Abstract
Background
Quercetin, a natural flavonoid compound, is a potent cancer therapeutic agent widely found in fruit and vegetables. It has been reported to induce growth inhibition and apoptosis in both A549 and H1299 human lung cancer cells. However, the effect of quercetin‐induced autophagy on apoptosis and the possible autophagy mechanism in A549 and H1299 cells have not yet been critically examined.
Methods
A549 and H1299 cells were treated with different concentrations of quercetin for 24 hours. Cell growth was measured by cell counting kit‐8 (CCK‐8) assay, whereas apoptosis was assessed by western blotting analysis of apoptotic proteins. The levels of proteins and genes involved in autophagy were determined by western blotting and reverse transcription polymerase chain reaction (RT‐PCR), respectively. Autophagosomes were also observed by transmission electron microscopy (TEM) and LC3 immunofluorescence.
Results
Quercetin inhibited cell viability and induced mitochondria‐dependent apoptosis in both A549 and H1299 cells in a dose‐dependent. Moreover, quercetin also promoted the expression of LC3‐II and beclin 1 and suppressed the expression of p62. The mRNA levels of LC3‐II, beclin 1, Atg5, Atg7, and Atg12 were upregulated by quercetin treatment. Autophagy inhibition with 3‐methyladenine could effectively inhibit quercetin‐induced apoptosis. In addition, quercetin dose‐dependently elevated the levels of SIRT1 protein and the pAMPK–AMPK ratio. Quercetin‐induced autophagy was attenuated by SIRT1 inhibitor EX527 and SirT1 knockdown by small interfering RNA (siRNA).
Conclusions
Quercetin‐induced autophagy contributes to apoptosis in A549 and H1299 lung cancer cells, which involved the SIRT1/AMPK signaling pathway.
Keywords: Apoptosis, autophagy, non‐small‐cell lung cancer cells, quercetin, SIRT1/AMPK pathway
The present study substantiated that quercetin induces pro‐apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell line A549 in vitro. Furthermore, our study might provide a more effective therapeutic strategy for lung cancer by a combination treatment with quercetin and autophagy agonist.
INTRODUCTION
Quercetin is a member of the flavonoid family and is one of the most important dietary antioxidants. It is widely distributed in vegetables and fruits, tea, and various kinds of food supplements. Quercetin has antioxidant, anti‐inflammatory and immunomodulatory effects and has attracted much attention in recent years because of its anticancer effects in many types of cancer. 1 , 2 , 3 , 4
Autophagy is a dynamic process through the lysosome pathway. In this process, cytoplasmic material is isolated as autophagosomes and fused with lysosomes to form autolysosomes. 5 Autophagy is a survival pathway activated in response to nutrient deprivation and other stressful stimuli. 6 Autophagy is intricately involved in many aspects of human health and disease, including neurodegeneration, liver disease, inflammation, type 2 diabetes, and cancer. 7 , 8 , 9 Autophagy is a double‐edged sword in tumorigenesis, acting both as a tumor suppressor and a protector of cancer cell survival. 10
Non‐small‐cell lung cancer (NSCLC), which includes adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and several other types, is a significant global health problem presently. As one of the most common malignancies, NSCLC remains the leading cause of cancer‐related death worldwide. Although great improvements have been achieved in early detection and the treatments for NSCLC, the prognosis for NSCLC is still poor. Therefore, searching for new and effective treatment is an urgent need for NSCLC patients.
Quercetin has been reported to induce both apoptosis and autophagy in A549 cells 11 , 12 , 13 , 14 , 15 and trigger growth inhibition and apoptosis in H1299 cells. 16 Therefore, quercetin is emerging as a promising new drug against NSCLC. However, the effect of quercetin‐induced autophagy on apoptosis and the mechanism through which quercetin activated autophagy in A549 and H1299 cells remained critically unknown so far. In this study, we examined the effect of quercetin‐induced autophagy on A549 and H1299 cell apoptosis and the underlying molecular mechanisms. Our findings indicated that quercetin‐induced autophagy contributes to apoptosis via the SIRT1/AMPK signaling pathway.
