Abstract
Objectives
The aim of this meta-analysis is to comprehensively evaluate the effects of lean mass on all-cause mortality across different cancer types.
Methods
This is a meta-analysis. Cohort studies on lean mass and mortality published before December 20, 2017 were obtained by systematic search on PubMed, Cochrane Library, and Embase. Inclusion criteria were cohort studies reporting lean mass measurements by dual-energy X-ray absorptiometry, bioimpedance analysis or computed tomography, and with all-cause mortality as the study outcome. Exclusion criteria were studies using muscle mass surrogates, anthropometric measurement of muscle, rate of change in muscle mass, and sarcopenia defined by composite criteria. Hazard ratios (HRs) and 95% confidence intervals (CIs) of low/reduced lean mass on cancer mortality were pooled with a random-effects model. Subgroup analysis stratifying studies according to cancer type and measurement index was performed.
Results
Altogether 100 studies evaluated the association between lean mass and cancer mortality. The overall pooled HR on cancer mortality was 1.41 (95% CI, 1.24 to 1.59) for every standard deviation decrease in lean mass and 1.69 (95% CI, 1.56 to 1.83) for patients with sarcopenia (binary cutoffs). Overall mortality was also significantly associated with sarcopenia in across various cancer types, except for hematopoietic, breast, ovarian and endometrial, and prostate cancer.
Conclusions
The robust association of decreased lean mass with increased mortality further justified the importance of developing clinical guidelines for managing sarcopenia in cancer patients. Public health initiatives aiming at promoting awareness of muscle health in susceptible individuals are urgently needed.
Keywords: Sarcopenia, Lean mass, Cancer
1. Introduction
Sarcopenia has been an emerging theme in clinical oncology, not only because it is an important prognostic factor of cancer, but also it is associated with increased economic burden to individuals and society [1,2]. Sarcopenia is a hallmark of cachexia [3,4]. It reduces tolerance to anti-cancer treatments [5,6] and increases susceptibility to infection, immobility, and other comorbidities. A pre-clinical study showed that reversal of muscle wasting led to prolonged survival in a cancer cachexia model [7], and randomized controlled trials (RCTs) demonstrated that muscle mass in cancer cachexia could be improved by pharmacological agents [8]. Thus, it is now increasingly important to recognize sarcopenia as a modifiable condition in patients with cancer.
Previous meta-analyses have suggested that sarcopenia is associated with a higher mortality in cancer patients [[9], [10], [11]]. However, these meta-analyses focussed on certain type(s) of cancer, and whether these results could be generalized to a wider spectrum of cancer types remains uncertain. The role of lean mass in cancer prognosis is still largely unclear. Furthermore, multiple methods have been proposed to detect presence and severity of sarcopenia, but thus far none has showed a clear superiority. Although lean mass per se is strongly correlated with body mass index (BMI), a commonly used marker of general health, BMI and body size (such as body surface area, BSA) are often misleading and cannot directly reflect lean mass [12]. BMI does not discriminate between fat and lean mass, which carry different implications on clinical outcomes including mortality [13]. On the other hand, measurement of L3 skeletal muscle mass index in computed tomography (CT) has been a recommended method to detect sarcopenia [14,15]. The L3 psoas index has been proposed as a simplified alternative [16], but this approach has yet to be validated [4,17]. The association between mortality and lean mass measured by these 2 indexes remains unclear.
In this meta-analysis, we aim to evaluate the role of lean mass on mortality in different types of cancer. In addition, we also evaluated the specific association of 2 most used muscle indexes, L3 skeletal muscle mass index and L3 psoas index, on mortality in cancer.
