Abstract
Aims/Introduction
The prevalence of obesity is rising in Japan and represents a considerable unmet medical need. The Awareness, Care and Treatment in Obesity MaNagement – International Observation (ACTION‐IO) study was designed to identify the perceptions, attitudes and barriers to obesity care among people with obesity (PwO) and healthcare professionals (HCPs) in Japan.
Materials and Methods
An online, cross‐sectional survey was carried out in 11 countries, including Japan.
Results
The survey was completed by 2,001 PwO and 302 HCPs in Japan. Fewer PwO (58%) than HCPs (85%) perceived obesity as a chronic disease. Most PwO (81%) thought that weight loss was their own responsibility, and waited a considerable time before seeking support from their HCP (mean 6 years). Most PwO (64%) had made one or more serious weight loss attempt in the past. In contrast, a serious attempt at losing weight was reported by HCPs for just 21% of their patients. Just 24% of PwO had weight discussions with an HCP in the past 5 years; of those, 56% expressed positive feelings after such a conversation, and just 2% felt offended. Lack of patient motivation (68%) and patient disinterest (61%) were reported by HCPs as barriers to weight management conversations. A higher proportion of obesity specialists (37%) than non‐specialists (22%) thought their patients were motivated to lose weight.
Conclusions
Our Japanese dataset shows a need to raise awareness of the pathophysiological basis and clinical management of obesity among PwO and HCPs. The largely positive feelings expressed by PwO after weight loss conversations should encourage HCPs to initiate earlier discussions before obesity‐related complications occur.
Keywords: Japan, Obesity, Obesity management
Assumptions and misperceptions among people with obesity (PwO) and healthcare professionals (HCPs) may pose barriers to the therapeutic approach to obesity. This study evaluated the perceptions, attitudes and barriers to obesity care among Japanese PwO and HCPs from the global ACTION‐IO study. The study revealed that there is a need to raise awareness of the pathophysiological basis and clinical management of obesity in Japan and that HCPs should initiate timelier weight management discussions without fear of causing offence.

Introduction
Obesity is a serious, chronic progressive disease with an increasing prevalence worldwide 1 , 2 . Obesity represents a significant public health concern, because it is associated with multiple comorbidities that increase the risk of disability and mortality 3 , 4 , 5 ; it is also thought to be a risk factor for COVID‐19‐related mortality 6 , 7 . Furthermore, individuals with obesity experience a decline in health‐related quality of life, and often face stigmatization and discrimination, resulting in low emotional and mental well‐being 5 , 8 , 9 , 10 . Like many developed countries, Japan is facing an increase in the incidence of obesity; results from the latest National Health and Nutrition Survey in Japan showed that 32.2% of males and 21.9% of females were affected by obesity in 2018 11 . Importantly, a high body mass index (BMI) has been associated with an increased mortality rate in Japan 12 , and even those with mild obesity tend to suffer from obesity‐related complications 13 .
The Japan Society for the Study of Obesity guidelines define obesity as a disease, and recommend lifestyle interventions, including dietary, exercise and behavioral therapies, as the first lines of treatment for obesity disease 14 . The Japanese national health insurance system aims to execute annual health checks, and provide guidance to reduce the risk factors and medical expenses associated with lifestyle‐related chronic diseases 15 , 16 . The health checks evaluate metabolic risk factors and specifically focus on abdominal obesity 15 , 16 . As a part of this initiative, specific health guidance is provided to individuals with obesity or metabolic syndrome or those at high risk of developing a chronic lifestyle‐related disease, and includes lifestyle counseling and interventions 15 , 16 , 17 . Pharmacotherapy might be considered only after these step‐by‐step treatments have been trialed, and it is only recommended if individuals do not achieve or maintain a definitive target reduction of ≥3% bodyweight after 3–6 months and/or if rapid weight loss is required due to the presence of serious comorbidities 14 . In such cases, people with obesity (PwO) with BMI ≥25 kg/m2 and visceral fat area ≥100 cm2 accompanied by two or more comorbidities or PwO with BMI ≥35 kg/m2 presenting with one or more comorbidities can initiate pharmacotherapy 14 . Despite the availability of evidence‐based policy guidelines, limited pharmacotherapy options are available in Japan; currently only mazindol is available for use in patients with a BMI of ≥35 kg/m2 14 . The patient selection criteria for surgical treatment are equally strict 18 . Bariatric surgery is intended for weight reduction, and might only be suggested for PwO with a BMI of ≥35 kg/m2 who are non‐responsive to pharmacotherapy. In contrast, metabolic surgery is intended to address obesity‐related complications, and is only recommended for those with a BMI of ≥32 kg/m2 presenting with type 2 diabetes or at least two obesity‐related comorbidities 19 , 20 . As of 2016, laparoscopic sleeve gastrectomy is the only bariatric surgical procedure covered by the Japanese national health insurance system 18 .
To provide a comprehensive understanding of the perceptions, attitudes and barriers to obesity care among PwO and health‐care professionals (HCPs) in Japan, we analyzed the Japanese data collected in the Awareness, Care and Treatment in Obesity MaNagement – International Observation (ACTION‐IO) study.
Methods
Methodology for the ACTION‐IO survey has been reported previously 21 . Briefly, it was a cross‐sectional, non‐interventional, descriptive study that collected data through an online survey carried out in 11 countries, including Japan. Japanese PwO and HCPs completed the survey in their native language or in English between 4 June 2018 and 26 July 2018.
The two questionnaires were developed by an international steering committee of experts in obesity, including representatives from each participating country and three medical doctors who were employed by Novo Nordisk (study sponsor). Questionnaire items were phrased and presented in the same order for each respondent. Items presented in a list were shown in alphabetical, categorical, chronological or random order, as deemed relevant for each set of responses. The KJT Group (Honeoye Falls, NY, USA) carried out the online survey and managed the collection and analysis of de‐identified data. The questionnaires were approved by an ethics/independent review board in Japan. The study was carried out in accordance with the Guidelines for Good Pharmacoepidemiology Practices 22 and the Declaration of Helsinki 23 , and was registered with ClincalTrials.gov (NCT03584191).
