Abstract
Background
The Ghent Older People’s Prescriptions community Pharmacy Screening (GheOP3S)-tool was developed in 2016 as a screening tool to detect drug-related problems (DRPs) and to help in performing medication reviews in older people (≥ 65 years).
Objective
This study aimed to revise and update the GheOP3S-tool.
Methods
Users’ comments were collected to improve the usability and appropriateness of the original GheOP3S-tool, followed by a two-round modified Delphi process according to the RAND/UCLA appropriateness method. This included a literature review, a round zero meeting, a first written round (with 15 international and multidisciplinary experts) and a second face-to-face round (with 11 experts) to change, delete or add GheOP3S-criteria. An additional third round with 14 community pharmacists was organised to preserve criteria applicable in the current community pharmacy practice.
Results
The updated GheOP3S-tool consists of five lists of DRPs and a new addendum containing medications that should be avoided or used with caution in older people with reduced renal function. During the first two rounds, related criteria were grouped, 14 criteria were added and 17 criteria were deleted from the original tool. All criteria were deemed applicable in round 3. This led to a final tool (version 2) with 64 GheOP3S-criteria.
Conclusion
GheOP3S-criteria were revised and updated according to experts’ agreement on their clinical relevance and recent scientific evidence. Future studies should investigate the impact of pharmacist-led medication reviews with GheOP3S-tool version 2 on clinical, humanistic and economic outcomes in primary care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40266-021-00862-6.
Key Points
| The GheOP3S‐tool can be applied without the need for extra clinical patient data, yet if the patient’s renal function is available, a GheOP3S-tool addendum can support the detection of additional drug-related problems, since the tool was expanded with an addendum containing medications that should be avoided or used with caution in older people with reduced renal function. |
| The new criteria “Combination of fall-risk-increasing drugs” should increase the awareness of the risks of these drugs and help pharmacists to contribute to fall prevention. |
| The updated GheOP3S‐tool can help community pharmacists to initiate medication reviews in primary care and to recommend evidence-based interventions to physicians to optimise the older patient’s medication use in an interprofessional setting. |
| Future research should correlate the updated GheOP3S-criteria with clinical outcomes to further consolidate their clinical relevance. In addition, the impact of pharmacist-led medication reviews with GheOP3S-tool version 2 on clinical, humanistic and economic outcomes in primary care should be investigated. |
Introduction
Prescribing for older patients is challenging in daily practice, considering that benefits and risks of all medications should be carefully weighed for every individual patient. Increased life expectancy together with multimorbidity increases the risk of polypharmacy and consequently the risk of drug-related problems (DRPs) and potentially inappropriate prescribing (PIP) [1–4]. Not only is the PIP prevalence among older patients in primary care high (i.e. pooled prevalence rate of 33%; 95% CI 30–37) [5], but PIP can also be associated with a range of negative health-related outcomes such as adverse drug events [6, 7], functional decline [7], reduced health-related quality of life [7], emergency room visits [7] and a higher rate of hospital admissions [7–10]. These outcomes can put a burden on patient safety and healthcare costs. Avoiding medication-related harm due to polypharmacy inappropriateness has therefore become a global public health issue [11]. Community pharmacists are well positioned to prevent, detect, discuss and solve DRPs because of their accessibility for all patients, their overview of prescribed and over-the-counter medications and because they are experts in medication use. Consequently, primary care interventions in older patients, such as pharmacist-led medication reviews performed with tools to detect DRPs or inappropriate medication use [12–14], have become a key strategy to prevent and to reduce these harms [5, 12].
Pazan et al. recently (2019) identified 76 tools to optimise medication in older people [15], with the Ghent Older People's Prescriptions community Pharmacy Screening (GheOP3S)‐tool as an example of a screening tool to detect DRPs or PIP with high clinical relevance for older patients (≥ 65 years) in the community pharmacy setting. This tool was developed after an extensive literature search and a Research ANd Development/University of California Los Angeles (RAND/UCLA) [16] process with a multidisciplinary panel of 11 European experts, and was first published in 2016 [17].
The GheOP3S-tool has several advantages compared with other tools to optimise medication in older people. First, the tool can be easily applied to medication dispensing data available in the community pharmacy (which includes over-the-counter medications, medications prescribed by general practitioners and by specialists), without the need for additional clinical patient information (only diagnoses or comorbidities that can be unambiguously derived from the medication history are included), as community pharmacists (in Belgium) have limited access to this information. Second, this evidence-based tool not only covers the whole range of PIP [18, 19], namely overprescribing (prescribing more medication than clinically needed), misprescribing (incorrectly prescribing medication that is needed) and underprescribing (failure to prescribe medication that is needed) of medication (in an explicit manner i.e. lists 1–4, see Table 1), but it also covers pharmaceutical care-related criteria (in an implicit manner, i.e. list 5, see Table 1). Furthermore, in 2018, a GheOP3S-tool addendum was developed containing medications that should be avoided or used with caution in older people with a reduced renal function (12 PIP criteria of Renally Excreted Active Drugs, READs) [20]. Third, the GheOP3S-tool is interchangeable between European countries as it consists of European-marketed medications or medication classes. Fourth, a rationale, alternative therapeutic options (‘alternatives’) and supporting literature are provided to facilitate pharmacists’ recommendations to physicians and to aid in initiating and performing a medication review. Fifth and last, since the tool predominantly consists of explicit criteria, it has the potential to be incorporated in pharmacy software systems to facilitate screening in daily practice (e.g. as part of a simple review [21, 22] based on medication dispensing data). The rather implicit criteria in list 5 could be used during a more in-depth review (intermediate or advanced medication review [21, 22]) of the patient’s medication use, as a patient interview is essential to check for the majority of these criteria.
Table 1.
Organisation of the GheOP3S-tool
| List 1 | Potentially inappropriate medication for older people |
| List 2 | Potentially inappropriate medication for older people, dependent on comorbidities |
| List 3 | Potentially omitted medication in older people |
| List 4 | Drug–drug interactions especially relevant in older people |
| List 5 | Pharmaceutical care-related criteria for older people to be addressed in the community pharmacy |
| Addendum | Medications that should be avoided or used with caution (need for reduction in dose or dosing frequency) in older people with a reduced renal function |
Recent research has demonstrated a high PIP prevalence among older patients in primary care when medication reviews were performed with the GheOP3S-tool [23–28]. A potential impact on the number of potentially inappropriate medications and the anticholinergic/sedative medication burden was shown after multidisciplinary medication reviews with the GheOP3S-tool [25, 26].