METHOD
Reagents and antibodies
Quercetin and EX527 were obtained from MedChemExpress. 3‐Methyladenine (3‐MA) was obtained from Sigma‐Aldrich Co. Cell counting kit‐8(CCK‐8) was purchased from Beyotime Institute of Biotechnology (China). Antibodies against LC3, beclin‐1, p62, p‐AMPK, AMPK, SIRT1, bax, bcl‐2, cleaved caspase‐3, and β‐actin as well as horseradish peroxidase (HRP)‐conjugated secondary antibodies were obtained from Cell Signaling Technology. Three small interfering RNAs (siRNAs) specific for SIRT1 and a scrambled control were synthesized by public protein/plasmid library (China).
Cell culture
A549 and H1299 cell lines were obtained from Tianjin Lung Cancer Institute of Tianjin Medical University General Hospital (Tianjin, China). Cells were cultured in RPMI‐1640 complete culture medium (Gibco) supplemented with 10% fetal bovine serum (Biological Industries) containing 100 U/mL penicillin and 100 U/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. All experiments were performed only when the cells were growing during the exponential phase.
Cell proliferation assay
The CCK‐8 assay was used to assess A549 and H1299 cell viability. A total of 5 × 103 cells were seeded in 96‐well plates. Following overnight incubation at 37°C, the cells were treated with different concentrations of quercetin (12.5, 25, 50, and 100 μM) for 24 hours. Next, 10 μL of CCK‐8 solution was added to the cultures and incubated for 2 hours at 37°C. Subsequently, the absorbance was measured at 450 nm using a microplate spectrophotometer.
Transmission electron microscopy (TEM)
A549 and H1299 cells were collected by centrifugation and washed with PBS, then fixed with 2.5% glutaraldehyde, post‐fixed with 1% perosmic acid, and dehydrated with acetone. Ultrathin sections were placed on 400‐mesh grids and double stained with uranyl acetate and lead citrate. Sections were observed under transmission electron microscopy (TEM: JEM‐1010; Jeol, Tokyo, Japan).
Quantitative real‐time PCR (qRT‐PR) for mRNA expression analysis
A549 and H1299 cells were treated with different concentrations of quercetin for 24 hours. Total RNA was extracted with Trizol reagent. RNA purity was detected, and RNA from each sample was used for cDNA synthesis using Prime Script RT reagent Kit. Primers used in the qRT‐PCR evaluation were designed; LC‐3: GATGTCCGACTTATTCGAGAGC, TTGAGCTGTAAGCGCCTTCTA; Beclin‐1 TTGAGCTGTAAGCGCCTTCTA, TTGAGCTGTAAGCGCCTTCTA; ATG5: AAAGATGTGCTTCGAGATG TGT, CACTTTGTCAGTTACCAACGTCA; ATG7: CAGTTTGCCCCTTTTAGTA GTGC, CCAGCCGATACTCGTTCAGC; ATG12: CTGCTGGCGACACCAAGAAA, CGTGTTCGCTCTACTGCCC; and β‐actin: CCTGGCACCCAGCACAAT, GGGCCGGACTCGTCATAC. Changes in the expression of target gene were measured relative to the mean critical threshold (CT) values of β‐actin gene.
Western blotting
Total protein was extracted from a total of 5 × 106 A549 or H1299 cells using a RIPA lysis buffer according to the manufacturer's protocol. The same amount of proteins of each sample was separated by 10% or 12% SDS‐PAGE gel and then transferred into polyvinylidene fluoride membranes. After blocking with 5% not‐fat milk in TBST buffer for 1 hour at room temperature, the membrane was incubated with specific primary antibodies overnight at 4°C followed by incubation with HRP‐conjugated secondary antibodies for 1 hour at room temperature, and signals were detected by electrochemical luminescence. Band density was quantified using Image J software. The relative levels of proteins were normalized to β‐actin.
