Abstract
Objectives
To investigate the perioperative outcomes of patients who underwent uniport video‐assisted thoracoscopic (VATS) segmentectomy for identifying the intersegmental boundary line (IBL) by the near‐infrared fluorescence imaging with the intravenous indocyanine green (ICG) method or the modified inflation‐deflation (MID) method and assess the feasibility and effectiveness of the ICG fluorescence (ICGF)‐based method.
Methods
We retrospectively analyzed the perioperative data in total 198 consecutive patients who underwent uniport VATS segmentectomy between February 2018 and August 2020. With the guidance of a preoperative imaging interpretation and analysis system (IQQA‐3D), the targeted segment structures could be precisely identified and dissected, and then the IBL was confirmed by the ICGF‐based method or the MID method. The clinical effectiveness and postoperative complications of the two methods were evaluated.
Results
An IBL was visible in 98% of patients in the ICGF‐based group, even with low doses of ICG. The ICGF‐based group was significantly associated with a shorter IBL clear presentation time (23.6 ± 4.4 vs. 23.6 ± 4.4 s) (p < 0.01) and operative time (89.3 ± 31.6 vs. 112.9 ± 33.3 min) (p < 0.01) compared to the MID group. The incidence of postoperative prolonged air leaks was higher in the MID group than in the ICGF‐based group (8/100, 8% vs. 26/98, 26.5%, p = 0.025). There were no significant differences in bleeding volume, chest tube duration, postoperative hospital stays, surgical margin width, and other postoperative complications.
Conclusion
The ICGF‐based method could highly accurately identify the IBL and make anatomical segmentectomy easier and faster, and therefore has the potential to be a feasible and effective technique to facilitate the quality of uniport VATS segmentectomy.
Keywords: indocyanine green, intersegmental boundary line, segmentectomy, surgery
Short abstract
Based on a large amount of clinical data, we found that the identification rate of IBL via ICGF‐based method is comparable to the traditional MID method which is the globally accepted. Our study shows that the ICGF‐based method can highly accurately identify the IBL and make anatomical segmentectomy easier and faster, and it helps to ensure sufficient resection margins, and therefore has the potential to be a feasible and effective technique to facilitate the quality of uniport VATS segmentectomy.
INTRODUCTION
Lung cancer has become the malignant tumor with the highest worldwide morbidity and mortality, and is a serious life‐threatening disease. 1 With the development of high‐resolution chest computed tomography (CT) and the recent generalization of physical examination, more and more pulmonary nodules are detected, and a large number of them are highly suspected to be precancerous lesions or early‐stage lung cancer, which require surgical resection. 2 , 3 Uniport video‐assisted thoracoscopic (VATS) anatomic segmental resection, which respects the rules of sufficient oncologic requirements while preserving pulmonary function, has become one of the reliable surgical procedures for early‐stage non‐small cell lung cancer (NSCLC) with small size. 4 , 5 , 6
Moreover, several advantages of anatomic segmental resection on sparing the postoperative lung function or facilitating the quality of a patient's life have been reported. 7 , 8 However, segmentectomy is considered to be a more complicated procedure compared with standard lobectomy, especially when performed through a complete uniport VATS approach, as the segmental structures are prone to individual variations, and the identification of intersegmental boundary line (IBL) between pulmonary segments is challenging regarding technical imperatives. Although several methods for the identification of IBL allowing thoracic surgeons to identify this demarcation during segmentectomy have been reported, there are two main methods to create an intersegmental boundary: using the modified inflation‐deflation (MID) 9 , 10 , 11 or near‐infrared fluorescence imaging with intravenous indocyanine green (ICG). 12 , 13 , 14 Due to narrowing of the surgical field of view leading to the lack of working space in the context of uniport VATS by the MID method, the ICG fluorescence (ICGF)‐based method has been considered advantageous for delineating the IBL without lung reinflation. In our previous study, we demonstrated that the IBL created by the ICGF‐based method, which is based on the degree of blood flow, was highly concordant with the MID method matching the real segmental fissure. 15 However, to our knowledge, there were no retrospective studies or prospective trials to report the perioperative results of these two methods in uniport VATS segmentectomy. Therefore, we conducted a retrospective study to compare the perioperative outcomes of patients who underwent uniport VATS segmentectomy for identifying the IBL by these two methods in our institution and assess the feasibility and effectiveness of the ICGF‐based method.
