Abstract
Adult T‐cell leukemia‐lymphoma (ATL) is caused by human T‐cell leukemia virus type 1 (HTLV‐1) infection. Among HTLV‐1 encoded genes, HTLV‐1 bZIP factor (HBZ) and tax are critical for the leukemogenesis of ATL. Adult T‐cell leukemia‐lymphoma needs a long latent period before onset, indicating that both viral genes and alterations (genetic and epigenetic) of the host genome play important roles for leukemogenesis. Viral genes influence genetic and epigenetic changes of the host genome, indicating that the virus is of primary importance in leukemogenesis. HBZ is expressed in all ATL cases, whereas Tax expression is heterogeneous among ATL cases. Different patterns of viral gene expression in tumors are also observed for Epstein‐Barr virus. We propose three subtypes of ATL cases based on Tax expression: high, intermittent, and lost expression. HBZ is detected in all ATL cases. Approximately 25% of all ATL cases lost Tax expression at infection of HTLV‐1, indicating that HBZ is the only viral gene responsible for leukemogenesis in addition to genetic and epigenetic changes of the host genes in these ATL cases. The host immune responses to Tax are also implicated in the heterogeneity of ATL. Thus, ATL is a heterogeneous disease in terms of its viral gene expression, which is important for pathogenesis of this intractable lymphomatous neoplasm.
Keywords: ATL, CCR4, CTL, EBV, HBZ, HTLV‐1, Tax
In this review, we describe the heterogeneity of adult T‐cell leukemia‐lymphoma (ATL) in regard to viral gene expression, and propose three subtypes of ATL. These findings lead to an understanding of pathogenesis by human T‐cell leukemia virus type 1 and new therapeutic strategies for ATL.

Abbreviations
- ATL
adult T‐cell leukemia‐lymphoma
- auto‐HSCT
autologous hematopoietic stem cell transplantation
- BART
BamHI‐A rightward transcript
- CADM1
cell adhesion molecule 1
- CCR4
CC chemokine receptor 4
- CTCL
cutaneous T‐cell lymphoma
- EBER
EBV‐encoded small RNA
- EBNA
EBV nuclear antigen
- EBV
Epstein‐Barr virus
- GVHD
graft‐versus‐host disease
- HBV
hepatitis B virus
- HBZ
HTLV‐1 bZIP factor
- HPV
human papilloma virus
- HTLV‐1
human T‐cell leukemia virus type 1
- LMP
latent membrane protein
- LTR
long terminal repeat
- TGF‐β
transforming growth factor‐β
- Treg
regulatory T cell
1. INTRODUCTION
Approximately 13% of cancers are caused by infection with viruses, bacterium, or parasites. 1 Human T‐cell leukemia virus type 1 is the causative agent of ATL as well as inflammatory diseases including HTLV‐1‐associated myelopathy/tropical spastic paraparesis. The presence of HTLV‐1 is essential for the development of ATL. The same scenario is observed in oncogenesis by other viruses: EBV, HPV, and HBV. In this review, we redefine ATL in terms of viral gene expression.
2. VIRAL INFECTION AND CANCERS
The IARC, an agency of WHO, has defined several viruses, bacteria, and parasites as carcinogenic agents in humans and reports 2.2 million incidences of cancer caused by infection with these pathogens. 1 Seven viruses were defined as carcinogenic agents in humans. Of these viruses, there are three different types. First are viruses that productively replicate in the host, causing inflammation that leads to the development of cancer. This type includes HBV and hepatitis C virus. Second are viruses that cause latent (often asymptomatic) infection, but become tumorigenic in vivo through genetic and epigenomic alterations. This group includes HTLV‐1, EBV, Kaposi’s sarcoma‐associated herpesvirus, and HPV. Although transcription of viral genes is observed in these virus‐associated tumors, viral replication is not active in these tumors. Finally, HIV‐1 does not directly cause cancer. However, it promotes cancerous growth by causing immunodeficiency in the host.
Adult T‐cell leukemia‐lymphoma is a poor prognostic T‐cell lymphoma caused by HTLV‐1 infection. 2 The first human retrovirus discovered, HTLV‐1 was found to cause ATL and chronic inflammatory diseases. 3 , 4 There are an estimated 10‐20 million individuals with HTLV‐1 infection worldwide, including endemic areas such as southwestern Japan, the Caribbean Islands, Africa, and South America. 5 The virus is mainly transmitted by breast feeding, sexual intercourse, blood transfusion, and needle sharing. 6 Recently, it was reported that approximately 4000 people are newly infected annually by horizontal infection routes in Japan. 7 After a long latent period, ATL develops in 4%‐6% of men and 2.6% of women with HTLV‐1 infection. 8
Among T‐cell lymphomas, ATL cells are similar to CTCL cells. Both malignant cells are CD4+CADM1+. 9 , 10 The frequency of CTCL is reported to be quite low in Japan. 11 However, the risk of development of ATL among HTLV‐1 carriers (~5%) is quite high. Taken together, these observations show that, although the oncogenic risk of CD4+CADM1+ T cells is low compared with mature B cells, HTLV‐1 infection dramatically increases the risk of cancer in this T cell subpopulation. Next, we will address why and how this virus transforms this type of T cell.
