACGCTCGA |
| P7–22 | CAAGCAGAAGACGGCATACGAGATACGTATCAGTGACTGGAGT TCAGACG*T | 22 | ACGTATCA |
| P7–23 | CAAGCAGAAGACGGCATACGAGATGGTAGCACGTGACTGGAGT TCAGACG*T | 23 | GGTAGCAC |
| P7–24 | CAAGCAGAAGACGGCATACGAGATGTCCAACCGTGACTGGAGT TCAGACG*T | 24 | GTCCAACC |
| P7–25 | CAAGCAGAAGACGGCATACGAGATAGATCGCAGTGACTGGAGT TCAGACG*T | 25 | AGATCGCA |
| P7–26 | CAAGCAGAAGACGGCATACGAGATAGCAGGAAGTGACTGGAG TTCAGACG*T | 26 | AGCAGGAA |
| P7–27 | CAAGCAGAAGACGGCATACGAGATAGTCACTAGTGACTGGAGT TCAGACG*T | 27 | AGTCACTA |
| P7–28 | CAAGCAGAAGACGGCATACGAGATATCCTGTAGTGACTGGAGT TCAGACG*T | 28 | ATCCTGTA |
| P7–29 | CAAGCAGAAGACGGCATACGAGATATTGAGGAGTGACTGGAGT TCAGACG*T | 29 | ATTGAGGA |
| P7–30 | CAAGCAGAAGACGGCATACGAGATCAACCACAGTGACTGGAGT TCAGACG*T | 30 | CAACCACA |
| P7–31 | CAAGCAGAAGACGGCATACGAGATCAAGACTAGTGACTGGAGT TCAGACG*T | 31 | CAAGACTA |
| P7–32 | CAAGCAGAAGACGGCATACGAGATCAATGGAAGTGACTGGAGT TCAGACG*T | 32 | CAATGGAA |
| P7–33 | CAAGCAGAAGACGGCATACGAGATCACTTCGAGTGACTGGAGT TCAGACG*T | 33 | CACTTCGA |
| P7–34 | CAAGCAGAAGACGGCATACGAGATCAGCGTTAGTGACTGGAGT TCAGACG*T | 34 | CAGCGTTA |
| P7–35 | CAAGCAGAAGACGGCATACGAGATCATACCAAGTGACTGGAGT TCAGACG*T | 35 | CATACCAA |
| P7–36 | CAAGCAGAAGACGGCATACGAGATCCAGTTCAGTGACTGGAGT TCAGACG*T | 36 | CCAGTTCA |
| P7–37 | CAAGCAGAAGACGGCATACGAGATGTTGTGCCGTGACTGGAGT TCAGACG*T | 37 | GTTGTGCC |
| P7–38 | CAAGCAGAAGACGGCATACGAGATCCGTGAGAGTGACTGGAGT TCAGACG*T | 38 | CCGTGAGA |
| P7–39 | CAAGCAGAAGACGGCATACGAGATCCTCCTGAGTGACTGGAGT TCAGACG*T | 39 | CCTCCTGA |
| P7–40 | CAAGCAGAAGACGGCATACGAGATCGAACTTAGTGACTGGAGT TCAGACG*T | 40 | CGAACTTA |
| P7–41 | CAAGCAGAAGACGGCATACGAGATCGACTGGAGTGACTGGAGT TCAGACG*T | 41 | CGACTGGA |
| P7–42 | CAAGCAGAAGACGGCATACGAGATCGCATACAGTGACTGGAGT TCAGACG*T | 42 | CGCATACA |
| P7–43 | CAAGCAGAAGACGGCATACGAGATCTCAATGAGTGACTGGAGT TCAGACG*T | 43 | CTCAATGA |
| P7–44 | CAAGCAGAAGACGGCATACGAGATCTGAGCCAGTGACTGGAGT TCAGACG*T | 44 | CTGAGCCA |
| P7–45 | CAAGCAGAAGACGGCATACGAGATCTGGCATAGTGACTGGAGT TCAGACG*T | 45 | CTGGCATA |
| P7–46 | CAAGCAGAAGACGGCATACGAGATGAATCTGAGTGACTGGAGT TCAGACG*T | 46 | GAATCTGA |
| P7–47 | CAAGCAGAAGACGGCATACGAGATGACTAGTAGTGACTGGAGT TCAGACG*T | 47 | GACTAGTA |
| P7–48 | CAAGCAGAAGACGGCATACGAGATGAGCTGAAGTGACTGGAGT TCAGACG*T | 48 | GAGCTGAA |
- phosphorothioate bond
Reagents for yeast i-BLESS
-
EDTA powder (BioShop, cat. no. EDT001.1)
!CAUTION This reagent is toxic if inhaled, may cause respiratory tract, eye and skin irritation, may be harmful if swallowed. Avoid breathing, use only in a well-ventilated area.
-
Lyticase from Arthrobacter Luteus (Sigma, cat. no. L2524–200KU)
!CAUTION This reagent may cause allergy, asthma symptoms or breathing difficulties if inhaled. Avoid breathing dust.
Potassium phosphate, dibasic, anhydrous (K2HPO4, BioShop, cat. no. PPD303.500)
Potassium phosphate, monobasic, anhydrous (KH2PO4, BioShop, cat. no. PPM302.500)
Glycerol (POCH, cat. no. 443320113)
-
β-mercaptoethanol (Calbiochem, cat. no. 444203)
!CAUTION This reagent is toxic if swallowed or if inhaled, may cause damage to organs (liver, heart) through prolonged or repeated exposure if swallowed, is fatal in contact with skin, causes skin irritation, may cause an allergic skin reaction, causes serious eye damage and is very toxic to aquatic life. Avoid inhalation, wear eye protection and avoid release to the environment.
FastDigest NotI (Thermo Scientific, cat. no. FD0595) or other rare cutting enzymes and their corresponding buffers. See Experimental design: Spike-in DSB induction (qDSB-Seq DSB quantification) for more details.
FastDigest Buffer (10X, Thermo Scientific, cat. no. B64)
Reagents for mammalian i-BLESS
10x DPBS buffer (Sigma, cat. no. D1408)
SgrDI (5 U/μL, Thermo Scientific, cat. no. ER2031) or other rare cutting enzymes and their corresponding buffers. See Experimental design: Spike-in DSB induction (qDSB-Seq DSB quantification) for more details.