METHODS
Patients
From February 2018 to August 2020, 198 consecutive patients who underwent uniport VATS segmentectomy for identifying the IBL by the MID method or the ICGF‐based method in our institution were retrospectively reviewed. The eligibility criteria were as follows: (a) pulmonary nodules were conformed with indications for segmentectomy according to National Comprehensive Cancer Network guidelines; (b) normal cardiopulmonary tests; and (c) informed consent was obtained after introducing the risks and benefits of uniport VATS segmentectomy. The exclusion criteria were as follows: (a) severe pleural adhesions; (b) a history of iodine or ICG allergies; and (c) multiple nodules were not in the same lung segment. This study was approved by the Ethics Committee of Nanjing Chest Hospital, Jiangsu, China (approval number: 2020‐KY093‐01).
Preoperative evaluation
Patients underwent computed tomography angiography (CTA) scanning after admission. Image data in DICOM format were reconstructed three‐dimensionally to locate the pulmonary nodules and identify the targeted segment structures by the IQQA system (EDDA Technology). Centering on the suspected malignant nodules, the adequacy of the simulated resection range was evaluated according to the distance from intersegmental veins, which should ensure a sufficient resection margin ≥2 cm or the diameter of the tumor. Simulation of the target segment resection was then performed on the reconstructed dynamic three‐dimensional (3D) images. The target segmental structures, including segmental bronchi, arteries, and intrasegmental veins, were determined, and the resection sequence was performed according to the anatomical variation characteristics. Moreover, the intersegmental vein was a marker to assist in the identification of IBL, which should be preserved during segmentectomy. Finally, the operative plan was created after optimization of the personalized virtual segmentectomy.
Operative procedure
All patients received general anesthesia with double‐cavity endotracheal intubation and were placed in a lateral position. A uniport VATS anatomical segmentectomy was performed through a 3 cm skin incision between the 4th or 5th intercostal space on the midaxillary line using single‐lung ventilation.
During the operation, the reconstructed 3D images were displayed on another computer in front of the thoracoscopic screen to guide the observation of segmental structures and realize accurate navigation, the dominant arteries and intrasegmental veins of the resecting segment were generally ligated with #4 suture or cut with an ultrasonic scalpel, and the dominant bronchus of the resecting segment were transected with stapling devices. In the ICGF‐based method, with the processing of segmental structures, ICG (Eisai Liaoning Pharmaceutical; drug approval number H20045514, China Food and Drug Administration) at 5 mg/body was rapidly injected into a peripheral vein and the lung was observed using infrared fluorescence thoracoscopy (PINPOINT Endoscopic Fluorescence Imaging System). Thus, the reserved lung stained with ICG was displayed in green while the resecting segment remained in the physiological color on the monitor. The IBL was visualized by the difference between the two colored areas and then marked on the visceral pleura using electrocautery (Figure 1). If necessary, ICG was injected again, to a maximum dose of 25 mg/body. In the MID method, after the division of the target segmental structures, the deflated lung of the operation side was completely re‐expanded with pure oxygen via double‐lung ventilation under the controlled airway pressure of 20 cmH2O, followed by one lung ventilation of the healthy side. 9 Thus, the targeted segment would still inflate and the preserving lung would deflate completely. According to the irregularly curved transitional zone on the membrane pleura between the inflated target segment and the deflated surrounding lung tissue, the IBL was formed clearly and labeled with an electrocoagulation hook (Figure 2). For the management of the tailoring IBL, we combined the visceral pleura marking and peeled along the intersegmental veins up to the outer third of the intersegmental parenchyma with an ultrasonic scalpel or electrocautery, and the peripheral IBL was divided by stapling devices on the basis of the visceral pleura marking.
FIGURE 1.
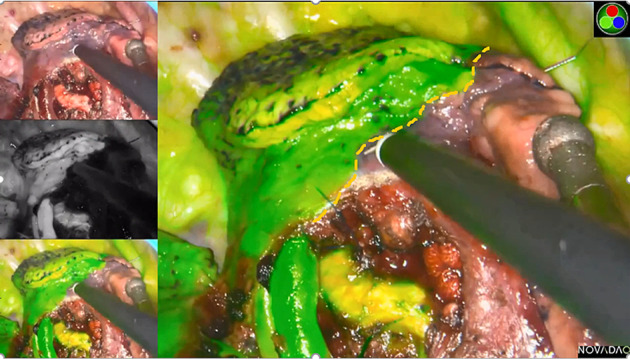
The intersegmental boundary line was clearly identified via the near‐infrared fluorescence imaging with the intravenous indocyanine green method using the PINPOINT endoscopic fluorescence imaging system
FIGURE 2.
