Abstract
Background
This multicentre, open‐label study evaluated the efficacy and safety of antiprogrammed death ligand 1 antibody SHR‐1316 plus liposomal irinotecan and 5‐fluorouracil as the first‐line treatment for patients with advanced esophageal squamous cell carcinoma (ESCC).
Methods
Eligible patients received SHR‐1316 (10 mg/kg), liposomal irinotecan (60 mg/m2 for the first cycle, 80 mg/m2 thereafter), and 5‐fluorouracil (2400 mg/m2) every 14 days until disease progression, intolerable toxicity or withdrawal of consent. The primary endpoint was progression‐free survival (PFS). Secondary endpoints were objective response rate (ORR), disease control rate (DCR), overall survival (OS), and safety.
Results
We enrolled 23 patients between 11 March 2019 and 31 May 2019. The median follow‐up duration was 15.2 months (95% CI 14.2–16.2). The median PFS was 8.5 months (95% CI 1.2–15.8), and ORR and DCR were 52.2% (95% CI 30.1–74.3) and 73.9% (95% CI 54.5–93.3), respectively. The median OS was 11.6 months (95% CI 6.7–16.6). The most common treatment‐related grade 3–4 adverse events (AEs) were neutropenia (17.4%), nausea (13.0%), and anorexia (13.0%). Treatment‐related serious AEs occurred in two patients. No treatment‐related deaths occurred.
Conclusions
SHR‐1316 plus liposomal irinotecan and 5‐fluorouracil has a promising efficacy and manageable safety profile, and could be a new first‐line treatment approach for patients with unresectable locally advanced or distant metastatic ESCC.
Keywords: anti‐PD‐L1 antibody, chemotherapy, esophageal squamous cell carcinoma, liposomal irinotecan
In this open‐label, multicentre, phase 2 trial, anti‐PD‐L1 antibody (SHR‐1316) plus liposomal irinotecan and 5‐fluorouracil resulted in an impressive median progression‐free survival of 8.5 months, median overall survival of 11.6 months, objective response rate of 52.2%, disease control rate of 73.9%, and median duration of response of 11.2 months. Adverse events were also manageable.
INTRODUCTION
Esophageal cancer is one of the most aggressive malignancies, ranking as the seventh most common cancer and the sixth leading cause of cancer death worldwide. 1 Asia accounts for 75% of the world's burden of esophageal cancer and China contributes almost half of esophageal cancer cases globally. 2 Esophageal squamous cell carcinoma (ESCC) remains the predominant histological subtype in Asia. 2 , 3 However, the optimal first‐line treatment for advanced or distant metastatic ESCC patients has not been established.
In past decades, chemotherapy has been the recommended treatment for advanced or distant metastatic ESCC in the upfront setting. To our knowledge, cisplatin, 5‐fluorouracil, taxanes, and irinotecan have been evaluated as first‐line treatment for advanced ESCC. 4 , 5 , 6 , 7 , 8 , 9 , 10 Objective response rates (ORRs) with the combinations of these drugs ranged between 30% and 56.5%. 4 , 5 , 6 , 7 , 8 , 9 , 10 Specifically, the ORRs with irinotecan‐based chemotherapy in the first‐line setting were between 30% and 42.9%. 8 , 9 , 10 The survival benefits of these traditional first‐line regimens remain unsatisfactory, with typical median progression‐free survival (PFS) of 4.4–6.1 months 6 , 7 , 8 , 9 , 10 and median overall survival (OS) of 6.7–17.0 months. 4 , 5 , 6 , 7 , 8 , 9 , 10
Recently, immune checkpoint inhibitors (ICIs) targeting the programmed cell death protein 1 (PD‐1) or its ligand programmed death ligand 1 (PD‐L1) has been reported to be effective in many kinds of malignancies. 11 , 12 , 13 Several anti‐PD‐1 antibodies have demonstrated promising efficacy and manageable safety in the treatment of advanced ESCC patients. 14 , 15 , 16 , 17 In two randomized phase 3 trials, both nivolumab and camrelizumab were associated with significant improvements in OS compared with chemotherapy in the second‐line treatment of patients with unresectable advanced or recurrent ESCC. 18 , 19 In the first‐line setting, pembrolizumab plus chemotherapy versus chemotherapy plus placebo provided a statistically significant and clinically meaningful improvement in OS in the phase 3 KEYNOTE‐590 study, suggesting a synergistic effect of chemotherapy in combination with ICIs. 20 Although a number of anti‐PD‐L1 antibodies have offered similar benefits in combination with chemotherapy for patients with other malignancies, 21 , 22 , 23 no report has addressed the treatment outcomes of advanced or distant metastatic ESCC patients with an anti‐PD‐L1 antibody combined with chemotherapy.
