Abstract
Objectives
Sarcopenia is recognized to be a health problem which is as serious as obesity, but its relevance to mortality is unclear. We conducted a meta-analysis of cohort studies on lean mass and mortality in populations with different health conditions.
Methods
In this study, a systematic search of PubMed, Cochrane Library and Embase was performed for cohort studies published before Dec 20, 2017 which examined the relationship between lean mass and mortality. We included studies reporting lean mass measurement by dual-energy X-ray absorptiometry, bioimpedance analysis or computed tomography, as continuous (per standard deviation [SD] decrease) or binary variables (using sarcopenia cutoffs). We excluded studies which used muscle mass surrogates, anthropometric measurement of muscle, rate of change in muscle mass, and sarcopenia defined by composite criteria. The primary study outcome was all-cause mortality. Pooled hazard ratio estimates were calculated using a random effects model.
Results
A total of 9602 articles were identified from the systematic search, and 188 studies with 98 468 participants from 34 countries were included in the meta-analysis. Of the 68 studies included in the present meta-analysis, the pooled HR was 1.36 and 1.74 for every SD decrease in lean mass and in people with low lean mass (cutoffs), respectively. Significant associations were also observed in elderly and all disease subgroups, irrespective of the measurement modalities.
Conclusions
Lower lean mass is robustly associated with increased mortality, regardless of health conditions and lean mass measurement modalities. This meta-analysis highlighted low lean mass as a key public health issue.
Keywords: Sarcopenia, Mortality, Chronic diseases, Meta-analysis, Systematic review
1. Introduction
Sarcopenia is a prevalent health problem worldwide and it is known to be significantly associated with increased risk of morbidity, immobility, and mortality [1,2]. It was recently endorsed as an independent clinical condition by the International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code (M62.84). Sarcopenia was first defined as age-related loss in muscle mass as introduced by Rosenberg in 1989 [2]. Since then, several operational definitions of sarcopenia were proposed, mainly including muscle strength (eg, grip strength) and function (eg, gait speed) [1,3]. Nevertheless, muscle mass is still one of the core phenotypes in defining sarcopenia; the Asian Working Group on Sarcopenia defined sarcopenia as “age-related loss of skeletal muscle mass plus loss of muscle strength and/or reduced physical performance” [1].
Muscle loss is not only due to age-related factors, but it is also caused by other reasons, such as comorbidities [1]. There are several known risk factors of muscle loss. Malnutrition (especially protein imbalance) [1], physically inactivity [1], increased inflammation [1], reduced anabolism (or increased catabolism) [4], insulin resistance [5], and polypharmacy [6] are known to be associated with increased risk of sarcopenia. Indeed, these factors are common risk factors of many comorbidities, including cancer and cardiovascular disease (CVD) [1]. Thus, lean mass in comorbidities may not only reflect the muscle health, but it also potentially indicates the severity of comorbidities. Previous meta-analyses have shown that sarcopenia is associated with mortality, but these studies were largely restricted to a single medical condition, such as liver cirrhosis [7,8]. This meta-analysis aims to evaluate the association of lean mass with all-cause mortality in multiple clinical conditions.
2. Methods
The materials and methods have been described in Cheung et al [9] in the same issue. In brief, from PubMed, Cochrane Library and Embase, a systematic search was performed for cohort studies published before Dec 20, 2017 which examined the relationship between lean mass and mortality. Studies reporting lean mass measurement by dual-energy X-ray absorptiometry, bioimpedance analysis or computed tomography, as continuous (per standard deviation decrease) or binary variables (using sarcopenia cut-offs) were included. Studies which used muscle mass surrogates, anthropometric measurement of muscle, rate of change in muscle mass, and sarcopenia defined by composite criteria were excluded. The primary study outcome was all-cause mortality. Pooled hazard ratio estimates were calculated using a random effects model.