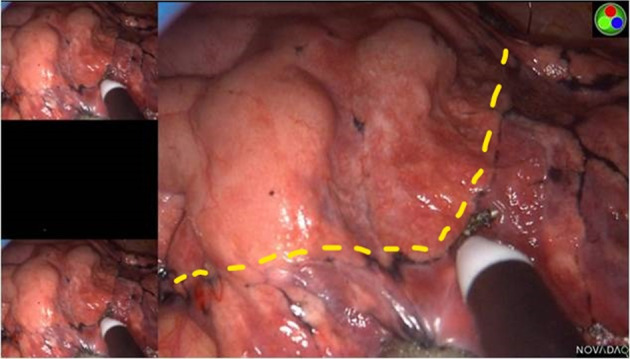
The intersegmental boundary line was clearly identified via the modified inflation‐deflation method using the PINPOINT endoscopic fluorescence imaging system
According to the rapid‐freezing pathology diagnosis, hilar and mediastinal lymph nodes dissection or sampling should be performed in malignant cases. Next, lung leakage testing was implemented. If a small amount of air leakage in the lung tissues was found, the wound surface could be covered with polyglycolic acid reinforcement (NEOVEIL) or fibrin glue spray, while if obvious air leakage caused by the damaged bronchus was found, a 3–0 prolene suture was required. A 22F thoracic drainage tube was placed through the single incision, to be removed as soon as possible when a good pulmonary expansion, no air leakage, and a drainage volume less than 200 ml were confirmed. In this study, the pathologic staging was based on the 8th edition Tumor Node Metastasis (TNM) Classification.
Analyzing evaluation data
General characteristics and perioperative data were collected. Perioperative data included operative time, bleeding volume, chest tube duration, postoperative hospital stays, identification rate, presentation time, surgical margin width, and complications. Postoperative complications related to surgery mainly consisted of pulmonary infection, atelectasis, hemoptysis, and prolonged air leak (>7 days). Operative time was defined as the time from skin incision to skin closure. In the ICGF‐based method, the presentation time was defined as the time from ICG injection to IBL visualization. In the MID method, the presentation time was defined as the time from entire lung re‐expansion to IBL visualization.
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used for statistical analysis. The continuous data were expressed as mean ± standard deviation (SD), and the comparison between group differences was performed by Student's t‐test. The categorical variables were expressed by frequency and percentage, and the comparison between group differences was performed using χ 2 or Fisher's exact test. p < 0.05 was statistically significant. All comparisons were two‐tailed, p < 0.05 was considered statistically significant.
RESULTS
General characteristics
Of the 198 consecutive patients who underwent uniport VATS segmentectomy, 100 were treated by the ICGF‐based method and 98 were treated by the MID method. Data on patient age, gender, smoking status, pulmonary function, comorbidity, tumor size, pleural adhesions, and pathological diagnosis are summarized in Table 1. There were no significant differences in the observed variables between the groups (all p > 0.05). The positions and cases of segmentectomies in the two groups are shown in Table 2.
TABLE 1.
Clinical characteristics of patients of the two groups
| Characteristics | ICGF‐based group (n = 100) | MID group (n = 98) | p value |
|---|---|---|---|
| Age, years, mean ± SD | 59.8 ± 10.5 | 60.6 ± 7.9 | 0.500 |
| Gender, n (%) | 0.242 | ||
| Male | 43 (43) | 48 (49) | |
| Female | 57 (57) | 50 (51) | |
| Smoking status, n (%) | 0.402 | ||
| Ever | 80 (80) | 76 (77.6) | |
| Never | 20 (20) | 22 (22.4) | |
| FEV1, L, mean ± SD | 2.5 ± 0.6 | 2.4 ± 0.7 | 0.789 |
| COPD, n (%) | 0.391 | ||
| Yes | 10 (10) | 12 (12.2) | |
| No | 90 (90) | 86 (87.8) | |
| Tumor size, cm, mean ± SD | 11.6 ± 3.9 | 11.6 ± 3.7 | 0.893 |
| Pleural adhesions, n (%) | 0.439 | ||
| Yes | 86 (86) | 12 (12.2) | |
| No | 14 (14) | 86 (87.8) | |
| Pathological diagnosis, n (%) | 0.579 | ||
| AAH | 4 (4) | 1 (1) | |
| AIS | 34 (34) | 37 (38) | |
| MIA | 52 (52) | 51 (52) | |
| IAC | 10 (10) | 9 (9) |
Abbreviations: AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; IAC, invasive adenocarcinoma; MIA, minimally invasive adenocarcinoma; SD, standard deviation.