We therefore initiated a prospective phase 2 trial to investigate the efficacy and safety of an anti‐PD‐L1 antibody combined with chemotherapy in the first‐line treatment of patients with unresectable locally advanced or distant metastatic ESCC. The ICI adopted in the current trial was SHR‐1316, a recombinant fully humanized IgG4 monoclonal antibody with high affinity and specificity for PD‐L1. In the dose escalation phase 1 trial of SHR‐1316, three dose‐escalating groups were designed (3 mg/kg, 10 mg/kg, 20 mg/kg), the results showed that patients treated with the dosage of 20 mg/kg did not experience dose‐limiting toxicity and maximum tolerated dose was not reached. In addition, we referred to the dose design of PD‐L1 antibody avelumab (10 mg/kg every 2 weeks) in the JAVELIN Ovarian 200 Phase III study, in which avelumab was administered alone or in combination with chemotherapy in patients with platinum‐resistant/refractory ovarian cancer. 24 We therefore used the SHR‐1316 at a dose of 10 mg/kg every 2 weeks in the present study. For the chemotherapy backbone, we used 5‐fluorouracil plus liposomal irinotecan. Liposomal irinotecan comprises irinotecan in liposome particles, thereby increasing tumor exposure to irinotecan and its active metabolite (SN‐38), and reducing treatment‐related adverse events (AEs). 25 We assumed that the substitution of the traditional irinotecan with the novel liposomal form may result in less toxicity, and the addition of SHR‐1316 to this effective regimen may further improve the outcomes.
METHODS
Study design and participants
In this prospective, multicentre, open‐label, phase 2 trial we recruited patients aged between 18 and 75 years old who had histologically/cytologically proven unresectable locally advanced or distant metastatic ESCC and had not received systemic therapies. Additional eligibility criteria included having at least one measurable lesion per the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, a life expectancy of at least 12 weeks, and adequate hematological, hepatic, and renal function assessed by complete blood count and blood chemistry tests.
Patients were excluded if they presented with body mass index <18 kg/m2, had central nervous system metastases, had uncontrolled pleural effusion, pericardial effusion, or ascites that required repeated drainage, had active or history of autoimmune disease, had received immunosuppressants within 2 weeks before enrolment, had previously been treated with anti‐PD‐1 or anti‐PD‐L1 antibody, had uncontrolled hypertension or clinically significant heart disease, or presented with active infection before 4 weeks of enrolment. Patients who had previously received neoadjuvant or adjuvant chemotherapy were eligible if the last treatment was at least 6 months prior to recurrence or progression.
The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The research protocol was reviewed and approved by the institutional review board or ethics committee at each participating institution. All patients provided written informed consent before the study treatment.
Drug administration
Eligible patients received SHR‐1316 (Jiangsu Hengrui Medicine Co. Ltd) intravenously at a dose of 10 mg/kg on day 1, liposomal irinotecan (Jiangsu Hengrui Medicine Co. Ltd) intravenously on day 1 (60 mg/m2 for the first cycle, 80 mg/m2 thereafter), and 5‐fluorouracil (Shanghai Xudong Haipu Pharmaceutical Co. Ltd) 2400 mg/m2 continuous infusion for 46 hours from day 1. All treatments were repeated every 14 days until disease progression, intolerable toxicity or withdrawal of consent, whichever occurred first. In consideration of the possibility of pseudoprogression, patients could continue the study treatment beyond initial disease progression at the discretion of the investigator(s). SHR‐1316 administration could be temporarily suspended or permanently discontinued for suspected or confirmed immune‐related AEs. Dose reductions were not permitted for SHR‐1316, while dose adjustments for liposomal irinotecan and 5‐fluorouracil were allowed.
Evaluation of disease
Baseline tumor imaging via CT or MRI scan was performed within 4 weeks before the initiation of the study treatment. Subsequent imaging evaluations were conducted every 6 weeks until disease progression. Response evaluation was performed according to RECIST version 1.1.
Monitoring safety
Laboratory tests including standard complete blood counts, blood biochemistry, and electrocardiograms were repeated every 2 weeks, while coagulation function, thyroid function tests, serum cortisol, adrenocorticotropic hormone, and urine and fecal routine tests were repeated every 4 weeks. The AEs were monitored throughout the study treatment, and treatment‐related AEs were collected until 90 days after the last dose of the study treatment. All AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI‐CTCAE), version 4.03. Patients were followed up via telephone every month after the discontinuation of the study treatment until death.
PD‐L1 expression
Tumor cell PD‐L1 expression was assessed using archival or newly obtained tumor samples at a central laboratory using a human PD‐L1 immunohistochemistry kit and the 6E8 antibody (Shuwen Biotech Co. Ltd). The membrane expression of PD‐L1 on tumor cells was determined by two independent pathologists blinded to the clinical data. PD‐L1 positivity was defined as PD‐L1 expression on at least 1% of tumor cells. For cases in which the tissue sample had not been optimally collected or prepared or in which PD‐L1 expression could not be assessed, the PD‐L1 status was categorized as unevaluable.