Immunofluorescence assay
The A549 and H1299 cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature washed with PBS, and permeabilized and blocked for 60 minutes, then incubated with the LC3 antibody at 4°C overnight. A fluorescent secondary antibody at a dilution of 1:200 was used for incubation at room temperature for 1 hour. Finally, the cells were incubated with DAPI for 10 minutes after PBS washing and were then covered with glycerin.
Transfection of siRNA
The target sequences of SIRT1 siRNA were si‐SIRT1‐1 (GGAAAUAUAUCCUGGA CAATT), si‐SIRT1‐2 (GGCAAUUAAUGAAGCUAUATT), and si‐SIRT1‐3 (UGUCA GAUAAGGAAGGAAATT). The cells were transfected using Lipofectamine 3000 reagent (Invitrogen, USA) in Opti‐MEM Reduced Serum Medium (Gibco, USA) according to the standard protocol. After 24 hours, the cells were harvested and the transfection efficiency was analyzed by western blotting.
Statistical analysis
GraphPad Prism software was used for the data analyses. All data are represented as mean ± standard deviation (SD). Student's t‐test was used to compare the mean values of two groups, and one‐way analysis of variance was used for comparing the mean values of multiple samples. Values of p < 0.05 were considered statistically significant.
RESULTS
Quercetin inhibits proliferation and induces apoptosis in A549 and H1299 cells
To determine the role of quercetin on the cell growth, A549 and H1299 cells were treated with various concentrations of quercetin (0, 12.5, 25, 50, and 100 μM) for 24 hours, and then cell viability was measured by CCK‐8 assay. As shown in Figure 1(a), quercetin caused a dose‐dependent inhibition of cell proliferation compared with the control group (* p < 0.05), where it was observed that 100 μM quercetin was the most effective concentration.
FIGURE 1.
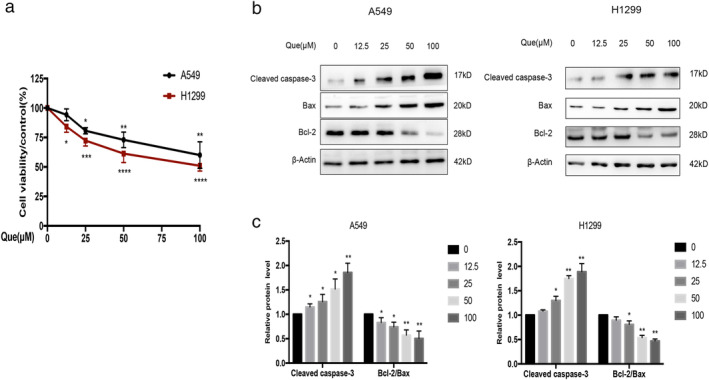
Que inhibits proliferation and induces apoptosis in A549 and H1299 cells. (a) A549 and H1299 cells were treated with different concentrations of Que for 24 hours, and then evaluated for cell viability by CCK‐8 assay. (b,c) A549 and H1299 cells were treated with different concentrations of Que, and western blot analysis was used for analyzing the expression of cleaved caspase‐3, bcl‐2, and bax. β‐actin was used as an internal control for equal amounts of protein applied. Columns indicate mean ± SD of three experiments, * p < 0.05, ** p < 0.01 versus respective control cells
To define whether quercetin‐induced cell death is associated with apoptosis, we treated A549 and H1299 cells with various concentrations of quercetin, then analyzed apoptosis‐associated markers. Western blotting analysis showed that quercetin significantly increased cleaved caspase‐3 protein expression and decreased bcl‐2–bax ratio compared with the control group (Figure 1(b)). All these findings suggested that quercetin could inhibit viability and induce apoptosis in A549 and H1299 cells.