2. Methods
The detailed materials and methods have been described in Lee et al. in this same issue. This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
We searched PubMed, Cochrane Library, and Embase for articles published up to December 20, 2017. We also checked for inclusion of additional literatures from the reference lists of systematic reviews and meta-analyses. Studies reporting the association between all-cause mortality and muscle mass (measured by CT, dual-energy X-ray absorptiometry [DXA] or bioelectrical impedance analysis [BIA]) reported as “reduced lean mass” (ie, lean mass was treated as continuous variable) or “low lean mass” (ie, lean mass was treated as a binary variable, low vs normal lean mass) were included. The pre-specified exclusion criteria were as follows: non-human studies; studies using the following exposures: other surrogates of muscle mass (estimated glomerular filtration rate [eGFR], creatinine level, or lean mass ratio), anthropometric measurement of muscle mass (such as skinfold measurement, mid-arm circumference, etc), rate of change in muscle mass, sarcopenia defined using composite criteria (low lean mass in combination with muscle strength and physical performance); and studies with insufficient data for meta-analysis (studies reporting lean mass as continuous variable without providing standard deviation (SD] for standardized hazard ratio (HR] calculation and studies providing P-values only).
Study quality appraisal was done using a modified Newcastle Ottawa Scale (NOS) by J.M and cross-checked by G.K.L (Table S3). A modified NOS was applied because some questions were not applicable in the current study, such as selection of non-exposed cohort, and demonstration that outcome of interest (ie, mortality in the current study) was not present at start of the study. In the current meta-analysis, studies of good quality were defined as 2 stars in selection domain AND 1/2 stars in comparability domain AND 1/2 stars in outcome/exposure domain; studies of fair quality were defined as 1 star in selection domain AND 1/2 stars in comparability domain AND 1/2 stars in outcome/exposure domain, and poor quality was defined as studies not meeting the criteria of good or fair quality. Any discrepancy in the data extraction and quality appraisal was addressed by discussion and consensus, with involvement of another author if necessary.
The overall summary statistics of reduced lean mass (defined as analysing lean mass as continuous variable) and low lean mass (defined using pre-specified cut-off/definitions in the included study) on mortality were adopted from Lee et al (2020) in this meta-analysis series. We further performed additional analyses to evaluate if the pooled HR estimates would differ in studies with or without adjustment for major confounding factors (age, sex, ethnicity, height, weight or BMI, and others, as these factors might be independently linked to low lean mass and cancer mortality, hence confounding the association) and in studies with or without testing for proportional hazard assumptions. Furthermore, we performed subgroup analyses by stratifying studies according to types of cancer and compared the respective HRs. Studies were further stratified according to measurement index (L3 skeletal muscle index vs L3 psoas index) in order to evaluate the agreement between these 2 indexes. In the ‘Leave-one-out’ analysis, the most influential study in each subgroup was removed, and the heterogeneity (assessed using I [2]) before and after the removal was compared. Publication bias was investigated using funnel plots.
Compared to other diseases, methods in studying lean mass in cancer is more consistent. For example, the majority of them used CT in evaluating lean mass (n = 95 using CT vs n = 5 using non-CT), studied binary lean mass instead of continuous lean mass (83 studied binary lean mass, 10 studied continuous lean mass, 7 studied both binary and continuous lean mass), and studied L3 skeletal muscle index (n = 70) or L3 psoas index (n = 11) instead of other lean mass derived index (n = 14). Thus, to further reduce heterogeneity, we included 81 cancer studies that studied binary lean mass using either L3 skeletal muscle index or L3 psoas index in the subsequent analysis.
The HR and 95% CI of each study were entered into Review Manager 5.3 (RevMan, Cochrane, United Kingdom) by G.K.L and cross-checked by P.C.A. If there was mismatch in the 95% CI between the calculated values in RevMan and those reported in the study publication, either upper or lower 95% CI was chosen as reference according to the calculated P-value in RevMan, with the one with a P-value closest to the P-value reported in the study chosen.