3. Results
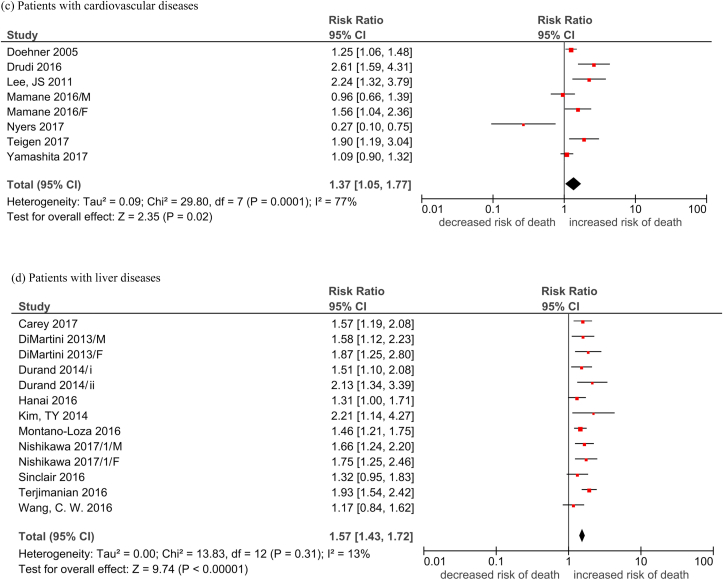
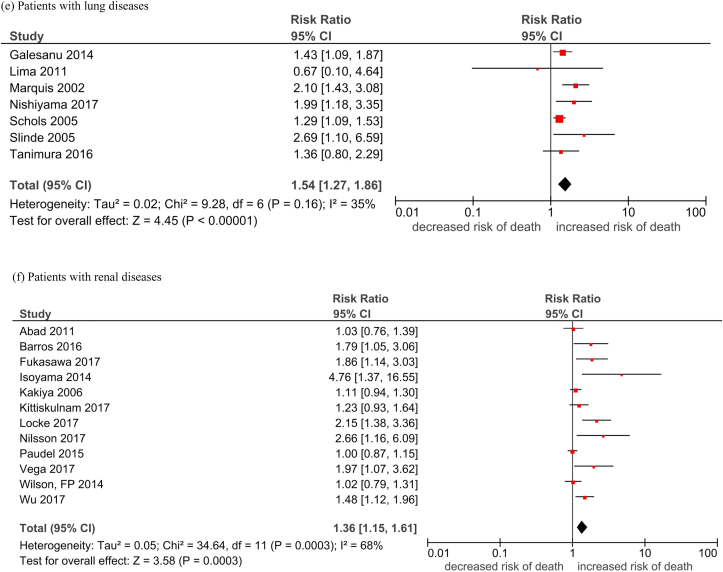
The detailed descriptive information for each study is provided in Cheung et al in the same issue. A total of 68 studies with reduced lean mass measurements were included in the analysis. The pooled hazard ratio (HR) of reduced lean mass with mortality was 1.36 (95% CI, 1.29–1.44; Table 1, Fig. 1). Among these studies, 17 (25%), 12 (17.6%), 10 (14.7%), 9 (13.2%), 7 (10.3%), 7 (10.3%), and 6 (8.8%) were conducted in cancer, renal diseases, liver diseases, elderly, CVD, lung diseases, and other diseases, respectively. The pooled HR was apparently the highest in people with liver diseases (HR 1.57) and lung diseases (HR 1.54), followed by people with cancer (HR 1.41), CVD (HR 1.37), and renal diseases (HR 1.36). The pooled HR was the lowest in the elderly population (HR 1.21). The funnel plots revealed low publication bias (Supplementary Fig. 1).
Table 1.
