Abstract
Aims/Introduction
This study aimed to evaluate the association between time in range (TIR) obtained from continuous glucose monitoring and the prevalence and degree of painful diabetic neuropathy.
Materials and Methods
A total of 364 individuals with diabetic peripheral neuropathy were enrolled in this study. Sensor‐based flash glucose monitoring systems were used to monitor the participants’ glucose levels, and the glycemic variability metrics were calculated, including the TIR, glucose coefficient of variation, standard deviation and the mean amplitude of glycemic excursions. The participants were asked to record any form of pain during the 2 weeks of monitoring, and score the pain every day on a numerical rating scale. Based on the numerical rating scale, the patients were divided into the pain‐free group, mild pain group and moderate/severe pain group.
Results
Overall, 51.92% (189/364) of the participants were diagnosed with painful diabetic neuropathy. Compared with the pain‐free group, the level of TIR decreased significantly in the mild pain and moderate/severe pain groups (P < 0.05). The prevalence of mild pain and moderate/severe pain decreased with increasing TIR quartiles (all P < 0.05). Multiple linear regression analysis showed that TIR was significantly negatively correlated with the numerical rating scale score after adjustment for glycated hemoglobin, glycemic variability indicators and other risk factors (P < 0.05). Logistic regression analysis showed that a decreasing level of TIR was significantly associated with an increasing risk of any pain and moderate/severe pain (P < 0.05).
Conclusions
TIR is correlated with painful diabetic neuropathy and is underscored as a valuable clinical evaluation measure.
Keywords: Continuous glucose monitoring, Painful diabetic neuropathy, Time in range
Time in range is correlated with the degree of painful diabetic neuropathy independently of the glycated hemoglobin level, other glycemic variability metrics and risk factors among diabetes patients. Furthermore, time in range was a valuable clinical evaluation indicator for patients with diabetes.

Introduction
The worldwide prevalence of diabetes mellitus has reached epidemic proportions, and its long‐term complications constitute a burden on human health 1 . Diabetic neuropathy (DN) is one of the most common complications. Diabetic peripheral neuropathy (DPN) is the most prevalent form of DN, and might affect approximately half of the people with diabetes 2 . DPN is symmetric, starts distally and gradually spreads proximally in a glove‐and‐stocking distribution. Approximately 15–25% of individuals with DPN present with neuropathic pain, also known as painful diabetic neuropathy (PDN) 3 . PDN can negatively affect a patient’s daily activities, functionality, mood and health‐related quality of life 4 . The reported risk factors for PDN include being female, increasing age, high alcohol intake, cigarette smoking, duration of diabetes mellitus, obesity and elevated glycated hemoglobin (HbA1c) levels 5 , 6 . The HbA1c level has been reported to be strongly associated with vascular complications of diabetes and is recommended as the “gold standard” for long‐term glycemic control 7 . However, because HbA1c does not incorporate the glycemic variability (GV), the level of HbA1c level alone does not sufficiently represent glycemic control 8 . Recently, research regarding the relationship between GV and chronic complications of diabetes has suggested that GV is an independent risk factor for chronic complications of diabetes, including DPN 9 . One study also found that GV was associated with the risk of PDN in type 2 diabetes mellitus 10 .
Continuous glucose monitoring (CGM) using a sensor provides the glucose profile over a number of days, and this might be the best way to assess an individual’s current GV status 11 . Time in range (TIR), a metric of CGM, refers to the proportion of time spent within the glucose range of 3.9–10.0 mmol/L during a 24‐h period, and can be used as a key indicator for the evaluation of short‐term glycemic control 12 . Emerging pieces of evidence have shown that TIR is a risk factor for diabetic retinopathy and diabetic cardiovascular autonomic neuropathy independent of HbA1c level 13 , 14 . However, to the best of our knowledge, no study has assessed the association between TIR and PDN. In the present study, we examined the association between TIR obtained through CGM with the prevalence and degree of PDN.
Methods
Study design and population
This was a cross‐sectional study of 364 adult individuals (age >18 years) with DPN recruited at the Department of Endocrinology and Metabolism, Henan Provincial People’s Hospital, Zhengzhou, China, from July 2018 to May 2019. The exclusion criteria were: (i) other causes of neuropathy, such as osteoarthritis, cervical and lumbar diseases, connective tissue disease, peripheral vascular disease, tumors, herpes zoster infection, abnormal thyroid function, and severe malnutrition or vitamin B12 deficiency; (ii) coexisting major psychiatric disorders; (iii) severe pain from a cause other than DPN; (iv) central nervous system lesions; and (v) pregnancy.
During a 1‐week run‐in period, all drugs for DPN treatment including analgesics were stopped. Concurrently, all patients were given dietary guidance according to the Diabetic Diet Guidelines in Chinese (2017). Then, a 2‐week CGM was carried out with all the eligible participants to assess their GV. An 11‐step numerical rating scale (NRS) was also used to evaluate the severity of neuropathic pain within the 2‐week period 15 .
