Abstract
Aims/Introduction
As a low‐carbohydrate diet and the use of sodium–glucose transporter‐2 inhibitors are both known to increase D‐beta‐hydroxybutyrate levels, the effect of these levels on glucose metabolism has attracted attention. We investigated the acute effects of ketone monoester (KM) ingestion on blood glucose levels during the 75‐g oral glucose tolerance test (OGTT) in participants with impaired glucose tolerance.
Materials and Methods
Nine Japanese adults aged 48–62 years (4 men, 5 women) with impaired glucose tolerance participated in this study. After participants fasted overnight, we carried out OGTT for 180 min with and without KM ingestion on two separate days in a randomized cross‐over design. We compared the area under the curve (AUC) of D‐beta‐hydroxybutyrate, glucose, insulin, C‐peptide, glucagon and free fatty acids during OGTT.
Results
The AUC of D‐beta‐hydroxybutyrate during OGTT was significantly higher with KM than without KM (KM 5995.3 ± 1257.1 mmol/L·h; without KM 116.1 ± 33.9 mmol/L·h, P < 0.0001), and the AUC of glucose with KM was significantly lower than that without KM (KM 406.6 ± 70.6 mg/dL·h; without KM 483.2 ± 74.3 mg/dL·h, P < 0.0001). This improved glucose excursion was associated with enhanced AUC of insulin during the first half (0–90 min) of OGTT, even though the AUC of C‐peptide during this period was unchanged. In contrast, the AUC of insulin, C‐peptide, glucagon and free fatty acids during 180 min of OGTT were similar in both conditions.
Conclusion
The ingestion of KM decreased the AUC of glucose during 75‐g OGTT in Japanese individuals with impaired glucose tolerance, and the mechanism might involve elevated levels of circulating early phase insulin.
Keywords: Impaired glucose tolerance, Insulin secretion, Ketone
Ketone monoester lowers glucose level during the oral glucose tolerance test in individuals with impaired glucose tolerance. This improvement was associated with decreased insulin clearance and elevated early phase insulin level.

Introduction
Ketone bodies, namely D‐beta‐hydroxybutyrate (βHB) and acetoacetate (AcAc), are produced mainly by the liver during periods of low glucose availability, such as prolonged fasting and a low‐carbohydrate and high‐fat diet (ketogenic diet) 1 . Although the normal blood ketone level in humans is <0.1 mmol/L in the fasting state, prolonged fasting and a ketogenic diet increase blood ketone levels by 1.0–5.0 mmol/L 2 . It is notable that administration of an oral hypoglycemic agent, such as a sodium–glucose transporter‐2 inhibitor, also increases blood ketone levels to approximately 0.1–0.15 mmol/L 3 . A short‐term ketogenic diet with calorie restriction has been shown to improve insulin resistance, high blood pressure and dyslipidemia 4 , 5 , 6 . These beneficial effects suggest that ketones might be a potential treatment for patients with obesity and diabetes mellitus. However, the aforementioned metabolic changes have also been observed with non‐ketogenic calorie restriction 7 , 8 ; thus, the direct effects of ketone bodies on glucose metabolism during a ketogenic diet are not yet fully understood.
The oral administration of ketone supplements, such as sodium acetoacetate, βHB salts and βHB esters, can acutely increase the concentration of circulating ketone bodies in the blood in the absence of a ketogenic diet 2 , 9 , 10 , 11 , 12 . Thus, oral administration of βHB is also expected to improve glucose metabolism, but to the best of our knowledge, just three studies have investigated the effect of oral ketone administration on glucose metabolism during an oral glucose tolerance test (OGTT). In 1970, Jenkins et al. 12 reported that ingestion of sodium acetoacetate 30 min before OGTT in young males increased plasma insulin levels during OGTT compared with the control condition, whereas blood glucose levels remained unchanged. In contrast, (R)‐3‐hydroxybutyl (R)‐3‐hydroxybutyrate (hereafter referred to as ketone monoester [KM]) supplementation is safe 9 , and has been shown to increase circulating βHB levels enough to induce a physiological effect 10 . The KM is hydrolyzed in the small intestine by non‐specific gut esterases 13 to βHB and (R)‐1,3‐butanediol. These metabolites are transported to the liver, where butanediol is metabolized to βHB 14 , and then βHB is released into the circulation. In 2018, Myette‐Côté et al. 15 showed that consuming a KM supplement 30 min before OGTT increased circulating βHB levels to 1–3 mmol/L and reduced the area under the curve (AUC) of glucose during OGTT without affecting insulin levels. Very recently, this group showed a similar effect of KM supplementation in obese participants 16 . These were the first studies showing that KM supplementation might be a potential therapeutic option for individuals with impaired glucose tolerance. However, the participants in these studies had normal glucose tolerance. Thus, the effect of KM supplementation on postprandial hyperglycemia has not yet been elucidated.