Endpoints and statistical analysis
The primary endpoint of the study was PFS in the intention‐to‐treat population, defined as the time period between treatment initiation and the first documented disease progression or death of any cause, with censoring for patients alive and progression‐free at data cut‐off.
Secondary endpoints included ORR, disease control rate (DCR), OS, and safety. ORR was defined as the percentage of patients achieving a best response of complete response (CR) or partial response (PR) as per RECIST version 1.1, DCR was the percentage of patients with a best response of CR, PR, or stable disease (SD). OS was defined as the time period between the start of the study treatment and death of any cause, censored for patients alive at data cut‐off.
The exploratory endpoint of the study was the investigation of the correlation between the PD‐L1 expression in tumor samples and response to the study treatment.
The efficacy analysis was performed in the intention‐to‐treat population; the safety analysis was assessed in all patients who received at least one dose of any of the study drugs. The Kaplan–Meier method was used to estimate time‐to‐event variables. The differences in rate were compared using Fisher's exact test. We used SPSS (version 22) for statistical analyses.
RESULTS
Patient characteristics
Twenty‐three eligible patients were enrolled between 11 March 2019 and 31 May 2019. The median age was 63 years (range: 44–75 years) and 18 of the patients (78.3%) were male (Table 1). Twenty‐two patients (95.7%) presented with metastatic disease. For patients with recurrent disease, prior treatment before the onset of the recurrent disease included surgical resection in nine (39.1%) patients, adjuvant or neoadjuvant chemotherapy in five (21.7%) patients, and radiotherapy in four (17.4%) patients.
TABLE 1.
Baseline characteristics of patients (n = 23)
| Characteristics | Number of patients [cases (%)] |
|---|---|
| Age, year [median (range)] | 63 (44–75) |
| <60 | 10 (43.5) |
| ≥60 | 13 (56.5) |
| Gender | |
| Male | 18 (78.3) |
| Female | 5 (21.7) |
| ECOG PS score | |
| 0 | 15 (65.2) |
| 1 | 8 (34.8) |
| Histologic grade | |
| G1 | 1 (4.3) |
| G2 | 9 (39.1) |
| G3 | 5 (21.7) |
| Unknown | 8 (34.8) |
| Number of organs with metastasis | |
| 1 | 11 (47.8) |
| ≥2 | 12 (52.2) |
| Site of metastases | |
| Lymph node | 20 (87.0) |
| Lung | 8 (34.8) |
| Liver | 3 (13.0) |
| Bone | 3 (13.0) |
| Others | 4 (17.4) |
| PD‐L1 expression | |
| <1% | 2 (8.7) |
| ≥1% | 16 (69.6) |
| <5% | 5 (21.7) |
| ≥5% | 13 (56.5) |
| <10% | 6 (26.1) |
| ≥10% | 12 (52.2) |
| <25% | 14 (60.9) |
| ≥25% | 4 (17.4) |
| Unevaluable | 5 (21.7) |
| Previous treatment | |
| Surgery | 9 (39.1) |
| Radiotherapy | 4 (17.4) |
| Adjuvant or neoadjuvant chemotherapy | 5 (21.7) |
| Extent of disease | |
| Unresectable locally advanced | 1 (4.3) |
| Metastatic disease | 22 (95.7) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Patient disposition
The median follow‐up duration was 15.2 months (95% CI 14.2–16.2) as of the data cut‐off date (31 July 2020) and 21 patients had discontinued the study treatment. The study treatment was ongoing in two patients. The reasons for treatment discontinuation were radiographic disease progression (12/21, 57.1%), treatment‐related AEs (2/21, 9.5%), treatment‐unrelated AEs (2/21, 9.5%), and withdrawal of consent (5/21, 23.8%).
Treatment exposure
The median duration of treatment with SHR‐1316 was 4.2 months (range 0.0–14.9 months) (median number of doses 10 [range 1–26]). The median duration of treatment with chemotherapy was 3.1 months (range 0.0–13.6 months) (median number of cycles 7 [range, 1–24]). Moreover, 18 patients received liposomal irinotecan at a preplanned dosage of 80 mg/m2 after the first cycle (median number of cycles 3.5 [range 1–22]).
Anti‐tumor activity
All 23 patients were included in the efficacy analysis. The median PFS was 8.5 months (95% CI 1.2–15.8), and the ORR and DCR were 52.2% (95% CI 30.1–74.3) and 73.9% (95% CI 54.5–93.3), respectively (Table 2). Among the responders, 11 patients had a confirmed PR and one patient achieved confirmed CR. Additionally, one patient achieved an unconfirmed PR after withdrawal of consent and discontinuation of study treatment prior to receiving any additional anticancer treatment. Fifteen (65.2%) patients experienced a reduction in target lesion burden from the baseline (Figure 1(a)), and the response was durable in most patients (Figure 1(b)). The median time to initial response was 1.4 months (range 1.3–7.0) in the 12 patients with an objective response (Figure 1(c)), and the median duration of response (DoR) was 11.2 months (95% CI 6.5–15.8). At the time of data cut‐off, 15 deaths had occurred and the median OS was 11.6 months (95% CI 6.7–16.6).