This study was approved by the ethics committee of the Henan Provincial People’s Hospital. Written informed consent was obtained from all participants before enrolment.
DPN diagnosis
DPN was confirmed according to the following diagnostic criteria: (i) more than one typical symptom (such as numbness, tingling, poor balance and pain) of diabetic distal symmetric polyneuropathy at least 3 months after diagnosis of diabetes, and with at least one sign (such as reduced/absent ankle reflexes and vibration perception); (ii) abnormal Toronto Clinical Scoring System (TCSS) scores; and/or (iii) abnormal nerve conduction test (NCT). The TCSS score was evaluated by an experienced and constant investigator, with an abnormal score defined as ≥5 15 . The NCT included the examination of amplitude, sensory nerve conduction velocities, and motor nerve conduction velocities of the distal median, common peroneal nerves and posterior tibial nerves under electromyography (MEB‐9400C, Nihon Kohden Corporation, Tokyo, Japan). Abnormal NCT was defined as a shorter amplitude or lower NCV level than the normal reference range as judged by the operator.
Pain assessment
The severity of neuropathic pain was quantified using an 11‐step NRS 16 . The DPN participants were asked to record any form of pain, such as a burning sensation; feeling of electric shocks, or a stabbing sensation in the toes, feet or legs every day during the 2‐week monitoring period. If the pain became unbearable and required medication, the observation was terminated at any time. After the observation period, the mean level of NRS was calculated, and the definition and severity of pain were rated according to the average NRS, with a score of 0 indicating no pain. PDN was defined as an NRS score of ≥1: 1–3 indicated mild pain and 4–10 indicated moderate/severe pain. The participants were divided into three subgroups based on the NRS score as the pain‐free group (score 0), mild pain group (score 1–3) and moderate/severe pain group (score 4–10).
CGM technique and data collection
We measured the glucose levels using a sensor‐based flash glucose monitoring system (FreeStyle Libre; Abbott Diabetes Care, Witney, UK). In brief, the patient attached the sensor, which uses a special wired enzyme, to the back of the upper arm to estimate the interstitial glucose levels continuously. The glucose data were automatically recorded and saved every 5 min. After 2 weeks of monitoring, the data were downloaded to a computer and analyzed 17 . The GV metrics were calculated, including the TIR, glucose coefficient of variation (CV), standard deviation (SD) and the mean amplitude of glycemic excursions (MAGE). The results with monitoring data of <3 days were excluded in the analysis.
Anthropometric and biochemical measures
A questionnaire survey was used to record the demographic characteristics, lifestyle factors and previous medical history. Antidiabetic medications included insulin and other oral hypoglycemic agents (OHA). The height and weight were assessed, and the body mass index (BMI) was calculated by dividing the weight (in kg) by the square of the height (in m). Blood pressure was measured three times using a standard mercury sphygmomanometer, and the mean value was recorded. Venous blood samples were collected on the first day of the CGM period after at least 8 h of fasting. Fasting plasma glucose (FPG) levels were assayed by the glucose oxidase method using a biochemical analyzer (ADVIA2400; Siemens, Berlin, Germany). HbA1c levels were measured by high‐performance liquid chromatography with a VARIANT II Hemoglobin A1c analyzer (Bio‐Rad Laboratories, Hercules, CA, USA). Blood C‐peptide levels were measured using a chemiluminescence immunoassay analyzer (Bayer ADVIA Centaur; Bayer, Leverkusen, Germany). The levels of total cholesterol (TC), triglyceride, high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C) and creatinine were determined by standard enzymatic methods using a biochemical analyzer (ADVIA2400; Siemens, Nuremberg, Germany). The estimated glomerular filtration rate (eGFR) was calculated using the creatinine level according to the Chronic Kidney Disease Epidemiology Collaboration equation 18 .
Statistical analysis
Continuous variables with a normal distribution were expressed as the mean ± SD. Variables with a non‐normal distribution were presented as the median (25–75th percentile). Categorical variables were presented as frequencies and proportions. One‐way analysis of variance was used to evaluate the differences between groups. Variables with a non‐normal distribution were subjected to log10 transformation, and the Kruskal–Wallis H‐test was used to compare continuous variables that could not be log‐transformed. The χ2‐test was used to assess the rates among the groups. Pearson correlation analysis was used to assess the TIR levels and other clinical variables. Multiple linear regression analysis was used to estimate the association between TIR and the NRS score. Multinomial logistic regression analysis was carried out to evaluate the independence of association of TIR with different stages of PDN after controlling for clinical risk factors including sex, age, BMI, diabetes mellitus duration, HbA1c level, fasting C‐peptide level, TC level, LDL‐C level, eGFR level, smoking status, drinking status, TCSS score, NCT and antidiabetic agents, as well as GV metrics, when indicated. The independence of association between TIR and the presence of any PDN was assessed by binary logistic regression analysis. Statistical analyses were carried out using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Analysis items with P < 0.05 were considered to show statistical significance.