Since evidence, guidelines and clinical expertise change over time, tools containing explicit criteria need to be updated regularly. Therefore, with this study, we aimed to revise and update the original GheOP3S-tool.
Methods
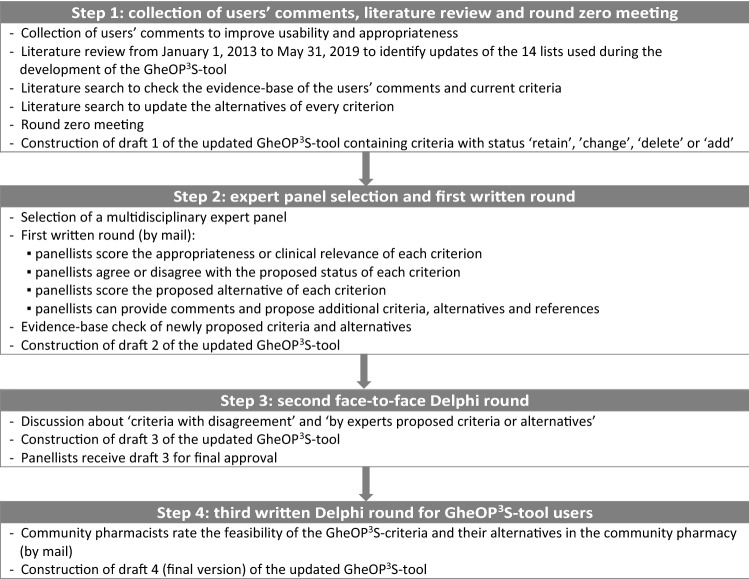
In analogy with the development of the original GheOP3S-tool [17], the update process followed a four-step approach based on the RAND/UCLA appropriateness method (i.e. a literature review, a round zero meeting and a two-round modified Delphi process) [16]. Every step led to a preliminary version (draft) of the updated GheOP3S-tool (Fig. 1: Study flowchart).
Fig. 1.
Study flowchart
Step 1: Collection of Users’ Comments, Literature Review and Round Zero Meeting (Draft 1)
Collection of Users’ Comments
A call (using Facebook, the local pharmacists association and email to our university pharmacy practice network) was launched for GheOP3S-tool users to provide comments and suggestions to improve the usability and appropriateness of the original tool. Users could propose new criteria, removal of criteria, alterations to existing criteria (e.g. criteria that are not clear or need additional information) or practical/structural amendments to the tool.
Literature Review
PubMed® and Embase® were searched from January 1, 2013 (the end of the previous search) to May 31, 2019 to identify published updates (or adapted versions) of the tools that were incorporated during the development of the original GheOP3S-tool [17]. For tools with multiple updates, only the most recent version was included. Criteria concerning medications not available on the European market or medications of which only the bulk product was available (for pharmaceutical compounding) were not taken into account.
New or altered criteria in these updated tools were considered to be ‘added’ to the GheOP3S-tool or considered to ‘change’ already existing criteria, in accordance with current literature. So, criteria proposed to be added to the GheOP3S-tool could be derived from users’ comments or the updated tools. Drug–drug interactions (DDIs) were only proposed to be added when they were present in (i) Stockley’s Drug Interactions [29], (ii) Lexicomp® Drug Interactions [30] and (iii) Clinical Pharmacology (level 1 ‘Severe’ & level 2 ‘Major’) [31]. The GheOP3S-table containing “Medications with high risk for anticholinergic adverse effects” was supplemented with substances also mentioned by the Belgian Commented Drugs Repertory [32].
In addition, the evidence base of all users’ comments and all original GheOP3S-criteria with their alternatives were checked in scientific literature. We focused on the first four layers (systems, summaries, synopses of syntheses and syntheses) of the 6S model of Haynes [33] to perform this search. At least two information sources from the highest possible layer were used.
Round Zero Meeting
The research team (AC, AS, EM, KB, KF, LDB) convened to discuss which users’ comments and criteria should (not) be withheld and to clarify the description of the criteria and alternatives. This was done in accordance with the ‘scope of the GheOP3S-tool’, which is based on following principles: (i) criteria should be targeted to older people (≥ 65 years); (ii) the tool aims to detect ‘low-hanging fruit’, these are potential DRPs that the community pharmacist can (and should) easily detect and try to resolve; (iii) for list 2 “Potentially inappropriate medication for older people, dependent on comorbidities”, only comorbidities that can be unambiguously derived from the actual medication use (e.g. medication history) should be withheld; (iv) criteria that require extra clinical information such as laboratory results will not be withheld, except for the addendum “Medications that should be avoided or used with caution in older people with a reduced renal function”, since access to renal functions in Belgium could be provided in the future; and (v) criteria should only contain medications that are available on the European market. Subsequently, all GheOP3S-criteria were listed with their alternatives (in ‘track changes’) and provided with a proposed status of ‘retain’, ‘change’, ‘delete’ or ‘add’ accompanied with their supporting evidence. This eventually resulted in draft 1 of the updated GheOP3S-tool.
Step 2: Expert Panel Selection and First Written Round (Draft 2)
Expert Panel Selection
In September 2019, the research team invited a 20-membered international and multidisciplinary Delphi panel with expertise in all decision-making disciplines involved in geriatric care and/or pharmacy practice, acknowledged leadership in their speciality, no conflicts of interest, good comprehension of the English language, geographic diversity and diversity of practice setting.
First Written Round
In November 2019, all participating panellists received (by mail) detailed rating instructions, a scoring form and draft 1 of the updated GheOP3S-tool accompanied by supporting evidence.