Quercetin induces autophagy in A549 and H1299 cells
To evaluate whether quercetin induces autophagy, we treated A549 and H1299 cells with various concentrations of quercetin, then examined molecular markers of autophagy by western blotting analysis. The results revealed that quercetin treatment significantly increased the protein expression level of beclin 1 and the LC3II–LC3I ratio, and significantly reduced p62 protein expression compared with the control group (Figure 2(a), (b)). Similar results were observed in qRT‐PCR analysis that these autophagy‐related genes (LC3‐II, beclin‐1, Atg5, Atg7, and Atg12) were increased with quercetin treatment (Figure 2(c)). In addition, the increased numbers of the autophagic vacuoles and autophagosomes were observed after quercetin treatment by TEM (Figure 2(d)). These results indicate that quercetin may promote autophagy in A549 and H1299 cells.
FIGURE 2.
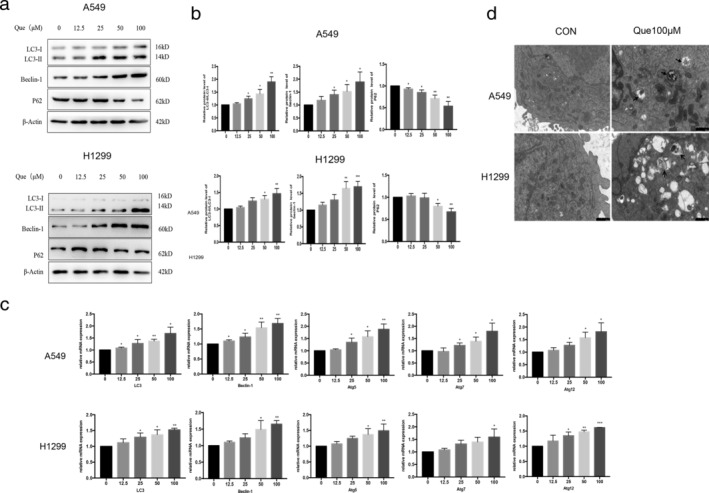
Que induces autophagy in A549 and H1299 cells. (a,b) A549 and H1299 cells were treated with different concentrations of Que for 24 hours, and western blot analysis was used for analyzing the expression of LC3II/I, beclin‐1and p62. (c) A549 and H1299 cells were treated with Que for 24 hours, and quantitative RT‐PCR was carried out for the primers of LC3‐II, Beclin‐1, Atg5, Atg7, and Atg12 gene levels, respectively. (d) A549 and H1299 cells were treated with or without Que (100 μM), and autophagosomes or autolysosomes (shown by arrows) were observed using TEM. The histogram represents quantification analysis based on three independent experiments. Columns indicate mean ± SD of three experiments,* p < 0.05,** p < 0.01 versus respective control cells
The autophagy inhibitor (3‐MA) alleviated quercetin‐mediated apoptosis effect
Our data shows that quercetin induces both autophagy and apoptosis in A549 and H1299 cells. To further delineate the effect of autophagy on apoptosis, we first examined the effect of the autophagy inhibitor 3‐MA on autophagy. From Figure 3(a)–(c), we can conclude that 3‐MA can inhibit autophagy compared with the quercetin group. Next, we evaluated the levels of cleaved caspase‐3 and bcl‐2–bax ratio after treatment with 3‐MA. As shown in Figure 3(d) and (e), 3‐MA decreased the expression of cleaved caspase‐3 and increased the bcl‐2–bax ratio with quercetin treatment combined with 3‐MA (# p < 0.05). From these data, we concluded that quercetin‐induced autophagy contributed to apoptosis in A549 and H1299 cells.
FIGURE 3.