TABLE 2.
Antitumor activity of the study treatment
| Efficacy variables | Number of patients [cases (%)] |
|---|---|
| Best overall response | |
| Complete response | 1 (4.3) |
| Partial response | 11 (47.8) |
| Stable disease | 5 (21.7) |
| Progressive disease | 5 (21.7) |
| Unconfirmed partial response a | 1 (4.3) |
| Objective response | 12 (52.2) |
| Disease control | 17 (73.9) |
| Median progression‐free survival | 8.5 months (95% CI:1.2–15.8) |
| Median overall survival | 11.6 months (95% CI:6.7–16.6) |
One patient at follow‐up after study treatment discontinuation due to consent withdrawal achieved an unconfirmed partial response prior to receiving any additional anticancer treatments.
FIGURE 1.
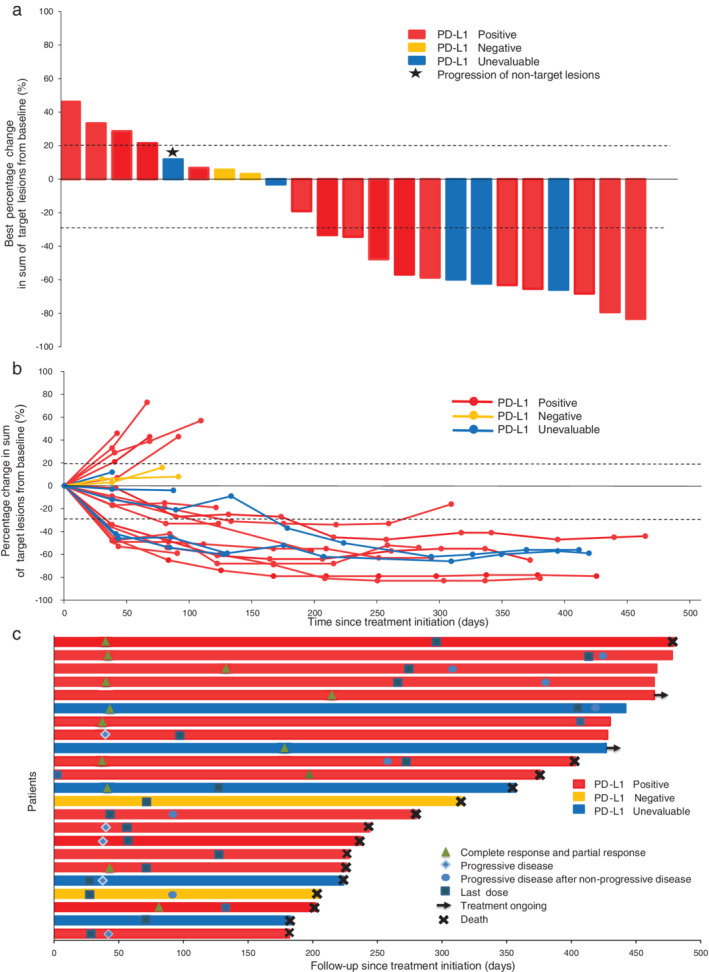
Activity of the study treatment. (a) Best change of target lesions from baseline. The asterisk indicates progression of nontarget lesions. (b) Longitudinal change of target lesions from baseline. (c) Treatment period and duration of response. The length of each bar represents the time to the last follow‐up
Safety
Safety analyses were based on the total of 23 patients. Treatment‐related AEs, as determined by the investigator(s), occurred in all patients. The grade 3–4 treatment‐related AEs were observed in 10 patients (43.5%) with neutropenia (4/23, 17.4%), nausea (3/23, 13.0%), anorexia (3/23, 13.0%), and leukopenia (2/23, 8.7%) occurring in ≥5% patients (Table 3). Treatment‐related AEs led to dose reductions of chemotherapy in 11 patients (47.8%) and discontinuation of SHR‐1316 in two patients having neutropenia (one case of patient refusal and one case of physician's decision). In addition, three patients (13.0%) refused chemotherapy without dose reduction because of treatment‐related AEs, and two of them received SHR‐1316 monotherapy afterwards.
TABLE 3.