TABLE 2.
Position of target segments in the two groups
| Segmentectomy position | ICGF‐based group (n = 100) | MID group (n = 98) |
|---|---|---|
| Right lobe | ||
| Single segment | ||
| S1 | 10 (10) | 13 (13.3) |
| S2 | 14 (14) | 4 (4.1) |
| S3 | 7 (7) | 19 (19.4) |
| S6 | 7 (7) | 4 (4.1) |
| S8 | 3 (3) | 1 (1) |
| Subsegment | ||
| S8a | 3 (3) | 2 (2) |
| Combined segmentectomy | ||
| S1 + 2 | 0 | 2 (2) |
| S2b + S3a | 4 (4) | 2 (2) |
| S7 + 8 | 3 (3) | 5 (5.1) |
| S9 + 10 | 3 (3) | 3 (3.1) |
| Left lobe | ||
| Single segment | ||
| S3 | 7 (7) | 6 (6.1) |
| S6 | 8 (8) | 6 (6.1) |
| S8 | 3 (3) | 1 (1) |
| Subsegment | ||
| S8a | 2 (2) | 1 (1) |
| Combined segmentectomy | ||
| S1 + 2 | 5 (5) | 9 (9.2) |
| S1 + 2 + 3 | 7 (7) | 7 (7.1) |
| S4 + 5 | 5 (5) | 7 (7.1) |
| S7 + 8 | 7 (7) | 6 (6.1) |
| S9 + 10 | 2 (2) | 0 |
Perioperative outcomes
No adverse events related to ICG injection occurred in the ICGF‐based group and neither 30‐day perioperative death nor readmission was observed in either group. The perioperative outcomes are summarized in Table 3, including the operative time, bleeding volume, chest tube duration, postoperative hospital stays, identification rate, presentation time, surgical margin width, and postoperative complications.
TABLE 3.
Perioperative data of the two groups
| Variable | ICGF‐based group (n = 100) | MID group (n = 98) | p value |
|---|---|---|---|
| Operative time, min, mean ± SD | 89.3 ± 31.6 | 112.9 ± 33.3 | <0.01 |
| Bleeding volume, ml, mean ± SD | 57.2 ± 41.7 | 67.4 ± 56.2 | 0.146 |
| Presentation time, s, mean ± SD | 23.6 ± 4.4 | 1027.7 ± 322.5 | <0.01 |
| Identification rate (%) | 98% | 89.80% | 0.015 |
| Surgical margin width, cm, mean ± SD | 2.5 ± 0.5 | 2.4 ± 0.5 | 0.199 |
| Chest tube duration, days, mean ± SD | 4.3 ± 1.8 | 4.7 ± 2.1 | 0.166 |
| Postoperative complications, n (%) | |||
| ICG‐related complications | 0 | — | |
| Prolonged air leaks (>7 days) | 8 (8) | 26 (26.5) | 0.025 |
| Pulmonary infection | 2 (2) | 1 (1) | 0.508 |
| Hemoptysis | 6 (6) | 9 (9) | 0.282 |
| Atelectasis | 4 (4) | 2 (2) | 0.351 |
| Postoperative hospital stays, days, Mean ± SD | 5.5 ± 1.7 | 5.9 ± 2.1 | 0.184 |
Abbreviations: ICG, indocyanine green; SD, standard deviation.