Results
Participant characteristics according to the presence and severity of PDN
The mean participant age was 53 years (range 46–60 years), and the mean duration of diabetes was 8 years (range 3–14 years). The clinicodemographic characteristics of the participants are shown in Table 1. The average TCSS score was 8 (range 7–10), and 322 (88.5%) patients had an abnormal NCT. There were 189 (51.92%) and 177 (93.7%) patients diagnosed with PDN and abnormal NCT, respectively.
Table 1.
Characteristics of the overall cohort and according to the presence and severity of painful diabetic neuropathy
| Characteristics | All participants (n = 364) | Pain‐free (n = 175) | Mild pain (n = 125) | Moderate/severe pain (n = 64) | P‐value |
|---|---|---|---|---|---|
| Age (years) | 53 (46–60) | 51 (45–57) | 54 (48–61) | 58 (47–61) | <0.001 |
| Female, n (%) | 119 (32.7) | 46 (26.4) | 47 (37.3) | 26 (40.6) | 0.040 |
| DM duration (years) | 8 (3–14) | 6 (2–10) | 10 (4–15) | 10 (2–18) | 0.001 |
| BMI (kg/m2) | 24.8 (23.1–26.9) | 24.5 (22.3–26.4) | 25.3 (23.2–27.1) | 25.0 (23.6–27.6) | 0.091 |
| Type 2 diabetes, n (%) | 341 (93.7) | 168 (96.0) | 116 (92.8) | 57 (89.1) | 0.131 |
| Hypertension, n (%) | 165 (45.3) | 72 (41.1) | 58 (46.4) | 35 (54.7) | 0.169 |
| Systolic BP (mmHg) | 130 (120–141) | 130 (120–140) | 132 (121–141) | 130 (120–143) | 0.592 |
| Diastolic BP (mmHg) | 80 (76–89) | 81 (76–90) | 80 (76–87) | 80 (75–88) | 0.458 |
| FPG (mmol/L) | 7.54 (6.54–8.59) | 7.21 (6.07–8.15) | 7.85 (6.84–9.06) | 8.88 (7.40–9.60) | <0.001 |
| HbA1c (%) | 7.35 (6.73–8.02) | 7.12 (6.54–7.59) | 7.55 (6.91–8.30) | 8.16 (7.27–8.65) | <0.001 |
| Fasting C‐peptide (ng/mL) | 1.62 ± 0.78 | 1.72 ± 1.05 | 1.67 ± 0.92 | 1.41 ± 0.71 | 0.057 |
| Dyslipidemia, n (%) | 146 (40.1) | 70 (40.0) | 46 (36.8) | 30 (46.9) | 0.409 |
| TC (mmol/L) | 4.36 (3.61–5.15) | 4.20(3.41–5.10) | 4.65 (3.76–5.38) | 4.39 (3.71–5.22) | 0.009 |
| TG (mmol/L) | 1.69 (1.16–2.63) | 1.58 (1.16–2.52) | 1.86 (1.23–2.87) | 1.71 (1.12–2.68) | 0.193 |
| HDL‐C (mmol/L) | 1.03 (0.88–1.20) | 1.01 (0.87–1.19) | 1.04 (0.89–1.18) | 1.12 (0.88–1.31) | 0.238 |
| LDL‐C (mmol/L) | 2.36 ± 0.41 | 2.30 ± 0.79 | 2.46 ± 0.07 | 2.35 ± 0.96 | 0.138 |
| eGFR (mL/min/1.73 m2) | 86.04 ± 18.78 | 90.45 ± 22.76 | 87.66 ± 21.64 | 84.89 ± 23.43 | 0.034 |
| TIR (%) | 78 (65–85) | 80 (74–87) | 76 (60–85) | 65 (49–73) | <0.001 |
| SD (mmol/L) | 2.21 (1.81–2.75) | 2.12 (1.78–2.61) | 2.30 (1.81–2.76) | 2.79 (2.08–3.21) | 0.001 |
| CV (%) | 0.30 (0.26–0.34) | 0.30 (0.26–0.34) | 0.29 (0.25–0.34) | 0.32 (0.27–0.37) | 0.066 |
| MAGE (mmol/L) | 5.13 (4.29–6.14) | 4.86 (4.03–5.82) | 5.14 (4.28–6.14) | 5.73 (4.91–6.95) | 0.001 |
| Smoking, n (%) | 158 (43.4) | 76 (43.4) | 49 (39.2) | 33 (51.6) | 0.268 |
| Drinking, n (%) | 182 (50.0) | 86 (49.1) | 60 (48.0) | 36 (56.3) | 0.535 |
| Use of antidiabetic agents, n (%) | 362 (99.5) | 174 (99.4) | 124 (99.2) | 64 (100) | 0.779 |
| OHA | 171 (47.3) | 94 (54.0) | 53 (42.8) | 24 (37.5) | 0.036 |
| Insulin | 53 (14.6) | 24 (13.8) | 17 (13.7) | 12 (18.7) | 0.591 |
| Both | 138 (38.1) | 56 (32.2) | 54 (43.5) | 28 (43.8) | 0.082 |
| TCSS score | 8 (7–10) | 7 (6–9) | 8 (7–10) | 9 (7–11) | <0.001 |
| Abnormal NCT, n (%) | 322 (88.5) | 145 (82.9) | 117 (93.6) | 60 (93.8) | <0.001 |
| Abnormal amplitude | 65 (20.2) | 38 (26.2) | 17 (14.6) | 10 (16.7) | 0.049 |
| Abnormal NCV | 78 (24.2) | 43 (29.7) | 29 (24.7) | 6 (10.0) | 0.011 |
| Both | 179 (55.6) | 64 (44.1) | 71 (60.7) | 44 (73.3) | <0.001 |
Values are presented as the mean ± standard deviation, median with interquartile range or frequency and proportion. One‐way anova was used to evaluate the samples with a normal distribution between groups. The Kruskal–Wallis H‐test was used to compare the variables with non‐normal distributions. The χ2‐test was used to examine the rates among the groups. BMI, body mass index; BP, blood pressure; CV, coefficient of variation; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MAGE, mean amplitude of glucose excursions; NCT, nerve conduction test; NCV, nerve conduction velocities; OHA, oral hypoglycemic agents; SD, standard deviation; TC, total cholesterol; TCSS, Toronto Clinical Scoring System; TG, triglyceride; TIR, time in range.