Panellists evaluated (i) the appropriateness or clinical relevance of every criterion by deciding on the question “How do you rate the added clinical value of a community pharmacist checking this criterion for an older patient (≥ 65 years)”; (ii) the proposed status of the criterion, by answering (and comment on): “Do you agree with the proposed status of the criterion?”; and (iii) the appropriateness of the proposed alternative (“How do you rate the proposed alternative?”). Panellists were instructed to use their own best clinical judgement and to consider the scientific evidence from the literature review to rate these questions. Practical aspects (e.g. the organisation of community pharmacies in the panellist’s country, cost implications and access to patients’ clinical records at the community pharmacy) were asked not to be taken into account during scoring.
All criteria were scored (for question 1 and 3) on a scale ranging from 1 to 9, with 1 indicating that checking for this criterion in the community pharmacy has no added clinical value or that the proposed alternative was not appropriate. A score of 9 indicated that checking for this criterion in the community pharmacy had a high added clinical value or that the proposed alternative was highly appropriate.
Afterwards, all panellists’ individual ratings were listed. Panel medians of 7–9 were classified as ‘appropriate’ and these criteria were eventually retained, changed, deleted or added (according to their proposed status) to the preliminary GheOP3S-tool. Furthermore, the dispersion of panel ratings to indicate (dis)agreement with a certain criterion or alternative was calculated using the Interpercentile Range Adjusted for Symmetry (IPRAS) method described in the RAND/UCLA appropriateness method user’s manual [16]. A criterion or alternative was rated with disagreement if the interpercentile range (IPR) (between the 30th and 70th percentile) was greater than the IPRAS of that same criterion or alternative.
Experts could also provide additional comments, criteria, alternatives and references in the scoring form. This step resulted in draft 2 of the updated GheOP3S-tool.
Step 3: Second Face-to-Face Round with Dutch-Speaking Panellists (Draft 3)
To avoid language barriers during the discussion, only Dutch-speaking panellists were invited to participate in this round (as suggested by the RAND/UCLA appropriateness method user’s manual [16]). Due to COVID-19 precautions, the face-to-face meeting was replaced by an online video meeting. Two weeks prior to this meeting, all participating Dutch-speaking panellists received draft 2. During this meeting, the moderator (KF) focused the discussion on ‘criteria with disagreement’ and ‘criteria, alternatives or comments that were proposed by experts’, to reach agreement between panellists. Draft 3 was then sent to these panellists for approval.
Step 4: Third Written Round with Community Pharmacists (Draft 4)
In the last step, community pharmacists (other than in step 1) rated the feasibility of the updated GheOP3S-criteria and alternatives (draft 3) in the community pharmacy practice. They had to decide on the following questions: “How do you rate the feasibility of checking this criterion in the current community pharmacy practice?” and “How do you rate the feasibility of the proposed alternative strategy?” by scoring them ranging from 1 (‘absolutely not feasible’) to 9 (‘absolutely feasible’). For list 4 (DDIs) and the addendum with READs, the pharmacists were asked whether they found them useful in daily practice (“Do you find this list/addendum useful in daily practice?”) and to provide comments to explain their answers. Pharmacists were instructed to consider practical aspects of the pharmacy workflow and cost implications rather than assess the clinical importance of each criterion. When criteria or alternatives were rated as ‘not feasible in the current community pharmacy practice’, the panellists from step 3 were asked to give feedback or rediscuss these criteria. All experts received draft 4 for final approval.
Results
Step 1: Collection of Users’ Comments, Literature Review and Round Zero Meeting (Draft 1)
Twelve Belgian community pharmacists who already had experience with the GheOP3S-tool provided comments on the original GheOP3S-tool. In total, eight criteria were proposed to be added, 19 were proposed to be deleted and ten were proposed to be changed. The literature search revealed three updated screening tools since 2013, namely Screening Tool of Older Persons’ Prescriptions/Screening Tool to Alert to Right Treatment (STOPP/START) criteria version 2 [34], Norwegian General Practice Nursing Home (NORGEP-NH) criteria [35] and the 2019 updated American Geriatrics Society (AGS) Beers criteria® [36]. The flow of GheOP3S-criteria throughout the study is provided in electronic supplementary material 1 (ESM1).
Step 2: Expert Panel Selection and First Written Round (Draft 2)
Fifteen of the 20 invited experts eventually participated in the first written Delphi round. The expert panel consisted of six geriatricians, two academics with backgrounds in clinical pharmacy and geriatric medicine, two clinical pharmacists, two general practitioners, one emergency physician, one community pharmacist, and one nurse, representing seven different countries. All criteria were classified as ‘appropriate’ (panel median 7–9), except (panel median < 7) the following ten criteria for which there was also ‘disagreement’ (according to the IPRAS method) in draft 1 (ESM1): the original criteria concerning (1) liquid paraffin, (2) pentazocine, (3) rivaroxaban/apixaban, and (4) a potential new criterion regarding desmopressin (list 1); (5) “Oral corticosteroids > 1 week in case of hypertension” (list 2); (6) “The patient has an elevated risk for osteoporosis and is not prescribed calcium/vitamin D supplementation” (list 3); (7) “Oral antidiabetic with risk of hypoglycaemia/insulin + cardioselective β-blocker”, (8) “Calcium + Quinolone/Tetracycline”, (9) “VKA + Macrolides (excluding azithromycin)”, (10) “Metformin + Renin-angiotensin-aldosterone system (RAAS) inhibitor + diuretics” (list 4). All ten of these criteria had the proposed status of ‘delete’ in draft 1. Further analysis revealed that some experts provided high scores for criteria with the status of ‘delete’, although they agreed to delete the criterion (leading to ‘disagreement’ for these criteria). A new table (Table B in ESM2) containing 14 medication classes of fall-risk-increasing drugs (FRIDs) according to the European Screening Tool of Older Persons Prescriptions in older adults with high fall risk (STOPPFall) [37] was proposed to be added to support the detection of a newly proposed criterion in list 4 “Combination of FRIDs”.
Step 3: Second Face-to-Face Round with Dutch-Speaking Panellists (Draft 3)
Eleven Dutch-speaking panellists (three geriatricians, two clinical pharmacists, two general practitioners, one emergency physician, one community pharmacist, one hospital pharmacist and one nurse) participated in this round. Based on the criteria with ‘disagreement’ and the additional panellists’ comments in draft 2, we focused on 35 criteria during the second round (ESM1). This round resulted in a tool with 64 GheOP3S-criteria (lists 1–5), with Table A (111 medications with high risk for anticholinergic adverse effects), Table B (14 FRIDs) and the addendum containing 17 criteria with READs (medications that should be avoided or used with caution in older people with a reduced renal function).