Against this background, in the present study we evaluated the impact of KM supplementation on post‐glucose load hyperglycemia during the 75‐g OGTT (primary outcome) in participants with impaired glucose tolerance (IGT). This is the first report to investigate the effects of KM in participants with IGT.
Methods
Participants
In the present study, we recruited candidate Japanese participants through clinical research companies (3H Medi Solution, Inc., Tokyo, Japan, and SOUKEN, Tokyo Japan); all potential participants were aged between 20 and 64 years, with a body mass index between 18.5 and 25.0 kg/m2. The exclusion criteria were as follows: history of diabetes, hypertension or dyslipidemia, history of food allergy or drug hypersensitivity, habitual drinker (ethanol ≥30 g/day), history of digestive system disease or digestive surgical operation, liver dysfunction or hepatitis B virus or hepatitis C virus infection, history of heart disease, chronic renal failure (creatinine level ≥1.3 mg/dL), recent weight changes or attempts to lose weight (3 kg weight gain or loss, within past 1 month), low‐carbohydrate diet (carbohydrate <150 g/day) and current smoker. We also excluded participants taking medicines or supplements that might affect glucose or lipid metabolism. Between March and April 2019, we carried out the 75‐g OGTT in the 72 eligible candidates after overnight fasting, and nine participants (4 men, 5 women) with IGT who fulfilled the criteria were included in the present study. The recruitment flowchart is presented in Figure 1.
Figure 1.
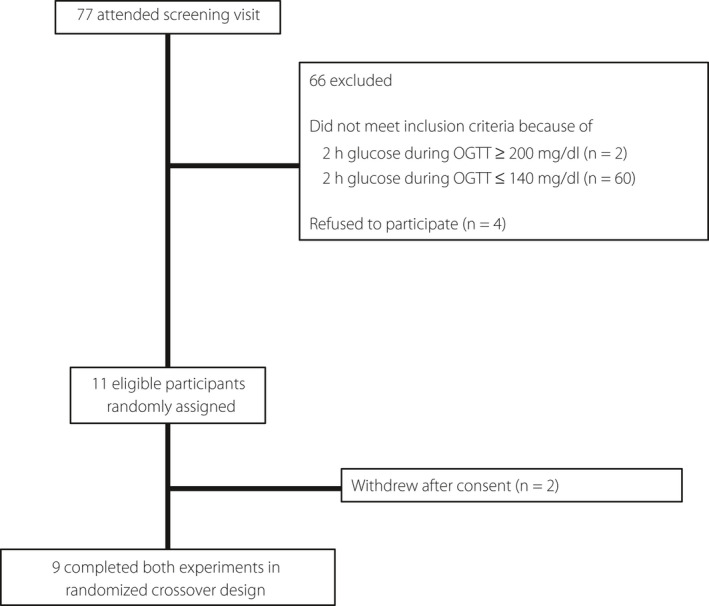
CONSORT flow diagram for cross‐over randomized trials. OGTT, oral glucose tolerance test.
Before the study, all participants provided written consent to participate after receiving information on the procedures and purpose of the study. The study protocol was approved by the Research Ethics Review Board of Juntendo University (18‐061 and 18‐222). This study was carried out in accordance with the principles outlined in the Declaration of Helsinki, and was registered with the Japan Clinical Trials Registry (UMIN000037787).