Respondents were recruited by e‐mail (PwO, HCPs) and by telephone or in‐person (HCPs), and completed the survey in Japanese. All respondents were required to provide electronic informed consent before initiation of the screening questions and survey. Five‐point end‐anchored Likert scales were used to assess agreement, where a rating of 1 meant “do not agree at all”, and a rating of 5 meant “completely agree”. Ratings of 4 or 5 were grouped and reported as “agree”, unless stated otherwise. For the Japanese dataset, a subgroup analysis of obesity specialists versus non‐specialists was carried out to evaluate the effect that formal obesity training has on the perceptions, attitudes and behaviors of HCPs toward PwO. An obesity specialist is defined as a qualified HCP who sees ≥50% of their patients for the management of obesity or excess weight, and/or has received advanced formal training in obesity treatment beyond medical school, and/or perceives themselves as an obesity expert or works in an obesity service clinic.
To reduce sampling bias, a stratified sampling approach was used for PwO, whereby the outbound samples were sent in accordance with predetermined demographic targets, including sex, age, household income, education and region. To achieve a nationally representative sample, targets were monitored throughout data collection. Before participation, PwO were not informed of the specific study goals, but only that the purpose was “to determine treatment experiences of patients with a specific condition”.
Respondent eligibility was based on predetermined demographic targets and was determined using a series of screening questions. BMI inclusion criteria for Japan differed from the global dataset due to differences in the definitions of obesity classes in Asia 24 . Class I obesity was defined as BMI 25–29.9 kg/m2, and class II obesity as BMI ≥30 kg/m2 24 . Eligible Japanese PwO were aged ≥18 years, with a current BMI ≥25 kg/m2 based on self‐reported height and weight. Eligible HCPs were medical practitioners with ≥2 years of practice experience who spend ≥70% of their time in direct patient care and who had seen ≥100 patients during the past month, at least 10 of whom had a BMI ≥25 kg/m2. HCPs specializing in general, plastic or bariatric surgery were excluded. Respondents who met these eligibility criteria and other study criteria completed the full survey.
Analysis of de‐identified data was carried out by the KJT Group using SPSS (version 23.0; IBM Corp., Armonk, NY, USA), Stata (version IC 14.2; StataCorp LLC, College Station, TX, USA) and Excel (version 2016; Microsoft, Redmond, WA, USA). Results are presented using descriptive statistics (means, medians, frequencies). The final PwO sample, including respondents who were not eligible to take part in the full survey, was subsequently weighted to representative demographic targets (age, sex, household income, education and region). HCP data were monitored for specialist types, but were not weighted.
Results
Demographics
In total, 2,001 PwO and 302 HCPs completed the ACTION‐IO survey in Japan (Table 1). The response rates for PwO and HCPs were 23% and 58%, respectively; the eligibility rates were 15% and 44%, respectively. The majority of PwO (74%) had class I obesity (BMI 25–29 kg/m2), and just 17% had class II obesity (BMI 30–34 kg/m2). Overall, 66% of PwO had at least one comorbidity. Of the Japanese HCPs, approximately one‐third (36%) considered themselves an obesity specialist.
Table 1.
Sample demographics and characteristics
|
PwO (n = 2,001) |
HCPs (n = 302) |
|
|---|---|---|
| Mean age, years (range) | 50 (18–87) | 52 (29–81) |
| Sex, n (%) | ||
| Male | 1,090 (54) | 272 (90) |
| Female | 910 (45) | 30 (10) |
| Other | 1 (<1) | 0 (0) |
| BMI classification, n (%) | ||
| Respondents | 2,001 (100) | 254 (84) † |
| BMI <25 kg/m2 | 0 (0) | 199 (78) |
| Obesity class I (25–29.9 kg/m2) | 1,459 (74) | 48 (19) |
| Obesity class II (30–34.9 kg/m2) | 356 (17) | 6 (2) |
| Obesity class III (35–39.9 kg/m2) | 89 (5) | 0 (0) |
| Obesity class IV (≥40 kg/m2) | 97 (5) | 1 (<1) |
| No. comorbidities, n (%) | ||
| 0 | 681 (34) | – |
| 1 | 534 (27) | – |
| 2 | 355 (18) | – |
| 3 | 251 (13) | – |
| ≥4 | 180 (9) | – |
| HCP category, n (%) | ||
| PCP | – | 150 (50) |
| Specialist | – | 152 (50) |
| Cardiologist | – | 38 (13) |
| Gastroenterologist | – | 36 (12) |
| Diabetologist | – | 31 (10) |
| Internal medicine (non‐PCP) | – | 23 (8) |
| Other | – | 17 (6) |
| Endocrinologist | – | 6 (2) |
| Bariatrics/obesity medicine ‡ | – | 1 (<1) |
| Obesity specialist § | ||
| Yes | – | 108 (36) |
| No | – | 194 (64) |
For people with obesity (PwO), all numbers (n) and demographic percentage values (age, sex) were unweighted, whereas all non‐demographic percentage values were weighted. Healthcare professional (HCP) data, including all numbers (n) and percentage values, were not weighted.
PCP, primary care physician.
Of the 302 HCPs, 48 declined to provide their height and/or weight measurements, so body mass index (BMI) classification was not determined for these respondents.
Bariatric surgeons were ineligible per protocol‐prespecified criteria.
A qualified HCP who sees ≥50% of their patients for the management of obesity or excess weight, and/or has received advanced formal training in obesity treatment beyond medical school, and/or perceives themselves as an obesity expert or works in an obesity service clinic.
Perception of obesity as a chronic disease
A similar proportion of PwO (74%) and HCPs (68%) believed that obesity has a large impact on overall health, and that a 5–10% reduction in bodyweight would be extremely beneficial (73% PwO; 88% HCPs; Figure S1a). Despite recognizing that obesity poses a serious health concern, a lower proportion of PwO (58%) perceived obesity as a chronic disease compared with HCPs (85%; Figure S1b).
Attitudes and barriers to weight loss
Most PwO (81%) reported that weight loss was their own responsibility, with only a small proportion (19%) believing that HCPs should also actively contribute (Figure 1, items 1 and 8). Almost half (49%) of HCPs felt that the responsibility to lose weight rested solely on PwO, whereas 58% acknowledged their own responsibility to contribute to their patients’ efforts to lose weight (Figure 1, items 1 and 8). Just 27% of HCPs attested to their patients’ motivation to lose weight, whereas 48% of PwO said they were motivated (Figure 1, item 3). In terms of barriers to weight loss, PwO and HCPs considered lack of exercise (81% PwO; 89% HCPs) and unhealthy eating habits (57% PwO; 93% HCPs) as key contributors. Just 31% of PwO and 56% of HCPs regarded genetic factors as a barrier to losing weight.