Pooled hazard ratio of reduced lean mass with mortality.
| Sub-groups | Overall (HR [95% CI]); I2 |
|---|---|
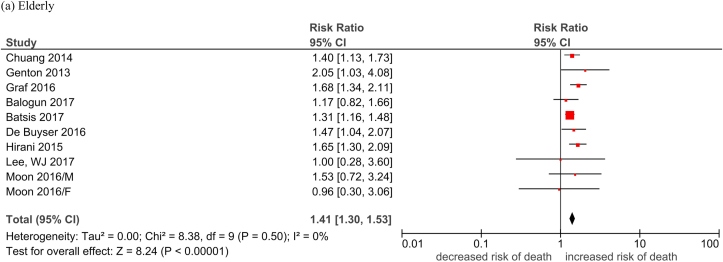
| Elderly | 1.21 [1.09, 1.34]; 55% |
| Cancer patients | 1.41 [1.24, 1.59]; 75% |
| People with cardiovascular diseases | 1.37 [1.05, 1.77]; 77% |
| People with liver diseases | 1.57 [1.43, 1.72]; 13% |
| People with lung diseases | 1.54 [1.27, 1.86]; 35% |
| People with renal diseases | 1.36 [1.15, 1.61]; 68% |
| People with other conditions | 1.12 [0.93, 1.34]; 54% |
| Overall | 1.37 [1.29, 1.45]; 68% |
Fig. 1.
Forest plots of the association of reduced lean mass (per SD decrease) with all-cause mortality by subgroups.
Legend: M: Male F: Female Durand 2014/i: 2002–2007 cohortDurand 2014/ii: 2007–2011 cohort Nishikawa 2017/1: Refer to Cheung et al Supplement Reference (119) in the same issue Nishikawa 2017/2: Refer to Cheung et al Supplement Reference (120) in the same issue Nishikawa 2017/3: Refer to Cheung et al Supplement Reference (121) in the same issue Nishikawa 2017/4: Refer to Cheung et al Supplement Reference (122) in the same issue Okumura 2017/1: Refer to Cheung et al Supplement Reference (127) in the same issue Okumura 2017/2: Refer to Cheung et al Supplement Reference (128) in the same issue Fukushima 2016/1: Refer to Cheung et al Supplement Reference (41) in the same issue Fukushima 2016/2: Refer to Cheung et al Supplement Reference (42) in the same issue.
(a) Elderly; (b) cancer patients; (c) patients with cardiovascular diseases; (d) patients with liver diseases; (e) patients with lung disease; (f) patients with renal disease; (g) patients with other conditions.
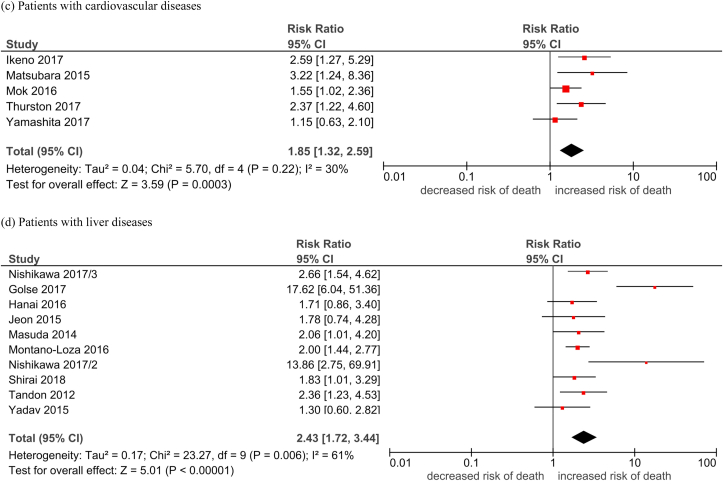
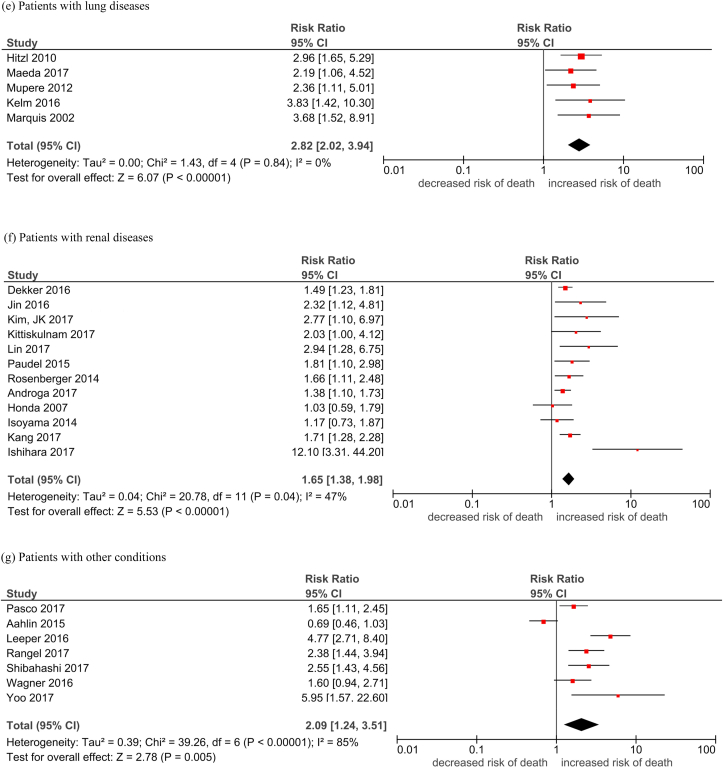
Table 2 shows the pooled HR of mortality in people with low lean mass, which was defined by a lean mass below a pre-defined cutoff point. The overall pooled HR of low lean mass with mortality was 1.74 (95% CI, 1.63–1.85, Table 2, Fig. 2). The majority of the studies were conducted in people with cancer (n = 90, 65.2%). The overall HR across all platforms was apparently the highest in people with lung diseases (HR 2.82) and liver diseases (HR 2.43), followed by people with other diseases (HR 2.09), CVD (HR 1.85), cancer (HR 1.69), and renal diseases (HR 1.65). The pooled HR of mortality was the lowest in the elderly population (HR 1.41). The funnel plots revealed low publication bias (Supplementary Fig. 2).