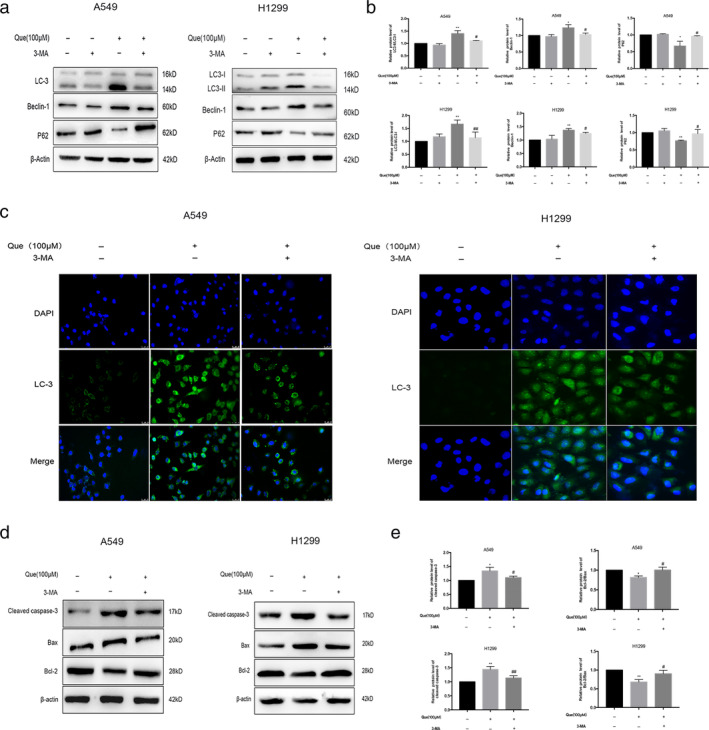
Treatment with the autophagy inhibitor, 3‐MA, eliminates the autophagy and inhibits apoptosis of Que in A549 and H1299 cells. The cells were treated with 100 μM Que with or without cotreatment with 10 mM 3‐MA for 24 hours. (a,b) Western blot analysis was used for analyzing the expression of LC3II/I, beclin‐1and p62. (c) Immunofluorescence was used for analyzing the expression of the autophagy marker, LC3. (c,d) Western blot analysis was used for analyzing the expression of cleaved caspase‐3,bcl‐2, and bax. Columns indicate mean ± SD of three experiments,* p < 0.05,** p < 0.01 versus respective control cells, # p < 0.05,## p < 0.01 versus Que
Quercetin activation SIRT1/AMPK signaling in A549 and H1299 cells
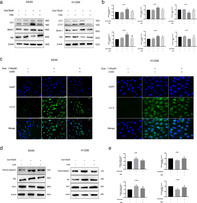
SIRT1 and AMPK are potent stimulators of cellular autophagy in most cells under various stress conditions. Western blot analysis was used to investigate whether the quercetin‐induced autophagy involved SIRT1/AMPK signaling pathway in A549 and H1299 cells. The protein levels of SIRT1 and phosphorylated AMPK were significantly increased in A549 cells after quercetin treatment compared with control group (Figure 4(a),(b)). To further confirm that autophagy induced by quercetin involves the SIRT1/AMPK pathway, we then combined EX527, a SIRT1 inhibitor, with quercetin to treat A549 and H1299 cells. The SIRT1/AMPK pathway and the LC3II–LC3I ratio were clearly inhibited compared with cells treated with quercetin only (Figure 4(c),(d)). Furthermore, we transfected A549 and H1299 cells with SIRT1 siRNA to decrease the expression of SIRT1 (Figure 5(a)–(d)). We observed that SIRT1 siRNA could counteract the increased levels of autophagy‐related protein induced by quercetin significantly. From these data, we conclude that the activation of SIRT1/AMPK pathway is involved in quercetin‐induced autophagy in A549 and H1299 cells.
FIGURE 4.