Figure 1.
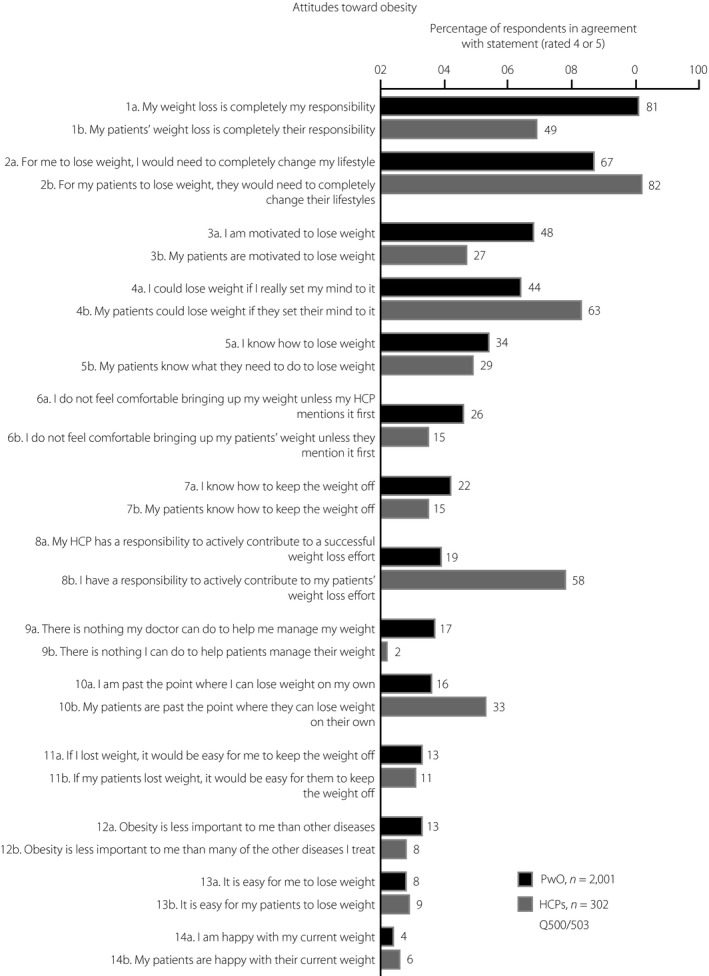
People with obesity (PwO) and healthcare professionals’ (HCPs) attitudes toward weight loss in Japan. Rated on a scale of 1–5, where 1 means “do not agree at all” and 5 means “completely agree”. Agreement was defined as a rating of 4 or 5. Black, PwO; dark gray, HCPs.
Weight loss efforts and outcomes
In total, 64% of PwO reported that they had already made at least one serious weight loss attempt in the past (Figure 2a). However, HCPs perceived that, on mean average, just 21% of their patients had made a serious effort to lose weight (Figure 2b); of these, just 30% were considered by HCPs as responding successfully. The majority of PwO intended to lose weight, with just 28% reporting that they had no weight loss plans within the next 6 months when asked to describe their concerns with weight (Figure 2c). Furthermore, close to half of PwO (44%) thought that if they really put their mind to it, they could lose weight (Figure 1, item 4). Few PwO (31%) reported a weight loss of at least 5% bodyweight over the past 3 years, and of those, just 10% were able to maintain the weight loss for ≥1 year (3.1% of all PwO in total).
Figure 2.
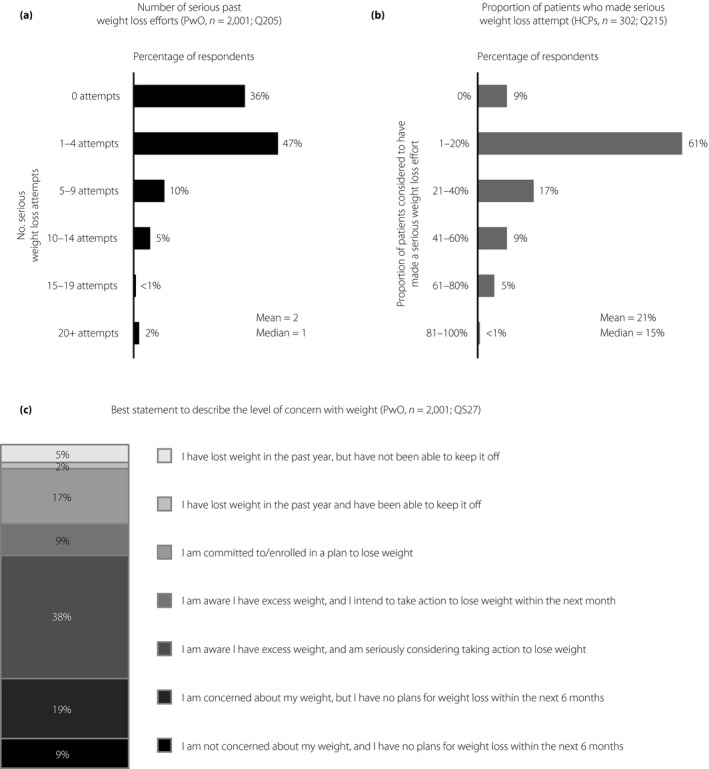
Weight loss efforts and level of concern regarding weight in Japanese people with obesity (PwO). (a) Number of serious attempts made in the past to lose weight, as reported by PwO. (b) Proportion of PwO considered to have made a serious past effort to lose weight, as reported by healthcare professionals (HCPs). (c) Level of concern with weight and weight loss plans among PwO at the time of completing the survey, as evaluated from agreement with the selected statements.
Weight management discussions
Fewer than one‐quarter (24%) of PwO had weight management discussions with an HCP in the past 5 years (Figure 3a). Furthermore, of the PwO who discussed weight with an HCP, 33% reported that they had initiated the conversation themselves. During these conversations, 23% of PwO discussed excess weight and 19% discussed losing weight with their HCP; interestingly, just 9% had discussed weight loss plans with an HCP in the past 6 months (Figure 3b). Of the PwO who had discussed weight with an HCP, there was a mean time delay of 6 years between starting to struggle with their weight and when they first discussed their weight with an HCP (Figure 3c). HCPs reported that weight loss conversations were had with 57% of their patients, and that 36% of patients had initiated these discussions.
Figure 3.