Experimental design
The present study was carried out using a randomized cross‐over design. OGTT both with and without ingestion of an exogenous KM supplement (HVMN, San Francisco, CA, USA) was carried out on two separate days separated by either a 7‐day or 14‐day interval. Participants were randomized in terms of whether KM supplementation was given at the first or second OGTT procedure by computer‐generated random numbers. All participants were asked to maintain their physical activity and dietary intake during the study period. In addition, they were asked to avoid exercise 72 h before the day of the OGTT. On the testing day, we measured each participant’s height and bodyweight, and estimated their body fat percentage using the impedance method (Inbody 730; Biospace, Tokyo, Japan) after overnight fasting (12 h). The duration of the 75‐g OGTT was 180 min. In the KM condition, participants simultaneously consumed the KM supplement (482 mg/kg bodyweight, in accordance with a previous study 15 ) and 75 g glucose; whereas in the control condition, the same amount of water was consumed instead of KM. All participants completed both experiments between April 2019 and July 2019.
Primary and secondary outcome variables and sample size
The primary outcome of the present study was the AUC of glucose during the 75‐g OGTT with and without KM ingestion. With regard to the secondary outcomes, we also evaluated changes in βHB, insulin, C‐peptide, C‐peptide/insulin ratio 17 , C‐peptide/glucose ratio 18 , glucagon, free fatty acids (FFA), Matsuda Index 19 , and the insulinogenic index during OGTT with and without KM ingestion. The sample size was calculated with GPower 3.1.3 (Dusseldorf, Germany) based on the effect size of 1.1 estimated by a previous study showing a 17% reduction in glucose level by KM ingestion 15 . We set α = 0.05 and power (1 – β) = 0.8 for this a priori power analysis model, and the required sample size of nine was determined.
OGTT and blood sampling
A standard 75‐g OGTT was carried out after individuals had fasted overnight. Venous blood samples were obtained before the ingestion of 75 g of glucose (0 min), and at 15, 30, 60, 90, 120 and 180 min afterward. The insulinogenic index was calculated using the following equation: (insulin at 30 min − fasting insulin) / (plasma glucose at 30 min − fasting plasma glucose). We evaluated levels of βHB, plasma glucose, serum insulin, FFA, glucagon and C‐peptide at each time point. The AUCs of these parameters during OGTT were calculated by the trapezoidal method.
Blood analysis
Blood samples were sent to a laboratory (SRL Inc., Tokyo, Japan) for biochemistry analysis. The circulating levels of βHB, plasma glucose, serum insulin, FFA, glucagon, C‐peptide, serum lipids (total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, FFA and triglycerides) and liver function tests (aspartate aminotransferase, alanine aminotransferase and gamma‐glutamyl transpeptidase) were measured by standardized methods (SRL Inc.). With regard to glucagon measurement, blood samples were collected in 3‐mL tubes containing ethylenediaminetetraacetic acid 2Na+ and aprotinin, then immediately centrifuged at 4ºC, and plasma was stored at −80ºC.
Statistical analysis
PASW Statistics version 20.0 (SPSS, IBM Corp., Armonk, NY, USA) was used for statistical analysis. All data are presented as the mean ± standard deviation. A paired t‐test was carried out to determine significant differences between the two experiments. The level of statistical significance was set at 0.05.
Results
Baseline demographic characteristics of the participants are shown in Table 1. No adverse effects were observed with in the present study, all participants successfully completed OGTT with and without βHB. Figure 2 shows the mean ± standard deviation values of parameters during OGTT under each condition. The βHB level in the KM condition increased immediately 15 min after the oral load; the peak was 2.4 ± 0.7 mmol/L at 90 min into OGTT, and the concentration remained similar throughout the remainder of the experiment (Figure 2). In contrast, no significant change in βHB level was observed in the control condition. Thus, compared with the control condition, the KM condition resulted in a significant increase in the βHB level at each time point after glucose ingestion, as well as a significantly higher AUC of βHB during the OGTT (Table 2). Fasting plasma glucose and FFA levels were slightly lower in the KM condition than in the control condition (fasting plasma glucose 105.2 ± 10.0 vs 100.9 ± 9.4 mg/dL, P = 0.024; FFA 663.1 ± 186.9 vs 578.8 ± 191.5 μEq/L, P = 0.029), whereas other parameters were similar in the fasting state in both conditions (Figure 2). As shown in Figure 1 and Table 2, glucose levels at 60, 90 and 120 min after glucose ingestion, and the AUC of glucose during OGTT were significantly lower in the KM condition than in the control condition. This difference was also significant when the AUC of glucose was calculated based on the incremental change in glucose concentration relative to baseline (data not shown). This improvement in the AUC of glucose seemed to be associated with elevated insulin levels during the first half of the OGTT (0–90 min; Figure 2). Indeed, insulin levels at 30 and 90 min after glucose ingestion and the AUC of insulin during the first half of OGTT were higher in the KM condition than in the control condition (Table 2). This elevation was not accompanied by changes in C‐peptide levels at 0, 30, 60 or 90 min after glucose ingestion or by changes in the AUC of C‐peptide (Figure 2, Table 2). Accordingly, the C‐peptide : insulin ratio was reduced by KM ingestion during the first half of the OGTT. In contrast, C‐peptide/glucose ratios were increased by KM ingestion during the OGTT. Other parameters were not significantly changed by KM ingestion (Table 2).