3. TAX AND HBZ
The HTLV‐1 provirus is integrated into the host genome and encodes several regulatory and accessory genes such as p12, p13, p30, rex, tax, and HBZ. 12 The most striking feature of HTLV‐1 is that this virus infects target cells exclusively by cell‐to‐cell contact through a specialized structure, the virological synapse, between an infected cell and a target cell. 13 Therefore, to promote transmission, HTLV‐1 must increase the number of infected cells in vivo. It does so by two routes: (i) de novo infection of cells (the infectious route); and (ii) clonal proliferation of infected cells (the mitotic route). 14 After primary infection by HTLV‐1, de novo infection occurs and afterwards, mitotic division is predominant in vivo. At the chronic phase, clonal proliferation of infected cells maintains a relatively steady state of infected cells. As most infected cells contain one provirus per cell, the copy number of the provirus (proviral load) reflects the number of infected cells. 15
Viral genes are implicated in viral replication and the proliferation of infected cells. Of these genes, tax is essential for de novo infection as Tax is indispensable for activation of the sense‐strand transcription of the provirus (Figure 1). HBZ plays important roles for the proliferation, survival, and special immunophenotype (CD4+CD45RO+CCR4+) of infected cells and ATL cells. 14 Transgenic mice expressing Tax or HBZ develop malignant diseases, indicating that both viral genes are oncogenic. 16 , 17 Tax induces different cancers depending on the promoter. With the lck promoter, Tax causes T‐cell lymphomas, although their immunophenotypes differ from those of ATL cells. 18 In contrast, HBZ causes T‐cell lymphomas with an immunophenotype resembling that of ATL cells.
FIGURE 1.
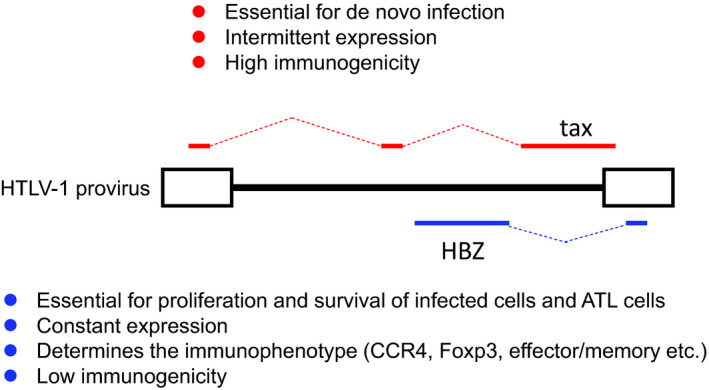
Characteristics of Tax and human T‐cell leukemia virus type 1 (HTLV‐1) bZIP factor (HBZ) in HTLV‐1 infection and adult T‐cell leukemia‐lymphoma (ATL). Tax is essential for de novo infection and intermittent expression of almost all viral proteins, and has high immunogenicity. HBZ is essential for proliferation, and shows constant expression and low immunogenicity
Although Tax is critical for viral replication, it is targeted by CTLs due to the high immunogenicity of the Tax protein. 19 , 20 Therefore, Tax is transiently expressed in ATL cells, allowing expression of the immunogenic viral protein to be minimized. 21 It was once believed that Tax promotes the proliferation of T cells. However, there are only a few reports to support this idea. 22 In contrast, Tax expression is reported to suppress S phase of the cell cycle and induce senescence. 21 , 23 As Tax expression of one quarter of ATL cases is lost at infection, Tax is not involved in the clonal proliferation of ATL cells. Rather, Tax induces genetic instability, which contributes to oncogenesis. 24
The proliferation of expressing cells is promoted by HBZ through upregulation of E2F1. 17 Approximately 40% of transgenic mice expressing HBZ developed T‐cell lymphomas and inflammatory diseases. 17 , 25 Both ATL and HTLV‐1 infected cells are CD4+CD45RO+ effector/memory T cells, and most of them are Foxp3+. 26 , 27 , 28 HBZ is responsible for these immunophenotypes. 29 For example, HBZ increases transcription of the Foxp3 gene through enhancing the TGF‐β/Smad pathway. 30 In contrast, Tax suppresses TGF‐β/Smad signaling and Foxp3 expression. 31 , 32 Furthermore, HBZ induces the expression of CCR4 and TIGIT, which are expressed on ATL cells and HTLV‐1 infected cells. 33 , 34 Thus, the immunophenotypes of these cells are attributed to the actions of HBZ. HBZ converts expressing cells to Treg‐like cells, which means Foxp3+CCR4+ cells. Foxp3 is a master gene of Treg cells and induces the expression of many immunosuppressive molecules including GITR, CTLA4, and CD39, which are also expressed on ATL cells. 35 , 36 This character helps infected cells and ATL cells to evade the host immune system. Thus, Treg‐like cells are suitable vehicles for this virus to hide from host immunosurveillance and transfer to breast milk and semen to enable its transmission.