Table 2.
Pooled hazard ratio of mortality in people with low lean mass.
| Study group | Overall (HR [95% CI]), I2 |
|---|---|
| Elderly | 1.41 [1.30, 1.53]; 0% |
| Cancer patients | 1.69 [1.56, 1.83]; 63% |
| People with cardiovascular diseases | 1.85 [1.32, 2.59]; 30% |
| People with liver diseases | 2.43 [1.72, 3.44]; 61% |
| People with lung diseases | 2.82 [2.02, 3.94]; 0% |
| People with renal diseases | 1.65 [1.38, 1.98]; 47% |
| People with other conditions | 2.09 [1.24, 3.51]; 85% |
| Overall | 1.74 [1.63, 1.85]; 63% |
Fig. 2.
Forest plots of the association of low lean mass with all-cause mortality by subgroups.
Legend: M: MaleF: Female Durand 2014/i: 2002–2007 cohort Durand 2014/ii: 2007–2011 cohort Nishikawa 2017/1: Refer to Cheung et al Supplement Reference (119) in the same issue Nishikawa 2017/2: Refer to Cheung et al Supplement Reference (120) in the same issue Nishikawa 2017/3: Refer to Cheung et al Supplement Reference (121) in the same issue Nishikawa 2017/4: Refer to Cheung et al Supplement Reference (122) in the same issue Okumura 2017/1: Refer to Cheung et al Supplement Reference (127) in the same issue Okumura 2017/2: Refer to Cheung et al Supplement Reference (128) in the same issue Fukushima 2016/1: Refer to Cheung et al Supplement Reference (41) in the same issue Fukushima 2016/2: Refer to Cheung et al Supplement Reference (42) in the same issue.
(a) Elderly; (b) cancer patients; (c) patients with cardiovascular diseases; (d) patients with liver diseases; (e) patients with lung disease; (f) patients with renal disease; (g) patients with other conditions.
4. Discussion
Although the risk of mortality in people with low lean mass across a wide range of populations has appeared to be consistent, whether the relationship is altered by measurement methods and statistical analysis is unknown. Robust evidence can now be drawn from the current meta-analysis involving 188 studies with data of approximately 98 000 participants. To the best of our knowledge, this is the first study which comprehensively investigated the relationship between lean mass and mortality with the unique analyses in people with different health conditions.