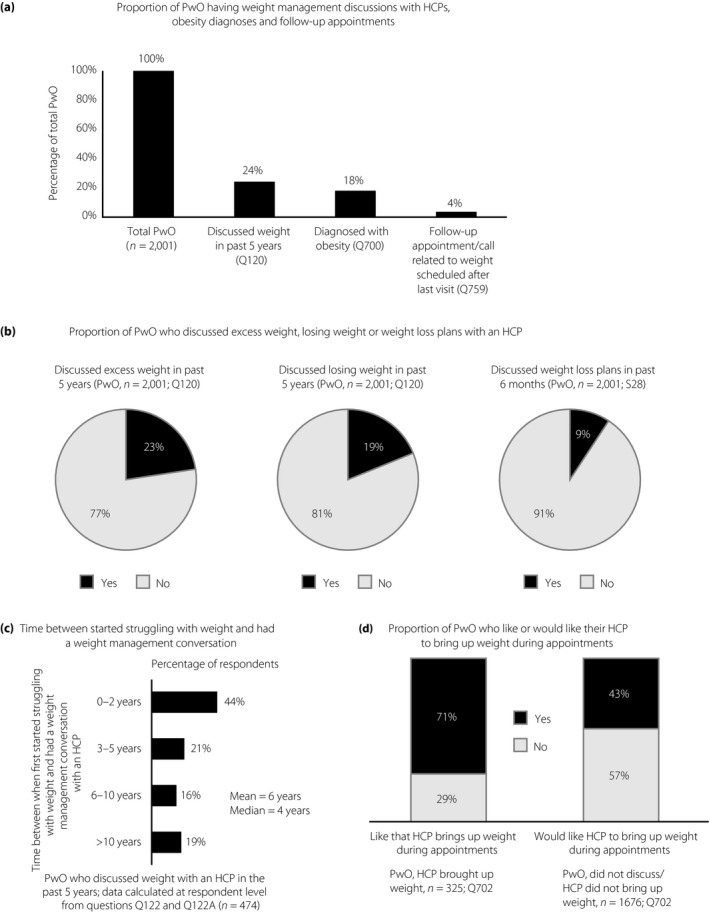
Weight management conversations among Japanese people with obesity (PwO) and healthcare professionals (HCPs). (a) Proportion of Japanese PwO discussing excess weight or losing weight with their HCP, receiving an obesity diagnosis and having follow‐up appointments or calls scheduled. (b) Proportion of PwO who reported discussing excess weight or losing weight with their HCP in the past 5 years or weight loss plans with an HCP in the past 6 months. (c) Proportion of PwO who discussed their weight with an HCP less than 2 years, 3–5 years, 6–10 years or >10 years after they first started struggling with their weight. (d) Proportion of PwO who prefer that their HCP raises the subject of weight during appointments.
Most PwO (73%) who had discussed their weight with an HCP reported receiving a formal obesity diagnosis (18% of all PwO in total; Figure 3a). In contrast, HCPs reported telling 62% of PwO that they have a diagnosis of obesity; 13% of HCPs reported never informing their patients about the diagnosis. PwO were willing to attend follow‐up appointments after their weight management discussions; nearly all PwO (97%) reported that they went or were planning to go to these appointments, if scheduled. However, among PwO who had discussions about their weight with HCPs, just 15% were scheduled a follow‐up appointment or call after their last visit (4% of all PwO in total; Figure 3a). HCPs reported scheduling follow‐up appointments for 31% of their patients, of whom 59% reportedly attended these appointments all or most of the time.
When assessing their weight management discussions with HCPs, most PwO (71%) liked that their HCP had brought up the subject of weight during appointments. However, few PwO (23%) found these conversations very or extremely helpful. For PwO who did not discuss their weight with an HCP, fewer than half (43%) stated they would like their HCPs to raise the subject (Figure 3d). After weight management discussions, 56% of PwO expressed positive feelings; slightly fewer PwO (48%) expressed negative feelings after such a conversation (Figure S2). Just 2% of PwO felt offended (Figure S2).
Barriers to weight loss conversations
The top reason cited by PwO for not discussing their weight with an HCP was the assumption that losing weight was completely their own responsibility (PwO 48%, HCPs 9%; Figure 4). In contrast, HCPs reported lack of patient motivation (HCPs 68%, PwO 13%) and patient disinterest (HCPs 61%, PwO 7%) as the top reasons for not discussing weight with their patients (Figure 4). More than half (57%) of HCPs also considered limited appointment time as a contributing factor to not having weight loss conversations with their patients, as they felt rushed (Figure 4).
Figure 4.
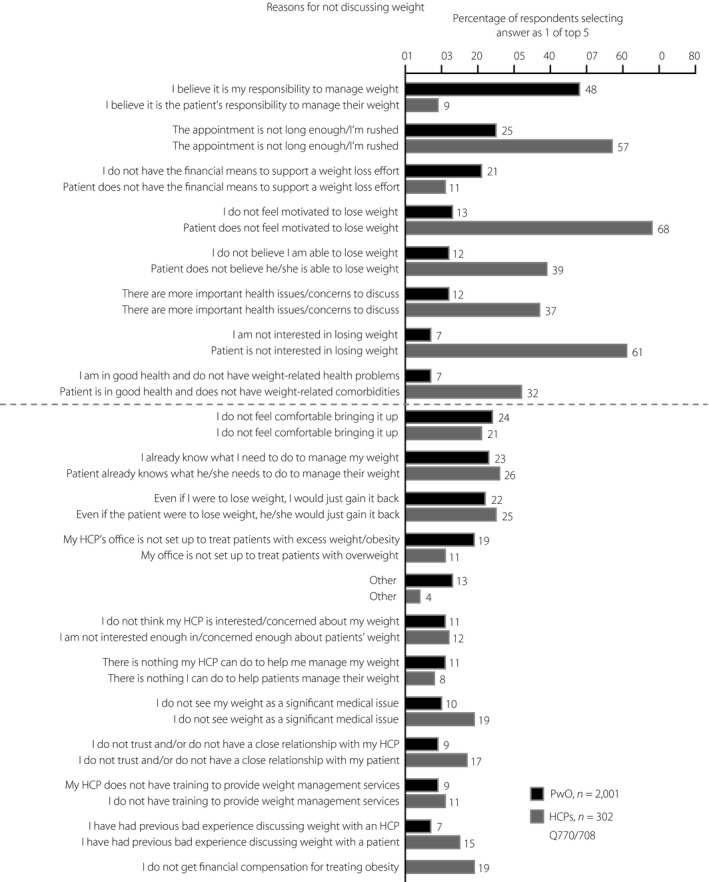
Top five reasons provided by Japanese people with obesity (PwO; black) and healthcare professionals (HCPs; dark gray) for not initiating conversations about weight with their HCP or patient, respectively. Reasons with at least 10% difference between HCPs and PwO are shown above the dashed line, all other reasons are shown below the dashed line.