4. VIRAL GENES IN ATL CASES
The expression pattern and immunogenicity of Tax and HBZ are contrasting. 37 HBZ is constantly expressed with low immunogenicity. Cytotoxic T‐lymphocyte responses are weak due to low immunogenicity and low expression level of HBZ protein. 38 Contrary to HBZ, the highly immunogenic viral protein Tax is transiently expressed (Figure 2A). Among ATL cases, Tax expression is lost in approximately half of all cases due to three mechanisms 39 : (a) deletion of the 5′‐LTR 40 ; (b) nonsense mutations or deletions/insertions in the tax gene 41 , 42 ; and (c) DNA methylation of the 5′‐LTR (Figure 2B). 43 , 44 Importantly, most nonsense mutations in the tax gene are generated by APOBEC3G during reverse transcription. 42 Furthermore, approximately half of proviruses lacking the 5′‐LTR are generated before their integration. 45 Taken together, these observations mean that approximately 25% of ATL cases are derived from infected cells that could not produce Tax from the moment of their infection of HTLV‐1, indicating that these ATL cells transform without Tax. In these cases, leukemogenesis is clearly not dependent on Tax. In contrast, Tax expression is silenced or lost during progression to ATL in approximately one quarter of ATL cases. This loss of Tax expression is caused by DNA methylation and/or deletion of the 5′‐LTR. In this type of ATL, Tax might be necessary at the early stage of leukemogenesis, and then ATL cells lose Tax expression to escape from host immune surveillance.
FIGURE 2.
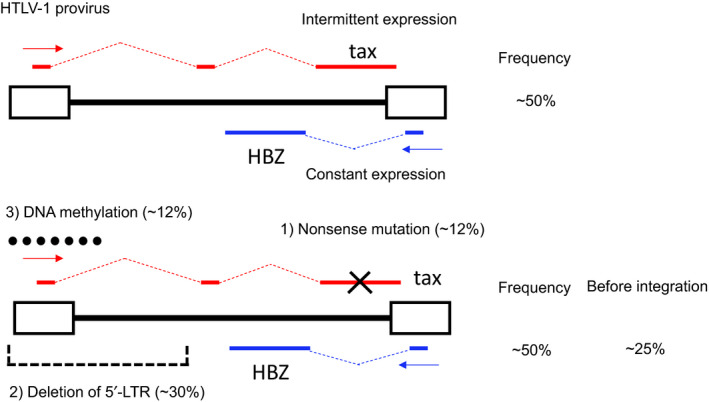
Human T‐cell leukemia virus type 1 (HTLV‐1) provirus and the expression pattern of Tax. A, Complete HTLV‐1 provirus. Adult T‐cell leukemia‐lymphoma (ATL) cells with intact provirus can intermittently express Tax along with constant expression of HTLV‐1 bZIP factor (HBZ). B, Provirus with inactivated Tax expression. Tax expression is absent due to: (a) nonsense mutation of the tax gene; (b) deletion of the 5′‐long terminal repeat (LTR); or (c) DNA methylation of the 5′‐LTR. ATL cells with these proviruses cannot express Tax, whereas they constantly express HBZ. The frequencies of the different kinds of proviruses among ATL cases are shown on the right
The other half of ATL cases can express Tax. Among these cases, there are two different Tax expression patterns. First, Tax is highly expressed in a small number of ATL cases. 39 , 46 Second, most ATL cells maintain the provirus structure that can express Tax but appear to express it only intermittently or at very low levels. It has been reported that Tax is transiently expressed in ATL cell lines and HTLV‐1 infected cell lines. 21 Therefore, it is speculated that ATL cells with an intact tax gene and unmethylated 5′‐LTR express Tax intermittently, like MT‐1 cells do.
These data indicate that there are three ATL subtypes with reference to Tax expression (Figure 3 and Table 1). In contrast, the HBZ coding region and 3′‐LTR remain intact in all ATL cases. 45 , 46 HBZ is critical for the proliferation and survival of ATL cells and the determination of their immunophenotype.
FIGURE 3.