Table 1.
Participants’ demographic characteristics
| M/F | 5/4 |
| Age (years) | 55.0 ± 4.0 |
| Height (cm) | 166.0 ± 11.8 |
| Weight (kg) | 64.5 ± 11.7 |
| Body mass index (kg/m2) | 23.2 ± 1.3 |
| Percent body fat (%) | 31.6 ± 4.7 |
| Fasting plasma glucose (mg/dL) | 109.2 ± 17.2 |
| Plasma glucose at 120 min during OGTT (mg/dL) | 167.9 ± 18.4 |
| Insulin (μU/mL) | 7.2 ± 2.7 |
| HbA1c (%) | 6.0 ± 0.2 |
| Free fatty acids (μEq/L) | 565.0 ± 126.9 |
| Triglycerides (mg/dL) | 106.7 ± 53.2 |
| High‐density lipoprotein cholesterol (mg/dL) | 74.1 ± 20.3 |
| Low‐density lipoprotein cholesterol (mg/dL) | 150.3 ± 23.1 |
| Aspartate aminotransferase (U/L) | 21.3 ± 4.9 |
| Alanine aminotransferase (U/L) | 19.8 ± 6.0 |
| Gamma‐glutamyl transferase (U/L) | 22.4 ± 6.8 |
| Blood urea nitrogen (mg/dL) | 15.0 ± 3.4 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 72.2 ± 15.6 |
Total n = 9. Data are expressed as mean ± standard deviation.
OGTT, oral glucose tolerance test.
Figure 2.
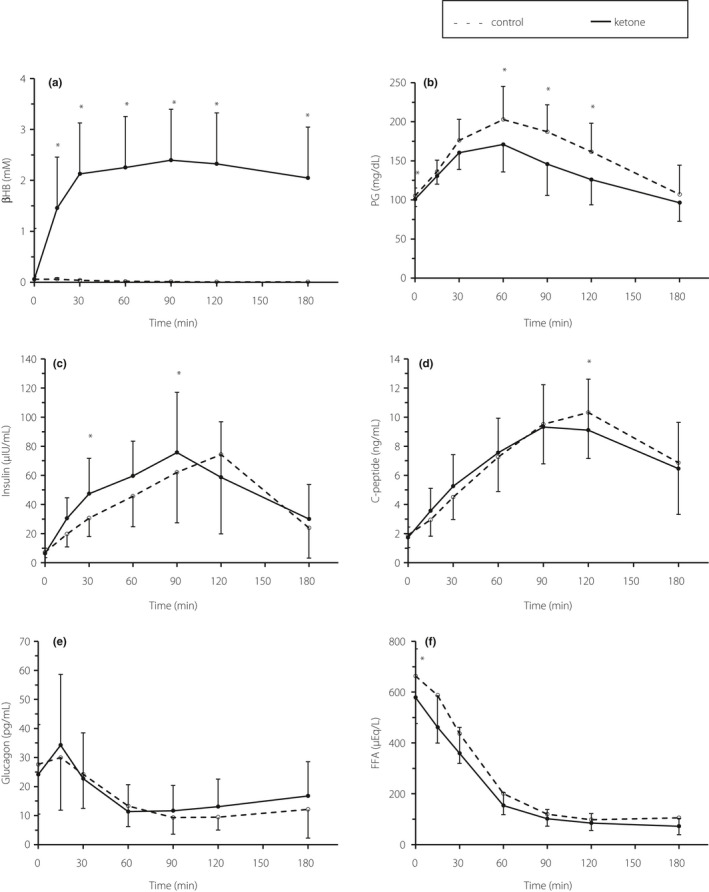
Temporal changes in blood parameters during a 75‐g oral glucose tolerance test. (a) D‐beta‐hydroxybutyrate (βHB), (b) plasma glucose, (c) insulin, (d) C‐peptide, (e) glucagon and (f) free fatty acids (FFA). The dotted lines indicate the control condition, and the solid lines indicate the ketone condition. Each datapoint indicates the mean value ± standard deviation with n = 9, except n = 8 for insulin at 180 min. *P < 0.05, significant difference between the conditions.