An IBL was visible in 98% of patients by the ICGF‐based method, even with low doses of ICG, and it was not clear in two cases with right apical segment (RS 1 ) resection combined with emphysema. The identification rate of IBL was 90% by the MID method and no clear boundary line was found in 10 cases after the prolonged waiting time, including four cases of RS 1 resection, two cases of anterior segment (S3) resection, three cases of superior segment (S6) resection, and one case of left apico‐posterior segment (LS1 + 2). Among them, there were six cases combined with emphysema and four cases combined with pleural adhesions. The IBL clear presentation time of the ICGF‐based group was decreased significantly compared with the MID group (23.59 ± 4.47 vs. 1026.80 ± 318.34 s) (p < 0.001). The operative time in the ICGF‐based group was shorter than that in the MID group (89.3 ± 31.6 vs. 112.9 ± 33.3 min) (p < 0.01). Under the guidance of the reconstructed 3D images, the surgical margin width showed no difference between the ICGF‐based group and the MID group (2.5 ± 0.5 vs. 2.4 ± 0.5 cm) (p = 0.199) and both groups met the adequacy of surgical resection range, which ensured a sufficient resection margin ≥2 cm or the diameter of the tumor. The ICGF‐based group exhibited trends toward less bleeding volume, shorter chest tube duration, and postoperative hospital stays, but the differences between each group were not statistically significant (p > 0.05).
Table 3 also records the postoperative complications for each group. The incidence of postoperative prolonged air leaks in the MID group was higher than in the ICGF‐based group, and the differences were statistically significant (8/100, 8% vs. 26/98, 26.5%, p = 0.025). Prolonged air leaks were observed in 26 (13.1%) patients, eight of whom were patients in the ICGF‐based group and 18 were patients in the MID group. Three patients had pulmonary infection (1.4%), with two patients in the ICGF‐based group, and one patient in the MID group. Hemoptysis occurred in 15 patients, with six patients in the ICGF‐based group and nine in the MID group. Atelectasis occurred in six cases, four in the ICGF‐based group and two in the MID group. No significant differences were found between each group in the postoperative complications of pulmonary infection, atelectasis, and hemoptysis (p > 0.05), and all patients with the above postoperative complications, including prolonged air leaks, were recovered after conservative management without reoperation.
DISCUSSION
Recently, several retrospective studies have shown that thoracoscopic anatomic segmental resection appears to be a minimally invasive procedure to treat early‐stage lung cancer, especially when the diameter is less than 2 cm, with similar oncologic outcomes to those of lobectomy procedures and the benefits of a small surgical incision and excellent preservation of normal lung tissue function. 16 , 17 , 18 , 19 However, even for experienced thoracic surgeons, thoracoscopic anatomic segmental resection is a demanding technique with many difficulties and limitations, especially in the uniport VATS approach. Thoracoscopic segmentectomy remains a significant challenges due to the sophisticated anatomical variations of segmental structures and the technical requirements for IBL identification.
For lung segmentectomy, the identification of the IBL is a critical step in the intraoperative procedure, which is directly related to adequate resection margins and fewer postoperative complications. Failure to precisely identify the IBL during lung segmentectomy may lead to an insufficient distance between the resection margins and lesion, residual target segment or lesion, excessive resection of the lung parenchyma and even conversion to lobectomy, iatrogenic intersegmental vein injury, and postoperative complications such as pulmonary infection, atelectasis, hemoptysis, postoperative prolonged air leak,s and even re‐insertion of thoracic catheter. However, precise dissection of target segmental structures based on the reconstructed dynamic 3D images is the prerequisite for presenting an accurate IBL. In our center, the software IQQA system was used to reconstruct 3D images of segmental arteries, veins, and bronchi of candidate patients undergoing segmentectomy for preoperative surgical simulation. 15 In addition, the reconstructed dynamic 3D images could accurately identify the anatomical variation characteristics and the incidence of postoperative complications could be significantly reduced. 20 In addition, recognition of target segmental structures based on the experience was essential as the conditions of the lung were slightly different between the intraoperative discernment and intraoperatively. After comprehensively reviewing relevant literature, although several methods for the identification of IBL allowing thoracic surgeons to identify this demarcation during segmentectomy have been reported, there are two main methods to create an IBL: using a MID 9 , 10 , 11 or near‐infrared fluorescence imaging with intravenous ICG. 12 , 13 , 14 However, to our knowledge, there have been no retrospective studies or prospective trials to report the perioperative results of these two methods in uniport VATS segmentectomy.