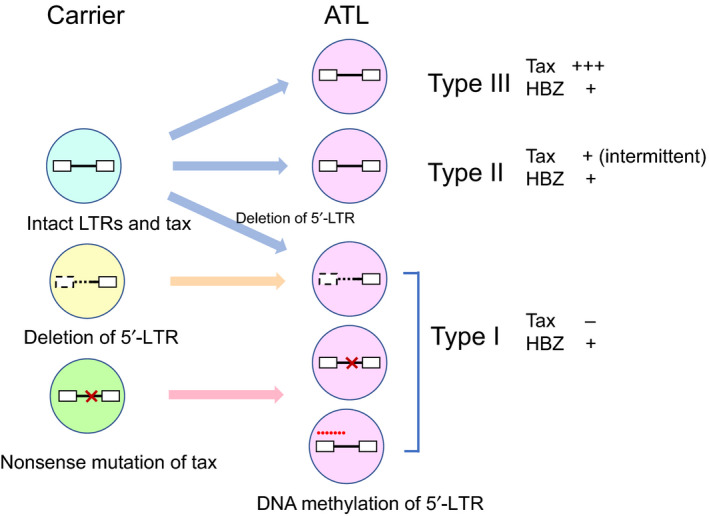
Different human T‐cell leukemia virus type 1 (HTLV‐1) proviruses and expression patterns of tax and HTLV‐1 bZIP factor (HBZ) in carriers and adult T‐cell leukemia‐lymphoma (ATL). In carriers, multiple different proviruses exist. In ATL, Tax expression is disrupted in approximately half of cases by one of three mechanisms: (a) nonsense mutation of the tax gene; (b) deletion of the 5′‐long terminal repeat (LTR); or (c) DNA methylation of the 5′‐LTR. In most other cases, Tax is transiently expressed as it is in MT‐1 cells
TABLE 1.
Three subtypes of adult T‐cell leukemia‐lymphoma
| Type I | Type II | Type III | |
|---|---|---|---|
| Tax | − | ±~+ | ++ |
| HBZ | + | + | + |
Tax expression is quantified by semiquantitative PCR as reported previously. 38
Abbreviation: HBZ, human T‐cell leukemia virus type 1 bZIP factor.
5. SIMILARITY BETWEEN EBV AND HTLV‐1 IN ONCOGENESIS
Epstein‐Barr virus latently infects most human populations. 47 Epstein‐Barr virus causes lymphomas, nasopharyngeal cancers, and gastric cancers in a limited number of infected individuals. After infecting B cells, EBV resides in the nucleus in the form of an episome. Epstein‐Barr virus encodes viral genes associated with latency: EBNA‐1, ‐2, ‐3A, ‐3B, ‐3C, and ‐LP, LMP‐1 and ‐2, BARTs, EBERs, and other genes. The latency of EBV is classified into four subtypes (0, I, IIa, b, and III) (Table 2). 48 , 49 Infected B cells express highly antigenic virus‐associated genes such as EBNA‐1, ‐2, ‐3A, ‐B, and ‐3C for proliferation, and are eliminated by host immune surveillance. This expression pattern is known as Latency III. Some infected B cells become memory B cells that express only nonprotein‐coding genes (Latency 0). Latency I is characterized by the expression of EBNA1, EBER, and BART‐miRNA, whereas the expression of these genes plus LMP‐1, LMP‐2A, and LMP‐2B is observed in Latency IIa. Loss of LMP‐1 expression in Latency IIa is classified as Latency IIb (Table 2). The EBV‐associated cancers are classified based on this latency pattern. Hodgkin lymphoma has a Latency IIa pattern of EBV infection, and Burkitt lymphoma has a Latency I pattern. 50 , 51
TABLE 2.
Latency pattern of EBV genes expression in hematological neoplasms
| Latency I | Latency IIa | Latency IIb | Latency III | |
|---|---|---|---|---|
| EBERs | + | + | + | + |
| EBNA 1 | + | + | + | + |
| EBNA 2 | − | − | + | + |
| EBNA 3s | − | − | + | + |
| EBNA LP | − | − | + | + |
| LMP‐1 | − | + | − | + |
| LMP‐2A/B | − | + | − | + |
| BART miRNA | + | + | + | + |
| Disease | BL | HL | HIV‐associated lymphoma | LPD |
Abbreviations: BART, BamHI‐A rightward transcript; BL, Burkitt lymphoma; EBER, EBV‐encoded small RNA; EBNA, EBV nuclear antigen; HL, Hodgkin lymphoma; LMP, latent membrane protein; LPD, lymphoproliferative disorder.
As described above, ATL cases can be similarly classified into three subtypes according to Tax expression (Table 1). HBZ is expressed in all three subtypes. Tax is highly expressed in a small number of cases (type III ATL cases), which correspond to the Latency III pattern of EBV‐associated tumors. Approximately half of ATL cases (type II ATL cases) maintain the provirus that can express Tax (intact 5′‐LTR and coding region), yet their tax gene transcripts are barely detected. This phenomenon is reminiscent of the intermittent expression pattern observed in MT‐1 cells. 21 It is speculated that Tax is intermittently expressed in these ATL cases. The third ATL subtype, type I, is that in which Tax expression is disrupted by one of the three mechanisms described above (Figure 2B). Importantly, half of proviruses without 5′‐LTR and most nonsense mutations in the tax gene occur prior to integration (Figure 2B). Accordingly, half of type I ATL cases are likely generated during the clinical course of leukemogenesis and/or ATL. In sum, there are three expression patterns of Tax in ATL and in HTLV‐1 infected cells in the carrier state. Thus, ATL is heterogenous with regard to the expression pattern of the tax gene.