Buffer R (10X, Thermo Scientific, cat. no. BR5)
Equipment
500-mL centrifugation tubes (Beckman, cat. no. 355607)
500-, 250- and 100-mL conical flasks
50- and 15-mL centrifuge tubes (Corning, cat. nos. 430829 and 430791, respectively)
5-mL centrifuge tubes, PCR clean (Eppendorf, cat. no. 0030119460)
1.5-mL DNA LoBind Tubes, Eppendorf, cat. no. 0030108051)
PCR tubes (Molecular Bioproducts, cat. no. 3412)
5-mL clear glass screw thread vials (Chromacol, cat. no. 5-SV)
13 mm Autosampler Vial Screw Thread Caps (Thermo Fisher Scientific™, cat. no. C4015–66A)
microTUBE AFA Fiber Pre-Slit Snap-Cap 6×16mm (Covaris, cat. no. 520045)
5-mL, 1,000-, 200-, 100-, 20-, 10- and 2,5-μL Pipettes (Eppendorf, cat. nos. 3120000070, 3120000062, 3120000054, 3120000046, 3120000038, 3120000020 and 3120000011, respectively)
5,000-, 1,000-, 200-, 100-, 20- and 10-μL Filter tips (Eppendorf, cat. nos. 0030077580, 0030077571, 0030077555, 0030077547, 0030077539 and 0030077504, respectively)
Cell counter (Countess II Automated Cell Counter, Life Technologies, cat. no. AMQAX1000)
Ultracentrifuge (Avanti J-30I with JA-10 rotor, Beckman Coulter, cat. no. 363120)
Centrifuge with cooling (5810R, Eppendorf, cat. no. 5811000420)
Mini centrifuge (Centrifuge MiniSpin, Eppendorf, cat. no. 5453000015)
Orbital shaker incubator with heating (BioSan, cat. no. ES-20)
Magnetic stirrer (IKA, cat. no. C-MAG MS 10)
Thermomixer (ThermoMixer C, Eppendorf, cat. no. 5382000015)
Rocker (Biosan, cat. no. MR-1)
Focused-ultrasonicator (Covaris S220, Covaris, cat. no 500217)
Electrophoresis instrument (Bioanalyzer 2100, Agilent, cat. no. G2939BA)
Qubit 2.0 Fluorometer (Thermo Fisher Scientific, cat. no. Q32866)
Magnetic rack (Dynamag-2 magnet, Thermo Fisher Scientific, cat. no. 12321D)
Tube rotator (Stuart SB3, Fisher Scientific, cat. no. 15199712)
Water purification system (Milli-Q® Integral Water Purification System, Millipore, cat. no. C72876)
Thermal cycler (C1000 Touch, Bio-Rad, cat. no. 1851148)
Cooling rack for 1.5- and 2-mL tubes (IsoTherm-System, Eppendrof, cat. no. 3880001166)
Cooling rack for 0.2-mL tubes (PCR-Cooler 0.2-mL, Eppendrof, cat. no. 3881000031)
Gloves (Protects Clinic, Sempermed, cat. nos. PROLPF S-XL)
Reagent Setup
SE buffer
SE buffer is 5M NaCl, 500 mM EDTA and pH 7.5, sterilized by autoclaving. It can be stored at 4°C for several months.
LMP agarose in SE
LMP agarose in SE is 1% (wt/vol) Agarose, Low Melting Point, Analytical Grade in SE buffer. After dissolving the agarose, prepare 5 mL aliquots in 5-mL centrifugation tubes, they can be stored at RT for several months. Prior to usage incubate needed number of aliquots at 90°C until agarose melts, then equilibrate at 40°C.
LMP agarose in DPBS
LMP agarose in DPBS is 1% (wt/vol) Agarose, Low Melting Point, Analytical Grade in DPBS buffer. After dissolving agarose, prepare 2 mL aliquots in 5-mL centrifugation tubes, they can be stored at RT for several months. Prior to usage incubate needed number of aliquots at 90°C until agarose melts, then equilibrate at 40°C.
Lyticase storage buffer
Lyticase storage buffer is 0.1 M phosphate potassium pH 7.5, 0.1 M NaCl and 50% (vol/vol) glycerol. It can be stored at RT for several months.
Lyticase solution
Lyticase solution is 200 U/μL Lyticase from Arthrobacter Luteus in Lyticase storage buffer. It can be stored at −20°C for several months.
PMSF stock
PMSF stock is 100 mM PMSF in isopropanol. It can be stored at 4°C for several months.
10 μM Adapter P
10 μM Adapter P is 10 μM Adapter P in 1x T4 ligase buffer (e.g. 80 μL H2O, 10 μL of 100 μM Adapter, 10 μL 10x T4 ligase buffer), heated for 5 min at 95°C and then cooled immediately for 5 min on ice. It can be stored at −20°C in 100 μl aliquots for several months.
CRITICAL Avoid repeated freezing and thawing of aliquots.
10 μM Adapter D
10 μM Adapter D is 10 μM Adapter D in 1x T4 ligase buffer (e.g. 80 μL H2O, 10 μL of 100 μM Adapter, 10 μL 10x T4 ligase buffer), heated for 5 min at 95°C and then cooled immediately for 5 min on ice. It can be stored at −20°C in 100 μl aliquots for several months.
CRITICAL Avoid repeated freezing and thawing of aliquots.
TE buffer
TE buffer is 10 mM Tris, pH 8.0 and 1 mM EDTA. It can be stored at 4°C for several months.
Proteolytic buffer
Proteolytic buffer is 1% sarkosyl (wt/vol), 25 mM EDTA and pH 8.0. It can be stored at RT for several months.
10x Blunting buffer
10x Blunting buffer is 1M Tris-HCl, 500 mM NaCl, 100 mM MgCl2, 0.25% (vol/vol) Triton X-100, 50 mM DTT, pH 7.5. It can be stored at −20°C for several months. Prior to usage incubate at 70°C for 5 min to inactivate residual endonucleases.
Tris buffer
Tris buffer is 10mM Tris, pH 8.5. It can be stored at 4°C for several months.
2x W&B buffer
2x W&B buffer is 10 mM Tris pH 7.5, 1 mM EDTA and 2 M NaCl. It can be stored at RT for several months.
1x W&B buffer with Triton X-100
1x W&B buffer is 1x W&B buffer with 0.01% Triton X-100 (vol/vol).
CRITICAL Add Triton X-100 fresh on the day of usage of 1x W&B buffer with Triton X-100.
80% (vol/vol) Ethanol
80% (vol/vol) Ethanol is prepared by diluting four parts of 99.8% ethanol with one part of molecular biology-grade water.
CRITICAL Always prepare fresh on the day of DNA purification with AMPure beads.
Procedure
CRITICAL All centrifugation steps are performed at RT, unless otherwise specified.
Cells encapsulation and lysis •Timing 16–24 h
-
1
For preparation of yeast cells follow option A, for preparation of human cells follow option B.
(A) Yeast cells
Culture yeast cells to ca. 1×107 cells/mL density in 250 mL of appropriate medium in a 2-L conical flask. Transfer cell culture to 500-mL centrifugation tube placed on ice and pellet the cells gently at 700 g for 5 min at 4°C.
Wash the cells by resuspending them in 20 mL of ice-cold SE buffer. Transfer the suspension to 50-mL tube and centrifuge at 700 g for 5 min at 4°C.
-
Discard the supernatant and resuspend the cells in 5 mL of SE buffer. Place 20 mL of liquid paraffin in a 250-mL conical flask and melt 5 mL of LMP agarose in SE. Equilibrate yeast suspension, liquid paraffin and melted LMP agarose in SE for 15 min at 40°C, with gentle agitation (100 rpm).
CRITICAL STEP Make sure that agarose is completely melted and that all ingredients are warm to prevent premature solidification upon their mixing.
-
Mix cells with melted LMP agarose in SE and quickly add mixture to liquid paraffin, shake vigorously by hand for 1 min.
CRITICAL STEP After 1 min of vigorous shaking emulsion should form, if after that time emulsion is not stable (i.e. layers start to separate once shaking is completed), time of shaking should be extended.
-
Quickly pour the solution into ice-cold 200 mL of SE buffer in 500-mL conical flask placed on the magnetic stirrer. Stir for 5 min at 700 rpm at 4°C.
CRITICAL STEP To maintain low temperature during stirring, the conical flask should be placed in an ice bucket, cold room or refrigerated incubator.
-
Transfer the mixture to five 50-mL centrifugation tubes and centrifuge at 500 g for 10 min at 4°C. Remove the paraffin layer formed at the top.
CRITICAL STEP During removal of paraffin layer pay attention to not remove the floating beads that may sometimes be present directly under the paraffin layer. After all the paraffin is removed, disperse the beads that have not pelleted by pipetting.