Treatment‐related adverse events (TRAEs)
| Events | Number of patients [cases (%)] | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Nausea | 12 (52.2) | 4 (17.4) | 3 (13.0) | 0 (0.0) |
| Anorexia | 11 (47.8) | 3 (13.0) | 3 (13.0) | 0 (0.0) |
| Fatigue | 9 (39.1) | 5 (21.7) | 0 (0.0) | 0 (0.0) |
| Vomiting | 7 (30.4) | 5 (21.7) | 1 (4.3) | 0 (0.0) |
| Diarrhea | 7 (30.4) | 7 (30.4) | 1 (4.3) | 0 (0.0) |
| Weight loss | 4 (17.4) | 2 (8.7) | 1 (4.3) | 0 (0.0) |
| Fever | 2 (8.7) | 2 (8.7) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 4 (17.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Leukopenia | 4 (17.4) | 6 (26.1) | 2(8.7) | 0 (0.0) |
| Neutropenia | 3 (13.0) | 3 (13.0) | 3 (13.0) | 1 (4.3) |
| Anemia | 1 (4.3) | 2 (8.7) | 1 (4.3) | 0 (0.0) |
| Thrombocytopenia | 4 (17.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ALT level increase | 3 (13.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AST level increase | 3 (13.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Blood bilirubin level increase | 1 (4.3) | 0 (0.0) | 1 (4.3) | 0 (0.0) |
| Hyperthyroidism | 4 (17.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hyponatremia | 1 (4.3) | 0 (0.0) | 1 (4.3) | 0 (0.0) |
Note: TRAEs observed in ≥10% of the patients and all the TRAEs of grade 3 or higher are listed; there were no grade 5 TRAEs.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Treatment‐related serious AEs were reported in two patients (8.7%), including one patient (4.3%) with grade 3 diarrhea and grade 2 fever, and one patient (4.3%) with grade 1 fever. All treatment‐related serious AEs resulted in the suspension of study treatment and were managed with appropriate medical care. There were no treatment‐related deaths.
AEs that were potentially immune‐related, as determined by the investigator(s), occurred in five patients (21.7%), including hyperthyroidism (n = 4, 17.4%), hypothyroidism (n = 1, 4.3%), pruritus (n = 1, 4.3%), and rash (n = 1, 4.3%) (Table 4). No grade ≥3 immune‐related AEs occurred. All these immune‐related AEs were managed with proper medical care and did not result in treatment discontinuation in any patient.
TABLE 4.
Immune‐related adverse events
| Events | Number of patients [cases (%)] | |
|---|---|---|
| Grade 1 | Grade 2 | |
| Hyperthyroidism | 4 (17.4) | 0 (0.0) |
| Hypothyroidism | 0 (0.0) | 1 (4.3) |
| Pruritus | 0 (0.0) | 1 (4.3) |
| Rash | 1 (4.3) | 0 (0.0) |
Note: No grade ≥3 immune‐related adverse events occurred.
PD‐L1 expression and efficacy
Eighteen patients provided evaluable tissue samples for the assessment of baseline tumor cell PD‐L1 expression, among which 16 (88.9%), 13 (72.2%), 12 (66.7%), and four (22.2%) patients had PD‐L1 expression ≥1%, ≥5%, ≥10%, and ≥25%, respectively. The ORR was 56.3% (9/16), 61.5% (8/13), 66.7% (8/12), and 75.0% (3/4), and the DCR was 68.8% (11/16), 76.9% (10/13), 75.0% (9/12), and 75.0% (3/4) in the four subgroups, respectively (Table 5). The differences in ORR and DCR in subgroups according to PD‐L1 expression level were not statistically significant, as shown in Table 5.
TABLE 5.
Responses according to PD‐L1 expression
| PD‐L1 expression | Objective response | p value | Disease control | p value |
|---|---|---|---|---|
| <1% | 0.0% (0/2) | 0.471 | 100.0% (2/2) | 1.000 |
| ≥1% | 56.3% (9/16) | 68.8% (11/16) | ||
| <5% | 20.0% (1/5) | 0.294 | 60.0% (3/5) | 0.583 |
| ≥5% | 61.5% (8/13) | 76.9% (10/13) | ||
| <10% | 16.7% (1/6) | 0.131 | 66.7% (4/6) | 1.000 |
| ≥10% | 66.7% (8/12) | 75.0% (9/12) | ||
| <25% | 42.9% (6/14) | 0.576 | 71.4% (10/14) | 1.000 |
| ≥25% | 75.0% (3/4) | 75.0% (3/4) |
Treatments after discontinuing study therapy
Among the 21 patients who had discontinued the study treatment, 13 patients received subsequent anticancer therapies, including radiotherapy (4/21, 19.0%), chemoradiotherapy (1/21, 4.8%), and chemotherapy (8/21, 38.1%).
DISCUSSION
To our knowledge, this is the first study reporting the antitumor activity and safety of PD‐L1 antibody (SHR‐1316) in combination with chemotherapy in patients with untreated advanced ESCC. Our findings indicate that SHR‐1316 combined with liposomal irinotecan and 5‐fluorouracil has promising efficacies and manageable safety profiles in the first‐line treatment of advanced ESCC. This novel combination regimen resulted in an impressive median PFS of 8.5 months, median OS of 11.6 months, ORR of 52.2%, DCR of 73.9%, and durable responses with a median DoR of 11.2 months. The safety profile was manageable and most of the treatment‐related AEs were of grade 1 or 2. Currently, this prospective, multicentre, phase 2 study is the first one to evaluate anti‐PD‐L1 antibody combined with chemotherapy in advanced ESCC.