Based on the NRS score, there were 175 patients in pain‐free group, 125 patients in mild pain group and 64 patients in moderate/severe pain group. Compared with the pain‐free group, the severe pain group were older; included a significantly higher proportion of female patients; had a longer diabetes mellitus duration; recorded higher levels of FPG, HbA1c and TC; and used more OHA (P < 0.05). In contrast, the eGFR, TIR, SD and MAGE showed a decreasing tendency in both the mild pain and moderate/severe pain groups (P < 0.05). With respect to neurological parameters, the TCSS score was significantly higher in the moderate/severe group and mild group than that in the pain‐free group (P < 0.001). In addition, the percentage of patients with abnormal NCT was significantly higher in the moderate/severe pain group (93.8%) and the mild pain group (93.6%) than that in the control group (82.9%; P < 0.001). The patients with more severe PDN were also more likely to have both amplitude and NCV abnormalities (P < 0.001).
Clinical characteristics of the participants according to the TIR quartiles
The participants were classified into four groups according to their TIR values. The 25th, 50th and 75th percentiles for TIR were 65.0, 78.0 and 85.0%, respectively. Accordingly, quartile 1 (Q1) comprised those with TIR ≤65.0%; quartile 2 (Q2), TIR 65.0–78.0%; quartile 3 (Q3), TIR 78.0–85.0%; and quartile 4 (Q4), TIR >85.0%. Compared with the Q1 group, the prevalence of both mild pain and moderate/severe pain was significantly lower in the Q2, Q3 and Q4 groups (P < 0.001; Figure 1). For the degree of severity of PDN, the NRS score decreased as the TIR quartile increased (P < 0.05; Table 2). Meanwhile, the TCSS score and percentage of abnormal NCT showed a similar decreasing tendency in the higher quartile of TIR (P < 0.05; Table 2). Diabetes mellitus duration, BMI, systolic blood pressure, diastolic blood pressure, FPG, HbA1c and fasting C‐peptide significantly differed among the four groups (P < 0.05; Table 2). Notably, other GV measures, including SD, CV and MAGE, were significantly lower in the higher TIR quartile groups (P < 0.001; Table 2). Interestingly, compared with the Q1 group, the percentage of only OHA for diabetes treatment was significantly higher in the Q2, Q3 and Q4 groups. However, the ratio of OHA plus insulin use for diabetes treatment showed a decreasing tendency (P < 0.001; Table 2).
Figure 1.
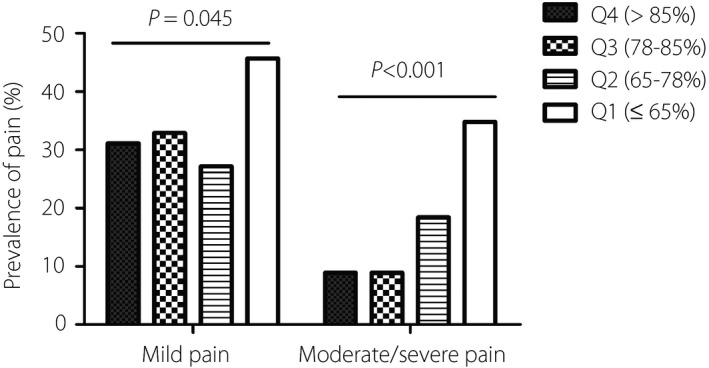
Prevalence of painful diabetic neuropathy in different quartiles (Q1–Q4) of time in range. The χ2‐test was used to examine the rates among the groups.
Table 2.