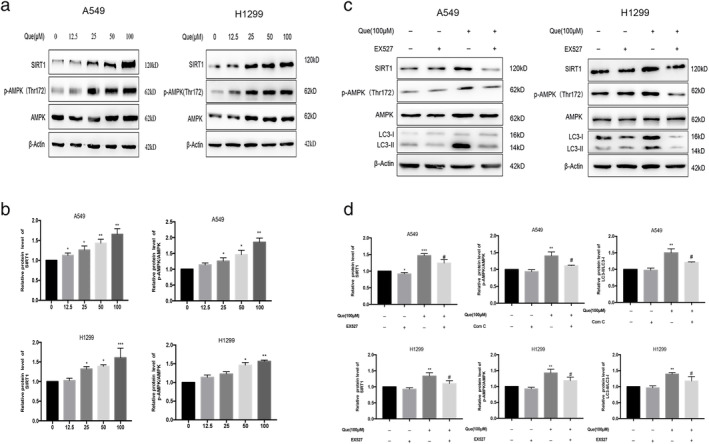
Que activated SIRT1/AMPK signaling in A549 and H1299 cells. (a,b) A549 and H1299 cells were treated with different concentrations of Que for 24 hours, and western blot analysis was used for analyzing the expression of SIRT1 and p‐AMPK/AMPK. (c,d) A549 and H1299 cells were treated with 100 μM Que with or without cotreatment with 10 μM EX527 for 24 hours, and western blot analysis was used for analyzing the expression of SIRT1, p‐AMPK/AMPK, and LC3‐II. Columns indicate mean ± SD of three experiments,* p < 0.05, ** p < 0.01 versus respective control cells, # p < 0.05 versus Que
FIGURE 5.
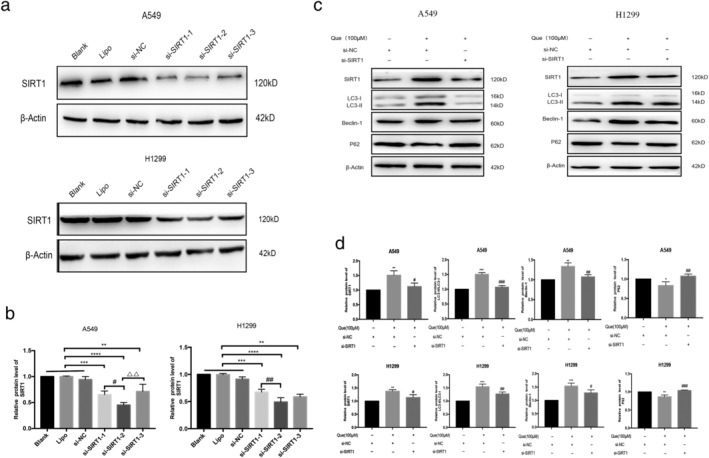
SIRT1 knockdown inhibited Que‐induced autophagy. (a,b) Western blot analysis was used for analyzing the expression of SIRT1 in cells transfected with si‐SIRT1‐1, si‐SIRT1‐2, and si‐SIRT1‐3. ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus control (blank, lipo, or si‐NC); # p < 0.05,## p < 0.01 versus si‐SIRT1‐1;ΔΔ p < 0.01 versus si‐SIRT1‐3. (c,d) Western blot analysis was used for analyzing the expression of SIRT1, LC3‐II, beclin‐1, and p62 level in control/SIRT1 knockdown in A549 and H1299 cells treated with or without Que. Columns indicate mean ± SD of three experiments.* p < 0.05, ** p < 0.01,*** p < 0.001 versus si‐NC; # p < 0.05, ## p < 0.01,### p < 0.001 versus Que + si‐NC
DISCUSSION
Quercetin is a polyphenolic flavonoid compound and is practically ubiquitous in plants. It has been studied as a promising chemopreventive component in a variety of cancer models. 17 , 18 Consistent with previous reports, 11 , 12 , 13 , 16 the current study revealed that quercetin inhibited growth and promoted apoptosis in A549 and H1299 human lung cancer cells.
Autophagy is an intracellular recycling process that maintains basal levels of metabolites and biosynthetic intermediates under starvation or other forms of stress and therefore becomes an important mechanism for metabolism adaptation in cancer cells. 5 In our study, quercetin treatment upregulated the protein expression of LC3‐II/I and beclin‐1, and downregulated p62 expression levels in A549 and H1299 cells. Furthermore, consistent with the western blotting results, qRT‐PCR analysis provided additional data showing the elevated mRNA levels of LC3, beclin‐1, Atg5, Atg7, and Atg12 in response to quercetin treatment, demonstrating that quercetin induced autophagy flux in A549 and H1299 cells. It is not surprising, because quercetin has been recently identified as an autophagy inducer in various tumor studies. 19 In fact, Zhang et al. 14 have reported that quercetin could increase the ratio of LC3II–LC3I in A549 cells after 24‐hour treatment. Moon et al. 15 also reported quercetin treatment for 12 hours induced autophagy flux by assessing LC3B production and p62 expression in A549 cells. Consistent with these data, our results further confirmed that quercetin dose‐dependently promoted autophagy after 24‐hour treatment with additional evidence by estimating mRNA levels of autophagy‐related genes.