-
Pellet the beads by centrifugation at 400 g for 10 min at 4°C. Remove all of the supernatant (including non-pelleted beads) by pouring off, combine the beads in one 50-mL tube and resuspend in 10 mL of TE buffer.
CRITICAL STEP As the bead pellet might be loose, during removal of the supernatant pay attention to not discard any beads. Alternatively, the supernatant can be removed by pipetting.
CRITICAL STEP As the bead pellet is difficult to aspirate by pipetting, to combine the beads one can use 5 mL of TE to resuspend the pellet in one tube, then transfer the suspension to another tube, resuspend and repeat for the remaining tubes until the whole pellet is in one tube. Then wash all the tubes with 5 mL of TE to collect leftover beads and add the suspension to the suspended beads.
-
Pellet the beads at 200 g for 10 min at 4°C. Remove supernatant and wipe the inside of the tube to remove any excess paraffin. Wash the pellet twice by resuspending in 10 mL of TE buffer and pellet at 200 g for 10 min at 4°C.
CRITICAL STEP Collect 10 μl of bead pellet and check under microscope if the cells are properly encapsulated in agarose beads. Most agarose beads should contain multiple immobilized cells (see Fig. 2a), single beads might be empty.
?TROUBLESHOOTING
-
Remove and discard the supernatant, add 0.5mL of β-mercaptoethanol, 20μL of Lyticase solution and 5 mL of SE, and resuspend by pipetting. Incubate the suspension at 30°C for 1 h with gentle agitation (100 rpm). The final volume should be around 10 mL.
CRITICAL STEP After 1 h, collect 10 μl of bead pellet and check the progress of spheroplasting under a microscope (see Fig. 3a for an example of proper spheroplasting). If the spheroplasting is not complete, check the progress every 15 min.
When spheroplasting is complete, pellet the beads at 200 g for 10 min at 4°C. Resuspend the beads in 10 mL of Proteolytic buffer, incubate for 10 min at RT and pellet at 200 g for 10 min.
Resuspend the beads in 10 mL of Proteolytic buffer. Add Proteinase K to final concentration of 50 μg/mL. Incubate the tube at 50°C overnight (12–18 h).
(B) Human cells
Transfer 1.2×107 cells from a fresh culture to 50 mL centrifugation tubes. For adherent cells, detach cells by trypsinization. Pellet the cells at 800 g for 5 min at 4°C and wash two times with 15 mL of 1x DPBS buffer. Pellet the cells again at 800 g for 5 min at 4°C.
-
Resuspend the cells in 2 mL of 1x DPBS buffer. Place 8 mL of liquid paraffin in 100-mL conical flask and melt 2 mL of LMP agarose in DPBS. Equilibrate cells suspension, liquid paraffin and melted LMP agarose in DPBS for 15 min at 40°C, with gentle agitation (100 rpm).
CRITICAL STEP Make sure that agarose is completely melted and that all ingredients are warm to prevent premature solidification upon their mixing.
-
Mix cells with melted LMP agarose in DPBS and quickly add mixture to liquid paraffin, shake vigorously by hand for 1 min.
CRITICAL STEP After 1 min of vigorous shaking an emulsion should form. If after that time emulsion is not stable (i.e. layers start to separate once shaking is completed), the time of shaking should be extended.
-
Quickly pour the solution into ice-cold 80 mL of 1x DPBS buffer in a 250-mL conical flask placed on the magnetic stirrer. Stir for 5 min at 700 rpm at 4°C.
CRITICAL STEP To maintain low temperature during stirring, the conical flask should be placed in an ice bucket, cold room or refrigerated incubator.
-
Transfer the mixture to two 50-mL centrifugation tubes and centrifuge at 500 g for 10 min at 4°C. Remove the paraffin layer at the top.
CRITICAL STEP During removal of the paraffin layer, pay attention to not remove the floating beads that may sometimes be present directly underneath. After all the paraffin is removed, disperse the beads that have not pelleted by pipetting.
-
Pellet the beads by centrifugation at 500 g for 10 min at 4°C. Remove all of the supernatant (including non-pelleted beads) by pipetting, combine the beads in one tube and resuspend in 10 mL of TE buffer.
CRITICAL STEP If beads will not pellet, leave suspension for 15 min to allow beads to settle on the bottom of the tube, then collect supernatant by pipetting.
CRITICAL STEP As the bead pellet is difficult to aspirate by pipetting, to combine the beads one can use 5 mL of TE to resuspend the pellet in one tube, then transfer the suspension to the second tube. Then wash the first tube with 5 mL of TE to collect leftover beads and add the suspension to the suspended beads.
?TROUBLESHOOTING
Pellet the beads at 500 g for 10 min at 4°C. Remove and discard the supernatant and wipe the inside of the tube to remove any excess paraffin.
-
Wash the pellet by resuspending in 10 mL of TE buffer and centrifuge at 500 g for 10 min at 4°C, then remove most of supernatant, leaving approximately 5 mL of bead pellet. Transfer beads to a 5-mL centrifugation tube and pellet at 200 g for 10 min.
CRITICAL STEP The border between pellet and supernatant might be barely visible. In this case, in this step and all washes in Steps 1B(ix)-2B(ii) aspirate 3 mL of liquid from the top to remove supernatant.
CRITICAL STEP Collect 10 μl of bead pellet and check under the microscope if the cells are properly encapsulated in agarose beads. Most agarose beads should contain multiple immobilized cells (see Fig. 2c), single beads might be empty.
?TROUBLESHOOTING
Wash the beads by resuspending in 3 mL of TE buffer and centrifuge at 200 g for 10 min at 4°C. Repeat one more time.
Pellet the beads at 200 g for 10 min at 4°C. Resuspend the beads in 3 mL of Proteolytic buffer, incubate for 10 min at RT and pellet at 200 g for 10 min.
Resuspend the beads in 3 mL of Proteolytic buffer. Add Proteinase K to a final concentration of 50 μg/mL. Incubate the tube at 50°C overnight (12–18 h).
Wash •Timing 2–3 h
-
2
For preparation of yeast cells follow option A, for preparation of human cells follow option B.
(A) Yeast cells
Pellet the beads at 200 g for 10 min at 4°C and resuspend in 10 mL of TE buffer with 0.1 mM PMSF. Incubate for 10 min at RT, gently rocking.
Pellet the beads at 200 g for 10 min, discard the supernatant and wash the beads twice by resuspending them with 10 mL of TE buffer. Pellet at 200 g for 10 min and discard the supernatant.
-
Resuspend the bead pellet in TE buffer to a final volume of 9 mL, divide each sample into six 1.5-mL centrifugation tubes. Pellet the beads at 200 g for 10 min and discard supernatant.
CRITICAL STEP The border between pellet and supernatant might be barely visible. In this case, in this step and all washes in Steps 3–10 aspirate 1 mL of liquid from the top of each tube to remove supernatant.
(B) Human cells
Pellet the beads at 200 g for 10 min at 4°C, discard the supernatant and resuspend the beads in 3 mL of TE buffer with 0.1 mM PMSF. Incubate for 10 min at RT, gently rocking.
Pellet the beads at 200 g for 10 min and wash twice by resuspending them with 3 mL of TE buffer. Pellet at 200 g for 10 min.
-
Resuspend the bead pellet in TE buffer to a final volume of 4.5 mL, divide each sample into three 1.5-mL centrifugation tubes. Pellet the beads at 200 g for 10 min and discard supernatant.
CRITICAL STEP The border between pellet and supernatant might be barely visible. In this case, in this step and all washes in Steps 3–10 aspirate 1 mL of liquid from the top of each tube to remove supernatant.