The analogy between ATL and EBV‐associated tumors reflects our thinking about the possible roles of the viruses in these cancers. These cancers are caused by exogenic viruses, which means that host immune surveillance plays a critical effect on oncogenesis. Interestingly, expression of EBERs and BART‐miRNA (for EBV) or HBZ (for HTLV‐1) is detected in all EBV‐ or HTLV‐1‐associated tumors. These viral genes function as RNA, which is not recognized by the host immune system. This is a foxy strategy of these oncogenic viruses.
6. IMMUNE RESPONSES AND TREATMENT
Because Tax is an immunogenic viral protein, immunotherapy that targets Tax protein is considered to be a novel therapy against ATL. Indeed, a Tax‐peptide pulsed dendritic cell vaccine effectively suppresses ATL. 52 It is speculated that this therapy is effective on type II and III ATL cases, as these ATL cases depend on Tax. Type I ATL cases are likely resistant to this therapy. It is noteworthy that Tax expression was lost in a relapsed case after the vaccination. 52 Thus, it could be important to consider the ATL subtypes regarding viral gene expression when contemplating a therapeutic approach.
Adult T‐cell leukemia‐lymphoma cells express CCR4, which reflects their Treg‐like character. 53 Mogamulizumab, a humanized mAb to CCR4, has been developed for the treatment of ATL, and shows significant clinical efficacy. 54 Mogamulizumab not only kills ATL cells through enhanced Ab‐dependent cell cytotoxicity, but also enhances antiviral immunity through suppression of Treg cells. 37 The clinical effects of mogamulizumab are sometimes long‐lasting, 54 which suggests that augmented immune responses to the viral antigens Tax and HBZ are closely associated with anti‐ATL effects. Enhanced CTL responses to Tax and HBZ were reported in mogamulizumab‐treated ATL patients. 37 Spontaneous remission of ATL has been reported. 55 Although the mechanism is unclear, increased CD8+ cells and higher CTL activity in ATL patients have been reported to associate with spontaneous remission, suggesting the involvement of an immunological mechanism. 56 , 57
Combination regimens of various anticancer drugs have been used for aggressive ATLs. However, the median survival period is very limited (~13 months), and only approximately 10% of patients show a long‐term response even with the most effective regimen. 58 Therefore, high‐dose chemotherapy with auto‐HSCT has been attempted. It was tried for only 10 cases, all of whom relapsed early or died from infection. 59 , 60 , 61 These findings indicate that this intensive chemotherapy is not effective for ATL, unlike other types of malignant lymphoma or multiple myeloma. As auto‐HSCT suppresses acquired immune responses to pathogens, impaired immune responses to HTLV‐1 could possibly be associated with the deterioration of ATL patients. In contrast to auto‐HSCT, allogeneic HSCT is effective for ATL patients. In a recent systematic review of 18 studies, the overall survival rate after transplantation was approximately 40% and the relapse rate was 36%. 62 Adult T‐cell leukemia‐lymphoma patients with chronic GVHD show a better prognosis than those without chronic GVHD, indicating that host immune responses play critical roles. 63 Thus, immune responses are critical for treatment of ATL.
7. CONCLUSION
In this review, we redefined ATL as a viral disease. Tax and HBZ are implicated in leukemogenesis by HTLV‐1. However, Tax expression is diverse among ATL cases whereas HBZ is constantly expressed. We propose three ATL subtypes in terms of Tax expression. Furthermore, we emphasize the similarity between ATL and EBV‐related malignancies based on the expression pattern of viral genes. The heterogeneity of ATL is possibly linked to responsiveness to therapy. Further studies are needed to develop better strategies of treatment.
DISCLOSURE
Masao Matsuoka has received grant support from Kyowa Kirin Co., Ltd. The other authors have no conflict of interest.
ACKNOWLEDGMENTS
This research is supported by a grant from the Project for Cancer Research and Therapeutic Evolution (P‐CREATE) (20cm0106306h0005 to M. M.), the Research Program on Emerging and Re‐emerging Infectious Diseases (20fk0108088h0002 to M. M.) from the Japan Agency for Medical Research and Development (AMED), and JSPS KAKENHI (19H03689 to MM). This study was also supported in part by the JSPS Core‐to‐Core Program A, Advanced Research Networks (integrative approach for normal and leukemic stem cells).