Accumulating evidence indicated that autophagy played a controversial role in regulating cell death and survival under various stimuli. Autophagy can enhance or attenuate apoptosis in cancer cells. The protective effects of quercetin‐induced autophagy to apoptosis have been reported in several cell lines. 20 , 21 , 22 On the contrary, quercetin induced autophagy also promoted TRAIL‐mediated apoptosis in lung cancer cells. 15 Therefore, the effect of quercetin‐activated autophagy on apoptosis is complicated. However, to the best of our knowledge, whether autophagy induced by quercetin exert pro‐apoptotic or anti‐apoptotic effect in A549 and H1299 cells has yet to be investigated clearly. Therefore, we assessed if quercetin‐induced apoptosis was regulated by the inhibition of autophagy using 3‐MA. The results indicated that 3‐MA markedly alleviated the quercetin‐induced apoptosis, as demonstrated by the apoptotic marker levels of reduced cleaved caspase 3 and increased bcl‐2–bax ratio. Our studies suggest that autophagy inhibition is able to resist quercetin‐induced apoptosis in A549 and H1299 cells. These findings might support using both quercetin and autophagy agonist as a new therapeutic strategy to treat lung cancer.
SIRT1 is an NAD+‐dependent HDAC, which is involved in multiple disease processes including cancer, vascular disease, and neurodegenerative disorders. SIRT1 can induce autophagy through deacetylation of beclin‐1 and other autophagy mediators. Similarly, we observed that quercetin treatment upregulated the expression of SIRT1 and induced autophagy significantly, indicating that quercetin induced autophagy through activating SIRT1 in A549 and H1299 cells. Adding SIRT1 inhibitor EX527 further verified our results that decreased expression of SIRT1 reduced autophagy. Moreover, knockdown of SIRT1 reduced autophagy significantly. These results suggested that quercetin acted as an SIRT1 activator to induce autophagy in A549 and H1299 cells.
AMPK, a downstream effector of SIRT1, is the key signaling modulators of autophagy. To clarify the molecular mechanism of autophagy of quercetin in A549 and H1299 cells, the effect of quercetin on the protein expression of AMPK was observed by western blotting. Our results showed that the levels of p‐AMPK significantly increased by quercetin in a dose‐dependent manner. However, the upregulation of LC3II–LC3I ratio and p‐AMPK was significantly inhibited by EX527 and siSIRT1, indicating that quercetin activated autophagy via SIRT1/AMPK signaling pathway in A549 and H1299 cells.
In conclusion, the present study substantiated that quercetin induces pro‐apoptotic autophagy via SIRT1/AMPK signaling pathway in non‐small‐cell lung cancer cell lines in vitro. Furthermore, our study might provide a more effective therapeutic strategy for lung cancer by a combination treatment with quercetin and autophagy agonist. However, further studies in other lung cancer cell line and animals are need to fully determine the potential of quercetin a promising anticancer agent for the treatment of lung cancer.
DISCLOSURE
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This study was funded by the grants from the National Natural Science Foundation of China (81600067, 81670084, and 81970084) and National Key Research and Development Program (2016YFC1304502).