3. Results
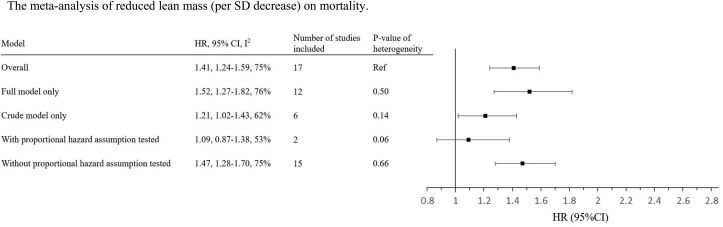
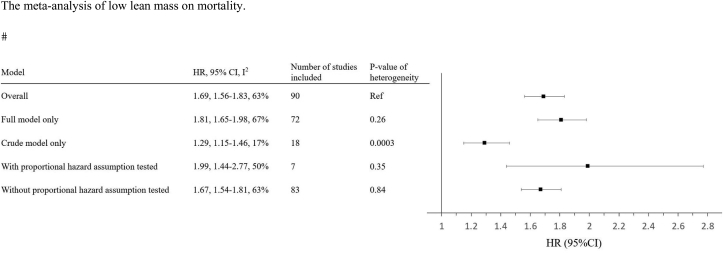
The overall association of “reduced lean mass” and “low lean mass” on mortality are shown in Fig. 1 and Fig. 2, respectively. The HRs of overall association of reduced lean mass and low lean mass on mortality were 1.41 (per SD decrease in lean mass) and 1.69, respectively, which have been described by Lee et al in the same issue of the journal. For reduced lean mass, the estimates were not significantly different in studies with or without adjustment for confounding factors or proportional assumption tested (Fig. 1). For low lean mass, the estimate was significantly lower in studies without adjustment for confounders, when compared with the analysis including all studies (Fig. 2).
Fig. 1.
The meta-analysis of reduced lean mass (per SD decrease) on mortality.
Keys: Full model refers to analysis adjusted for any of the followings (either alone or in combination): age, sex, ethnicity, height, weight or body mass index, and other confounding factors.
HR, hazard ratio; CI, confidence interval; SD, standard deviation.
Fig. 2.
The meta-analysis of low lean mass on mortality.
Keys: Full model refers to analysis adjusted for any of the followings (either alone or in combination): age, sex, ethnicity, height, weight or body mass index, and other confounding factors.
HR, hazard ratio; CI, confidence interval; SD, standard deviation.
There were 81 cancer studies that studied low lean mass using either L3 skeletal muscle index or L3 psoas index. Table 1 shows the pooled HR of low lean mass on mortality in people with different types of cancer. Among 81 studies included, gastrointestinal cancer was the most studied (n = 18), followed by liver and intrahepatic bile duct (n = 17), urinary tract (n = 12), pancreatic (n = 8), ovarian and endometrium (n = 6), lung (n = 5), multiple (n = 5), hematopoietic (n = 3), breast (n = 3), bile duct (n = 2), head and neck (n = 1), and prostate (n = 1). The forest plots and funnel plots of the meta-analysis of each cancer are provided in Supplementary Figures S1 and S2.
Table 1.
Pooled hazard ratios of low lean mass on mortality according to cancer type.
| Cancer type | Number of studies | Overall (HR [95% CI]), I2 |
|---|---|---|
| Bile duct (excludes intrahepatic) | 2 | 2.58 [1.82, 3.64], 0% |
| Breast | 3 | 1.69 [0.79, 3.58]; 61% |
| Gastrointestinal | 18 | 1.56 [1.36, 1.78]; 48% |
| Head and neck | 1 | 1.92 [1.19, 3.11]; NA |
| Hematopoietic | 3 | 1.34 [0.51, 3.53]; 73% |
| Liver and intrahepatic bile duct | 17 | 2.22 [1.86, 2.65]; 24%a |
| Lung | 5 | 2.19 [1.28, 3.75]; 60% |
| Ovarian and endometrium | 6 | 1.24 [0.91, 1.70]; 49%b |
| Pancreatic | 8 | 1.63 [1.44, 1.84]; 0% |
| Prostate | 1 | 0.90 [0.54, 1.50]; NA |
| Urinary tract | 12 | 1.88 [1.52, 2.34]; 41% |
| Mixed | 5 | 1.19 [1.03, 1.38]; 61% |
| Overall | 81 | 1.68 [1.55, 1.83]; 63% |
HR, hazard ratio; CI, confidence interval; NA, not applicable.