Table 2.
Blood parameters during oral glucose tolerance test in the two experimental conditions
| Variables | Control | Ketone ester | P‐value |
|---|---|---|---|
| AUC 0–180 min | |||
| βHB (mmol/L·h) | 116.1 ± 33.9 | 5995.3 ± 1257.1 | <0.001 |
| Glucose (mg/dL·h) | 483.2 ± 74.3 | 406.6 ± 70.6 | <0.001 |
| Insulin (μU/mL·h) | 130.4 ± 76.0 | 141.0 ± 64.6 | 0.310 |
| Glucagon (pg/mL·h) | 44.5 ± 22.4 | 49.8 ± 33.6 | 0.353 |
| C‐peptide (ng/mL·h) | 22.4 ± 5.0 | 21.0 ± 5.3 | 0.327 |
| Free fatty acids (μEq/L·h) | 638.8 ± 109.7 | 548.8 ± 74.1 | 0.170 |
| C‐peptide/glucose ratio | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.013 |
| C‐peptide/insulin ratio | 0.18 ± 0.06 | 0.15 ± 0.04 | 0.007 |
| AUC 0–90 min | |||
| βHB (mmol/L·h) | 104.2 ± 37.0 | 2720.3 ± 543.2 | <0.001 |
| Glucose (mg/dL·h) | 261.6 ± 37.1 | 227.4 ± 32.3 | 0.003 |
| Insulin (μU/mL·h) | 55.7 ± 22.6 | 74.9 ± 29.3 | 0.003 |
| Glucagon (pg/mL·h) | 29.0 ± 14.9 | 28.7 ± 19.9 | 0.925 |
| C‐peptide (ng/mL·h) | 8.6 ± 1.9 | 9.2 ± 2.5 | 0.164 |
| Free fatty acids (μEq/L·h) | 506.8 ± 101.6 | 413.2 ± 77.7 | 0.510 |
| C‐peptide/glucose ratio | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.014 |
| C‐peptide/insulin ratio | 0.17 ± 0.04 | 0.13 ± 0.03 | <0.001 |
| AUC 90–180 min | |||
| βHB (mmol/L·h) | 11.9 ± 7.1 | 2275.0 ± 775.3 | <0.001 |
| Glucose (mg/dL·h) | 221.6 ± 52.4 | 179.2 ± 43.5 | <0.001 |
| Insulin (μU/mL·h) | 78.96 ± 58.0 | 72.77 ± 45.4 | 0.515 |
| Glucagon (pg/mL·h) | 15.5 ± 8.5 | 21.1 ± 14.4 | 0.082 |
| C‐peptide (ng/mL·h) | 13.8 ± 3.9 | 11.7 ± 3.8 | 0.053 |
| Free fatty acids (μEq/L·h) | 131.9 ± 65.0 | 135.6 ± 37.2 | 0.065 |
| C‐peptide/glucose ratio | 0.06 ± 0.01 | 0.07 ± 0.02 | 0.011 |
| C‐peptide/insulin ratio | 0.20 ± 0.09 | 0.19 ± 0.07 | 0.409 |
| Matsuda index | 4.6 ± 1.9 | 5.2 ± 2.2 | 0.091 |
| Insulinogenic index | 0.37 ± 0.24 | 0.75 ± 0.48 | 0.067 |
Data are expressed as mean ± standard deviation. Fasting plasma glucose and free fatty acid levels differed significantly between the ketone and control conditions; therefore we calculated the changes in both parameters relative to the fasting level and compared the areas under the curve. βHB, D‐beta‐hydroxybutyrate; AUC, area under the curve;
Discussion
In the present study, we investigated glucose metabolism during OGTT with and without KM ingestion using a randomized cross‐over design in participants with IGT. The KM ingestion protocol increased circulating βHB levels (maximum 2.4 ± 0.7 mmol/L at 90 min into OGTT), and decreased AUC‐glucose (−15.9 ± 6.4%) during the OGTT compared with the control condition. Furthermore, insulin levels during the first half of the OGTT (0–90 min) were significantly higher, and glucose levels were significantly lower in the KM condition than in the control condition, whereas C‐peptide levels were comparable.