Guo H, Ding H, Tang X, et al. Quercetin induces pro‐apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac Cancer. 2021;12:1415–1422. 10.1111/1759-7714.13925
Funding information National key research and development program, Grant/Award Number: 2016YFC1304502; National Natural Science Foundation of China, Grant/Award Numbers: 81600067, 81670084, 81970084
REFERENCES
- 1. Ji Y, Li L, Ma YX, Li WT, Li L, Zhu HZ, et al. Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice. J Nutr Biochem. 2019;69:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jia L, Huang S, Yin X, Zan Y, Guo Y, Han L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt‐mTOR pathway mediated autophagy induction. Life Sci. 2018;208:123–30. [DOI] [PubMed] [Google Scholar]
- 3. Liu Y, Gong W, Yang ZY, Zhou XS, Gong C, Zhang TR, et al. Quercetin induces protective autophagy and apoptosis through ER stress via the p‐STAT3/Bcl‐2 axis in ovarian cancer. Apoptosis. 2017;22(4):544–57. [DOI] [PubMed] [Google Scholar]
- 4. Granato M, Rizzello C, Gilardini Montani MS, Cuomo L, Vitillo M, Santarelli R, et al. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J Nutr Biochem. 2017;41:124–36. [DOI] [PubMed] [Google Scholar]
- 5. Yu L, Chen Y, Tooze SA. Autophagy pathway: Cellular and molecular mechanisms. Autophagy. 2018;14(2):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Karantza‐Wadsworth V. Role and regulation of autophagy in cancer. Biochim Biophys Acta. 2009;1793(9):1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poillet‐Perez L, White E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 2019;33(11–12):610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maheswari U, Sadras SR. Mechanism and regulation of autophagy in cancer. Crit Rev Oncog. 2018;23(5–6):269–80. [DOI] [PubMed] [Google Scholar]
- 10. Chen N, Karantza V. Autophagy as a therapeutic target in cancer. Cancer Biol Ther. 2011;11(2):157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mukherjee A, Khuda‐Bukhsh AR. Quercetin down‐regulates IL‐6/STAT‐3 signals to induce mitochondrial‐mediated apoptosis in a non‐small‐cell lung‐cancer cell line, A549. J Pharm. 2015;18(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nguyen TTT, Tran E, Nguyen TH, Do PT, Huynh TH, Huynh H. The role of activated MEK‐ERK pathway in quercetin‐induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis. 2005;25(5):647–59. [DOI] [PubMed] [Google Scholar]
- 13. Zheng SY, Li Y, Jiang D, Zhao J, Ge JF. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A‐549. Mol Med Rep. 2012;5:822–6. [DOI] [PubMed] [Google Scholar]
- 14. Zhang G, Liu Z, Chen Y, Zhang Y. High serum HDGF levels are predictive of bone metastasis and unfavorable prognosis in non‐small cell lung cancer. Tohoku J Exp Med. 2017;242(2):101–8. [DOI] [PubMed] [Google Scholar]
- 15. Moon JH, Eo SK, Lee JH, Park SY. Quercetin‐induced autophagy flux enhances TRAIL‐mediated tumor cell death. Oncol Rep. 2015;34(1):375–81. [DOI] [PubMed] [Google Scholar]
- 16. Kuo P, Liu H, Chao J. Survivin and p53 modulate quercetin‐induced cell growth inhibition and apoptosis in human lung carcinoma cells. J Biol Chem. 2004;279(53):55875–85. [DOI] [PubMed] [Google Scholar]
- 17. Jeong JH, An JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: Cancer cell‐specific inhibition of cell cycle progression. J Cell Biochem. 2009;106(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deng S, Shanmugam MK, Kumar AP, Yap CT, Sethi G, Bishayee A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer. 2019;125(8):1228–46. [DOI] [PubMed] [Google Scholar]
- 19. Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J, et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin Cancer Biol. 2015;35(Suppl):S25–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim H, Moon JY, Ahn KS, Cho SK. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid Med Cell Longev. 2013;2013:596496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang K, Liu R, Li JY, Mao J, Lei Y, Wu J, et al. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt‐mTOR and hypoxia‐induced factor 1α‐mediated signaling. Autophagy. 2011;7:966–78. [DOI] [PubMed] [Google Scholar]
- 22. Qu L, Liang XC, Gu B, Liu W. Quercetin alleviates high glucose‐induced Schwann cell damage by autophagy. Neural Regen Res. 2014;9:1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]