Patients with advanced ESCC have a poor prognosis, and although combination chemotherapy is typically given as first‐line treatment, the benefit is limited. Currently, 5‐fluorouracil combined with platinum remains the most commonly recommended first‐line treatment regimen for advanced ESCC patients, while other cytotoxic drugs such as taxanes and irinotecan are also reasonable choices. In previous studies concerning the application of chemotherapy in the first‐line treatment of ESCC, the median PFS was 4.4–6.1 months, ORR was 30.0–56.5%, and the median OS was 6.7–17.0 months. 4 , 5 , 6 , 7 , 8 , 9 , 10 Among these studies, irinotecan‐based regimens including irinotecan plus 5‐fluorouracil resulted in median PFS of 4.4–4.5 months, ORR of 30.0–42.9%, and median OS of 8.8–10.0 months. 8 , 9 , 10 Liposomal irinotecan is associated with increased tumor exposure to irinotecan and its active metabolite, and decreased treatment‐related AEs. 25 We therefore chose the novel liposomal irinotecan in combination with 5‐fluorouracil as a chemotherapy backbone. The PFS in our study was superior to that recorded in previous studies that evaluated traditional chemotherapy, including irinotecan‐based regimens in the first‐line treatment of advanced ESCC. 6 , 7 , 8 , 9 The improvement of median DoR was also observed (11.2 months vs. 5.0–9.3 months). 4 , 5 , 8 , 9 The notable contrast in PFS and DoR reflects a durable response in our trial. Although no report exists concerning the application of liposomal irinotecan plus 5‐fluorouracil for advanced ESCC treatment, a phase 2 study evaluating the efficacy and safety of liposomal irinotecan plus 5‐fluorouracil versus paclitaxel as second‐line therapy in patients with advanced ESCC is ongoing in France. 26
Furthermore, ICIs have been evaluated in advanced ESCC patients in recent years. The randomized phase 3 trials ATTRACTION‐3 and ESCORT have shown that the PD‐1 antibodies nivolumab and camrelizumab are related to an improved OS compared with chemotherapy in the second‐line treatment of advanced ESCC patients; the ORR was 19.0–20.2% and median PFS was 1.9 months. 18 , 19 In first‐line treatment, findings of a recent phase 3 study, KEYNOTE‐590, have demonstrated that pembrolizumab combined with chemotherapy versus chemotherapy provided significantly superior PFS, OS, and ORR in patients with advanced esophageal cancer, including ESCC and esophageal adenocarcinoma. 20 The efficacy observed in our study was comparable to that of patients treated with pembrolizumab plus chemotherapy in KEYNOTE‐590. In the pembrolizumab plus chemotherapy arm, the median PFS and OS for ESCC patients were 6.3 and 12.6 months, respectively, and the median PFS, OS, ORR, and DoR for all patients were 6.3, 12.4 months, 45.0%, and 8.3 months, respectively. 20 The median PFS and DoR seemed longer in our study compared with those from KEYNOTE‐590. However, caution is needed when interpreting our results because of the relatively small sample size, different chemotherapy regimens, and the enrolment of only Chinese patients.
No reports ever addressed the role of liposomal irinotecan in the treatment of advanced ESCC before the present study. Therefore, we cannot affirm that the promising efficacy observed in our study was due to the fact that liposomal irinotecan itself is more effective or that SHR‐1316 is synergistic with liposomal irinotecan and 5‐fluorouracil, thus large randomized, multicentre studies to clarify the role of SHR‐1316 and liposomal irinotecan in advanced ESCC are warranted.