Nosaka K, Matsuoka M. Adult T‐cell leukemia‐lymphoma as a viral disease: Subtypes based on viral aspects. Cancer Sci. 2021;112:1688–1694. 10.1111/cas.14869
Funding information
Project for Cancer Research and Therapeutic Evolution (P‐CREATE), Grant/Award Number: 20cm0106306h0005; Research Program on Emerging and Re‐emerging Infectious Diseases from the Japan Agency for Medical Research and Development (AMED), Grant/Award Number: 20fk0108088h0002; JSPS KAKENHI, Grant/Award Number: 19H03689; This study was also supported in part by the JSPS Core‐to‐Core Program A, Advanced Research Networks (integrative approach for normal and leukemic stem cells).
Contributor Information
Kisato Nosaka, Email: knosaka@kumamoto-u.ac.jp.
Masao Matsuoka, Email: mamatsu@kumamoto-u.ac.jp.
REFERENCES
- 1. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180‐e190. [DOI] [PubMed] [Google Scholar]
- 2. Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481‐492. [PubMed] [Google Scholar]
- 3. Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T‐cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415‐7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T‐cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982;79:2031‐2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV‐1 infection. Front Microbiol. 2012;3:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maayan S, Shufman EN, Engelhard D, Shouval D. Exposure to hepatitis B and C and to HTLV‐1 and 2 among Israeli drug abusers in Jerusalem. Addiction. 1994;89:869‐874. [DOI] [PubMed] [Google Scholar]
- 7. Satake M, Iwanaga M, Sagara Y, Watanabe T, Okuma K, Hamaguchi I. Incidence of human T‐lymphotropic virus 1 infection in adolescent and adult blood donors in Japan: a nationwide retrospective cohort analysis. Lancet Infect Dis. 2016;16:1246‐1254. [DOI] [PubMed] [Google Scholar]
- 8. Iwanaga M. Epidemiology of HTLV‐1 infection and ATL in Japan: an update. Front Microbiol. 2020;11:1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sasaki H, Nishikata I, Shiraga T, et al. Overexpression of a cell adhesion molecule, TSLC1, as a possible molecular marker for acute‐type adult T‐cell leukemia. Blood. 2005;105:1204‐1213. [DOI] [PubMed] [Google Scholar]
- 10. Yuki A, Shinkuma S, Hayashi R, et al. CADM1 is a diagnostic marker in early‐stage mycosis fungoides: Multicenter study of 58 cases. J Am Acad Dermatol. 2018;79:1039‐1046. [DOI] [PubMed] [Google Scholar]
- 11. Chihara D, Ito H, Matsuda T, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsuoka M, Jeang KT. Human T‐cell leukaemia virus type 1 (HTLV‐1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270‐280. [DOI] [PubMed] [Google Scholar]
- 13. Igakura T, Stinchcombe JC, Goon PK, et al. Spread of HTLV‐I between lymphocytes by virus‐induced polarization of the cytoskeleton. Science. 2003;299:1713‐1716. [DOI] [PubMed] [Google Scholar]
- 14. Bangham CRM, Matsuoka M. Human T‐cell leukaemia virus type 1: parasitism and pathogenesis. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732):20160272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cook LB, Rowan AG, Melamed A, Taylor GP, Bangham CR. HTLV‐1‐infected T cells contain a single integrated provirus in natural infection. Blood. 2012;120:3488‐3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grossman WJ, Kimata JT, Wong FH, Zutter M, Ley TJ, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T‐cell leukemia virus type I. Proc Natl Acad Sci U S A. 1995;92:1057‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV‐I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci U S A. 2006;103:720‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohsugi T, Kumasaka T. Low CD4/CD8 T‐cell ratio associated with inflammatory arthropathy in human T‐cell leukemia virus type I Tax transgenic mice. PLoS One. 2011;6:e18518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV‐I pX in patients with HTLV‐I associated neurological disease. Nature. 1990;348:245‐248. [DOI] [PubMed] [Google Scholar]
- 20. Kannagi M, Harada S, Maruyama I, et al. Predominant recognition of human T cell leukemia virus type I (HTLV‐I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV‐I‐infected cells. Int Immunol. 1991;3:761‐767. [DOI] [PubMed] [Google Scholar]
- 21. Mahgoub M, Yasunaga J‐I, Iwami S, et al. Sporadic on/off switching of HTLV‐1 Tax expression is crucial to maintain the whole population of virus‐induced leukemic cells. Proc Natl Acad Sci U S A. 2018;115:E1269‐E1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iwanaga R, Ohtani K, Hayashi T, Nakamura M. Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T‐cell leukemia virus type I. Oncogene. 2001;20:2055‐2067. [DOI] [PubMed] [Google Scholar]