Higashi 2016 performed subgroup analysis, hepatocellular carcinoma subgroup was chosen (appendix p27, ref 58).
Rutten 2017 included both lean mass measurements, L3 Skeletal Muscle Index was chosen (appendix p36, ref 145).
The HR of the overall association of low lean mass with mortality in cancer was 1.68 (95% CI, 1.55–1.83, Table 1). Among 12 types of cancer studied (Table 1), significant association of low lean mass with mortality was observed in 8 types of cancer, but not in breast cancer (HR, 1.69; 95% CI, 0.79–3.58), hematopoietic cancer (HR, 1.34; 95% CI, 0.51–3.53), ovarian and endometrium (HR, 1.24; 95% CI, 0.91–1.70), and prostate (HR, 0.90; 95% CI, 0.54–1.50). Among the 8 types of cancer showing significant association, the highest HR was observed with bile duct cancer (HR, 2.58; 95% CI, 1.82–3.64), while the lowest HR was observed with multiple types of cancer (HR, 1.19; 95% CI, 1.03–1.38). In general, a moderate to high heterogeneity was observed in each specific cancer meta-analysis, with the highest and lowest I [2] being observed for hematopoietic cancer (I [2] = 73%) and pancreatic cancer (I [2] = 0%), respectively.
Stratifying by measurement index (L3 skeletal muscle index vs single-muscle measurement L3 psoas index), 70 and 11 studies conducted the lean mass CT measurement at L3 skeletal muscle and L3 psoas muscle, with an HR of 1.66 (95% CI, 1.52 to 1.82) and 1.70 (95% CI, 1.38 to 2.08), respectively. The difference between these HR was not statistically significant (P > 0.05). In the ‘Leave-one-out’ analysis, heterogeneity was reduced in most subgroups (Table S1).
4. Discussion
In this study, we showed that low lean mass was significantly associated with mortality in most of the cancers, except hormone-related cancers (breast cancer, ovarian cancer, endometrial cancer, and prostate cancer) and hematopoietic cancers. Among all muscle indices, L3 skeletal muscle index and L3 psoas index were the most used. The association of these 2 indices with mortality had similar estimates. This study provides robust evidence that lean mass is an important prognostic factor of mortality in cancers.
In past literatures, lean mass was analyzed as a continuous and/or categorical variable. The units of continuous lean mass measured by different studies were different. Therefore, we derived a standardized HR that can be directly compared across different types of cancer and measurement platforms by estimating the effect for each SD decrease in lean mass. Notably, both low lean mass (low vs normal lean mass) and reduced lean mass (per SD decrease in lean mass) were significantly associated with mortality in cancer. This is in agreement with a previous meta-analysis in solid tumors (n = 7843) evaluated the impact of low lean mass, measured by CT, on mortality by pooling together studies with different cut-off values [9]. However, the earlier meta-analysis did not evaluate the effects of reduced lean mass (per SD decrease), and lean mass measured at different skeletal sites. Also, the association between lean mass and mortality in non-solid tumors was not evaluated. Furthermore, studies evaluating the association in breast, head and neck, lung, and prostate cancer were not included. The current study provided a more detailed and comprehensive investigation of the effect of lean mass on mortality in cancer than previous studies and reinforced the important role of lean mass as a prognostic factor of cancer.