Step 4: Third Written Round with Community Pharmacists (Draft 4)
Fourteen Belgian community pharmacists (8 Dutch and 6 French-speaking) scored the feasibility of the GheOP3S-criteria and alternatives (draft 3) in the community pharmacy practice. No criteria needed to be rediscussed by the experts from the previous step, since all criteria were classified as ‘appropriate’ (according to their panel medians) and since there was no disagreement according to the IPRAS method. The majority of pharmacists found list 4 (79% of pharmacists) and the addendum with READs (86% of pharmacists) useful in daily practice. Based on pharmacists’ comments, minor changes were made (e.g. providing examples of ‘strong CYP3A4 inhibitors’, relocating indications from ‘criterion’ to ‘alternative’ for three criteria). Further, criteria were arranged according to the classification of the Belgian Commented Drugs Repertory [32], which is based on physiological systems (e.g. cardiovascular system, gastrointestinal system, respiratory system).
Step 4 resulted in the final (draft 4) GheOP3S-tool with a total of 64 GheOP3S-criteria (lists 1–5), with Table A (111 medications with high risk for anticholinergic adverse effects), Table B (14 medication classes of FRIDs) and the addendum containing 17 criteria with READs.
Overall, the following general changes were made to the original GheOP3S-tool: all alternatives were rewritten (to clarify them, to streamline their descriptions and/or to adapt them to the current scientific literature); the alternatives of list 4 were deleted and replaced with a general alternative “Check national guidelines or drug interaction checkers for specific recommendations”; list 5 was expanded and completed with alternatives; Table A containing medications with high risk for anticholinergic adverse effects was expanded with medications present in the 2019 updated AGS Beers criteria® and the Belgian Commented Drugs Repertory [32]; the addendum with READs and an additional table with FRIDs were added to the tool. Eventually, 14 criteria were added (Table 2) and 17 criteria were deleted (Table 3) from the tool. GheOP3S-tool version 2 can be found in ESM2.
Table 2.
New (n = 14) GheOP3S-criteria (in version 2)
| List | No. | Criterion | Reason |
|---|---|---|---|
| 1 | 3 | Acetylsalicylic acid >100 mg/day | Proposed by GheOP3S-tool user, partially derived from STOPP/START v2 |
| 1 | 13 | Desmopressin | Based on 2019 updated AGS Beers criteria® |
| 1 | 16 | Bisphosphonates for >5 years | Proposed by experts |
| 1 | 21 | Nitrofurantoin >6 months | Based on 2019 updated AGS Beers criteria® |
| 1 | 23 | Oral elemental iron >200 mg/day | Based on STOPP/START v2 |
| 3 | 37 | Osteoporotic treatment (e.g. bisphosphonates, denosumab, selective estrogen receptor modulator, teriparatide) without adequate calcium/vitamin D | Proposed by GheOP3S-tool user, partially derived from STOPP/START v2 |
| 3 | 39 | Older patients with high risk of pneumococcal infection (e.g. important comorbidity or immunosuppression) without pneumococcal vaccination (double vaccination with pneumococcal conjugate vaccine [PCV]13 and pneumococcal polysaccharide vaccine [PPV]23 according to national guidelines) at least once after age 65 years | Based on STOPP/START v2 |
| 4 | 41 | β-blocker (including eye drops) + verapamil/diltiazem | Based on STOPP/START v2 |
| 4 | 45 | Combination of QT prolonging drugs or combination of QT prolonging drug and drug that inhibits metabolism of this drug | Proposed by GheOP3S-tool user |
| 4 | 49 | Phosphodiesterase type-5 inhibitors (e.g. sildenafil, tadalafil, vardenafil) + nitrate | Based on STOPP/START v2 |
| 4 | 52 | Combination of fall-risk-increasing drugs | Based on 2019 updated AGS Beers criteria® and STOPP/START v2 |
| 5 | 60 | The patient is taking medication with a questionable efficacy and/or unfavourable safety profile (with examples) | Proposed by experts |
| 5 | 61 | Medication is being prescribed to treat an adverse effect of another medication (i.e. prescribing cascade) | Proposed by GheOP3S-tool user |
| 5 | 62 | The patient's renal function has not been taken into consideration in the dosing regimen of renally cleared medications | Based on 2019 updated AGS Beers criteria® and STOPP/START v2, GheOP3S-tool user |
| Table A | New molecules: aclidinium, amoxapine, benztropine, biperiden, carbinoxamine, clidinium-chlordiazepoxide, desipramine, doxylamine, fesoterodine, glycopyrronium, ipratropium, maprotiline, nefopam, perphenazine, propiverine, prothipendyl, protriptyline, solifenacin, tiotropium, trifluoperazine, trospium, umeclidinium | Based on 2019 updated AGS Beers criteria® and the Belgian Commented Drugs Repertory | |
AGS American Geriatrics Society, GheOP3S Ghent Older People’s Prescriptions Community Pharmacy Screening, NORGEP-NH Norwegian General Practice Nursing Home, STOPP/START Screening Tool of Older Persons’ Prescriptions/Screening Tool to Alert to Right Treatment
Table 3.