The current comprehensive study investigated the effect of lean mass in more detail than previous studies. Previous meta-analyses evaluated the impact of lean mass on mortality in a particular population, such as people awaiting liver transplantation [7,8], or people with liver cirrhosis [10], tumors [[11], [12], [13], [14]], and post-operation [[15], [16], [17], [18], [19]]. Some studies included less validated methods, such as phase angle [14] and mid-arm muscle [8] in the meta-analysis. The risk estimates identified in these studies were largely consistent with the estimated identified in the subgroup analysis of the present meta-analysis. However, the association of lean mass with mortality in all people, as well as people with many other health conditions remain understudied. Most, if not all, of these meta-analyses did not evaluate the role of reduced lean mass on mortality. In view of this, we produced a pooled estimate of reduced lean mass by deriving a standardized HR in each included study such that the estimate for each SD decrease in lean mass can be directly compared across different measurement modalities.
Low lean mass is universally associated with increased mortality in all populations. Among all subgroups of different health conditions, the HR between low lean mass and mortality was the lowest in the elderly. In particular, the difference in HR was statistically significant in comparison with people with cancer, liver, and lung diseases. Those studies included in the subgroup analysis of elderly population comprised healthy and community-dwelling old people. Whereas, other populations were rather sick and had a higher risk of mortality. For example, cachexia is commonly observed in people with cancer [20], chronic obstructive pulmonary disease [21], and end-stage liver disease [22], with muscle wasting as the hallmark feature. Although the risk of death due to low lean mass in elderly patients is relatively small, the population size of elderly is the largest among different age groups and it is therefore expected that a huge number of elderly is at higher risk of mortality due to low lean mass. Thus, more research should be conducted in this particular area to reduce the burden of low lean mass. On the other hand, clinicians should be aware of potential decrease in lean mass while prescribing drugs for their patients, which may subsequently affect prognosis; a recent review concluded that there are increasing evidence on how common-prescribed drugs, such as statins and antidiabetic agents, can be associated with development of sarcopenia [23]. Further studies on optimising the cutoff points and establishing prognosis of low lean mass in populations with different health conditions are warranted.
Several limitations should be considered. First, caution must be taken when interpreting the summary estimates because of the heterogeneity of studies. The absence of internationally agreed definitions of low lean mass, different adjustment models, and study protocol are likely to explain the high heterogeneity. Therefore, we used the more conservative random-effects method and followed the recommendations where meta-analyses should be pursued while acknowledging heterogeneity [24]. Second, other lean mass measurements such as anthropometry and serum creatinine levels were not investigated, since these are generally not recommended when compared to lean mass measured by the 3 modalities examined in the current study. Third, like many other meta-analyses, other eligible articles may be missed by our screening algorithms. For example, some literature titles or abstracts did not include the keywords within the algorithms but they may have data related to lean mass and survival. These articles may be unintentionally missed in our analysis. Nevertheless, the number of studies included in the current meta-analysis was large, and the result was highly significant. Therefore, it is expected that the conclusion remains the same even if there are missing literature. Fourth, meta-analysis of observational studies cannot infer causality and unmeasured confounders may affect the estimates, especially in studies without adjustment for any confounding factors. Fifth, although we reported the association of lean mass with mortality in the elderly population, whether lean mass can predict mortality in the younger population is unknown. Since most, if not all, of the studies evaluating the relationship between lean mass and mortality were conducted in the elderly population, future long-term follow up study is required to clarify this issue if lean mass in younger age is also a predictor of mortality.
Our study has several strengths. Firstly, this is the largest and the most comprehensive evaluation of lean mass and all-cause mortality. The study also compared the different definitions of low lean mass by cutoff points, measurement modalities, and strength of association in different populations. Secondly, the available literature in this area was reviewed using a comprehensive search strategy, covering most of the independent studies reporting lean mass and mortality data. Thirdly, the study investigated lean mass measurements as both binary and continuous variables, adding more dimension to the analysis. Lastly, relationship of lean mass and all-cause mortality were compared in various disease groups, which offers more clinical insights for physicians when treating patients.