Just 10% of all HCPs felt very or extremely comfortable during weight management conversations with their patients (Figure 5a). Interestingly, the level of comfort among HCPs did not appear to differ between obesity specialists and non‐specialists, with 10% of obesity specialists or non‐specialists finding weight loss conversations very or extremely comfortable (Figure 5a). In contrast, 22% of obesity specialists and 26% of non‐specialists were not at all comfortable discussing weight with their patients (Figure 5a). In accordance with these findings, weight management conversations were perceived as being very helpful or extremely helpful by just 11% of all HCPs (Figure 5b). Notably, a higher percentage of obesity specialists (15%) perceived these conversations as helpful, when compared with non‐specialists (8%; Figure 5b).
Figure 5.
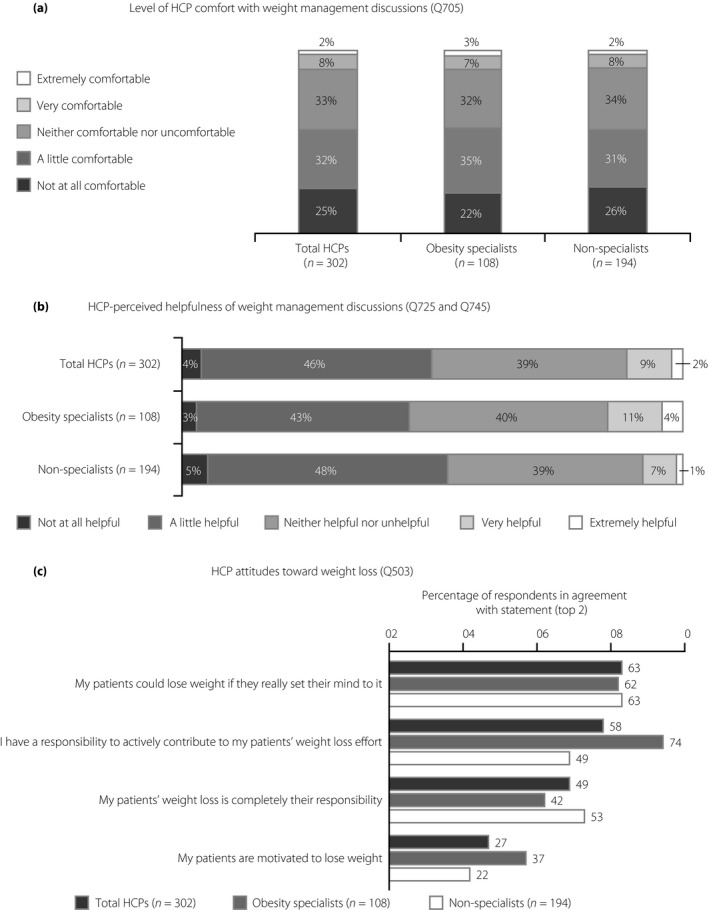
Perceptions and attitudes toward weight management discussions and weight loss among Japanese healthcare professionals (HCPs). (a) Level of comfort with weight management discussions among all HCPs, obesity specialists and non‐specialists. (b) Perceived helpfulness of weight management discussions among all HCPs, obesity specialists and non‐specialists. (c) Attitudes toward weight loss among all HCPs, obesity specialists and non‐specialists, rated on a scale of 1–5. An obesity specialist is defined as a qualified HCP who sees ≥50% of their patients for the management of obesity or excess weight, and/or has received advanced formal training in obesity treatment beyond medical school, and/or perceives themselves as an obesity expert or works in an obesity service clinic.
Differences in attitudes toward weight loss were also observed between obesity specialists and non‐specialists (Figure 5c). Many obesity specialists (74%) believed that they have a responsibility to actively contribute to their patients’ weight loss, whereas fewer non‐specialists (49%) agreed with this statement (Figure 5c). In turn, a higher proportion of non‐specialists (53%) believed that their patients are responsible for their own weight loss, when compared with obesity specialists (42%; Figure 5c). Additionally, a higher proportion of obesity specialists (37%) than non‐specialists (22%) felt that their patients were motivated to lose weight (Figure 5c).
Weight management strategies
Elimination diets (PwO 32%, HCPs 26%), general improvements in eating habits (PwO 26%, HCPs 42%) and increasing physical activity levels (PwO 24%, HCPs 33%) were the weight management methods most frequently discussed with or recommended by HCPs (Figure 6a). Specific diet or exercise programs, tracking, weight loss medications, bariatric surgery and referrals to specialists were not frequently discussed or recommended by HCPs during conversations about weight (Figure 6a). Most PwO and HCPs regarded general improvements in eating habits (PwO 77%, HCPs 66%) and physical activity levels (PwO 79%, HCPs 46%) as the most effective weight management methods (Figure 6b). Very few HCPs acknowledged the effectiveness of weight loss treatments, including over‐the‐counter (2%) and prescription (9%) weight loss medications, and bariatric surgery (9%; Figure 6b). Visiting an obesity specialist (14%), behavioral therapy (18%) and specific diet or exercise programs (18% each) were also considered by HCPs to be less effective weight management methods (Figure 6b).
Figure 6.