- 23. Kuo YL, Giam CZ. Activation of the anaphase promoting complex by HTLV‐1 tax leads to senescence. Embo J. 2006;25:1741‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chandhasin C, Ducu RI, Berkovich E, Kastan MB, Marriott SJ. Human T‐cell leukemia virus type 1 tax attenuates the ATM‐mediated cellular DNA damage response. J Virol. 2008;82:6952‐6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Satou Y, Yasunaga J‐I, Zhao T, et al. HTLV‐1 bZIP factor induces T‐cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011;7:e1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yasunaga J‐I, Sakai T, Nosaka K, et al. Impaired production of naive T lymphocytes in human T‐cell leukemia virus type I‐infected individuals: its implications in the immunodeficient state. Blood. 2001;97:3177‐3183. [DOI] [PubMed] [Google Scholar]
- 27. Toulza F, Nosaka K, Takiguchi M, et al. FoxP3+ regulatory T cells are distinct from leukemia cells in HTLV‐1‐associated adult T‐cell leukemia. Int J Cancer. 2009;125:2375‐2382. [DOI] [PubMed] [Google Scholar]
- 28. Satou Y, Utsunomiya A, Tanabe J, Nakagawa M, Nosaka K, Matsuoka M. HTLV‐1 modulates the frequency and phenotype of FoxP3+CD4+ T cells in virus‐infected individuals. Retrovirology. 2012;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanaka A, Matsuoka M. HTLV‐1 Alters T cells for viral persistence and transmission. Front Microbiol. 2018;9:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao T, Satou Y, Sugata K, et al. HTLV‐1 bZIP factor enhances TGF‐beta signaling through p300 coactivator. Blood. 2011;118:1865‐1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamano Y, Takenouchi N, Li H‐C, et al. Virus‐induced dysfunction of CD4+CD25+ T cells in patients with HTLV‐I‐associated neuroimmunological disease. J Clin Invest. 2005;115:1361‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grant C, Oh U, Yao K, Yamano Y, Jacobson S. Dysregulation of TGF‐beta signaling and regulatory and effector T‐cell function in virus‐induced neuroinflammatory disease. Blood. 2008;111:5601‐5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sugata K, Yasunaga J‐I, Kinosada H, et al. HTLV‐1 viral factor HBZ induces CCR4 to promote T‐cell migration and proliferation. Cancer Res. 2016;76:5068‐5079. [DOI] [PubMed] [Google Scholar]
- 34. Yasuma K, Yasunaga J‐I, Takemoto K, et al. HTLV‐1 bZIP factor impairs anti‐viral immunity by inducing co‐inhibitory molecule, T cell immunoglobulin and ITIM domain (TIGIT). PLoS Pathog. 2016;12:e1005372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shimauchi T, Kabashima K, Tokura Y. Adult T‐cell leukemia/lymphoma cells from blood and skin tumors express cytotoxic T lymphocyte‐associated antigen‐4 and Foxp3 but lack suppressor activity toward autologous CD8+ T cells. Cancer Sci. 2008;99:98‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagate Y, Ezoe S, Fujita J, et al. Ectonucleotidase CD39 is highly expressed on ATLL cells and is responsible for their immunosuppressive function. Leukemia. 2021;35(1):107‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sugata K, Yasunaga J‐I, Miura M, et al. Enhancement of anti‐STLV‐1/HTLV‐1 immune responses through multimodal effects of anti‐CCR4 antibody. Sci Rep. 2016;6:27150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hilburn S, Rowan A, Demontis M‐A, et al. In vivo expression of human T‐lymphotropic virus type 1 basic leucine‐zipper protein generates specific CD8+ and CD4+ T‐lymphocyte responses that correlate with clinical outcome. J Infect Dis. 2011;203(4):529‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takeda S, Maeda M, Morikawa S, et al. Genetic and epigenetic inactivation of tax gene in adult T‐cell leukemia cells. Int J Cancer. 2004;109:559‐567. [DOI] [PubMed] [Google Scholar]
- 40. Tamiya S, Matsuoka M, Etoh K, et al. Two types of defective human T‐lymphotropic virus type I provirus in adult T‐cell leukemia. Blood. 1996;88:3065‐3073. [PubMed] [Google Scholar]
- 41. Furukawa Y, Kubota R, Tara M, Izumo S, Osame M. Existence of escape mutant in HTLV‐I tax during the development of adult T‐cell leukemia. Blood. 2001;97:987‐993. [DOI] [PubMed] [Google Scholar]