Our study highlights that decrease in lean mass is an important prognostic indicator in the aging and common disease populations. Although body mass index (BMI) is commonly measured in clinical practice, it cannot differentiate lean mass from fat mass [25]. Given the findings from the current meta-analysis, it is encouraged to evaluate lean mass in addition to routine BMI measurement. Development of consensus of lean mass cutoff point is needed, especially in different health conditions specifically. Although low lean mass may be incorporated in overall health evaluation, like cachexia, the current study demonstrated lean mass alone may be able to predict mortality accurately. Therefore, it further echoes the importance of lean mass measurement in routine clinical practice.
5. Conclusions Lean mass decrease is a significant predictor of all-cause mortality across different health populations and measuring modalities. Future work on consensus of sarcopenia definition is needed and screening of lean mass decrease can be included in future clinical management guidelines.
CRediT author statement
Grace Koon-Yee Lee: Formal analysis, Resources, Writing - Original Draft, Writing - Review & Editing. Philip Chun-Ming Au: Formal analysis, Resources, Writing - Review & Editing. Gloria Hoi-Yee Li: Formal analysis, Resources, Writing - Review & Editing. Marcus Chan: Formal analysis, Resources, Writing - Review & Editing. Hang-Long Li: Writing - Review & Editing. Bernard Man-Yung Cheung: Writing - Review & Editing. Ian Chi-Kei Wong: Review & Editing. Victor Ho-Fun Lee: Review & Editing. James Mok: Formal analysis, Resources, Writing - Review & Editing. Benjamin Hon-Kei Yip: Review & Editing. Kenneth King-Yip Cheng: Review & Editing. Chih-Hsing Wu: Review & Editing. Ching-Lung Cheung: Conceptualization, Methodology, Writing - Review & Editing, Supervision.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
ORCID Grace Koon-Yee Lee: 0000-0002-9362-4319. Philip Chun-Ming Au: 0000-0002-0736-4726. Gloria Hoi-Yee Li: 0000-0003-0275-2356. Marcus Chan: 0000-0001-6072-7648. Hang-Long Li: 0000-0002-2294-2977. Bernard Man-Yung Cheung: 0000-0001-9106-7363. Ian Chi-Kei Wong: 0000-0001-8242-0014. Victor Ho-Fun Lee: 0000-0002-6283-978X. James Mok: 0000-0003-1974-0829. Benjamin Hon-Kei Yip: 0000-0002-4749-7611. Kenneth King-Yip Cheng: 0000-0002-7274-0839. Chih-Hsing Wu: 0000-0002-0504-2053. Ching-Lung Cheung: 0000-0002-6233-9144.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.afos.2021.02.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg I.H. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuggle N., Shaw S., Dennison E., Cooper C. Sarcopenia. Best Pract Res Clin Rheumatol. 2017;31:218–242. doi: 10.1016/j.berh.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleasby M.E., Jamieson P.M., Atherton P.J. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229:67–81. doi: 10.1530/JOE-15-0533. [DOI] [PubMed] [Google Scholar]
- 6.Konig M., Spira D., Demuth I., Steinhagen-Thiessen E., Norman K. Polypharmacy as a risk factor for clinically relevant sarcopenia: results from the Berlin Aging Study II. J Gerontol A Biol Sci Med Sci. 2017;73:117–122. doi: 10.1093/gerona/glx074. [DOI] [PubMed] [Google Scholar]
- 7.van Vugt J.L., Levolger S., de Bruin R.W., van Rosmalen J., Metselaar H.J., Jn I.J. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16:2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 8.Chang K.V., Chen J.D., Wu W.T., Huang K.C., Han D.S. Association of loss of muscle mass with mortality in liver cirrhosis without or before liver transplantation: a systematic review and meta-analysis. Medicine (Baltim) 2019;98 doi: 10.1097/MD.0000000000014373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung C.L., Lee G.K., Au P.C., Li G.H., Chan M., Cheung B.M. Systematic review and meta-analysis of lean mass and mortality: rationale and study description. Osteoporos Sarcopenia. 