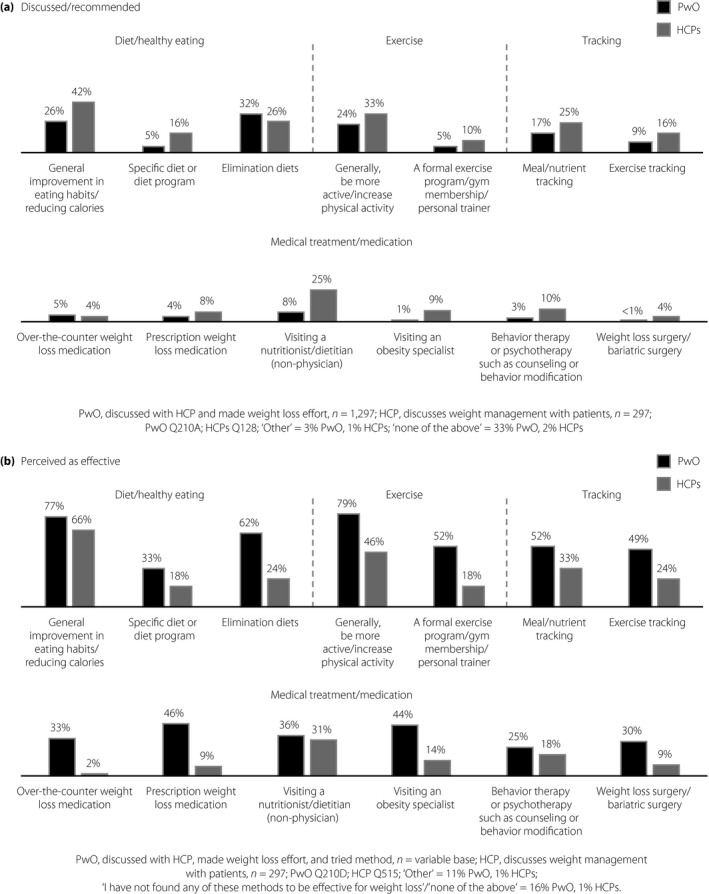
Weight management methods discussed/recommended and perceived as being effective by Japanese people with obesity (PwO) and healthcare professionals (HCPs). (a) Weight management methods discussed with an HCP (reported by PwO) and recommended by HCPs. (b) Weight management methods perceived as effective by PwO and HCPs. Only PwO who had ever tried the weight management method were asked about whether they found it to be effective for weight loss. Black, PwO; dark gray, HCPs.
Discussion
Results from the Japanese cohort of the ACTION‐IO study have identified several differences in the perceptions and attitudes toward obesity and its management among PwO and HCPs. Although the vast majority of respondents recognized that obesity has a large impact on overall health, fewer PwO (58%) than HCPs (85%) perceived obesity as a chronic disease. When compared with the other published ACTION‐IO cohorts, fewer PwO in Japan perceived obesity as a chronic disease (68% global cohort 21 , 78% South Korean cohort 25 and 62% Italian cohort 26 ), whereas the proportion of HCPs was about the same (88% global cohort 21 , 81% South Korean cohort 25 and 91% Italian cohort 26 ). The misperception by PwO in Japan regarding the disease status of obesity might explain why many PwO (81%) felt that weight loss was their own responsibility. Notably, <20% of PwO believed that HCPs have a role in contributing to their weight management. This is almost identical to the global, South Korean and Italian datasets, in which 81%, 81% and 84% of PwO thought that weight loss was their sole responsibility, and just 26%, 36% and 24% believed that HCPs had a duty to support their weight loss efforts, respectively 21 , 25 , 26 . Although PwO and HCPs perceived unhealthy eating habits and lack of exercise as key barriers to weight loss, fewer respondents acknowledged the genetic components underlying obesity disease as a weight loss barrier. Despite nearly half of PwO (48%) expressing their motivation to lose weight, and most having already made at least one serious weight loss attempt in the past, just 27% of HCPs acknowledged their patients’ motivation for weight loss. This lack of appreciation by HCPs might demotivate and discourage PwO from having honest, open discussions regarding their weight. When taken together, these findings show the need to raise awareness of the genetic and pathophysiological mechanisms that contribute to obesity to mitigate feelings of self‐blame among Japanese PwO and other barriers to effective obesity care. In addition, HCPs are encouraged to adopt a positive and engaging attitude toward their patients’ weight loss efforts to facilitate PwO–HCP communication.
Misalignment regarding weight loss efforts made by Japanese PwO was also evident in the present study. Nearly half of all PwO believed that they could lose weight if they set their mind to it; however, the vast majority struggled with losing weight and maintaining weight loss, suggesting that they have a limited response on their own. Considerably fewer Japanese PwO (24%) had discussed their weight with an HCP in the past 5 years, when compared with the global dataset (54% of PwO 21 ) and the Italian dataset (64% of PwO 26 ); however, it was only slightly fewer compared with the South Korean dataset (31% of PwO 25 ). The lack of awareness among Japanese PwO regarding the genetic and biological basis of obesity disease, and feelings of self‐blame might be preventing patients from discussing weight management with their HCP. Indeed, there was a mean time gap of 6 years between when PwO started to struggle with weight and when they first discussed their weight with an HCP; this was identical to the mean time gap reported for the global and Italian cohorts 21 , 26 , whereas the mean time gap in the South Korean cohort was shorter at 1 year 25 . Reducing this time gap by initiating earlier weight management discussions might be an effective strategy for reducing obesity and associated health conditions, and lessening the economic burden of the disease. The need for HCPs to proactively raise the topic before obesity‐related complications occur is further emphasized by the finding that most Japanese PwO selected reducing the risks or health conditions associated with excess weight as their top weight management goal.
Of the PwO who discussed weight with an HCP, just 33% reported initiating the conversation themselves. Furthermore, nearly 60% of PwO (57% vs 35% globally 21 , 26% in Italy 26 , 55% in South Korea 25 ) who had not previously discussed their weight with an HCP reported that they would not like their HCP to bring up the topic during appointments. For Japanese PwO, the main reason provided for not discussing weight with an HCP was their belief that losing weight is their own responsibility. These data suggest that Japanese PwO might be reluctant to initiate or participate in weight management discussions during appointments, which once again underscores the need for widespread disease awareness to encourage PwO to seek help from an HCP and to facilitate open discussions. Multiple studies have reported obesity stigmatization by HCPs 9 , 10 , 27 , which might be an additional contributing factor. In contrast, the present results suggest that many PwO who discussed their weight with an HCP (71%) liked that their HCP had raised the topic of weight during their appointments, and they mostly reported positive feelings after such a conversation. This positive attitude expressed by PwO should encourage Japanese HCPs to discuss the subject of weight during appointments with their patients.
In contrast with the PwO findings, Japanese HCPs cited lack of patient motivation and patient disinterest in losing weight as the main reasons preventing them from initiating weight management conversations with their patients. More than half of HCPs also regarded limited appointment time as a restricting factor for engaging in weight loss conversations. Effective implementation of current obesity guidelines and health policies in Japan, including nationwide health checks and counseling, might help remove these barriers. Furthermore, adopting a collaborative approach to obesity care, involving HCPs, nurses and paramedics certified by the Japan Society for the Study of Obesity and other medical associations, might facilitate the clinical management of obesity and help address the needs of Japanese PwO.