Based on a large amount of clinical data, we found that the identification rate of IBL via the ICGF‐based method is comparable to the traditional MID method, which is globally accepted. Furthermore, in our previous study we proved that the IBL created by the ICGF‐based method was highly concordant with the MID method and matched the real segmental fissure. 15 For patients with impaired lung function, such as emphysema or extensive pleural adhesions, the IBL was hard to identify using the MID method because of decreased lung compliance. 9 In this study, the IBL could not be identified in 10 patients via the MID method, and all of these patients had emphysema and pleural adhesions. However, the IBL could not be clearly identified only in two patients via the ICGF‐based method, which uses the basis of blood flow, and both of them had severe emphysematous regions. The blood flow of emphysematous regions is lower than of the normal lung, so it may be difficult to distinctly visualize the IBL by ICG in emphysematous lungs with the extreme reduction in blood flow. 21 However, well‐defined IBL was visualized in other patients with emphysematous regions, so further research is needed to explain this issue.
The present study showed that the mean presentation time of IBL in the ICGF‐based group was significantly shortened compared with the MID group, and the operative time of the ICGF‐based group was shorter than that of the MID group, with an average difference of about 23 minutes between the two groups. In addition, the shortened operative time was probably due to the good surgical view and clear IBL as the ICGF‐based method is based on blood flow and does not require lung reinflation. On the other hand, in the MID method, experienced anesthesiologists needed a high degree of engagement, which was beyond the control of the chief surgeon. Lung reinflation narrowed the surgical field of view, resulting in a lack of working space in the context of uniport VATS, which increased the difficulty of the procedure, especially in emphysematous lungs where the IBL could not be well defined and it may take a long time to deflate the lung. The reinflated lung parenchyma was not conducive to the removal of a small single‐port incision and was prone to fragmentation, resulting in difficulty in palpating the lesion. The prolonged incision may also increase postoperative chest pain in patients. Theoretically, shortening the operative time can generate better perioperative outcomes and accelerate patient recovery. The main complication causing the difference in perioperative outcomes between the two groups was prolonged air leaks. The diagnostic criteria for prolonged air leaks in this study was defined as a rate of >50 ml/min, lasting for more than 7 days. In fact, postoperative prolonged air leaks were more often observed in the MID group, especially in patients with an unclear IBL. As the IBL was not defined, the segmental hilar should be opened wider with an ultrasonic scalpel or electrocautery to peel along intersegmental veins with more intersegmental parenchyma incised. This may lead to more air leaks especially in patients with impaired lung function. 22 , 23 However, we found no significant differences in many other perioperative outcomes between the groups. Although the ICGF‐based group showed trends toward less bleeding volume, shorter time for chest tube duration, and postoperative hospital stays, the differences did not reach a statistical significance. In addition, the median chest tube duration in prolonged air leaks patients was 8.2 days, so prolonged air leaks would definitely extend the chest tube duration. This contradiction may be related to a relatively small proportion of prolonged air leaks and a limited sample size. Owing to the reconstructed dynamic 3D images, which were placed in front of the operator screen for real‐time intraoperative navigation, accidental injury of the blood vessels and bronchi could be minimized and the identification rate of clear IBL could be increased. This may explain that the small amount of bleeding volume and sufficient resection margins in both groups in this study.
This study has a few limitations. First, like any other retrospective series, the potential selection bias cannot be eliminated. Second, the PINPOINT endoscopic fluorescence imaging system is relatively expensive, about $290 000. However, the expensive device purchased by our center is not financially burdensome to patients. In our institution, the application of VATS in most thoracic operations can be performed by different surgical teams using the fluorescence equipment instead of standard thoracoscopic equipment, as the PINPOINT endoscopic fluorescence imaging system has almost the same specifications as a standard thoracoscopic system, reducing the cost of purchasing other thoracoscopic equipment. Third, several previous studies have confirmed that the ICG intravenous injection is safe and permissible in thoracic surgery. 14 , 24 In this study, we used a standard dose of ICG at 5 mg for every patient, irrespective of their bodyweight, which proved to be sufficient for clear IBL visualization without ICG‐related complications. In addition, the dose of ICG is still quite different to that in Japanese reports, 25 , 26 and we think this may be due to different fluorescence equipment with different sensibilities to identify near‐infrared light. We used a PINPOINT endoscopic fluorescence imaging system and the Japanese teams used Olympus fluorescence equipment (Olympus Co., Ltd). Moreover, this much fewer doses of ICG also enhanced the safety of the procedure without toxicity. A relatively short duration of ICG staining, as reported by the Japanese teams, was also found in our study. It is therefore very importatn to be ready to mark the transitional zone on the visceral pleura using electrocautery quickly after ICG injection. As the IBL was visualized by a difference in color, we did not find that only a few minutes to mark the transitional zone on the visceral pleura with electrocautery was a limitation. In addition, with the small dose of ICG we used, multiple injections are safe and do not show toxicity.