The subgroup analyses in our meta-analysis according to cancer type yielded results largely consistent results with previous meta-analyses. Previous literature have identified significant associations in patients with lung cancer [10], head and neck cancer [11,18], GI cancer [9,19,20], liver cancer [9,21,22], pancreatic cancer [23], and urinary tract cancer [24], with largely consistent effect sized identified in the current meta-analysis. However, other recently published meta-analyses by Zhang et al [25], Ubachs et al [26], and Jia et al [27] have identified a significant association between sarcopenia and survival in breast cancer, ovarian cancer, and hematological malignancies, respectively, contrary to insignificant association identified in the current meta-analysis. The inconsistencies are likely due to fewer studies being included in our subgroup analysis, which could be attributed to differences in search strategy. Meanwhile, the association between bile duct cancer and prostate cancer have not been studied. While our study provides insights into the association between sarcopenia and cancer mortality, further studies and meta-analyses are required to evaluate whether sarcopenia is associated with poor survival in breast, ovarian, hematological, prostate, and bile duct cancer.
In CT, L3 skeletal muscle surface measurement at the abdomen is recommended [14,15]. A simplified single-muscle approach using surface and/or density of the psoas muscle to represent total skeletal mass was proposed. This rationale for using this approach over measurement of skeletal muscle index was that it is more simple and convenient [16]. However, this approach has not been validated [4,17]. In the current study, we showed that the HRs derived from the L3 psoas index and L3 muscle index were similar for most of the cancers, except lung cancer (Table 2). It was reported that psoas muscle was correlated with overall lean mass and was less labour intensive to quantify compared to total muscle area at L3 [28]. Our findings supported that screening for low lean mass in solid cancers using single L3 psoas muscle could be a more practical and convenient option.
Table 2.
Pooled hazard ratios of low lean mass on mortality according to cancer type and measurement index.
| Cancer type | L3 Skeletal Muscle Index |
L3 Psoas Index |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (no. of studies) | I2 | HR | 95% CI | n (no. of studies) | I2 | HR | 95% CI | |||
| Bile duct (excludes intrahepatic) | 100 (1) | NA | 2.02 | [1.12, 3.65] | 207 (1) | NA | 2.92 | [1.91, 4.47] | ||
| Breast | 325 (3) | 61% | 1.69 | [0.79, 3.58] | NA | |||||
| Gastrointestinal | 8668 (18) | 48% | 1.56 | [1.36, 1.78] | NA | |||||
| Head and neck | 2840 (1) | NA | 1.92 | [1.19, 3.11] | NA | |||||
| Hematopoietic | 259 (3) | 73% | 1.34 | [0.51, 3.53] | NA | |||||
| Liver and intrahepatic bile duct | 2947 (15) | 28% | 2.30 | [1.89, 2.78] | 189 (2) | 0% | 1.71 | [1.03, 2.83] | ||
| Lung | 546 (4) | 49% | 2.69 | [1.50, 4.85] | 161 (1) | NA | 1.17 | [0.66, 2.08] | ||
| Ovarian and endometrium | 740 (4) | 54% | 1.18 | [0.85, 1.64] | 338 (2) | 38% | 1.25 | [0.73, 2.15] | ||
| Pancreatic | 1109 (5) | 0% | 1.65 | [1.38, 1.97] | 1381 (3) | 16% | 1.62 | [1.34, 1.97] | ||
| Prostate | 226 (1) | NA | 0.90 | [0.54, 1.50] | NA | |||||
| Urinary tract | 1529 (10) | 48% | 1.86 | [1.45, 2.38] | 254 (2) | 0% | 2.15 | [1.31, 3.51] | ||
| Mixed | 4293 (5) | 61% | 1.19 | [1.03, 1.38] | NA | |||||
| Overall | 23582 (70) | 65% | 1.66 | [1.52, 1.82] | 2530 (11) | 50% | 1.70 | [1.38, 2.08] | ||
n, number of subjects; HR, hazard ratio; CI, confidence interval; NA, not applicable.