Participant characteristics according to the time in range quartiles
| Characteristics | Q1 (≤65%) n = 92 | Q2 (65–78%) n = 103 | Q3 (78–85%) n = 79 | Q4 (>85) n = 90 | P‐value |
|---|---|---|---|---|---|
| TIR (%) | 55 (44–62) | 73 (69–76) | 82 (80–84) | 90 (88–92) | <0.001 |
| NRS score | 3 (2–5) | 0 (0–3) | 0 (0–1) | 0 (0–2) | <0.001 |
| Age (years) | 57.0 (45.5–61.8) | 53.0 (47.0–60.0) | 53.0 (45.0–58.0) | 51.0 (45.7–56.0) | 0.060 |
| Female, n (%) | 38 (41.3) | 36 (34.9) | 22 (27.8) | 23 (25.6) | 0.099 |
| DM duration (years) | 10.5 (4.0–18.0) | 7.0 (3.0–13.0) | 9.0 (3.0–13.0) | 6.0 (2.0–10.0) | 0.004 |
| BMI (kg/m2) | 25.1 (23.4–27.5) | 24.3 (22.3–26.0) | 24.9 (23.2–27.0) | 25.2 (23.3–27.2) | 0.044 |
| Type 2 diabetes, n (%) | 84 (91.3) | 95 (92.2) | 74 (93.7) | 88 (97.8) | 0.285 |
| Hypertension, n (%) | 47 (51.1) | 46 (44.7) | 34 (43.0) | 38 (42.2) | 0.622 |
| Systolic BP (mmHg) | 125 (120–136) | 130 (120–144) | 133 (124–145) | 130 (120–140) | 0.026 |
| Diastolic BP (mmHg) | 80.0 (74.0–84.8) | 82.0 (76.0–88.0) | 83.0 (79.0–90.0) | 80.0 (75.8–90.0) | 0.018 |
| FPG (mmol/L) | 9.40 (8.90–10.30) | 7.97 (7.43–8.42) | 7.02 (6.50–7.53) | 6.31 (5.95–6.93) | <0.001 |
| HbA1c (%) | 8.50 (8.20–9.10) | 7.63 (6.29–7.91) | 7.03 (6.70–7.35) | 6.58 (6.36–6.97) | <0.001 |
| Fasting C‐peptide (ng/mL) | 1.37 ± 0.74 | 1.68 ± 0.50 | 1.63 ± 0.41 | 2.17 ± 0.12 | 0.032 |
| Dyslipidemia, n (%) | 36 (39.1) | 37 (35.9) | 39 (49.4) | 34 (37.68) | 0.283 |
| TC (mmol/L) | 4.4 (3.7–5.2) | 4.3 (3.7–5.0) | 4.4 (3.4–5.4) | 4.3 (3.5–5.0) | 0.575 |
| TG (mmol/L) | 1.55 (1.12–2.32) | 1.63 (1.14–2.78) | 1.73 (1.22–2.61) | 1.88 (1.24–2.72) | 0.512 |
| HDL‐C (mmol/L) | 1.05 (0.85–1.20) | 1.01 (0.89–1.21) | 1.03 (0.85–1.23) | 1.02 (0.88–1.20) | 0.996 |
| LDL‐C (mmol/L) | 2.44 ± 0.81 | 2.28 ± 0.69 | 2.45 ± 0.91 | 2.29 ± 0.77 | 0.344 |
| eGFR (mL/min/1.73 m2) | 87.35 ± 7.18 | 102.66 ± 11.56 | 97.15 ± 9.83 | 103.65 ± 12.82 | 0.109 |
| SD (mmol/L) | 3.17 (2.71–3.64) | 2.51 (2.13–2.76) | 2.08 (1.92–2.23) | 1.55 (1.36–1.77) | <0.001 |
| CV (%) | 0.32 (0.29–0.38) | 0.33 (0.27–0.36) | 0.31 (0.27–0.33) | 0.24 (0.22–0.27) | <0.001 |
| MAGE (mmol/L) | 6.48 (5.57–7.57) | 5.45 (4.88–6.26) | 4.90 (4.46–5.37) | 3.82 (3.27–4.47) | <0.001 |
| Smoking (%) | 35 (38.0) | 45 (43.7) | 42 (53.2) | 36 (40.0) | 0.206 |
| Drinking (%) | 37 (40.2) | 50 (48.5) | 48 (60.8) | 47 (52.2) | 0.059 |
| Use of antidiabetic agents (%) | 92 (100) | 103 (100) | 78 (98.7) | 89 (98.9) | 0.505 |
| OHA | 26 (28.3) | 45 (43.7) | 44 (56.4) | 56 (62.9) | <0.001 |
| Insulin | 19 (20.7) | 12 (11.7) | 10 (12.8) | 12 (13.5) | 0.166 |
| Both | 47 (51.0) | 46 (44.6) | 24 (30.8) | 21 (23.6) | <0.001 |
| TCSS score | 9.5 (8.0–11.0) | 8.0 (7.0–9.0) | 8.0 (7.0–10.0) | 7.0 (6.0–8.0) | <0.001 |
| Abnormal NCT, n (%) | 89 (96.7) | 91 (88.4) | 71 (89.9) | 71 (78.9) | 0.002 |
| Abnormal amplitude | 15 (16.9) | 18 (19.8) | 13 (18.3) | 19 (26.8) | 0.443 |
| Abnormal NCV | 13 (14.6) | 23 (25.3) | 21 (29.6) | 21 (29.6) | 0.080 |
| Both | 61 (68.5) | 50 (54.9) | 37 (52.1) | 31 (43.6) | 0.011 |
Values are presented as the mean ± standard deviation, median with interquartile range or frequency and proportion. One‐way anova was used to evaluate the samples with a normal distribution between groups. The Kruskal–Wallis H‐test was used to compare the variables with non‐normal distributions. The χ2‐test was used to examine the rates among the groups. BMI, body mass index; BP, blood pressure; CV, coefficient of variation; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MAGE, mean amplitude of glucose excursions; NCT, nerve conduction test; NCV, nerve conduction velocities; NRS, numerical rating scale; OHA, oral hypoglycemic agent; SD, standard deviation; TC, total cholesterol; TCSS, Toronto Clinical Scoring System; TG, triglyceride; TIR, time in range.