Early phase insulin secretion is an important determinant of post‐load glucose level. Decreased early phase insulin secretion is observed in IGT participants, and is associated with enhanced endogenous glucose production during the postprandial state, causing postprandial hyperglycemia 20 . Indeed, restoration of impaired early insulin secretion by an insulin secretagogue in IGT or type 2 diabetes 21 , 22 , or by exogenous insulin administration in type 2 diabetes 23 , is known to improve postprandial hyperglycemia. Consistent with this fact, the present data showed that reduced the AUC of glucose in the KM condition was associated with elevated insulin levels during the first half of the OGTT. Taken together, the KM‐induced increase in circulating insulin levels during the first half of OGTT might have contributed to reduced glucose levels during the OGTT procedure.
A recent study using the hyperglycemic clamp technique showed that KM ingestion increased endogenous insulin secretion, glucose uptake and muscle glycogen synthesis in the presence of persistently high glucose concentrations 24 . Previous studies have also shown that ketones directly stimulate insulin secretion 25 , 26 , and this effect is glucose‐dependent 27 . Accordingly, KM administration resulted in a significant decrease in the glucose level, but no change in the C‐peptide level. Thus, the C‐peptide : glucose ratio, a marker of insulin secretion 18 , was elevated by the KM ingestion (Table 2). Similarly, Myette‐Côté et al. 15 , 16 reported that KM administration did not alter the insulin level, but decreased the blood glucose level during the OGTT. These data suggested that insulin secretion could be enhanced during OGTT by the KM ingestion.
The underlying mechanisms of enhanced insulin secretion by the KM ingestion remain unclear. It has been shown that short chain fatty acids, such as ketone, are ligands for G‐protein‐coupled receptor (GPR)41 and GPR43. GPR41 and GPR43 are expressed in L cells producing glucagon‐like peptide (GLP)‐1 28 . Thus, it is supposed that insulin secretion could be enhanced after the KM ingestion through increased GLP‐1 secretion from L cells. However, two previous studies using the same KM showed decreased GLP‐1 secretion by the KM ingestion 15 , 16 . Further study is required to clarify the mechanisms of enhanced insulin secretion after the KM ingestion. The circulating insulin level is enhanced not only by increased insulin secretion, but also by reduced insulin clearance. In the present study, the C‐peptide : insulin ratio during the OGTT was decreased by the KM ingestion, which might suggest that KM ingestion decreased insulin clearance. However, the AUC of the molar C‐peptide : insulin ratio after glucose challenge has several limitations 29 . In brief, the difference in the plasma half‐lives of C‐peptide (30 min) and insulin (4 min) complicated the interpretation of the molar C‐peptide : insulin ratio. Thus, the incremental areas under the C‐peptide and insulin concentration curves after stimuli could be precisely compared if the curves are followed until plasma levels return to baseline. However, after 3 h of orally administered glucose, the insulin and C‐peptide did not return basal level (Figure 2). Thus, further study is required to confirm whether the KM ingestion alter insulin clearance during OGTT.