Compared with the global, South Korean and Italian datasets, fewer Japanese HCPs acknowledged their responsibility to contribute to their patients’ weight loss efforts (80% global 21 , 79% South Korea 25 and 76% Italy 26 vs 58% Japan). Similarly, a lower proportion of Japanese HCPs felt very or extremely comfortable during weight management conversations (10% vs 54% globally [ACTION‐IO study steering committee, personal communication], 52% Italy 26 and 29% South Korea 25 ), and few considered these conversations to be helpful (11% vs 42% globally [ACTION‐IO study steering committee, personal communication] and 41% South Korea 25 ). Cultural differences might help explain the misalignment between the global or South Korean and Japanese datasets with regard to the level of comfort during weight management discussions; in our experience, Japanese HCPs might hesitate to raise the issue of excess weight or obesity, out of politeness and respect for their patients.
Similar to the global, Italian and South Korean studies, both PwO and HCPs perceived general improvements in eating habits and physical activity levels as effective weight management methods 21 , 25 , 26 . This is perhaps unsurprising, given that comprehensive education regarding weight management is provided to Japanese residents to encourage better dietary and exercise habits, and to improve mental health 17 . However, a smaller proportion of Japanese HCPs, relative to the global, Italian and South Korean datasets, considered treatments, such as weight loss medication (9% Japan vs 30% global 21 , 56% South Korea 25 and 14% Italy 26 ) or bariatric surgery (9% Japan vs 38% global 21 , 23% South Korea 25 , and 37% Italy 26 ), as effective weight management options, which might indicate a need to expand the knowledge regarding the latest data on weight management strategies 21 . A limited number of pharmacotherapy options are available in Japan, which might also impact on HCPs’ perceptions of evidence‐based treatments. The fact that a higher proportion of obesity specialists acknowledged their patients’ motivation to lose weight and believed that they have a responsibility to actively contribute to their patients’ weight loss, when compared with non‐specialists, further emphasizes the importance of expanding the knowledge of Japanese HCPs regarding the clinical management of obesity. Additionally, both PwO and HCPs should be more proactive about discussing obesity during appointments, and HCPs should also increase the frequency of follow‐up appointments and referrals to specialists to ensure that the most suitable treatment strategies are implemented as early as possible.
The strengths of the present study include the large number of respondents and scientific rigor in the design and implementation of the survey, including the stratified sampling approach used to achieve a nationally representative cohort. The limitations of this study include its exploratory and descriptive design, the reliance on self‐reported height and weight, the accuracy of respondent recall, and the low response rates, which are typical for survey‐based research.
Overall, the present data suggest that although fewer Japanese PwO recognize obesity as a chronic disease, they typically assume full responsibility for their own weight loss and place little importance on the role of Japanese HCPs, instead opting to self‐manage their disease. The misperception that PwO are disinterested in or not motivated enough to lose weight prevented HCPs from initiating timely weight management conversations with PwO due to inherent bias. However, that the PwO expressed self‐motivation for weight loss and their largely positive feelings after weight management conversations showed that HCPs could initiate earlier weight loss discussions without fear of causing offence and before obesity‐related complications occur. The results from the present study also show a need to enhance HCP knowledge about the physiology, causes and treatment of obesity.
Disclosure
MI reports receiving non‐financial support from Novo Nordisk during the study, and personal fees from Novo Nordisk outside the submitted work. TY reports receiving grants from AeroSwitch, Asahi Mutual Life Insurance Company, Mitsubishi Corporation Life Sciences, Nipro Corporation, NTT DOCOMO and Tosoh Corporation during the study and outside the submitted work; personal fees from Dojindo Molecular Technologies, Eli Lilly and Company, FUJIFILM Toyama Chemical, Johnson & Johnson, Kissei Pharmaceutical, Medtronic Japan (formerly Covidien Japan) and Nippon Becton Dickinson during the study and outside the submitted work; grants and personal fees from Astellas Pharma, AstraZeneca K.K., Boehringer Ingelheim, Daiichi Sankyo, Kowa Pharmaceutical, Merck & Co., Mitsubishi Tanabe Pharma, Novartis International AG, Novo Nordisk Pharma Ltd., Ono Pharmaceutical, Sanofi S.A., Sanwa Kagaku Kenkyusho, Shionogi & Co, Sumitomo Dainippon Pharma, Taisho Pharmaceutical and Takeda Pharmaceutical during the study; and grants and personal fees from Kyowa Kirin outside the submitted work. IS reports receiving grants, personal fees and non‐financial support from Novo Nordisk Pharma Ltd. during the study; grants from Eli Lilly Japan K.K. and Novartis Pharma K.K. outside the submitted work; personal fees from Amgen Astellas BioPharma K.K., AstraZeneca K.K., KOBAYASHI Pharmaceutical, Medtronic Japan (formerly Covidien Japan), Nippon Boehringer Ingelheim, Nippon Chemiphar, Rohto Pharmaceutical, Sanwa Kagaku Kenkyusho and Taisho Pharmaceutical outside the submitted work; and grants and personal fees from Astellas Pharma, Daiichi Sankyo, Kowa Pharmaceutical, Kyowa Kirin, Mitsubishi Tanabe Pharma, MSD K.K., Ono Pharmaceutical, Sanofi K.K., Sumitomo Dainippon Pharma, Takeda Pharmaceutical and Teijin Pharma outside the submitted work. KE is an employee of Novo Nordisk Pharma Ltd. YO declares no conflict of interest.
Supporting information
Figure S1 | PwO and HCP perceptions about obesity and weight management in Japan.
Figure S2 | PwO outcomes following recent weight management conversations with their HCP in Japan.