Spike-in •Timing 2–3 h
CRITICAL Apply the following steps both to treated and control samples. Other rare cutting enzymes and corresponding buffers might be used instead of NotI and SgrDI.
-
3
This step of the protocol is optional and required only if normalization between samples and/or quantification of absolute DSB number per cell is desired. If not, proceed to Step 4 (for “dirty” end repair) or to Step 5 (for all other cases). For preparation of yeast cells follow option A, for preparation of human cells follow option B.
(A) Yeast cells
Wash the pellet in each tube by resuspending in 1 mL of 1x FastDigest buffer (the amount of buffer used in this step is not a critical parameter, if the volume of bead pellet is higher than 0.5 mL, use lower volume of buffer to not exceed the maximum capacity of 1.5 mL tubes) and incubating for 10 min at RT, gently rocking. Pellet the beads at 200 g for 10 min.
- Remove the supernatant and resuspend each pellet in 500 μL of the following mix:
Component Amount Final Concentration 10x FastDigest buffer 50 μL 1x NotI FD (diluted 1000 fold in 1x FD buffer) 5 μL Water 445 μL Total 500 μL Mix gently and incubate for 1 h at 37°C.
Pellet the beads at 200 g for 10 min.
(B) Human cells
Wash the pellet in each tube by resuspending in 1 mL of 1x R buffer (the amount of buffer used in this step is not a critical parameter, if the volume of bead pellet is higher than 0.5 mL, use lower volume of buffer to not exceed the maximum capacity of 1.5 mL tubes) and incubating for 10 min at RT, gently rocking. Pellet the beads at 200 g for 10 min.
- Discard the supernatant and resuspend each pellet in 500 μL of the following mix:
Component Amount Final Concentration 10x R buffer 50 μL 1x SgrDI (5U/μL) 6 μL 0.06 U/μL Water 444 μL Total 500 μL Mix gently and incubate for 1 h at 37°C.
Pellet the beads at 200 g for 10 min.
Repair of “dirty” ends •Timing 3–4 h
CRITICAL Apply the following steps both to treated and control sample.
-
4
This step of the protocol is optional and required only if a substantial level of DSBs with “dirty” ends is expected (i.e. in case of irradiated samples or samples treated with radiomimetics). If high levels of DSBs with ‘dirty ends’ are not expected, proceed to Step 5. For preparation of yeast cells follow option A, for preparation of human cells follow option B.
(A) Yeast cells
Wash the pellet in each tube by resuspending in 1 mL of 1x NEBNext FFPE DNA Repair Buffer(the amount of buffer used in this step is not a critical parameter, if the volume of bead pellet is higher than 0.5 mL, use lower volume of buffer to not exceed the maximum capacity of 1.5 mL tubes) and incubating for 10 min at RT, gently rocking. Pellet the beads at 200 g for 10 min.
- Discard the supernatant and resuspend each pellet in 500 μL of the following mix:
Component Amount Final Concentration 10x NEBNext FFPE DNA Repair Buffer 50 μL 1x NEBNext FFPE DNA Repair Mix 8 μL Water 442 μL Total 500 μL Mix gently and incubate for 2 h at 20°C.
Pellet the beads at 200 g for 10 min.
(B) Human cells
Wash pellet in each tube by resuspending in 1 mL of 1x NEBNext FFPE DNA Repair Buffer (the amount of buffer used in this step is not a critical parameter, if the volume of bead pellet is higher than 0.5 mL, use lower volume of buffer to not exceed the maximum capacity of 1.5 mL tubes) and incubating for 10 min at RT, gently rocking. Pellet the beads at 200 g for 10 min.
- Discard the supernatant and resuspend each pellet in 500 μL of the following mix:
Component Amount Final Concentration 10x NEBNext FFPE DNA Repair Buffer 50 μL 1x NEBNext FFPE DNA Repair Mix 16 μL Water 434 μL Total 500 μL Mix gently and incubate for 2 h at 20°C.
Pellet the beads at 200 g for 10 min.
DNA ends blunting •Timing 3–4 h
-
5
For preparation of yeast cells follow option A, for preparation of human cells follow option B.
(A) Yeast cells
Wash pellet in each tube by resuspending in 1 mL of 1x Quick Blunting buffer (the amount of buffer used for washes in Steps 5–10 is not a critical parameter, if the volume of bead pellet is higher than 0.5 mL, use lower volume of buffer to not exceed the maximum capacity of 1.5 mL tubes). Incubate for 10 min at RT, gently rocking. Pellet the beads at 200 g for 10 min.
- Discard the supernatant and resuspend each pellet in 500 μL of the following mix:
Component Amount Final Concentration 10x Blunting buffer 50 μL 1x BSA, 20 mg/mL 2.5 μL 100 μg/mL dNTPs 1mM 15 μL 30 μM Blunting Enzyme mix 6 μL Water 426.5 μL Total 500 μL Mix gently and incubate for 2 h at 25°C.
Pellet the beads at 200 g for 10 min. Wash twice by resuspending in 1 mL of TE buffer and incubating for 10 min at RT, gently rocking, then pellet the beads at 200 g for 10 min.
-
Discard the supernatant and resuspend the beads in 1 mL of TE buffer.
PAUSE POINT The resuspended pellet can be stored at 4°C overnight.
(B) Human cells
Wash the pellet in each tube by resuspending in 1 mL of 1x Quick Blunting buffer (the amount of buffer used for washes in Step 5–10 is not a critical parameter, if the volume of bead pellet is higher than 0.5 mL, use lower volume of buffer to not exceed the maximum capacity of 1.5 mL tubes). Incubate for 10 min at RT, gently rocking. Pellet the beads at 200 g for 10 min.
- Discard the supernatant and resuspend each pellet in 500 μL of the following mix:
Component Amount Final Concentration 10x Blunting buffer 50 μL 1x BSA, 20 mg/mL 2.5 μL 100 μg/mL dNTPs 1mM 30 μL 60 μM Blunting Enzyme mix 12 μL Water 405.5 μL Total 500 μL Mix gently and incubate for 2 h at 25°C.
Pellet the beads at 200 g for 10 min. Wash twice by resuspending in 1 mL of TE buffer, incubate for 10 min at RT, gently rocking, pellet the beads at 200 g for 10 min.
-
Discard the supernatant and resuspend the beads in 1 mL of TE buffer.
PAUSE POINT The resuspended pellet can be stored at 4°C overnight.
DSB labeling •Timing 19–22 h
-
6
For preparation of yeast cells follow option A, for preparation of human cells follow option B.
(A) Yeast cells
Pellet the beads at 200 g for 10 min. Wash the pellet in each tube by resuspending in 1 mL of 1x dA-Tailing Reaction Buffer, incubating for 10 min at RT, gently rocking. Pellet the beads at 200 g for 10 min.
- Discard the supernatant and resuspend each pellet in 500 μL of the following mix:
Component Amount Final Concentration 10x dA-Tailing Reaction Buffer 50 μL 1x Klenow Fragment (3´→5´ exo-) 9 μL Water 441 μL Total 500 μL Mix gently and incubate for 1 h 20 min at 37°C.
Pellet the beads at 200 g for 10 min. Wash by resuspending in 1 mL of 1x T4 ligase buffer and incubating for 10 min at RT, gently rocking, then pellet the beads at 200 g for 10 min.
- Discard the supernatant and resuspend each pellet in 494 μL of the following mix:
Component Amount Final Concentration 10x T4 ligase buffer 50 μL 1x 10 μM Adapter P 6 μL 120 nM H2O 438 Total 494 μL Mix gently by pipetting.
Add 6 μL of T4 ligase to each tube, mix gently by pipetting, and incubate at 16°C overnight (16–18 h).