With respect to safety profile, compared with the results from the KEYNOTE‐590 trial, our study was associated with a decreased incidence of grade ≥3 treatment‐related AEs (43.5% vs. 72%). 20 The incidence rate of immune‐related AEs in our study (21.7%) was reduced when contrasted to that observed in patients treated with the PD‐L1 inhibitor atezolizumab plus chemotherapy, 21 , 22 and no grade ≥3 immune‐related AEs occurred in our study, indicating that SHR‐1316 is well tolerated. AEs potentially related to chemotherapy in our study were consistent with those in the previous NAPOLI‐1 study, in which gemcitabine refractory metastatic pancreatic cancer patients received nanoliposomal irinotecan plus 5‐fluorouracil regimen, and the most common grade 3–4 treatment‐emergent AEs were neutropenia (27%), diarrhea (13%), vomiting (11%), fatigue (14%), and anemia (9%), 27 suggesting that the addition of SHR‐1316 did not increase the toxicity of chemotherapy. Moreover, the incidence of grade 3–4 hematological toxicity and diarrhea in our study decreased in contrast to irinotecan plus cisplatin in the treatment of advanced or distant metastatic ESCC patients, especially neutropenia (17.4% vs. 40.7–50.0%). 8 , 9 Furthermore, grade 3–4 diarrhea seemed less frequent in our study relative to conventional irinotecan monotherapy (4.3% vs. 18.2–30%) and other liposomal irinotecan (4.3% vs. 27.3%) in the treatment of gastric adenocarcinoma. 25 , 29
PD‐L1 expression has been reported to be associated with the efficacy of other PD‐1/PD‐L1 inhibitors in many cancers. 29 , 30 , 31 However, the predictive role of PD‐L1 expression in the ICI treatment of patients with esophageal cancer is unclear. In the ATTRACTION‐3 trial, the OS benefit of nivolumab compared with chemotherapy in the second‐line treatment of ESCC patients was observed irrespective of tumor cell PD‐L1 expression. 19 Similarly, in the ESCORT trial, clinical benefits of camrelizumab were observed in all PD‐L1 expression subgroups, although patients with higher PD‐L1 expression appear to derive more benefit of OS than those with low PD‐L1 expression. 18 In the KEYNOTE‐590 trial, although the improvement in OS with pembrolizumab plus chemotherapy versus chemotherapy alone in untreated patients with ESCC and esophageal adenocarcinoma was independent of PD‐L1 CPS status, patients with CPS ≥10 achieved greater benefit (CPS ≥10 HR 0.62, 95% CI 0.49–0.78 vs. CPS < 10 HR 0.86, 95% CI 0.68–1.10). 20 In the present study, ORR was numerically higher in patients with higher tumor cell PD‐L1 expression, while we did not observe statistically significant differences in ORR and DCR in subgroups according to PD‐L1 expression. When PD‐L1 antibody is combined with chemotherapy, the predictive role of PD‐L1 expression in response in ESCC patients becomes more intricate, and the relatively small sample size of our study and the complex tumor‐immune microenvironment may also complicate the results.
The limitations of the present study include the single‐arm design and the relatively small sample size. Therefore, we are unable to compare our findings directly with results of chemotherapy treatment. In particular, both SHR‐1316 and liposomal irinotecan have not been reported in the treatment of ESCC, thus a randomized, double‐blind, controlled study is warranted. In addition, our study did not use iRECIST for tumor response assessment. Furthermore, our findings were conducted in an unselected cohort. Future studies should distinguish patients that would most likely benefit from this new combination strategy through certain biomarkers.
In summary, SHR‐1316 plus liposomal irinotecan and 5‐fluorouracil showed encouraging antitumor activity and a favorable safety profile in the first‐line treatment of patients with unresectable locally advanced or distant metastatic ESCC, and SHR‐1316 could be administered in combination with these two cytotoxic drugs.
CONFLICT OF INTEREST
Xiaopeng Wang is an employee of Jiangsu Hengrui Medicine Co. Ltd. The other authors declare no competing interests.
ACKNOWLEDGMENTS
This study was sponsored and funded by Jiangsu Hengrui Medicine Co. Ltd. We thank the patients, their families, and the study personnel involved in this trial.
Mu L, Song Y, Zhao K, et al. SHR‐1316, an anti‐PD‐L1 antibody, plus chemotherapy as the first‐line treatment for advanced esophageal squamous cell carcinoma: A multicentre, phase 2 study. Thorac Cancer. 2021;12:1373–1381. 10.1111/1759-7714.13913
Clinical trial registration number: NCT03732508 (Clinicaltrials.gov).
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Malhotra GK, Yanala U, Ravipati A, Follet M, Vijayakumar M, Are C. Global trends in esophageal cancer. J Surg Oncol. 2017;115:564–79. [DOI] [PubMed] [Google Scholar]
- 3. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayashi K, Ando N, Watanabe H, Ide H, Nagai K, Aoyama N, et al. Phase II evaluation of protracted infusion of cisplatin and 5‐fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan esophageal oncology group (JEOG) trial (JCOG9407). Jpn J Clin Oncol. 2001;31:419–23. [DOI] [PubMed] [Google Scholar]
- 5. Bleiberg H, Conroy T, Paillot B, Lacave AJ, Blijham G, Jacob JH, et al. Randomised phase II study of cisplatin and 5‐fluorouracil (5‐FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer. 1997;33:1216–20. [DOI] [PubMed] [Google Scholar]