The robust association of lean mass with mortality in cancer could be attributed by its direct and indirect role in cancer management. Reduced lean mass is a hallmark of cancer cachexia. By comparing the effect of lean mass on mortality in people with health conditions of different levels of cachexia prevalence (lowest in elderly and highest in end-stage chronic kidney diseases [29]), we showed that lower lean mass was associated with increased mortality across multiple health conditions, including healthy elderly with a relatively low prevalence of cachexia. This suggested that lower lean mass could confer higher risk of mortality in both cachexia-dependent and -independent manners. Similarly, reduced lean mass is known to be associated with abnormal nutritional status, which is associated with increased mortality. Lean mass also plays an active role in treatment response. Lean mass was found to be an independent determinant of cancer treatment toxicity (including targeted therapy and chemotherapy) [5,6,30]. Animal studies have showed that reversal of muscle wasting led to prolonged survival in a cancer cachexia model [7], demonstrating that lean mass indeed played a causal role in affecting mortality in cancer. Therefore, lean mass is not only important for patients’ mobility and quality of life, but also for the therapeutic efficacy and toxicity of cancer therapy. Therefore, measuring lean mass in cancer patients is clinically important.
Several limitations in the current study should be considered. First, caution must be taken when interpreting the summary estimates because of the heterogeneity of studies. The absence of internationally agreed definition of low lean mass, different adjustment models, and different study protocols are likely to explain the high heterogeneity. High heterogeneity has also been observed in a recent meta-analysis of BMI, with reported I [2] > 90% [31]. Therefore, we used the more conservative random-effects method and acknowledged the possibility of heterogeneity [32]. To address the high heterogeneity issue, we conducted a ‘leave-one-out’ analysis and showed that the elevated I [2] could be explained by the most extreme study in most of the analyses (Supplementary Table 1). Second, different disease groups used different low lean mass definitions, therefore, effect estimates from different disease populations may not be readily comparable. Nevertheless, the use of standardized HRs allowed comparison across disease groups. Third, other lean mass measures, such as anthropometry and serum creatinine levels, were not included because they are generally not recommended. Fourth, this meta-analysis was observational in nature. No causality could be inferred. Fifth, the search strategy was different compared to more recent meta-analyses, giving rise to inconsistent associations in some subgroup analyses. Sixth, like many other meta-analyses, eligible literatures could be missed from our screening algorithms. However, the overall number of studies included in the current meta-analysis was large and the results were highly significant. Therefore, the conclusions were likely to remain the same even when there were missing literatures. Last, the current meta-analysis did a systematic search of the literature till the end of 2017, many recent related literatures were not included in this meta-analysis. However, the number of included studies is the largest up to date, the robust estimates observed suggesting that the conclusion may not change much even if we include more studies. Nevertheless, our study provides a solid background for further study.
Nevertheless, there were several strengths in our study. First, this was the largest and most comprehensive evaluation of the effect of low lean mass on all-cause mortality with sub-groups of different cancer types and muscle index used. Second, the existing literatures were reviewed using a comprehensive search strategy, covering most of the independent cohort studies reporting lean mass and mortality data. Third, we investigated lean mass measurements as both binary and continuous variables, adding more dimensions to the analysis.
5. Conclusions
Reduced lean mass is an important prognostic indicator for patients with different types of cancer. Promotion of muscle health awareness among these groups should be of clinical and public health importance. Our study provided robust evidence of the association of lean mass with mortality in cancer, which is critical for developing clinical guidelines and public health policies for managing sarcopenia in cancer patients.