- 42. Fan J, Ma G, Nosaka K, et al. APOBEC3G generates nonsense mutations in human T‐cell leukemia virus type 1 proviral genomes in vivo. J Virol. 2010;84:7278‐7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koiwa T, Hamano‐Usami A, Ishida T, et al. 5'‐long terminal repeat‐selective CpG methylation of latent human T‐cell leukemia virus type 1 provirus in vitro and in vivo. J Virol. 2002;76:9389‐9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taniguchi Y, Nosaka K, Yasunaga J, et al. Silencing of human T‐cell leukemia virus type I gene transcription by epigenetic mechanisms. Retrovirology. 2005;2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miyazaki M, Yasunaga J, Taniguchi Y, Tamiya S, Nakahata T, Matsuoka M. Preferential selection of human T‐cell leukemia virus type 1 provirus lacking the 5' long terminal repeat during oncogenesis. J Virol. 2007;81:5714‐5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304‐1315. [DOI] [PubMed] [Google Scholar]
- 47. Shannon‐Lowe C, Rickinson A. The global landscape of EBV‐associated tumors. Front Oncol. 2019;9:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Speck SH, Ganem D. Viral latency and its regulation: lessons from the gamma‐herpesviruses. Cell Host Microbe. 2010;8:100‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Price AM, Luftig MA. To be or not IIb: a multi‐step process for Epstein‐Barr virus latency establishment and consequences for B cell tumorigenesis. PLoS Pathog. 2015;11:e1004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Read SA, Douglas MW. Virus induced inflammation and cancer development. Cancer Lett. 2014;345:174‐181. [DOI] [PubMed] [Google Scholar]
- 51. Herbert KM, Pimienta G. Consideration of epstein‐barr virus‐encoded noncoding RNAs EBER1 and EBER2 as a functional backup of viral oncoprotein latent membrane protein 1. MBio. 2016;7:e01926‐01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suehiro Y, Hasegawa A, Iino T, et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide‐pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br J Haematol. 2014;169(3):356‐367. [DOI] [PubMed] [Google Scholar]
- 53. Yoshie O, Fujisawa R, Nakayama T, et al. Frequent expression of CCR4 in adult T‐cell leukemia and human T‐cell leukemia virus type 1‐transformed T cells. Blood. 2002;99:1505‐1511. [DOI] [PubMed] [Google Scholar]
- 54. Yamamoto K, Utsunomiya A, Tobinai K, et al. Phase I study of KW‐0761, a defucosylated humanized anti‐CCR4 antibody, in relapsed patients with adult T‐cell leukemia‐lymphoma and peripheral T‐cell lymphoma. J Clin Oncol. 2010;28:1591‐1598. [DOI] [PubMed] [Google Scholar]
- 55. Matsushita K, Arima N, Fujiwara H, et al. Spontaneous regression associated with apoptosis in a patient with acute‐type adult T‐cell leukemia. Am J Hematol. 1999;61:144‐148. [DOI] [PubMed] [Google Scholar]
- 56. Suzuki M, Uno H, Kiyomizu A, et al. Observation of T cell surface antigens in the clinical course of adult T‐cell leukemia: case report of a spontaneous remission. Acta Haematol. 1995;93:40‐45. [DOI] [PubMed] [Google Scholar]
- 57. Jinnohara T, Tsujisaki M, Sasaki S, Hinoda Y, Imai K. Cytotoxic activity in a case of adult T‐cell leukemia/lymphoma with spontaneous regression. Int J Hematol. 1997;65:293‐298. [DOI] [PubMed] [Google Scholar]
- 58. Fukushima T, Nomura S, Shimoyama M, et al. Japan Clinical Oncology Group (JCOG) prognostic index and characterization of long‐term survivors of aggressive adult T‐cell leukaemia‐lymphoma (JCOG0902A). Br J Haematol. 2014;166:739‐748. [DOI] [PubMed] [Google Scholar]
- 59. Tsukasaki K, Maeda T, Arimura K, et al. Poor outcome of autologous stem cell transplantation for adult T cell leukemia/lymphoma: a case report and review of the literature. Bone Marrow Transplant. 1999;23:87‐89. [DOI] [PubMed] [Google Scholar]
- 60. Nakane M, Ohashi K, Sato Y, et al. Molecular remission in adult T cell leukemia after autologous CD34+ peripheral blood stem cell transplantation. Bone Marrow Transplant. 1999;24:219‐221. [DOI] [PubMed] [Google Scholar]
- 61. Watanabe J, Kondo H, Hatake K. Autologous stem cell transplantations for recurrent adult T cell leukaemia/lymphoma using highly purified CD34+ cells derived from cryopreserved peripheral blood stem cells. Leuk Lymphoma. 2001;42:1115‐1117. [DOI] [PubMed] [Google Scholar]
- 62. Iqbal M, Reljic T, Klocksieben F, et al. Efficacy of Allogeneic Hematopoietic Cell Transplantation in Human T Cell Lymphotropic Virus Type 1‐Associated Adult T Cell Leukemia/Lymphoma: Results of a Systematic Review/Meta‐Analysis. Biol Blood Marrow Transplant. 2019; 25: 1695‐1700. [DOI] [PubMed] [Google Scholar]
- 63. Kanda J, Hishizawa M, Utsunomiya A, et al. Impact of graft‐versus‐host disease on outcomes after allogeneic hematopoietic cell transplantation for adult T‐cell leukemia: a retrospective cohort study. Blood. 2012;119:2141‐2148. [DOI] [PubMed] [Google Scholar]