Criteria deleted (n = 17) from the original GheOP3S-tool (version 1)
| List | Criterion | Reason |
|---|---|---|
| 1 | Any drug for arterial vascular disorders | Preference for a general (rather implicit) criterion “The patient is taking medication with a questionable efficacy and/or unfavourable safety profile” with some examples (see criterion 60 in list 5) |
| 1 | Any recently marketed drug (black triangles) | Outside scope of GheOP3S-tool |
| 1 | Dabigatran | Can be safely used in older people (recent evidence-base), DOACs are still present in the addendum with READs |
| 1 | Rivaroxaban or apixaban | Can be safely used in older people (recent evidence-base), DOACs are still present in the addendum with READs |
| 1 | Ginkgo biloba or Panax ginseng | Preference for a general (rather implicit) criterion “The patient is taking medication with a questionable efficacy and/or unfavourable safety profile” with some examples (see criterion 60 in list 5) |
| 1 | Pentazocine | No longer on market in most European countries |
| 1 | Ticlopidine, new prescription | Outside scope of GheOP3S-tool |
| 2 | Oral corticosteroids >1 week with hypertension | Weak or equivocal evidence |
| 3 | The patient has an elevated risk for osteoporosis (determined via FRAX® tool) and is not prescribed calcium/vitamin D supplementation | Weak or equivocal evidence, outside scope of GheOP3S-tool |
| 3 | The patient is taking oral corticosteroids for ≥1 month and is not prescribed calcium/vitamin D supplementation | Weak or equivocal evidence |
| 4 | Oral antidiabetics/insulin + cardioselective β-blocker | DDI not deemed relevant enough (according to the scope of the GheOP3S-tool) by the expert panel |
| 4 | First dose RAAS inhibitor at full dosage + pretreatment with diuretic | DDI not deemed relevant enough (according to the scope of the GheOP3S-tool) by the expert panel |
| 4 | Calcium + quinolones/tetracyclines | DDI not deemed relevant enough (according to the scope of the GheOP3S-tool) by the expert panel |
| 4 | Calcium + strontium ranelate | DDI not deemed relevant enough (according to the scope of the GheOP3S-tool) by the expert panel |
| 4 | Calcium + levothyroxine | DDI not deemed relevant enough (according to the scope of the GheOP3S-tool) by the expert panel |
| 4 | Bisphosphonate + calcium, magnesium, zinc, iron or aluminium | DDI not deemed relevant enough (according to the scope of the GheOP3S-tool) by the expert panel |
| 4 | VKA + vitamin K-containing drugs/supplements | DDI not deemed relevant enough (according to the scope of the GheOP3S-tool) by the expert panel |
DDI drug–drug interaction, DOAC direct oral anticoagulant, FRAX fracture risk assessment tool, GheOP3S Ghent Older People’s Prescriptions Community Pharmacy Screening, RAAS renin-angiotensin-aldosterone system, READ renally excreted active drug, VKA vitamin K antagonist
Discussion
In this study, we updated the original GheOP3S-tool according to the RAND/UCLA appropriateness method with an international multidisciplinary expert panel. The updated tool now consists of 64 criteria (instead of 83 in the original version), divided over (the same) five lists, and a new addendum with READs. Multiple criteria were grouped, 14 criteria were newly added and 17 criteria were deleted from the original tool.
Based on the number of modifications made to the original tool, it is clear that regular revisions and updates of tools containing explicit criteria are required. Most of these modifications were related to reformulation and clarification of criteria and/or alternatives (e.g. according to users’ comments and new evidence), grouping of criteria (e.g. benzodiazepines, contact laxatives, centrally-acting antihypertensives, alizapride and metoclopramide in case of Parkinson’s disease, list 4 DDIs), deleting criteria with weak or equivocal evidence or criteria outside the scope of the GheOP3S-tool, and adding new relevant criteria. We ended up with less criteria in version 2 of the tool, which underlines the aim of revising and updating the tool, rather than expanding the tool with too many criteria.
We asked community pharmacists whether they found list 4 with DDIs and the addendum with READs useful in daily practice. Even though most pharmacy software systems contain a DDI checker, most of the participating pharmacists declared that it was useful to have a short list of DDIs that are especially relevant for older people (e.g. leading to increased risk of hospital admissions). Therefore, we ‘grouped’ interactions leading to similar adverse effects (such as increased risk of digoxin and lithium toxicity, bleeding, QT prolongation) and we deleted DDIs that were outside the scope of the GheOP3S-tool. These deleted DDIs remain relevant in general, but are no longer included in the tool. Additionally, since the management of DDIs is regularly updated and may differ between different drug interaction checkers, we no longer provided alternatives for this list. A new criterion concerning the combination of FRIDs (with reference to an additional table with FRIDs based on the European STOPPFall [37]) was added to list 4. This was based on the fact that almost one-third of older people fall at least once a year and that 38% of fallers report that at least one fall required medical treatment or restricted their activity [38]. Furthermore, falling may lead to serious injuries such as fractures and head injuries, increased emergency department visits and hospital admissions, and increased healthcare costs [38]. The Centers for Disease Control and Prevention point out that healthcare professionals can play an important role in fall prevention by screening older people for fall risk, by reviewing and managing medications linked to falls, and by recommending vitamin D where appropriate [38]. Therefore, this new criterion can further increase the awareness for the risks of FRIDs and help pharmacists to focus on fall prevention.
Compared with the original GheOP3S-tool, we now provide an addendum containing medications (READs) that should be avoided or used with caution in older people with a reduced renal function. Patients with reduced renal functions often have complex medication regimens due to their comorbidities (e.g. cardiovascular diseases, hypertension, diabetes, and metabolic bone diseases such as osteoporosis) [39, 40], making them more prone to DRPs. Consequently, these patients have an increased risk of cardiovascular events, hospitalisation and even death [39]. Preventive actions (e.g. recommendations for dose or dosing frequency reductions) from pharmacists to reduce the potential nephrotoxic effect of READs therefore seem appropriate. The inclusion of some medications (e.g. thiazides) in the addendum could be considered controversial [41]. Nevertheless, their potential inappropriateness should be discussed with the treating physician for every patient individually. We also chose not to provide standard cut-off intervals or dose/frequency adaptations as these can vary widely between local guidelines. Although renal functions are not yet available as standard to Belgian community pharmacists, they declared that this addendum could be useful in those cases where the patient’s renal function is already available (e.g. when the patient provides his laboratory results to the pharmacist) or when these will become available in the future. This is also confirmed by the literature, since several studies have suggested positive effects (e.g. increased detection of DRPs [42]) on clinical (e.g. blood pressure, haemoglobin, parathyroid hormone, and creatinine clearance), humanistic (e.g. health-related quality of life, patient satisfaction) and economic outcomes when information on renal function was available to pharmacists [42, 43].
The main strength of this study was that the GheOP3S-tool was updated rigorously in different steps, with a broad input from practicing pharmacists, physicians, an international multidisciplinary panel with specific expertise in emergency medicine, family medicine, geriatric medicine, nursing science, pharmacy practice and research; and a thorough review of the recent literature. Accordingly, the GheOP3S-tool is a comprehensive, evidence-based, inexpensive and easy-to-use tool covering all aspects of PIP in an explicit way, combined with additional DRPs related to pharmaceutical care-related criteria for older people to be addressed in the community pharmacy in a rather (more patient-centred) implicit way.