(B) Human cells
Pellet the beads at 200 g for 10 min. Wash pellet in each tube by resuspending in 1 mL of 1x dA-Tailing Reaction Buffer and incubating for 10 min at RT, gently rocking. Pellet the beads at 200 g for 10 min.
- Resuspend each pellet in 500 μL of the following mix:
Component Amount Final Concentration 10x dA-Tailing Reaction Buffer 50 μL 1x Klenow Fragment (3´→5´ exo-) 18 μL Water 432 μL Total 500 μL Mix gently and incubate for 1 h 20 min at 37°C.
Pellet the beads at 200 g for 10 min. Wash by resuspending in 1 mL of 1x T4 ligase buffer and incubating for 10 min at RT, gently rocking, then pellet the beads at 200 g for 10 min.
- Discard the supernatant and resuspend each pellet in 492 μL of the following mix:
Component Amount Final Concentration 10x T4 ligase buffer 50 μL 1x 10 μM Adapter P 8 μL 120 nM H2O 434 Total 492 μL Mix gently by pipetting.
Add 8 μL of T4 ligase to each tube, mix gently by pipetting, incubate at 16°C overnight (16–18 h).
DNA isolation •Timing 5–7 h
-
7
Pellet the beads at 200 g for 10 min. Wash pellet in each tube by resuspending in 1 mL of Proteolytic Buffer and incubating for 10 min at RT, gently rocking. Pellet the beads at 200 g for 10 min.
-
8Discard the supernatant and resuspend each pellet in 500 μL of the following mix:
Component Amount Final Concentration Proteolytic buffer 497.5 μL Proteinase K 20 mg/ml 2.5 μL 100 μg/ml Total 500 μL -
9
Mix gently by pipetting and incubate for 1 h at 50°C.
-
10
Pellet the beads at 500 g for 10 min. Wash by resuspending in 1 mL of TE buffer and incubating for 10 min at RT, gently rocking, then pellet the beads at 500 g for 10 min and discard the supernatant.
-
11
Transfer beads from all centrifugation tubes to one or two 5-mL glass screw thread vials (if the volume of agarose beads is ≤ 2 mL use one vial, if it exceeds 2 mL, dispense the agarose beads evenly to two vials). Spin down at 800 g for 5 s and remove any remaining supernatant.
-
12
Sonicate using Covaris S220 with the following program settings: Peak Power: 175.0, Duty Factor: 10.0, Cycles/Burst: 200, Time: 600 s, Temperature: 5°C, Max Temperature: 7°C.
-
13
Spin vials briefly. Transfer the bead suspension to 15-mL centrifugation tube and measure its volume. Add 3 volumes of ADB buffer from Zymoclean™ Large Fragment DNA Recovery Kit, mix by pipetting and incubate for 10 min at 50°C.
CRITICAL STEP Transfer of bead suspension from a glass vial to a centrifugation tube is easier after adding e.g. 1 mL ADB buffer to bead suspension and mixing, which will result in most beads dissolving. The remaining volume of ADB buffer should then be added to the centrifugation tube. Divide total volume (expressed in mL) of thus obtained suspension by 2 and round up to obtain number of spin columns used in the next step, e.g. for total volume of 7 mL use 4 spin columns.
-
14
Transfer up to 900 μl of the melted agarose solution to each spin column from Zymoclean™ Large Fragment DNA Recovery Kit placed in a collection tube. Centrifuge for 1 min at 11,000–16,000 g and discard the flow-through. Add the remaining suspension to the same columns and centrifuge again, then discard the flow-through.
-
15
Add 200 μL of DNA Wash Buffer from Zymoclean™ Large Fragment DNA Recovery Kit to the columns and centrifuge for 30 s at 11,000–16,000 g. Discard the flow-through and repeat the wash step.
-
16
Place the spin column in 1.5-mL centrifugation tubes, to each column add 50 μL of Tris buffer heated to 60°C, incubate for 3 min at RT. Centrifuge for 30 s at 11,000–16,000 g.
-
17
Pool eluted DNA in one tube, add an equal volume of AMPure XP Beads and mix well by pipetting. Incubate for 15 min at RT.
CRITICAL STEP Equilibrate AMPure XP beads at RT for at least 30 min before use.
-
18
Place the tube on a magnetic stand until the solution becomes clear (5 min) and remove supernatant.
-
19
Without disturbing the beads add 600 μL of freshly prepared 80% (vol/vol) ethanol, incubate for 30 s at RT and remove the supernatant. Repeat the wash with 80% (vol/vol) ethanol one more time.
-
20
While keeping the tube on the magnetic stand, let the sample air dry at RT for 5–10 min. CRITICAL STEP Proceed to the next steps as soon as the beads are dry to prevent them from
-
21
over-drying, which decreases the amount of eluted DNA.
-
22
Resuspend the dried pellet with 130 μL of Tris buffer and incubate for 2 min at RT. Place the tube on the magnetic stand at RT until the liquid appears clear (5 min).
CRITICAL STEP Equilibrate Tris buffer at 50°C for 10 min before use for higher elution yield.
-
23
Transfer the supernatant to a new microTUBE.
-
24
Place the tube in Covaris S220 and sonicate with the following program settings: Peak Power: 140.0, Duty Factor: 10.0, Cycles/Burst: 200, Time: 81 s, Temperature: 5°C, Max Temperature: 7°C.
-
25
Transfer the sonicated DNA to a fresh 1.5-mL centrifugation tube.
-
26
Check DNA quality and fragment size using Bioanalyzer 2100 and Agilent High Sensitivity DNA Kit, following the instructions provided with the kit.
CRITICAL STEP The peak of DNA size should be around 350–400 bp.
?TROUBLESHOOTING
PAUSE POINT The cleaned DNA can be stored at −20°C for several months.
Distal adapter ligation •Timing 17–19 h
-
27
Measure DNA concentration using Qubit 2.0 with Qubit dsDNA HS Assay Kit following the instructions provided with the kit.
?TROUBLESHOOTING
-
28
Add 20 μL of Dynabeads MyOne C1 suspension to a fresh 1.5-mL DNA LoBind tube (for each DNA sample). Add 800 μL of 1x W&B buffer with Triton X-100 and mix well. Place the tube on a magnetic stand until the solution becomes clear (2 min) and remove supernatant. Wash beads with 800 μL of 1x W&B buffer with Triton X-100 one more time.
CRITICAL STEP Add TritonX-100 fresh on the day of usage of 1x W&B buffer.
-
29
To each tube, add 1 – 4 μg of DNA and an equivalent volume of 2x W&B buffer, then bring the volume up to 600 μL with 1x W&B buffer with Triton X-100 and mix well. Store unused gDNA at −20°C.
CRITICAL STEP Use the same amount of DNA for a given treated sample and the corresponding control. If you are using the qDSB-Seq variant of the protocol, retain a portion of gDNA sufficient for library preparation (e.g. at least 100 ng for library preparation with ThruPLEX DNA Kit by Rubicon Genomics).
-
30
Rotate at 7 rpm for 30 min at 4°C using rotator.
-
31
Spin briefly to remove bead solution from the cap. Place the tube on a magnetic stand until the solution becomes clear (2 min) and remove the supernatant.
-
32
Add 600 μL of 1x W&B buffer with Triton X-100 and mix well. Place the tube on a magnetic stand until the solution becomes clear (2 min) and remove supernatant. Wash with 600 μL of 1x W&B buffer with Triton X-100 two more times.
-
33To each sample add 65 μL of the following mix:
Component Amount Final Concentration 10x End repair reaction buffer 6.5 μL 1x End Prep Enzyme mix (NEB) 3 μL H2O 55.5 μL Total 65 μL -
34
Incubate for 30 min at 20°C followed by 30 min at 65°C, then put the tube on ice.