In conclusion, our study shows that the ICGF‐based method can highly accurately identify the IBL and make anatomical segmentectomy easier and faster, and it helps to ensure sufficient resection margins, therefore it has the potential to be a feasible and effective technique to facilitate the quality of uniport VATS segmentectomy. Further studies with a larger sample size are needed to verify these promising conclusions.
AUTHOR CONTRIBUTIONS
Conception and design: Y.S. and F.S. Administrative support: R.Y. and F.S. Provision of study materials and patients: R.Y. and F.S. Collection and assembly of data: Y.S. Data analysis and interpretation: Y.S. Manuscript writing: all authors. Final approval of manuscript: all authors.
DISCLOSURES
The authors declare that they have no conflicts of interest.
ETHICAL STATEMENT
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of Nanjing Chest Hospital, Jiangsu, China (approval number: 2020‐KY093‐01). Written informed consent was obtained from all patients before surgery.
ACKNOWLEDGMENTS
This work was supported by a key project of the Nanjing Medical Technology Development Fund, Government of Jiangsu Province, China (No. ZKX19046).
Sun Y, Zhang Q, Wang Z, Shao F, Yang R. Feasibility investigation of near‐infrared fluorescence imaging with intravenous indocyanine green method in uniport video‐assisted thoracoscopic anatomical segmentectomy for identifying the intersegmental boundary line. Thorac Cancer. 2021;12:1407–1414. 10.1111/1759-7714.13923
Funding information This work was supported by a key project of Nanjing Medical Technology Development Fund, Jiangsu Province, China, Grant/Award Number: ZKX19046; Government of Jiangsu Province
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. Ca‐Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horeweg N, Scholten ET, de Jong PA, van der Aalst CM, Weenink C, Lammers JWJ, et al. Detection of lung cancer through low‐dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol. 2014;15:1342–50. [DOI] [PubMed] [Google Scholar]
- 4. Liu C‐C, Shih C‐S, Pennarun N, Cheng C‐T. Transition from a multiport technique to a single‐port technique for lung cancer surgery: is lymph node dissection inferior using the single‐port technique? Eur J Cardiothorac Surg. 2016;49:i64–72. [DOI] [PubMed] [Google Scholar]
- 5. Yang W, Zhang G, Pan S, Wang Z, Li J, Ren W, et al. Comparison of the perioperative efficacy between single‐port and two‐port video‐assisted thoracoscopic surgery anatomical lung resection for non‐small cell lung cancer: a systematic review and meta‐analysis. J Thorac Dis. 2019;11:2763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu CF, Fernandez R, Mercedes dT, et al. Mid‐term survival outcome of single‐port video‐assisted thoracoscopic anatomical lung resection: a two‐Centre experience. Eur J Cardiothorac Surg. 2018;54:252–9. [DOI] [PubMed] [Google Scholar]
- 7. Tane S, Nishio W, Nishioka Y, Tanaka H, Ogawa H, Kitamura Y, et al. Evaluation of the residual lung function after thoracoscopic Segmentectomy compared with lobectomy. Ann Thorac Surg. 2019;108:1543–50. [DOI] [PubMed] [Google Scholar]
- 8. Nomori H, Shiraishi A, Cong Y, Sugimura H, Mishima S. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg. 2018;53:640–7. [DOI] [PubMed] [Google Scholar]
- 9. Wang J, Xu X, Wen W, Wu W, Zhu Q, Chen L. Modified method for distinguishing the intersegmental border for lung segmentectomy. Thorac Cancer. 2018;9:330–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roman M, Labbouz S, Valtzoglou V, Ciesla A, Hawari M, Addae‐Boateng E, et al. Lobectomy vs. segmentectomy. A propensity score matched comparison of outcomes. Eur J Surg Oncol. 2019;45:845–50. [DOI] [PubMed] [Google Scholar]