Previous research has shown that application of the GheOP3S-tool by the community pharmacist is feasible [24], yet integration of this tool in pharmacy software (e.g. as computerised or clinical decision support systems) [44] is recommended to limit the time investment of manually screening medication lists [26] and to improve the applicability in routine practice.
A limitation of our approach is that we did not provide additional information on the ‘quality of evidence’ and ‘strength of recommendations’ for every GheOP3S-criterion (as in the AGS Beers criteria®). The main reason for this is that proposed changes were based on recently adapted tools that were incorporated during the development of the GheOP3S-tool, namely STOPP/START criteria version 2 [34], NORGEP-NH [35] and the 2019 updated AGS Beers criteria® [36], which are all Delphi consensus validated lists. Furthermore, we only based our update on these recently adapted tools because our main goal was to update the criteria already present in the tool, rather than to expand the tool. However, participants did have the opportunity to propose additional criteria, which were checked for their evidence base.
It should also be noted that tools that address DRPs or PIP only act as surrogate markers (prescribing indicators or process measures) for polypharmacy inappropriateness. Since these tools do not provide support as to how treatment decisions should be prioritised for the individual patient, they can only be used as guidance to support clinical decision making [44, 45]. Hence, they are not a substitute for a comprehensive clinical patient evaluation or for rational clinical decision making tailored to the patient’s needs [44]. Consequently, a patient-centred and individualised approach [44] with shared decision making (considering the patient’s needs, preferences, values, therapeutic goals, treatment targets, time until benefit and life expectancy [46]) in collaboration with involved parties (e.g. healthcare professionals such as pharmacists, physicians and nurses, carers or family) remains essential to determine the clinical relevance of DRPs (i.e. potentially inappropriate versus actually inappropriate), to prioritise actions and to assure actual implementation of interventions (with adequate follow-up).
The question of whether the use of the updated GheOP3S-tool might have a positive impact on the outcomes of pharmacist-led medication reviews in community-dwelling older patients should be addressed in forthcoming research. Concerning this future research, several steps can be proposed. First, the updated GheOP3S-criteria should be clinically validated to further consolidate their clinical relevance. This can be performed in a large sample of older patients with polypharmacy, where GheOP3S-criteria can be correlated with clinical or health-related outcomes such as adverse drug reactions, drug-related hospital admissions, and patient-related outcomes [44, 47]. Second, the clinical relevance of pharmacists’ recommendations related to the detected DRPs using the updated GheOP3S-tool, should be further investigated in a large prospective trial taking into account patients’ characteristics (such as comorbidities, renal insufficiency, fall risk, etc.). An additional economic evaluation regarding pharmacist-led medication reviews with the GheOP3S-tool in primary care should also be conducted. Third, ideally, a randomised controlled trial should be performed to assess the impact of pharmacist-led medication reviews using the GheOP3S-tool in community-dwelling older patients. A core outcome set as proposed by Beuscart et al. (2018) [48] or Rankin et al. (2018) [49] can be used to report relevant outcomes.
To conclude, community pharmacists are ideally placed to address polypharmacy inappropriateness by initiating medication reviews with the help of this updated tool [50]. The updated tool facilitates evidence-based recommendations to physicians, and therefore facilitates interprofessional collaboration to optimise the patient’s medication use. The tool can be applied without the need for extra clinical patient data, yet if the patient’s renal function is also available, the new addendum with READs can further aid pharmacists to detect additional clinically relevant DRPs and to help in optimising medication use [42, 43, 51].
Conclusion
This study revised and updated all GheOP3S-criteria with their proposed alternatives in order to identify clinically relevant DRPs in older people (aged ≥65 years) and to provide evidence-based recommendations to physicians during pharmacist-led medication reviews. The updated tool consists of 64 criteria and can support pharmacists to consolidate their role as medication experts by reducing polypharmacy inappropriateness and/or by optimising patients’ medication use in primary care. Future studies should investigate the impact of pharmacist-led and interprofessional medication reviews with GheOP3S-tool version 2 on clinical, humanistic and economic outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are profoundly indebted to the following experts and community pharmacists who participated in the GheOP3S-tool update process: Belgian community pharmacists who provided users comments: Jan Berghmans, Leen Claes, Tom Goesaert, Leen Ghyzelings, Geert Heungens, Marina Lermytte, Katrien Louwagie, Joris Maesschalk, Eline Tommelein, Yves Van Bastelaere, Christoph Vandekerckhove and Chloé Zegers. Experts participating in the first written round: Thierry Christiaens (Belgium), Peter De Paepe (Belgium), Tinne Dilles (Belgium), Sirpa Hartikainen (Finland), Sarah Hilmer (Australia), Graziano Onder (Italy), Mirko Petrovic (Belgium), Anna Renom-Guiteras (Spain), Anne Spinewine (Belgium), Jennifer Stevenson (United Kingdom), Goedele Strauven (Belgium), Patricia van den Bemt (The Netherlands), Tischa van der Cammen (The Netherlands), Nathalie van der Velde (The Netherlands), Ellen Van Leeuwen (Belgium). Experts participating in the second round: Andreas Capiau (Belgium), Thierry Christiaens (Belgium), Peter De Paepe (Belgium), Tinne Dilles (Belgium), Mirko Petrovic (Belgium), Annemie Somers (Belgium), Goedele Strauven (Belgium), Patricia van den Bemt (The Netherlands), Tischa van der Cammen (The Netherlands), Nathalie van der Velde (The Netherlands), Ellen Van Leeuwen (Belgium). Belgian community pharmacists participating in the third round: Wim Aerts, Katty Dantoin, Isabel De Sutter, Nathalie Dujardin, Valérie Lacour, Arnaud Lambert, Véronique Maertens, Bieke Popelier, Anne Santi, Stéphanie Valentin, Nele Van Boxelaer, Karlien Van Heuverswyn, Veerle Van Overloop, Annelies Vantieghem.