-
35
Place the tube on a magnetic stand until the solution becomes clear (2 min) and remove the supernatant.
-
36
Add 600 μL of 1x W&B buffer with Triton X-100 and mix well. Place the tube on a magnetic stand until the solution becomes clear (2 min) and remove supernatant. Wash with 600 μL of 1x W&B buffer with Triton X-100 two more times.
-
37
Resuspend each sample in 37 μL of 1x T4 ligase buffer.
-
38
To each tube add 10 μL of Adapter D and 3 μL of T4 ligase. Mix well and incubate at 16°C overnight (14–16 h).
USER digestion of captured fragments •Timing 3 h
-
39
Place the tube on a magnetic stand until the solution becomes clear (2 min) and remove the supernatant.
-
40
Add 600 μL of 1x W&B buffer with Triton X-100 and mix well. Place the tube on a magnetic stand until the solution becomes clear (2 min) and remove the supernatant. Wash with 600 μL of 1x W&B buffer with Triton X-100 one more time.
-
41Resuspend the beads in 25 μL of the following mix:
Component Amount Final Concentration 10x Cut smart buffer 2.5 μL 1x USER 1 μL H2O 21.5 μL Total 25 μL -
42
Incubate at 37°C for 45 min followed by 20°C for 15 min.
-
43
Place the tube on a magnetic stand until the solution becomes clear (2 min) and transfer the supernatant to a new PCR tube.
-
44
Add 25 μL of water to the remaining beads, vortex for 5 s, spin down and place the tube on a magnetic stand. Transfer 25 μL of supernatant to the previously collected supernatant.
-
45
Add 50 μL of AMPure XP Beads to the pooled supernatants and mix well by pipetting. Incubate for 15 min at RT.
CRITICAL STEP Equilibrate AMPure XP beads at RT for at least 30 min before use.
-
46
Follow Steps 18–21 using 200 μL of freshly prepared 80% (vol/vol) ethanol for washing and 30 μL of Tris buffer for elution.
CRITICAL STEP Equilibrate Tris buffer at 50°C for 10 min before use for higher elution yield.
-
47
Transfer the supernatant to a new PCR tube. Add 30 μL of AMPure XP Beads and mix well by pipetting. Incubate for 15 min at RT.
-
48
Follow Steps 18–21 using 200 μL of freshly prepared 80% (vol/vol) ethanol for washing and 25 μL of Tris buffer for elution.
CRITICAL STEP Equilibrate Tris buffer at 50°C for 10 min before use for higher elution yield.
-
49
Transfer the supernatant to a new PCR tube.
PAUSE POINT The cleaned DNA can be stored at −20°C for several months.
Amplification of labeled fragments •Timing 2 h
-
50Prepare the following reagent mix for each sample:
Component Amount Final Concentration 5x Phusion HF buffer 10 μL 1x Primer P5 (10 μM) 2 μL 400 nM Primer P7 (10 μM) 2 μL 400 nM dNTPs mix (10 mM) 1 μL 200 μM Polymerase Phusion (2 U/μl) 0.5 μL 20 mU/μL H2O 9.5 μL DNA from Step 47 25 μL Total 50 μL Choose one from the 48 uniquely indexed Primers P7 provided in Table 3 based on a pooling scheme designed for sequencing.
CRITICAL STEP Follow the guidance of sequencer’s manufacturer for index pooling (for Illumina platforms see: Index Adapters Pooling Guide, https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/experiment-design/index-adapters-pooling-guide-1000000041074-10.pdf).
-
51Mix well and run the following program in a thermal cycler, repeating Steps b-d 18 times.
Step Duration Temperature a 60 s 98°C b 30 s 98°C c 20 s 53°C d 60 s 72°C e 10 min 72°C f Hold 4°C -
52
Check the size distribution of the prepared library using Bioanalyzer 2100 and Agilent High Sensitivity DNA Kit, following the instructions provided with the kit.
CRITICAL STEP Place the samples on ice until library assessment. If no product is visible, place the tubes back into a thermal cycler and run the above PCR program (Step 50) starting from Step b and with total of two cycles of amplification, then check the library once again.
-
53
Add 50 μL of AMPure XP Beads and mix well by pipetting. Incubate for 15 min at RT.
-
54
CRITICAL STEP Equilibrate AMPure XP beads at RT for at least 30 min before use.
-
55
Follow Steps 18–21 using 200 μL of freshly prepared 80% (vol/vol) ethanol for washing and 30 μL of Tris buffer for elution.
CRITICAL STEP Equilibrate Tris buffer at 50°C for 10 min before use for higher elution yield.
-
56
Transfer the supernatant to a new PCR tube.
-
57
Check the size distribution of the prepared library using Bioanalyzer 2100 and Agilent High Sensitivity DNA Kit, following the instructions provided with the kit.
CRITICAL STEP Make sure that the size of DNA fragments in your library is between 200 and 800 bp and that there are no free adapters, primers and primer dimers in your library (distinct peaks with length between 50 and 150 bp) as they can influence sequencing run efficiency and quality. In case of adapter or primer contamination, perform an additional AMPure XP bead cleanup using a 1x ratio of beads.
?TROUBLESHOOTING
Box 1 | Sequencing •Timing 1–2 d
Procedure
Check the quantity of prepared library using Qubit fluorimeter and Qubit™ dsDNA HS Assay Kit, following the instructions provided with the kit.
Normalize library concentrations according to recommendations for particular Illumina sequencing platform. Use vacuum concentrator if library concentration is too low.
-
Sequence prepared libraries using Illumina next generation sequencing platform according to particular instrument user guide. Set single-end sequencing run with at least 50 bp read length (single-end sequencing is sufficient as in our approach R1 reads are directly adjacent to DSBs).
(Optional) For libraries characterized by low initial sequence diversity and when using qDSB-Seq approach follow our modified protocols for generation of high-quality sequencing data from low-diversity samples51. When using qDSB-Seq approach and utilizing gDNA sequencing for calculation of cutting efficiency, perform paired-end sequencing of gDNA samples with at least 2×50 bp read length.
Box 2 | Data analysis •Timing 1–2 d
Procedure
Identification of DSBs
Data analysis of i-BLESS sequencing data can be performed using iSeq software (http://iseq.rowickalab.org). See 52 for more details.
-
1
Map i-BLESS reads to the appropriate reference genome, e.g. using bowtie.
-
2
Count i-BLESS read coverage from the first nucleotide of a sequencing read. The obtained read counts reflect relative abundance of DSBs at corresponding genomic location.
(Optional) Quantification of i-BLESS data
The following steps are optional and performed only when spike-in DSBs were induced.
Quantification can be performed using qDSB-Seq software (https://github.com/rowickalab/qDSB-Seq). See 34 for more details.
-
3Calculate cutting efficiency of the enzyme used to introduce spike-in DSBs. If gDNA sequencing is used to estimate cutting efficiency, follow option A; if qPCR is utilized, follow option B.
- Align paired-end gDNA sequencing reads to the reference genome and calculate the average enzyme cutting efficiency per cutting site based on the number of uncut fragments covering the cutting site and number of uncut fragments (i.e. fragments mapped within several nucleotides (e.g. up to 12 nt) of the cutting site).
- design PCR primers flanking a few selected cutting sites and perform qPCR reaction using gDNA isolated from untreated and enzyme-treated cells. To calculate enzyme cutting efficiency, compare the amount of PCR product amplified in both samples using the ΔCT method.