There were several limitations to the present study. First, the sample size of our study was small (5 men, 4 women, total of 9) and included only Japanese individuals. Thus, additional research is required in other populations (younger and/or older adults) to generalize this study. Previous studies showed that East Asians, including Japanese individuals, have a limited innate capacity for insulin secretion 30 , and therefore KM ingestion might be more effective than in individuals of other ethnicities, such as white individuals. Second, we only investigated the acute effects of KM ingestion; its chronic effects on glucose metabolism and β‐cell function remain unknown. Pujol et al. 31 reported that chronic βHB treatment improved β‐cell function in an animal model. In contrast, several animal models showed impaired insulin clearance, secondary induced adiposity and an insulin‐resistant state 32 . Thus, further studies are clearly required to confirm the efficacy and safety of βHB treatment. Fasting FFA level was slightly, but significantly, lower in the KM condition than in the control condition. It has been shown that acute (90 min) elevation of FFA by approximately twofold resulted in impaired insulin sensitivity and enhanced insulin secretion; however, chronic (48 h) FFA elevation did not enhance insulin secretion, whereas insulin sensitivity was reduced 33 . Thus, we cannot deny the possibility the difference in FFA concentration influenced the present results; however, the difference in FFA concentration between the conditions was small; therefore, the difference in FFA might not have a strong impact on the results. Finally, we did not measure other parameters related to glucose metabolism (e.g., GLP‐1 and sympathetic nerve activity). It has been shown that ketone body suppresses sympathetic nerve activity through GPR41 34 . As endogenous glucose production is enhanced by activated sympathetic nerve, suppression of sympathetic nerve activity might be involved in the mechanisms of improved glucose excursion after the KM ingestion.
In conclusion, KM ingestion decreased the AUC of glucose during the 75‐g OGTT in Japanese individuals with IGT. A possible mechanism is the enhancement of circulating insulin levels by enhanced insulin secretion.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank Miyuki Iwagami and Naoko Daimaru for their excellent technical assistance. This work was supported in part by the Strategic Research Foundation at Private Universities of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
J Diabetes Investig 2021; 12: 756–762
Clinical Trial Registry Japan Clinical Trials RegistryUMIN000037787
References
- 1. Balasse EO, Fery F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev 1989; 5: 247–270. [DOI] [PubMed] [Google Scholar]
- 2. Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol 2017; 595: 2857–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polidori D, Iijima H, Goda M, et al. Intra‐ and inter‐subject variability for increases in serum ketone bodies in patients with type 2 diabetes treated with the sodium glucose co‐transporter 2 inhibitor canagliflozin. Diabetes Obes Metab 2018; 20: 1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kosinski C, Jornayvaz F. Effects of ketogenic diets on cardiovascular risk factors: evidence from animal and human studies. Nutrients 2017; 9: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwingshackl L, Hoffmann G. Comparison of effects of long‐term low‐fat vs high‐fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta‐analysis. J Acad Nutr Diet 2013; 113: 1640–1661. [DOI] [PubMed] [Google Scholar]
- 6. Browning JD, Baker JA, Rogers T, et al. Short‐term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr 2011; 93: 1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low‐carbohydrate, mediterranean, or low‐fat diet. N Engl J Med 2008; 359: 229–241. [DOI] [PubMed] [Google Scholar]
- 8. Manzoni GM, Castelnuovo G, Molinari E. Weight loss with a low‐carbohydrate, mediterranean, or low‐fat diet. N Engl J Med 2008; 359: 2170; author reply 2171–2172. [PubMed] [Google Scholar]
- 9. Clarke K, Tchabanenko K, Pawlosky R, et al. Kinetics, safety and tolerability of (r)‐3‐hydroxybutyl (r)‐3‐hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol 2012; 63: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cox PJ, Kirk T, Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab 2016; 24: 256–268. [DOI] [PubMed] [Google Scholar]