Correlation between TIR level and clinical variables
As shown in Table 3, TIR was negatively correlated with NRS (r = −0.506, P < 0.001), TCSS score (r = −0.388, P < 0.001) and abnormal NCT (r = −0.245, P < 0.001). Furthermore, TIR was negatively correlated with female sex, age, diabetes mellitus duration, FPG, HbA1c and drinking (P < 0.05). For antidiabetic agents, TIR was positively associated with OHA monotherapy, but negatively associated with both OHA and insulin use (P < 0.05).
Table 3.
Correlation between time in range levels and other clinical variables
| Female sex | Age | DM duration | BMI | Systolic BP | Diastolic BP | FPG | HbA1c | OHA only | Insulin only | OHA and insulin | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TIR | |||||||||||
| r | −0.142 | −0.145 | −0.252 | 0.008 | 0.043 | 0.132 | −0.479 | −0.574 | 0.283 | −0.090 | −0.225 |
| P‐value | 0.007 | 0.006 | <0.001 | 0.913 | 0.415 | 0.012 | <0.001 | <0.001 | 0.001 | 0.087 | <0.001 |
| Fasting C‐peptide | TC | TG | HDL‐C | LDL‐C | eGFR | smoking | drinking | TCSS | abnormal NCT | NRS | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TIR | |||||||||||
| r | 0.088 | −0.067 | −0.024 | 0.010 | −0.068 | 0.095 | −0.068 | −0.159 | −0.388 | −0.245 | −0.506 |
| P‐value | 0.094 | 0.205 | 0.651 | 0.937 | 0.195 | 0.078 | 0.198 | 0.002 | <0.001 | <0.001 | <0.001 |
Pearson correlation analysis was used to evaluate the relationship between time in range (TIR) level and other clinical variables. BMI, body mass index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NCT, nerve conduction test; NRS, numerical rating scale; OHA, oral hypoglycemic agent; TC, total cholesterol; TCSS, Toronto Clinical Scoring System; TG, triglyceride.
Multiple linear regression analysis
Five models were constructed to analyze the association between the TIR(%) and NRS score (Table 4). A linear relationship between the dependent and independent variables in each model was confirmed. In model 1, we found that TIR was negatively correlated with NRS (P < 0.05). Furthermore, model 2 also showed a significant negative association between TIR and NRS score independent of sex, age, BMI, diabetes mellitus duration, FPG level, HbA1c level, TC level, LDL‐C level, eGFR, smoking status, drinking status, TCSS score, NCT and antidiabetic agents(P < 0.05). In models 3, 4 and 5, TIR remained to have a significant negative correlation with NRS scores after adjustment for other GV indicators, including SD, CV and MAGE (P < 0.05).
Table 4.
Multiple linear regression analysis of the relationship between time in range (%) and numerical rating scale
| Independent variable |
Mode 1 TIR (%) not adjusted |
Model 2 TIR (%) |
Model 3 TIR (%) SD |
Model 4 TIR (%) CV |
Model 5 TIR (%) MAGE |
|---|---|---|---|---|---|
| β Coefficient (95% CI) | −0.068 (−0.080 to −0.056) | −0.049 (−0.072 to −0.025) | −0.050 (−0.132 to −0.045) | −0.053 (−0.078 to −0.021) | −0.050 (−0.076 to −0.024) |
| P‐value | <0.001 | <0.001 | 0.001 | <0.001 | 0.001 |
Model 1 was not adjusted. Model 2 was adjusted for sex, age, body mass index, diabetes mellitus duration, fasting plasma glucose, glycated hemoglobin, fasting C‐peptide, total cholesterol, low‐density lipoprotein cholesterol, estimated glomerular filtration rate, smoking, drinking, Toronto Clinical Scoring System score, nerve conduction test and antidiabetic agents. Model 3 was adjusted for the variables adjusted for in model 1 + standard deviation (SD). Model 4 was adjusted for the variables adjusted for in model 1 + coefficient of variation (CV). Model 5 was adjusted for the variables adjusted for in model 1 + mean amplitude of glucose excursions (MAGE). CI, confidence interval; NRS, numerical rating scale; TIR, time in range.