-
4
Multiply average enzyme cutting frequency per site by the number of enzyme cutting sites used in the calculation to estimate the average number of spike-in DSBs per cell.
-
5
Compute the ratio between the number of introduced spike-in DSBs and the number of i-BLESS reads mapped to the enzyme cutting sites.
-
6
Multiply this ratio by the number of i-BLESS reads mapped to the whole genome to obtain average total number of DSBs per cell. To estimate DSB numbers in specific regions of interest, use only i-BLESS reads mapping to these regions.
Annotation of i-BLESS data
Annotation of i-BLESS data can be performed using iSeq software (http://iseq.rowickalab.org). See 52 for more details.
-
7
Identify DSB-enriched regions (regions with significant increase of labeled reads between studied and control samples), e.g. using hygestat_bless.
-
8
Annotate DSB-enriched regions to genomic features of interest, an/or perform other analysis, as desired, e.g. using hygestat_annotation.
Troubleshooting
Troubleshooting advices can be found in Table 4.
Table 4.
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 1A(viii) 1B(viii) |
Agarose beads are empty or contain few cells | Insufficient amount of starting material | Discard the beads and start from the beginning with a higher number of cells |
| Cells were damaged | Ensure that the cells are not damaged during their culture or harvest | ||
| Emulsion was not formed properly | Ensure that melted agarose, cell suspension and paraffin are all equilibrated at 40°C, while SE or DPBS buffers are ice-cold | ||
| Ensure proper emulsion mixing | |||
| 25 | DNA size is lower than required | DNA in cells was substantially fragmented | Remove apoptotic cells from the culture by low speed centrifugation (e.g. 200 g for 5 min for mammalian cells) after which most apoptotic cells should be present in the supernatant |
| DNA size is higher than required | DNA sonication was ineffective | Place the DNA into a new microTUBE and repeat Steps 23–25 | |
| 26 | Not enough DNA | Insufficient amount of starting material | Start from the beginning with more cells |
| A substantial part of agarose beads was lost during washes | Make sure not to aspirate bead pellet when removing supernatant during wash steps | ||
| No ethanol was added to the Wash buffer from Zymoclean Large Fragment DNA Recovery Kit and DNA was eluted during washing | If flow-through from washing was saved, condense it using a vacuum concentrator and clean using AMPure beads. If not, start from the beginning making sure that ethanol was added to the Wash buffer | ||
| The AMPure beads were over-dried | Avoid over-drying the beads in Step 20 | ||
| 55 | The library is underamplified | The number of amplification cycles was too low | Place the samples back in the thermocycler and run two more cycles |
| The library is overamplified | The number of amplification cycles was too high | Use the remaining amount of DNA from Step 25 and redo Steps 27–55 with an adjusted number of amplification cycles. In case of problems with establishing the optimal number of PCR cycles, instead of Steps 49–50 one can use an approach described by Buenrostro et al54, in which a small part of the library is amplified by qPCR to determine the number of cycles after which 0.25 of the maximum fluorescent intensity is achieved. Then, the remaining library is amplified using this number of cycles | |
| Additional signal for short oligos | Free adapters, primers or primer dimers contamination | Repeat AMPure beads clean up using 1x bead ratio | |
Timing
Step 1A or 1B - Cell encapsulation and lysis of yeast or human cells: 4–6 h, overnight incubation: 12–18 h
Step 2A or 2B - Wash: 2–3 h
Step 3A or 3B - Spike-in: 2–3 h
Step 4A or 4B - Repair of dirty ends: 3–4 h
Step 5A or 5B - DNA ends blunting: 3–4 h
Step 6A or 6B - DSB labeling: 3–4 h, overnight incubation: 16–18 h
Steps 7–25 - DNA isolation: 5–7 h
Steps 26–37 - Adapter D ligation: 3 h, overnight incubation: 14–16 h
Steps 38–48 - USER digestion of captured fragments: 3 h
Steps 49–55 - Amplification of labeled fragments: 2 h
Box 1: Sequencing: 1–2 d
Box 2: Data analysis: 1–2 d
Anticipated results
A typical expected i-BLESS library is shown in Fig 4a. After sequencing (Box 1), data analysis can be performed using our computational pipeline (http://iseq.rowickalab.org), as described briefly in Box 2 and in detail elsewhere52.
Mapped reads can be visualized using any available genome browser. Depending on the studied conditions the expected output may vary greatly, e.g. restriction enzyme digestion will result in sharp peaks at specific loci, while signal caused by other DSBs can appear in the form of peaks of different width and intensity, according to the mechanisms of DSB formation and the repair processes that they undergo. An example of mapping results uploaded to a genome browser is shown in Fig. 4b. This figure presents hydroxyurea-treated mec1–1 yeast cells, Zeocin-treated wild type cells, and control: untreated wild type cells. While it is easy to observe that hydroxyurea causes DSB induction around replication origins, breaks caused by Zeocin are more evenly distributed throughout the genome, resulting in signal similar to that observed in control cells. In such cases, proper data interpretation is impossible, and normalization of i-BLESS data using the qDSB-Seq approach needs to be performed, as described briefly in Box 2 and in detail elsewhere34 (Fig. 4c-d). However, as qDSB-Seq allows not only normalization but also quantification of absolute DSB numbers per cell, we recommend using it in all experiments. The expected frequency of DSBs at a given position ranges from 0.001% for ultra-rare DSBs (e.g. spontaneous breaks at G-quadruplexes in yeast) to almost 100% for in vitro treatment with restriction enzymes (in vivo digestion with endonucleases is not likely to result in such high cutting frequency due to restricted accessibility of chromatin).
To identify DSB-rich regions under a given condition, hygestat_bless (part of iSeq software suite, available from http://iseq.rowickalab.org) can be used52. hygestat_bless detects regions with a significant increase of read numbers in treatment versus control samples, within windows of selected size. Next, to determine whether a given genomic feature (e.g. gene promoters, transcription start and end sites, regions forming stable secondary structures) is enriched in DSB-rich regions, hygestat_annotation (part of iSeq software suite) can be used52.
Supplementary Material
Supplementary Movie 1. Encapsulation of human GM19239 cells in agarose beads.
Acknowledgements
We thank Karolina Jodkowska and Tomasz Biernacki for help with the preparation of Supplementary Movie 1. We also thank Andrzej Kudlicki for critical reading of the manuscript. This work was supported by the Foundation for Polish Science (TEAM to K.G.) and the Polish National Science Centre (2015/17/D/NZ2/03711 to M.S.) and an NIH grant R01GM112131 to M.R.
Footnotes
Competing interests
The authors declare no competing interests.
Data availability statement
The raw data underlying Fig. 4b-d are deposited to the NCBI Sequence Read Archive (SRA), accession codes SRP125409 and SRP189465.