- 11. Stubbs BJ, Cox PJ, Evans RD, et al. On the metabolism of exogenous ketones in humans. Front Physiol 2017; 8: 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jenkins DJ, Hunter WM, Goff DV. Ketone bodies and evidence for increased insulin secretion. Nature 1970; 227: 384–385. [DOI] [PubMed] [Google Scholar]
- 13. Van Gelder J, Shafiee M, De Clercq E, et al. Species‐dependent and site‐specific intestinal metabolism of ester prodrugs. Int J Pharm 2000; 205: 93–100. [DOI] [PubMed] [Google Scholar]
- 14. Desrochers S, David F, Garneau M, et al. Metabolism of R‐ and S‐1,3‐butanediol in perfused livers from meal‐fed and starved rats. Biochem J 1992; 285(Pt 2): 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myette‐Cote E, Neudorf H, Rafiei H, et al. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J Physiol 2018; 596: 1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Myette‐Côté É, Caldwell HG, Ainslie PN, et al. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: a randomized trial. Am J Clin Nutr 2019; 110: 1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamaki M, Fujitani Y, Hara A, et al. The diabetes‐susceptible gene slc30a8/znt8 regulates hepatic insulin clearance. J Clin Invest 2013; 123: 4513–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. den Biggelaar LJ, Sep SJ, Eussen SJ, et al. Discriminatory ability of simple OGTT‐based beta cell function indices for prediction of prediabetes and type 2 diabetes: the CODAM study. Diabetologia 2017; 60: 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 20. Mitrakou A, Kelley D, Mokan M, et al. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 1992; 326: 22–29. [DOI] [PubMed] [Google Scholar]
- 21. Hirose T, Mizuno R, Yoshimoto T. The effects of nateglinide following oral glucose load in impaired glucose tolerance subjects: rapid insulin stimulation by nateglinide in IGT subjects. Endocr J 2002; 49: 649–652. [DOI] [PubMed] [Google Scholar]
- 22. Uchino H, Niwa M, Shimizu T, et al. Impairment of early insulin response after glucose load, rather than insulin resistance, is responsible for postprandial hyperglycemia seen in obese type 2 diabetes: assessment using nateglinide, a new insulin secretagogue. Endocr J 2000; 47: 639–641. [DOI] [PubMed] [Google Scholar]
- 23. Bruttomesso D, Pianta A, Mari A, et al. Restoration of early rise in plasma insulin levels improves the glucose tolerance of type 2 diabetic patients. Diabetes 1999; 48: 99–105. [DOI] [PubMed] [Google Scholar]
- 24. Holdsworth DA, Cox PJ, Kirk T, et al. A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med Sci Sports Exerc 2017; 49: 1789–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madison LL, Mebane D, Unger RH, et al. The hypoglycemic action of ketones. Ii. Evidence for a stimulatory feedback of ketones on the pancreatic beta cells. J Clin Invest 1964; 43: 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balasse EO, Ooms HA, Lambilliotte JP. Evidence for a stimulatory effect of ketone bodies on insulin secretion in man. Horm Metab Res 1970; 2: 371–372. [DOI] [PubMed] [Google Scholar]
- 27. Goberna R, Tamarit J Jr, Osorio J, et al. Action of B‐hydroxy butyrate, acetoacetate and palmitate on the insulin release in the perfused isolated rat pancreas. Horm Metab Res 1974; 6: 256–260. [DOI] [PubMed] [Google Scholar]
- 28. Tolhurst G, Heffron H, Lam YS, et al. Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein‐coupled receptor FFAR2. Diabetes 2012; 61: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polonsky KS, Rubenstein AH. C‐peptide as a measure of the secretion and hepatic extraction of insulin: pitfalls and limitations. Diabetes 1984; 33: 486–494. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka Y, Atsumi Y, Matsuoka K, et al. Interesting insulin response to oral glucose load in young Japanese subjects with impaired glucose tolerance. Diabetes Care 2000; 23: 710–712. [DOI] [PubMed] [Google Scholar]
- 31. Pujol JB, Christinat N, Ratinaud Y, et al. Coordination of gpr40 and ketogenesis signaling by medium chain fatty acids regulates beta cell function. Nutrients 2018; 10: 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Najjar SM, Perdomo G. Hepatic insulin clearance: mechanism and physiology. Physiology (Bethesda) 2019; 34: 198–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carpentier A, Mittelman SD, Lamarche B, et al. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol 1999; 276: E1055–E1066. [DOI] [PubMed] [Google Scholar]
- 34. Kimura I, Inoue D, Maeda T, et al. Short‐chain fatty acids and ketones directly regulate sympathetic nervous system via G protein‐coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 2011; 108: 8030–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]