Logistic regression analysis
Binary logistic regression analysis was carried out to further explore the relationship between TIR level and PDN (Table 5). After adjustment for sex, age, BMI, diabetes mellitus duration, FPG, HbA1c, Fasting C‐peptide, TC, LDL‐C, eGFR, smoking status, drinking status, TCSS score, NCT and antidiabetic agents (model 1), the data showed that compared with Q4 TIR, the declining Q1 TIR significantly increased the risk of any pain (odds ratio [OR] 2.66, P = 0.021). Multinomial logistic regression analysis was carried out to assess the relationship between the TIR level and the severity of PDN (Table 5). Compared with Q4, the risk of moderate/severe pain, but not that of mild pain, increased as the quartile of TIR decreased in Q1 (OR 5.80, P = 0.003). After further adjusting the data for SD, CV and MAGE in models 2, 3 and 4, decreased Q1 TIR was still significantly associated with an increased risk of any pain (model 2 OR 2.76, P = 0.049; model 3 OR 3.51, P = 0.014; model 4 OR 2.88, P = 0.046) and moderate/severe pain (model 2 OR 5.04, P = 0.019; model 3 OR 6.09, P = 0.009; model 4 OR 5.19, P = 0.019).
Table 5.
Associations between quartiles of time in range and various stages of painful diabetic neuropathy after controlling for confounding factors
| Models | Independent variable | Mild pain | Moderate/severe pain | Any pain | |||
|---|---|---|---|---|---|---|---|
| OR | P‐value | OR | P‐value | OR | P‐value | ||
| Model 1 | TIR (%) | ||||||
| Q1 (≤65) | 2.11 (0.86–5.19) | 0.103 | 5.80 (1.82–18.53) | 0.003 | 2.66 (1.16–6.10) | 0.021 | |
| Q2 (65–78) | 0.62 (0.30–1.31) | 0.212 | 1.60 (0.57–4.48) | 0.369 | 0.82 (0.42–1.61) | 0.566 | |
| Q3 (78–85) | 0.77 (0.37–1.60) | 0.482 | 0.60 (0.18–1.97) | 0.402 | 0.65 (0.33–1.29) | 0.219 | |
| Q4 (>85) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| Model 2 | TIR (%) | ||||||
| Q1 (≤65) | 2.47 (0.83–7.37) | 0.104 | 5.04 (1.30–19.51) | 0.019 | 2.76 (1.00–7.60) | 0.049 | |
| Q2 (65–78) | 0.68 (0.30–1.51) | 0.341 | 1.49 (0.50–4.41) | 0.473 | 0.84 (0.40–1.74) | 0.634 | |
| Q3 (78–85) | 0.81 (0.38–1.73) | 0.581 | 0.59 (0.17–1.93) | 0.373 | 0.66 (0.32–1.34) | 0.246 | |
| Q4 (>85) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| SD | 0.88 (0.52–1.46) | 0.2609 | 1.14 (0.62–2.09) | 0.672 | 0.97 (0.60–1.57) | 0.903 | |
| Model 3 | TIR (%) | ||||||
| Q1 (≤65) | 2.98 (1.02–8.69) | 0.045 | 6.09 (1.57–23.72) | 0.009 | 3.51 (1.29–9.52) | 0.014 | |
| Q2 (65–78) | 0.80 (0.34–1.85) | 0.083 | 1.65 (0.52–5.17) | 0.394 | 1.00 (0.46–2.15) | 0.990 | |
| Q3 (78–85) | 0.91 (0.42–2.00) | 0.400 | 0.62 (0.18–2.11) | 0.441 | 0.74 (1.18–4.08) | 0.420 | |
| Q4 (>85) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| CV | 0.04 (0.00–6.74) | 0.233 | 0.66 (0.02–26.17) | 0.891 | 0.094 (0.01–9.66) | 0.317 | |
| Model 4 | TIR (%) | ||||||
| Q1 (≤65) | 2.72 (0.89–8.30) | 0.079 | 5.19 (1.32–20.49) | 0.019 | 2.88 (1.02–8.15) | 0.046 | |
| Q2 (65–78) | 0.73 (0.32–1.67) | 0.454 | 1.48 (0.48–4.51) | 0.494 | 0.86 (0.40–1.83) | 0.020 | |
| Q3 (78–85) | 0.85 (0.39–1.86) | 0.685 | 0.57 (0.17–1.94) | 0.368 | 0.67 (0.32–1.38) | 0.019 | |
| Q4 (>85) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| MAGE | 0.91 (0.72–1.51) | 0.431 | 1.05 (0.81–1.37) | 0.713 | 0.97 (0.78–1.21) | 0.804 | |
Model 1 was adjusted for sex, age, body mass index, diabetes mellitus duration, fasting plasma glucose, glycated hemoglobin, fasting C‐peptide, total cholesterol, low‐density lipoprotein cholesterol, estimated glomerular filtration rate, smoking, drinking, Toronto Clinical Scoring System score, nerve conduction test and antidiabetic agents. Model 2 was adjusted for the variables adjusted for in model1 + standard deviation. Model 3 was adjusted for the variables adjusted for in model 1 + coefficient of variation. Model 4 was adjusted for the variables adjusted for in model 1 + mean amplitude of glucose excursions. OR, odds ratio; TIR, time in range.