CRediT author statement
Philip Chun-Ming Au: Formal analysis, Resources, Writing – review & editing. Hang-Long Li: Writing – review & editing. Grace Koon-Yee Lee: Formal analysis, Resources. Gloria Hoi-Yee Li: Formal analysis, Resources. Marcus Chan: Formal analysis, Resources. Bernard Man-Yung Cheung: Writing – review & editing. Ian Chi-Kei Wong: Writing – review & editing. Victor Ho-Fun Lee: Writing – review & editing. James Mok: Formal analysis, Resources, Writing – review & editing. Benjamin Hon-Kei Yip: Writing – review & editing. Kenneth King-Yip Cheng: Writing – review & editing. Chih-Hsing Wu: Writing – review & editing. Ching-Lung Cheung: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
ORCID Philip Chun-Ming Au: 0000-0002-0736-4726. Hang Long Li: 0000-0002-2294-2977. Grace Koon-Yee Lee: 0000-0002-9362-4319. Gloria Hoi-Yee Li: 0000-0003-0275-2356. Marcus Chan: 0000-0001-6072-7648. Bernard Man-Yung Cheung: 0000-0001-9106-7363. Ian Chi-Kei Wong: 0000-0001-8242-0014. Victor Ho-Fun Lee: 0000-0002-6283-978X. James Mok: 0000-0003-1974-0829. Benjamin Hon-Kei Yip: 0000-0002-4749-7611. Kenneth King-Yip Cheng: 0000-0002-7274-0839. Chih-Hsing Wu: 0000-0002-0504-2053. Ching-Lung Cheung: 0000-0002-6233-9144.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.afos.2021.03.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.van Vugt J.L.A., Buettner S., Levolger S., Coebergh van den Braak R.R.J., Suker M., Gaspersz M.P. Low skeletal muscle mass is associated with increased hospital expenditure in patients undergoing cancer surgery of the alimentary tract. PloS One. 2017;12 doi: 10.1371/journal.pone.0186547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur S.T., Van Doren B.A., Roy D., Noone J.M., Zacherle E., Blanchette C.M. Cachexia among US cancer patients. J Med Econ. 2016;19:874–880. doi: 10.1080/13696998.2016.1181640. [DOI] [PubMed] [Google Scholar]
- 3.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 4.Hilmi M., Jouinot A., Burns R., Pigneur F., Mounier R., Gondin J. Body composition and sarcopenia: the next-generation of personalized oncology and pharmacology? Pharmacol Ther. 2019;196:135–159. doi: 10.1016/j.pharmthera.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Prado C.M., Baracos V.E., McCargar L.J., Mourtzakis M., Mulder K.E., Reiman T. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Canc Res. 2007;13:3264–3268. doi: 10.1158/1078-0432.CCR-06-3067. [DOI] [PubMed] [Google Scholar]
- 6.Antoun S., Baracos V.E., Birdsell L., Escudier B., Sawyer M.B. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. 2010;21:1594–1598. doi: 10.1093/annonc/mdp605. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X., Wang J.L., Lu J., Song Y., Kwak K.S., Jiao Q. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Advani S.M., Advani P.G., VonVille H.M., Jafri S.H. Pharmacological management of cachexia in adult cancer patients: a systematic review of clinical trials. BMC Canc. 2018;18:1174. doi: 10.1186/s12885-018-5080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shachar S.S., Williams G.R., Muss H.B., Nishijima T.F. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Canc. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Buentzel J., Heinz J., Bleckmann A., Bauer C., Röver C., Bohnenberger H. Sarcopenia as prognostic factor in lung cancer patients: a systematic review and meta-analysis. Anticancer Res. 2019;39:4603–4612. doi: 10.21873/anticanres.13640. [DOI] [PubMed] [Google Scholar]
- 11.Hua X., Liu S., Liao J.F., Wen W., Long Z.Q., Lu Z.J. When the loss costs too much: a systematic review and meta-analysis of sarcopenia in head and neck cancer. Front Oncol. 2019;9:1561. doi: 10.3389/fonc.2019.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyengar N.M., Arthur R., Manson J.E., Chlebowski R.T., Kroenke C.H., Peterson L. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: a secondary analysis of a randomized clinical trial and observational study. JAMA Oncol. 2019;5:155–163. doi: 10.1001/jamaoncol.2018.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee D.H., Keum N., Hu F.B., Orav E.J., Rimm E.B., Willett W.C. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. 2018;362:k2575. doi: 10.1136/bmj.k2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsiopoulos N., Baumgartner R.N., Heymsfield S.B., Lyons W., Gallagher D., Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1985;85:115–122. doi: 10.1152/jappl.1998.85.1.115. 1998. [DOI] [PubMed] [Google Scholar]