- 11. Fu HH, Feng Z, Li M, Wang H, Ren WG, Peng ZM. The arterial‐ligation‐alone method for identifying the intersegmental plane during thoracoscopic anatomic segmentectomy. J Thorac Dis. 2020;12:2343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Misaki N, Tatakawa K, Sung Soo C, Go T, Yokomise H. Constantrate intravenous infusion of indocyanine green leading to high fluorescence intensity in infrared thoracoscopic segmentectomy. J Thorac Cardiovasc Surg Tech. 2020;3:319–324. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iizuka S, Kuroda H, Yoshimura K, Dejima H, Seto K, Naomi A, et al. Predictors of indocyanine green visualization during fluorescence imaging for segmental plane formation in thoracoscopic anatomical segmentectomy. J Thorac Dis. 2016;8:985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Motono N, Iwai S, Funasaki A, Sekimura A, Usuda K, Uramoto H. Low‐dose indocyanine green fluorescencenavigated segmentectomy: prospective analysis of 20 cases and review of previous reports. J Thorac Dis. 2019;11:702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun Y, Zhang Q, Wang Z, Shao F, Yang R. Is the near‐infrared fluorescence imaging with intravenous indocyanine green method for identifying the intersegmental plane concordant with the modified inflation‐deflation method in lung segmentectomy? Thorac Cancer. 2019;10:2013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamel MK, Rahouma M, Lee B, Harrison SW, Stiles BM, Altorki NK, et al. Segmentectomy is equivalent to lobectomy in hypermetabolic clinical stage IA lung adenocarcinomas. Ann Thorac Surg. 2019;107:217–23. [DOI] [PubMed] [Google Scholar]
- 17. Tsubokawa N, Tsutani Y, Miyata Y, Handa Y, Misumi K, Hanaki H, et al. Segmentectomy versus lobectomy for Radiologically pure solid clinical T1a‐bN0M0 lung cancer. World J Surg. 2018;42:2493–501. [DOI] [PubMed] [Google Scholar]
- 18. Okada M, Mimae T, Tsutani Y, Nakayama H, Okumura S, Yoshimura M, et al. Segmentectomy versus lobectomy for clinical stage IA lung adenocarcinoma. Ann Cardiothorac Surg. 2014;3:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kodama K, Higashiyama M, Okami J, Tokunaga T, Imamura F, Nakayama T, et al. Oncologic outcomes of Segmentectomy versus lobectomy for clinical T1a N0 M0 non‐small cell lung cancer. Ann Thorac Surg. 2016;101:504–11. [DOI] [PubMed] [Google Scholar]
- 20. Qiu B, Ji Y, He H, Zhao J, Xue Q, Gao S. Three‐dimensional reconstruction/personalized threedimensional printed model for thoracoscopic anatomical partial‐lobectomy in stage I lung cancer: a retrospective study. Transl Lung Cancer Res. 2020;9:1235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guigard S, Triponez F, Bédat B, Vidal‐Fortuny J, Licker M, Karenovics W. Usefulness of near‐infrared angiography for identifying the intersegmental plane and vascular supply during video‐assisted thoracoscopic segmentectomy. Interact Cardiovasc Thorac Surg. 2017;25:703–9. [DOI] [PubMed] [Google Scholar]
- 22. Kim HK, Han KN. Uniportal video‐assisted thoracoscopic surgery Segmentectomy. Thorac Surg Clin. 2017;27:387–98. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura H, Taniguchi Y, Miwa K, Adachi Y, Fujioka S, Haruki T, et al. Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non‐small cell lung cancer. Thorac Cardiovasc Surg. 2011;59:137–41. [DOI] [PubMed] [Google Scholar]
- 24. Chang TI, Chen YS, Huang SC. Intraoperative indocyanine green fluorescence lymphography to detect chylous leakage sites after congenital heart surgery. J Thorac Cardiovasc Surg. 2014;148:739–40. [DOI] [PubMed] [Google Scholar]
- 25. Kasai Y, Tarumi S, Chang SS, Misaki N, Gotoh M, Go T, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2‐ and 1‐wavelength methods. Eur J Cardiothorac Surg. 2013;44:1103–7. [DOI] [PubMed] [Google Scholar]
- 26. Tarumi S, Misaki N, Kasai Y, Chang SS, Go T. Clinical trial of video‐assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg. 2014;46:112–5. [DOI] [PubMed] [Google Scholar]