- 6. Huang J, Zhou Y, Zhang H, Qu T, Mao Y, Zhu H, et al. A phase II study of biweekly paclitaxel and cisplatin chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma: ERCC1 expression predicts response to chemotherapy. Med Oncol. 2013;30:343. [DOI] [PubMed] [Google Scholar]
- 7. Gong Y, Ren L, Zhou L, Zhu J, Huang M, Zhou X, et al. Phase II evaluation of nedaplatin and paclitaxel in patients with metastatic esophageal carcinoma. Cancer Chemother Pharmacol. 2009;64:327–33. [DOI] [PubMed] [Google Scholar]
- 8. Kim M, Keam B, Kim TM, Kim HG, Kim JS, Lee SS, et al. Phase II study of irinotecan and cisplatin combination chemotherapy in metastatic, unresectable esophageal cancer. Cancer Res Treat. 2017;49:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee DH, Kim HT, Han JY, Lee SY, Yoon SJ, Kim HY, et al. A phase II trial of modified weekly irinotecan and cisplatin for chemotherapy‐naive patients with metastatic or recurrent squamous cell carcinoma of the esophagus. Cancer Chemother Pharmacol. 2008;61:83–8. [DOI] [PubMed] [Google Scholar]
- 10. Wolff K, Wein A, Reulbach U, Männlein G, Brückl V, Meier C, et al. Weekly high‐dose 5‐fluorouracil as a 24‐h infusion and sodium folinic acid (AIO regimen) plus irinotecan in patients with locally advanced nonresectable and metastatic adenocarcinoma or squamous cell carcinoma of the oesophagus: a phase II trial. Anticancer Drugs. 2009;20:165–73. [DOI] [PubMed] [Google Scholar]
- 11. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- 12. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE‐059 trial. JAMA Oncol. 2018;4:e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD‐L1 as cancer therapeutics. J Hematol Oncol. 2019;12:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous‐cell carcinoma: an open‐label, multicentre, phase 2 trial. Lancet Oncol. 2017;18:631–9. [DOI] [PubMed] [Google Scholar]
- 15. Doi T, Piha‐Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, et al. Safety and antitumor activity of the anti‐programmed death‐1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. 2018;36:61–7. [DOI] [PubMed] [Google Scholar]
- 16. Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, et al. Safety, activity, and biomarkers of SHR‐1210, an anti‐PD‐1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24:1296–304. [DOI] [PubMed] [Google Scholar]
- 17. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE‐180 study. JAMA Oncol. 2019;5:546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second‐line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open‐label, phase 3 study. Lancet Oncol. 2020;21:832–42. [DOI] [PubMed] [Google Scholar]
- 19. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION‐3): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20:1506–17. [DOI] [PubMed] [Google Scholar]
- 20. Kato K, Sun JM, Shah MA, Enzinger PC, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy as first‐line therapy in patients with advanced esophageal cancer: the phase 3 KEYNOTE‐590 study. Ann Oncol. 2020;31:S1192–3. [Google Scholar]
- 21. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. N Engl J Med. 2018;379:2108–21. [DOI] [PubMed] [Google Scholar]
- 22. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20:924–37. [DOI] [PubMed] [Google Scholar]
- 23. Nowak AK, Lesterhuis WJ, Kok PS, Brown C, Hughes BGM, Karikios DJ, et al. Durvalumab with first‐line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): a multicentre, single‐arm, phase 2 trial with a safety run‐in. Lancet Oncol. 2020;21:1213–23. [DOI] [PubMed] [Google Scholar]
- 24. Pujade‐Lauraine E, Fujiwara K, Dychter SS, Devgan G, Monk BJ. Avelumab (anti‐PD‐L1) in platinum‐resistant/refractory ovarian cancer: JAVELIN ovarian 200 phase III study design. Future Oncol. 2018;14:2103–13. [DOI] [PubMed] [Google Scholar]
- 25. Roy AC, Park SR, Cunningham D, Kang YK, Chao Y, Chen LT, et al. A randomized phase II study of PEP02 (MM‐398), irinotecan or docetaxel as a second‐line therapy in patients with locally advanced or metastatic gastric or gastro‐oesophageal junction adenocarcinoma. Ann Oncol. 2013;24:1567–73. [DOI] [PubMed] [Google Scholar]
- 26. Randrian V, Adenis A, Desrame J, Barbier E, di Fiore F, Lièvre A, et al. Nal‐IRI/LV5‐FU versus paclitaxel as second‐line therapy in patients with metastatic esophageal squamous cell carcinoma (OESIRI)‐PRODIGE 62: a multicentre, randomised, non‐comparative phase II study. Dig Liver Dis. 2020;52:347–50. [DOI] [PubMed] [Google Scholar]
- 27. Wang‐Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine‐based therapy (NAPOLI‐1): a global, randomised, open‐label, phase 3 trial. Lancet. 2016;387:545–57. [DOI] [PubMed] [Google Scholar]
- 28. Enzinger PC, Kulke MH, Clark JW, Ryan DP, Kim H, Earle CC, et al. A phase II trial of irinotecan in patients with previously untreated advanced esophageal and gastric adenocarcinoma. Dig Dis Sci. 2005;50:2218–23. [DOI] [PubMed] [Google Scholar]
- 29. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero‐Torres A, et al. Avelumab, an anti‐PD‐L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat. 2018;167:671–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Powles T, O'Donnell PH, Massard C, et al. Efficacy and safety of Durvalumab in locally advanced or metastatic Urothelial carcinoma: updated results from a phase 1/2 open‐label study. JAMA Oncol. 2017;3:e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]