Related links
Key references using this protocol
Biernacka, A. et al. Commun Biol 1, 181 (2018): https://doi.org/10.1038/s42003–018-0165–9
Zhu, Y. et al. Nat Commun 10, 2313 (2019): https://doi.org/10.1038/s41467–019-10332–8
Promonet, A. et al. Nat Commun 11, 3940 (2020): https://doi.org/10.1038/s41467–020-17858–2
References
- 1.Alt FW & Schwer B. DNA double-strand breaks as drivers of neural genomic change, function, and disease. DNA Repair (Amst) 71, 158–163, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gothe HJ, Minneker V. & Roukos V. Dynamics of Double-Strand Breaks: Implications for the Formation of Chromosome Translocations. Adv Exp Med Biol 1044, 27–38, (2018). [DOI] [PubMed] [Google Scholar]
- 3.Mitelman F, Johansson B. & Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer 7, 233–245, (2007). [DOI] [PubMed] [Google Scholar]
- 4.O’Driscoll M. Diseases associated with defective responses to DNA damage. Cold Spring Harb Perspect Biol 4, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White RR & Vijg J. Do DNA Double-Strand Breaks Drive Aging? Mol Cell 63, 729–738, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun 9, 1911, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll D. Genome engineering with zinc-finger nucleases. Genetics 188, 773–782, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma AC, Chen Y, Blackburn PR & Ekker SC TALEN-Mediated Mutagenesis and Genome Editing. Methods Mol Biol 1451, 17–30, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian X. et al. CRISPR/Cas9 - An evolving biological tool kit for cancer biology and oncology. NPJ Precis Oncol 3, 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin SA, Lord CJ & Ashworth A. DNA repair deficiency as a therapeutic target in cancer. Curr Opin Genet Dev 18, 80–86, (2008). [DOI] [PubMed] [Google Scholar]
- 11.Turinetto V. & Giachino C. Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic Acids Res 43, 2489–2498, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McManus KJ & Hendzel MJ ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol Biol Cell 16, 5013–5025, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F. & Higgins JM Histone modifications and mitosis: countermarks, landmarks, and bookmarks. Trends Cell Biol 23, 175–184, (2013). [DOI] [PubMed] [Google Scholar]
- 14.Leduc F. et al. Genome-wide mapping of DNA strand breaks. PLoS One 6, e17353, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baranello L. et al. DNA break mapping reveals topoisomerase II activity genome-wide. Int J Mol Sci 15, 13111–13122, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shastri N. et al. Genome-wide Identification of Structure-Forming Repeats as Principal Sites of Fork Collapse upon ATR Inhibition. Mol Cell 72, 222–238 e211, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gittens WH et al. A nucleotide resolution map of Top2-linked DNA breaks in the yeast and human genome. Nat Commun 10, 4846, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratto F. et al. DNA recombination. Recombination initiation maps of individual human genomes. Science 346, 1256442, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khil PP, Smagulova F, Brick KM, Camerini-Otero RD & Petukhova GV Sensitive mapping of recombination hotspots using sequencing-based detection of ssDNA. Genome Res 22, 957–965, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biernacka A. et al. i-BLESS is an ultra-sensitive method for detection of DNA double-strand breaks. Commun Biol 1, 181, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman EA, McCulley A, Haarer B, Arnak R. & Feng W. Break-seq reveals hydroxyurea-induced chromosome fragility as a result of unscheduled conflict between DNA replication and transcription. Genome Res 25, 402–412, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crosetto N. et al. Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat Methods 10, 361–365, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ran FA et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aymard F. et al. Genome-wide mapping of long-range contacts unveils clustering of DNA double-strand breaks at damaged active genes. Nat Struct Mol Biol 24, 353–361, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi W. et al. Ssb1 and Ssb2 cooperate to regulate mouse hematopoietic stem and progenitor cells by resolving replicative stress. Blood 129, 2479–2492, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu J. et al. Detecting DNA double-stranded breaks in mammalian genomes by linear amplification-mediated high-throughput genome-wide translocation sequencing. Nat Protoc 11, 853–871, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lensing SV et al. DSBCapture: in situ capture and sequencing of DNA breaks. Nat Methods 13, 855–857, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canela A. et al. DNA Breaks and End Resection Measured Genome-wide by End Sequencing. Mol Cell 63, 898–911, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mimitou EP, Yamada S. & Keeney S. A global view of meiotic double-strand break end resection. Science 355, 40–45, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada S. et al. Molecular structures and mechanisms of DNA break processing in mouse meiosis. Genes Dev, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiegand RC, Godson GN & Radding CM Specificity of the S1 nuclease from Aspergillus oryzae. J Biol Chem 250, 8848–8855, (1975). [PubMed] [Google Scholar]
- 32.Yan WX et al. BLISS is a versatile and quantitative method for genome-wide profiling of DNA double-strand breaks. Nat Commun 8, 15058, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Promonet A. et al. Topoisomerase 1 prevents replication stress at R-loop-enriched transcription termination sites. Nat Commun 11, 3940, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Y. et al. qDSB-Seq is a general method for genome-wide quantification of DNA double-strand breaks using sequencing. Nat Commun 10, 2313, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petek LM, Russell DW & Miller DG Frequent endonuclease cleavage at off-target locations in vivo. Mol Ther 18, 983–986, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai SQ et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol 33, 187–197, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabriel R. et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat Biotechnol 29, 816–823, (2011). [DOI] [PubMed] [Google Scholar]
- 38.Botstein D, Chervitz SA & Cherry JM Yeast as a model organism. Science 277, 1259–1260, (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bassett DE Jr., Boguski MS & Hieter P. Yeast genes and human disease. Nature 379, 589–590, (1996). [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y. et al. Integrated analysis of patterns of DNA breaks reveals break formation mechanisms and their population distribution during replication stress. Preprint at https://www.biorxiv.org/content/10.1101/171439v2, (2017).
- 41.Sriramachandran AM et al. Genome-wide Nucleotide-Resolution Mapping of DNA Replication Patterns, Single-Strand Breaks, and Lesions by GLOE-Seq. Mol Cell 78, 975–985 e977, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung WH, Zhu Z, Papusha A, Malkova A. & Ira G. Defective resection at DNA double-strand breaks leads to de novo telomere formation and enhances gene targeting. PLoS Genet 6, e1000948, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Symington LS Mechanism and regulation of DNA end resection in eukaryotes. Crit Rev Biochem Mol Biol 51, 195–212, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Caron P, Legube G. & Paull TT Quantitation of DNA double-strand break resection intermediates in human cells. Nucleic Acids Res 42, e19, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aymard F. et al. Transcriptionally active chromatin recruits homologous recombination at DNA double-strand breaks. Nat Struct Mol Biol 21, 366–374, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vieira Braga FA & Miragaia RJ Tissue Handling and Dissociation for Single-Cell RNA-Seq. Methods Mol Biol 1979, 9–21, (2019). [DOI] [PubMed] [Google Scholar]
- 47.Reichard A. & Asosingh K. Best Practices for Preparing a Single Cell Suspension from Solid Tissues for Flow Cytometry. Cytometry A 95, 219–226, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leelatian N. et al. Preparing Viable Single Cells from Human Tissue and Tumors for Cytomic Analysis. Curr Protoc Mol Biol 118, 25C 21 21–25C 21 23, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cannan WJ & Pederson DS Mechanisms and Consequences of Double-Strand DNA Break Formation in Chromatin. J Cell Physiol 231, 3–14, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li F. et al. Apn2 resolves blocked 3’ ends and suppresses Top1-induced mutagenesis at genomic rNMP sites. Nat Struct Mol Biol 26, 155–163, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitra A, Skrzypczak M, Ginalski K. & Rowicka M. Strategies for achieving high sequencing accuracy for low diversity samples and avoiding sample bleeding using illumina platform. PLoS One 10, e0120520, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitra A. et al. Analyzing and interpreting DNA double-strand break sequencing data. Preprint at https://www.biorxiv.org/content/10.1101/2020.03.05.977801v1, (2020).
- 53.Tiwari S, Wang S, Hagen G. & Guilfoyle TJ Transfection assays with protoplasts containing integrated reporter genes. Methods Mol Biol 323, 237–244, (2006). [DOI] [PubMed] [Google Scholar]
- 54.Buenrostro JD, Wu B, Chang HY & Greenleaf WJ ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr Protoc Mol Biol 109, 21 29 21–21 29 29, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie 1. Encapsulation of human GM19239 cells in agarose beads.