- 15.Prado C.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 16.Hanaoka M., Yasuno M., Ishiguro M., Yamauchi S., Kikuchi A., Tokura M. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis. 2017;32:847–856. doi: 10.1007/s00384-017-2773-0. [DOI] [PubMed] [Google Scholar]
- 17.Cesari M., Fielding R.A., Pahor M., Goodpaster B., Hellerstein M., van Kan G.A. Biomarkers of sarcopenia in clinical trials-recommendations from the international working group on sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:181–190. doi: 10.1007/s13539-012-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong A., Zhu D., Kraus D., Tham T. Laryngoscope; 2020. Radiologically defined sarcopenia affects survival in head and neck cancer: a meta-analysis. [DOI] [PubMed] [Google Scholar]
- 19.Su H., Ruan J., Chen T., Lin E., Shi L. CT-assessed sarcopenia is a predictive factor for both long-term and short-term outcomes in gastrointestinal oncology patients: a systematic review and meta-analysis. Canc Imag. 2019;19:82. doi: 10.1186/s40644-019-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun G., Li Y., Peng Y., Lu D., Zhang F., Cui X. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis. 2018;33:1419–1427. doi: 10.1007/s00384-018-3128-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G., Meng S., Li R., Ye J., Zhao L. Clinical significance of sarcopenia in the treatment of patients with primary hepatic malignancies, a systematic review and meta-analysis. Oncotarget. 2017;8:102474–102485. doi: 10.18632/oncotarget.19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang K.V., Chen J.D., Wu W.T., Huang K.C., Hsu C.T., Han D.S. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer. 2018;7:90–103. doi: 10.1159/000484950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mintziras I., Miligkos M., Wächter S., Manoharan J., Maurer E., Bartsch D.K. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: systematic review and meta-analysis. Int J Surg. 2018;59:19–26. doi: 10.1016/j.ijsu.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Li J., Deng Y., Zhang M., Cheng Y., Zhao X., Ji Z. Prognostic value of radiologically determined sarcopenia prior to treatment in urologic tumors: a meta-analysis. Medicine (Baltim) 2019;98 doi: 10.1097/MD.0000000000017213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X.-M., Dou Q.-L., Zeng Y., Yang Y., Cheng A.S.K., Zhang W.-W. Sarcopenia as a predictor of mortality in women with breast cancer: a meta-analysis and systematic review. BMC Canc. 2020;20:172. doi: 10.1186/s12885-020-6645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ubachs J., Ziemons J., Minis-Rutten I.J.G., Kruitwagen R., Kleijnen J., Lambrechts S. Sarcopenia and ovarian cancer survival: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10:1165–1174. doi: 10.1002/jcsm.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia S., Qiao R., Xiao Y., Qin D., Zhao W., Zhao Y. Prognostic value of sarcopenia in survivors of hematological malignances undergoing a hematopoietic stem cell transplantation: a systematic review and meta-analysis. Support Care Canc. 2020;28:3533–3542. doi: 10.1007/s00520-020-05359-3. [DOI] [PubMed] [Google Scholar]
- 28.Jones K.I., Doleman B., Scott S., Lund J.N., Williams J.P. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015;17:O20–O26. doi: 10.1111/codi.12805. [DOI] [PubMed] [Google Scholar]
- 29.von Haehling S., Anker S.D. Prevalence, incidence and clinical impact of cachexia: facts and numbers-update 2014. J Cachexia Sarcopenia Muscle. 2014;5:261–263. doi: 10.1007/s13539-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Go S.I., Park M.J., Song H.N., Kim H.G., Kang M.H., Lee H.R. Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle. 2016;7:567–576. doi: 10.1002/jcsm.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aune D., Sen A., Prasad M., Norat T., Janszky I., Tonstad S. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ioannidis J.P., Patsopoulos N.A., Rothstein H.R. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336:1413–1415. doi: 10.1136/bmj.a117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.