Declarations
Funding
No funding was received for conducting this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
Study concept and design (AS, EM, KB, KF, LDB and MP). Analysis and interpretation of data (AC, AS, EM, KB, KF, LDB and MP). Drafting the manuscript (KF) and final approval of submitted manuscript (AC, AS, EM, KB, KF, LDB and MP).
Contributor Information
Katrien Foubert, Email: katrien.foubert@ugent.be.
Andreas Capiau, Email: Andreas.Capiau@ugent.be.
Els Mehuys, Email: els.mehuys@ugent.be.
Leen De Bolle, Email: Leen.DeBolle@ugent.be.
Annemie Somers, Email: annemie.somers@ugent.be.
Mirko Petrovic, Email: mirko.petrovic@ugent.be.
Koen Boussery, Email: koen.boussery@ugent.be.
References
- 1.Kaufmann CP, et al. Determination of risk factors for drug-related problems: a multidisciplinary triangulation process. BMJ Open. 2015;5(3):e006376. doi: 10.1136/bmjopen-2014-006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wastesson JW, et al. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drug Saf. 2018;17(12):1185–1196. doi: 10.1080/14740338.2018.1546841. [DOI] [PubMed] [Google Scholar]
- 3.Cahir C, et al. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010;69(5):543–552. doi: 10.1111/j.1365-2125.2010.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nivya K, et al. Systemic review on drug related hospital admissions—a pubmed based search. Saudi Pharm J. 2015;23(1):1–8. doi: 10.1016/j.jsps.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liew TM, et al. The prevalence and impact of potentially inappropriate prescribing among older persons in primary care settings: multilevel meta-analysis. Age Ageing. 2020;49(4):570–579. doi: 10.1093/ageing/afaa057. [DOI] [PubMed] [Google Scholar]
- 6.Lund BC, et al. Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother. 2010;44(6):957–963. doi: 10.1345/aph.1M657. [DOI] [PubMed] [Google Scholar]
- 7.Liew TM, et al. Potentially inappropriate prescribing among older persons: a meta-analysis of observational studies. Ann Fam Med. 2019;17(3):257–266. doi: 10.1370/afm.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing XX, et al. Associations between potentially inappropriate medications and adverse health outcomes in the elderly: a systematic review and meta-analysis. Ann Pharmacother. 2019;53(10):1005–1019. doi: 10.1177/1060028019853069. [DOI] [PubMed] [Google Scholar]
- 9.Patel NS, et al. Hospitalizations due to preventable adverse reactions—a systematic review. Eur J Clin Pharmacol. 2017;73(4):385–398. doi: 10.1007/s00228-016-2170-6. [DOI] [PubMed] [Google Scholar]
- 10.Hyttinen V, Jyrkka J, Valtonen H. A systematic review of the impact of potentially inappropriate medication on health care utilization and costs among older adults. Med Care. 2016;54(10):950–964. doi: 10.1097/MLR.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO). Medication Without Harm - WHO Global Patient Safety Challenge. 2017 [cited 2020 12 August]; Available from: http://apps.who.int/iris/bitstream/handle/10665/255263/WHO-HIS-SDS-2017.6-eng.pdf;jsessionid=71CED9AAFB935471017FB9C2E67D1AB8?sequence=1. Accessed 12 Aug 2020.
- 12.World Health Organization. Medication Errors: Technical Series on Safer Primary Care. 2016 [cited 2020 13 July]; https://apps.who.int/iris/bitstream/handle/10665/252274/9789241511643-eng.pdf?sequence=1. Accessed 12 Aug 2020.
- 13.Tecklenborg S, et al. Interventions to reduce adverse drug event-related outcomes in older adults: a systematic review and meta-analysis. Drugs Aging. 2020;37(2):91–98. doi: 10.1007/s40266-019-00738-w. [DOI] [PubMed] [Google Scholar]
- 14.Anderson LJ, et al. A systematic overview of systematic reviews evaluating interventions addressing polypharmacy. Am J Health Syst Pharm. 2019;76(21):1777–1787. doi: 10.1093/ajhp/zxz196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pazan F, Kather J, Wehling M. A systematic review and novel classification of listing tools to improve medication in older people. Eur J Clin Pharmacol. 2019;75(5):619–625. doi: 10.1007/s00228-019-02634-z. [DOI] [PubMed] [Google Scholar]
- 16.Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, van het Loo M, McDonnell J, Vader J, Kahan JP. The RAND/UCLA appropriateness method user's manual. Santa Monica: RAND Corporation; 2001. [Google Scholar]
- 17.Tommelein E, et al. Older patients' prescriptions screening in the community pharmacy: development of the Ghent Older People's Prescriptions community Pharmacy Screening (GheOP(3)S) tool. J Public Health (Oxf) 2016;38(2):e158–e170. doi: 10.1093/pubmed/fdv090. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor MN, Gallagher P, O'Mahony D. Inappropriate prescribing: criteria, detection and prevention. Drugs Aging. 2012;29(6):437–452. doi: 10.2165/11632610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Spinewine A, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–184. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 20.Wazzan AAA, et al. Development and APplication of the GheOP(3)S-tool addendum on potentially inappropriate prescribing (PIP) of renally excreted active drugs (READs) in older adults with polypharmacy. Drugs Aging. 2018;35(4):343–364. doi: 10.1007/s40266-018-0530-x. [DOI] [PubMed] [Google Scholar]
- 21.Griese-Mammen N, et al. PCNE definition of medication review: reaching agreement. Int J Clin Pharm. 2018. [DOI] [PubMed]
- 22.Pharmaceutical Care Network Europe (PCNE). PCNE statement on medication review 2013. 2013 [cited 2020 28 March]; https://www.pcne.org/upload/files/150_20160504_PCNE_MedRevtypes.pdf. Accessed 12 Aug 2020.