Acknowledgment
This study was sponsored by Novo Nordisk, which also provided financial support for medical editorial assistance from Bhavika Modasia PhD and Ege Yildirim PhD, of Articulate Science. KE is an employee of Novo Nordisk Pharma Ltd. MI and TY are employees of the Department of Diabetes and Metabolic Diseases at The University of Tokyo. MI is a member of the ACTION‐IO study steering committee, and reports industrial links with Novo Nordisk. TY reports industrial links with AeroSwitch, Asahi Mutual Life Insurance Company, Astellas Pharma, AstraZeneca K.K., Boehringer Ingelheim, Daiichi Sankyo, Dojindo Molecular Technologies, Eli Lilly and Company, FUJIFILM Toyama Chemical, Johnson & Johnson, Kissei Pharmaceutical, Kowa Pharmaceutical, Kyowa Kirin, Medtronic Japan (formerly Covidien Japan), Merck & Co., Mitsubishi Corporation Life Sciences, Mitsubishi Tanabe Pharma, Nippon Becton Dickinson, Nipro Corporation, Novartis International AG, Novo Nordisk Pharma Ltd., NTT DOCOMO, Ono Pharmaceutical, Sanofi S.A., Sanwa Kagaku Kenkyusho, Shionogi & Co., Sumitomo Dainippon Pharma, Taisho Pharmaceutical, Takeda Pharmaceutical and Tosoh Corporation. IS is an employee of the Department of Metabolic Medicine at Osaka University, and reports industrial links with Amgen Astellas BioPharma K.K., Astellas Pharma, AstraZeneca K.K., Daiichi Sankyo, Eli Lilly Japan K.K., KOBAYASHI Pharmaceutical, Kyowa Kirin, Medtronic Japan (formerly Covidien Japan), Mitsubishi Tanabe Pharma, MSD K.K., Nippon Boehringer Ingelheim, Nippon Chemiphar, Novartis Pharma K.K., Ono Pharmaceutical, Rohto Pharmaceutical, Sanofi K.K., Sumitomo Dainippon Pharma, Sanwa Kagaku Kenkyusho, Sumitomo Dainippon Pharma, Taisho Pharmaceutical, Takeda Pharmaceutical and Teijin Pharma. YO is an employee of the Department of Medicine and Bioregulatory Science at Kyushu University, and has no industrial links to disclose.
J Diabetes Investig 2021; 12: 845–858
References
- 1. Bray GA, Kim KK, Wilding JPH, et al. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev 2017; 18: 715–723. [DOI] [PubMed] [Google Scholar]
- 2. NCD Risk Factor Collaboration . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitlock G, Lewington S, Sherliker P, et al. Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tremmel M, Gerdtham UG, Nilsson PM, et al. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health 2017; 14: pii: E435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Obesity Collaborators GBD, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. NEJM 2017; 377: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kassir R. Risk of COVID‐19 for patients with obesity. Obes Rev 2020; 21: e13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019 — COVID‐NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghanemi A, Yoshioka M, St‐Amand J. Broken energy homeostasis and obesity pathogenesis: The surrounding concepts. J Clin Med 2018; 7: pii: E453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sikorski C, Luppa M, Kaiser M, et al. The stigma of obesity in the general public and its implications for public health – a systematic review. BMC Public Health 2011; 11: 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med 2020; 26: 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ministry of Health Labour and Welfare . The National Health and Nutrition Survey in Japan (Japanese), 2018. https://www.mhlw.go.jp/content/000615325.pdf. Accessed March 26, 2020.
- 12. Sasazuki S, Inoue M, Tsuji I, et al. Body mass index and mortality from all causes and major causes in Japanese: results of a pooled analysis of 7 large‐scale cohort studies. J Epidemiol 2011; 21: 417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Examination Committee of Criteria for ‘Obesity Disease’ in Japan, Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. 2002; 66: 987–992. [DOI] [PubMed] [Google Scholar]
- 14. Japan Society for the Study of Obesity . Guideline for the Management of Obesity Disease. Himan Kenkyu 2016; 12: 1–91. [Google Scholar]
- 15. Ministry of Health Labour and Welfare . Specific Health Checkups and Specific Health Guidance. https://www.mhlw.go.jp/english/wp/wp‐hw3/dl/2‐007.pdf. Accessed March 26, 2020.
- 16. Ministry of Health Labour and Welfare . The Final Interim Report by the Work Group for Studying the Effects of the Specific Health Checkups and Specific Health Guidance on Health Care Expenditures, 2015. https://www.mhlw.go.jp/file/05‐Shingikai‐12401000‐Hokenkyoku‐Soumuka/0000123428.pdf. Accessed March 26, 2020.
- 17. Ministry of Health Labour and Welfare . Measures against Lifestyle‐Related Diseases through “Health Japan 21” and Promotion of “Shokuiku (food and nutrition education)”. https://www.mhlw.go.jp/english/wp/wp‐hw2/part2/p2c1s3.pdf. Accessed March 26, 2020.
- 18. Lim J, Cho YH, Yamamoto H, et al. Governmental or social support of bariatric surgery in the Asia‐Pacific region. J Obes Metab Syndr 2017; 26: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Society for the Surgery of Obesity and Metabolic Disorder . Guidelines for the Safe and Exceptional Surgical Treatment for Severe Obesity in Japan, 2013 (Japanese). http://plaza.umin.ne.jp/~jsto/gakujyutsu/updata/surgery_guideline_2013.pdf. Accessed March 26, 2020.
- 20. Sasaki A, Wakabayashi G, Yonei Y. Current status of bariatric surgery in Japan and effectiveness in obesity and diabetes. J Gastroenterol 2014; 49: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caterson ID, Alfadda AA, Auerbach P, et al. Gaps to bridge: misalignment between perception, reality and actions in obesity. Diabetes Obes Metab 2019; 21: 1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. International Society for Pharmacoepidemiology (ISPE) . Guidelines for Good Pharmacoepidemiology Practices (GPP). https://www.pharmacoepi.org/resources/policies/guidelines‐08027/. Accessed January 15, 2019.
- 23. WMA . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 24. International Association for the Study of Obesity (IASO) . The Asia‐Pacific perspective: redefining obesity and its treatment. https://apps.who.int/iris/bitstream/handle/10665/206936/0957708211_eng.pdf?sequence=1&isAllowed=y. Accessed January 30, 2020.
- 25. Lim S, Oh B, Lee SH, et al. Perceptions, attitudes, behaviors, and barriers to effective obesity care in South Korea: results from the ACTION‐IO study. J Obes Metab Syndr 2020; 29: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sbraccia P, Busetto L, Santini F, et al. Misperceptions and barriers to obesity management: Italian data from the ACTION‐IO study. Eat Weight Disord 2020. 10.1007/s40519-020-00907-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flint SW. Obesity stigma: Prevalence and impact in healthcare. Br J Obes 2015; 1: 14–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | PwO and HCP perceptions about obesity and weight management in Japan.
Figure S2 | PwO outcomes following recent weight management conversations with their HCP in Japan.