2021;7(S1):S3–S12. doi: 10.1016/j.afos.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim G., Kang S.H., Kim M.Y., Baik S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PloS One. 2017;12 doi: 10.1371/journal.pone.0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shachar S.S., Williams G.R., Muss H.B., Nishijima T.F. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Canc. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Sun G., Li Y., Peng Y., Lu D., Zhang F., Cui X., Zhang Q., Li Z. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis. 2018;33:1419–1427. doi: 10.1007/s00384-018-3128-1. [DOI] [PubMed] [Google Scholar]
- 13.Chang K.V., Chen J.D., Wu W.T., Huang K.C., Hsu C.T., Han D.S. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer. 2018;7:90–103. doi: 10.1159/000484950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buentzel J., Heinz J., Bleckmann A., Bauer C., Röver C., Bohnenberger H. Sarcopenia as prognostic factor in lung cancer patients: a systematic review and meta-analysis. Anticancer Res. 2019;39:4603–4612. doi: 10.21873/anticanres.13640. [DOI] [PubMed] [Google Scholar]
- 15.Jones K., Gordon-Weeks A., Coleman C., Silva M. Radiologically determined sarcopenia predicts morbidity and mortality following abdominal surgery: a systematic review and meta-analysis. World J Surg. 2017;41:2266–2279. doi: 10.1007/s00268-017-3999-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soud M., Alahdab F., Ho G., Kuku K.O., Cejudo-Tejeda M., Hideo-Kajita A. Usefulness of skeletal muscle area detected by computed tomography to predict mortality in patients undergoing transcatheter aortic valve replacement: a meta-analysis study. Int J Cardiovasc Imag. 2019;35:1141–1147. doi: 10.1007/s10554-019-01582-0. [DOI] [PubMed] [Google Scholar]
- 17.Antoniou G.A., Rojoa D., Antoniou S.A., Alfahad A., Torella F., Juszczak M.T. Effect of low skeletal muscle mass on post-operative survival of patients with abdominal aortic aneurysm: a prognostic factor review and meta-analysis of time-to-event data. Eur J Vasc Endovasc Surg. 2019;58:190–198. doi: 10.1016/j.ejvs.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Borggreve A.S., den Boer R.B., van Boxel G.I., de Jong P.A., Veldhuis W.B., Steenhagen E. The predictive value of low muscle mass as measured on ct scans for postoperative complications and mortality in gastric cancer patients: a systematic review and meta-analysis. J Clin Med. 2020;9:199. doi: 10.3390/jcm9010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weerink L.B.M., van der Hoorn A., van Leeuwen B.L., de Bock G.H. Low skeletal muscle mass and postoperative morbidity in surgical oncology: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11:636–649. doi: 10.1002/jcsm.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 21.Sanders K.J., Kneppers A.E., van de Bool C., Langen R.C., Schols A.M. Cachexia in chronic obstructive pulmonary disease: new insights and therapeutic perspective. J Cachexia Sarcopenia Muscle. 2016;7:5–22. doi: 10.1002/jcsm.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd K., Kimbell B., Murray S., Iredale J. Living and dying well with end-stage liver disease: time for palliative care? Hepatology. 2012;55:1650–1651. doi: 10.1002/hep.25621. [DOI] [PubMed] [Google Scholar]
- 23.Campins L., Camps M., Riera A., Pleguezuelos E., Yebenes J.C., Serra-Prat M. Oral drugs related with muscle wasting and sarcopenia. A review. Pharmacology. 2017;99:1–8. doi: 10.1159/000448247. [DOI] [PubMed] [Google Scholar]
- 24.Ioannidis J.P., Patsopoulos N.A., Rothstein H.R. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336:1413–1415. doi: 10.1136/bmj.a117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher D., Visser M., Sepulveda D., Pierson R.N., Harris T., Heymsfield S.B. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.