- 23.Tommelein E, et al. Potentially inappropriate prescribing in nursing home residents detected with the community pharmacist specific GheOP3S-tool. Int J Clin Pharm. 2016;38(5):1063–8. doi: 10.1007/s11096-016-0366-6. [DOI] [PubMed] [Google Scholar]
- 24.Tommelein E, et al. Community pharmacists' evaluation of potentially inappropriate prescribing in older community-dwelling patients with polypharmacy: observational research based on the GheOP(3)S tool. J Public Health (Oxf). 2017;39(3):583–592. doi: 10.1093/pubmed/fdw108. [DOI] [PubMed] [Google Scholar]
- 25.Foubert K, et al. Application of the GheOP(3)S-tool in nursing home residents: acceptance and implementation of pharmacist recommendations. Acta Clin Belg. 2020;75(6):388–396. doi: 10.1080/17843286.2019.1634323. [DOI] [PubMed] [Google Scholar]
- 26.Foubert K, et al. Pharmacist-led medication review in community-dwelling older patients using the GheOP(3) S-tool: General practitioners' acceptance and implementation of pharmacists' recommendations. J Eval Clin Pract. 2020;26(3):962–972. doi: 10.1111/jep.13241. [DOI] [PubMed] [Google Scholar]
- 27.Stojanovic M, et al. GheOP(3) S tool and START/STOPP criteria version 2 for screening of potentially inappropriate medications and omissions in nursing home residents. J Eval Clin Pract. 2020;26(1):158–164. doi: 10.1111/jep.13107. [DOI] [PubMed] [Google Scholar]
- 28.Harasani K, Xhafaj D, Qipo O. Prevalence and types of potentially inappropriate prescriptions among older and middle-aged community-dwelling Albanian patients. Int J Risk Saf Med. 2020;31(1):5–13. doi: 10.3233/JRS-195052. [DOI] [PubMed] [Google Scholar]
- 29.Baxter K; Preston CL (ed). Stockley’s Drug Interactions. [online]. 2019 [cited 2019 10 April]; http://www.medicinescomplete.com/. Accessed 10 Oct 2019.
- 30.UpToDate (Wolters Kluwer Clinical Drug Information). Lexicomp(R) Online - Drug Interactions. 2019 [cited 2019 10 October]; https://www.uptodate.com/drug-interactions/?source=responsive_home#di-druglist. Accessed 10 Oct 2019.
- 31.Elsevier. Clinical Pharmacology. 2019 [cited 2019 October 2019]. https://www.clinicalkey.com/pharmacology/. Accessed 10 Oct 2019.
- 32.Belgian Center for Pharmacotherapeutic Information (BCFI). Belgian Commented Drugs Repertory. 2019 [cited 2019 20 April]; http://www.bcfi.be/. Accessed 10 Oct 2019.
- 33.DiCenso A, Bayley L, Haynes RB, ACP Journal Club Editorial: accessing preappraised evidence: fine-tuning the 5S model into a 6S model. A Intern Med. 2009;151(6):Jc3-2. doi: 10.7326/0003-4819-151-6-200909150-02002. [DOI] [PubMed] [Google Scholar]
- 34.O'Mahony D, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyborg G, et al. The Norwegian General Practice-Nursing Home criteria (NORGEP-NH) for potentially inappropriate medication use: a web-based Delphi study. Scand J Prim Health Care. 2015;33(2):134–141. doi: 10.3109/02813432.2015.1041833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The 2019 American Geriatrics Society Beers Criteria® Update Expert Panel, American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019; 67(4): 674–94. [DOI] [PubMed]
- 37.Seppala LJ, et al. STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Age Ageing, 2020. [DOI] [PMC free article] [PubMed]
- 38.Bergen GSM, Burns ER. Falls and fall injuries among adults aged ≥ 65 years—United States, 2014. Morbidity and Mortality Weekly Report (MMWR) - Weekly / September 23, 2016 / 65(37);993–998 2014 [cited 2020 9 October]. https://www.cdc.gov/mmwr/volumes/65/wr/mm6537a2.htm?s_cid=mm6537a2_w#suggestedcitation. Accessed 10 Oct 2019. [DOI] [PubMed]
- 39.Go AS, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 40.Weir MR, Fink JC. Safety of medical therapy in patients with chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2014;23(3):306–313. doi: 10.1097/01.mnh.0000444912.40418.45. [DOI] [PubMed] [Google Scholar]
- 41.Hanlon JT, et al. Consensus guidelines for oral dosing of primarily renally cleared medications in older adults. J Am Geriatr Soc. 2009;57(2):335–340. doi: 10.1111/j.1532-5415.2008.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mongaret C, et al. The role of community pharmacists in the detection of clinically relevant drug-related problems in chronic kidney disease patients. Pharmacy (Basel, Switzerland) 2020;8(2):89. doi: 10.3390/pharmacy8020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al Raiisi F, et al. Clinical pharmacy practice in the care of Chronic Kidney Disease patients: a systematic review. Int J Clin Pharm. 2019;41(3):630–666. doi: 10.1007/s11096-019-00816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufmann CP, et al. Inappropriate prescribing: a systematic overview of published assessment tools. Eur J Clin Pharmacol. 2014;70(1):1–11. doi: 10.1007/s00228-013-1575-8. [DOI] [PubMed] [Google Scholar]
- 45.Cadogan CA, Ryan C, Hughes CM. Appropriate polypharmacy and medicine safety: when many is not too many. Drug Saf. 2016;39(2):109–116. doi: 10.1007/s40264-015-0378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmes HM, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166(6):605–609. doi: 10.1001/archinte.166.6.605. [DOI] [PubMed] [Google Scholar]
- 47.O'Mahony D. STOPP/START criteria for potentially inappropriate medications/potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol. 2020;13(1):15–22. doi: 10.1080/17512433.2020.1697676. [DOI] [PubMed] [Google Scholar]
- 48.Beuscart JB, et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med. 2018;16(1):21. doi: 10.1186/s12916-018-1007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rankin A, et al. Core outcome set for trials aimed at improving the appropriateness of polypharmacy in older people in primary care. J Am Geriatr Soc. 2018;66(6):1206–1212. doi: 10.1111/jgs.15245. [DOI] [PubMed] [Google Scholar]
- 50.Spinewine A, Fialova D, Byrne S. The role of the pharmacist in optimizing pharmacotherapy in older people. Drugs Aging. 2012;29(6):495–510. doi: 10.2165/11631720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 51.Pourrat X, et al. Community pharmacist intervention in patients with renal impairment. Int J Clin Pharm. 2015;37(6):1172–1179. doi: 10.1007/s11096-015-0182-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.