Discussion
The present study found that the levels of TIR were significantly decreased in diabetes patients with PDN. In addition, TIR was significantly negatively correlated with the NRS score. After adjusting for other GV metrics (including SD, CV and MAGE), a decreasing level of TIR was associated with an increasing risk of any pain and moderate/severe pain.
Neuropathic pain in diabetes distinctly presents as a burning, electric, sharp, aching or evoked pain. Compared with painless DPN, PDN is associated with increased distress and poor quality of life, and many patients also experience depression, anxiety and sleep disturbance 19 . However, not all cases of DPN progress to PDN. In the present study, PDN was prevalent in 51.92% of the patients, which is higher than those reported in previous studies 20 , 21 . This could be because the participants were recruited among hospitalized patients and they might have had more serious DPN than did outpatients. Understanding the risk factors for PDN will be beneficial for the appropriate management and prevention of this painful condition. In the present study, compared with pain‐free patients, those who had mild and moderate/severe pain were older; had a longer diabetes mellitus duration; included more women; and had higher levels of FPG, HbA1c and TC. Our findings were consistent with those of previous studies showing that hyperglycemia 22 , hyperlipidemia 20 , age 23 , sex 19 and diabetes mellitus duration 21 are risk factors of DPN.
In addition to our result that HbA1c and other GV indicators differed in PDN patients, another notable finding from the present study was that the levels of TIR were significantly decreased among patients with pain, particularly in the moderate/severe pain group. Furthermore, the NRS score, TCSS score and percentage of abnormal NCT were significantly higher in the groups of decreased TIR quartiles than that in the upper TIR quartile group. Furthermore, the prevalence rates of both mild pain and moderate/severe pain were the highest in the lowest TIR quartiles. These results indicated that the level of TIR might be related to the prevalence and degree of PDN.
As a GV indicator of the CGM system, TIR measurements add valuable information to assessing the degree of current glycemic control in addition to what is known from the HbA1c 12 . TIR is one of the commonly used standardized parameters of clinical outcome in the consensuses regarding type 1 diabetes mellitus published by several American medical societies 24 . Meanwhile, an online survey of type 1 diabetes mellitus, insulin‐treated type 2 diabetes mellitus and insulin‐free type 2 diabetes mellitus patients showed that TIR, which centers on glucose, was more or equally important than HbA1c 25 .
Recently, increasing clinical data suggest that GV contributes to the development of microvascular complications of diabetes 13 , 26 , and TIR is associated with diabetic retinopathy, carotid intima‐media thickness and diabetic cardiovascular autonomic neuropathy in type 2 diabetes mellitus patients 3 , 26 , 27 . In the present study, we found that the level of TIR was negatively associated with the NRS score. Furthermore, a decline in TIR quartile was associated with an increase in the risk of any pain and moderate/severe pain independent of the HbA1c level. To our knowledge, this is the first study to correlate TIR with PDN in diabetes patients.
GV was also influenced by several factors, such as medicine and β‐cell function 4 , 27 . We evaluated the factors that might be related to the TIR level, and found that its level was negatively associated with sex, age, diabetes mellitus duration, FPG, HbA1c, treatment with both OHA and insulin, and drinking. In contrast, the TIR was positively associated with OHA monotherapy. This might be because patients treated with OHA only had better islet function than those treated with OHA plus insulin. To reduce the influence of these factors on the results in the regression model, we adjusted for the covariates, and the results showed that TIR was still associated with PDN independent of HbA1c level and other risk factors among diabetes patients.
The present study assessed not only TIR as an index of GV, but also SD, CV and MAGE. Although the effects of SD and MAGE on the development of DPN and PDN have been suggested in recent years 27 , 28 , 29 , the results are still controversial. One study found that increasing MAGE was a significant independent contributor to DPN in type 2 diabetes mellitus patients 27 . In contrast, another study showed that MAGE and SD were not associated with DPN 29 . The conflicting results might be attributable to the different diagnostic criteria of DPN and different types of diabetes assessed. In the present study, we found that the level of SD and MAGE were more higher among patients with PDN. After adjusting for these factors in the multiple linear regression models, TIR was still negatively correlated with the NRS score. Furthermore, decreased TIR remained a risk factor for PDN independent of SD, CV and MAGE. Collectively, these results support that the level of TIR was associated with PDN independent of other GV metrics.
In the present study, the flash glucose‐sensing system was used, and TIR was obtained from the comprehensive glucose data without the need for user (finger prick) calibration. This system has been reported to be safer and more accurate, even in pregnant women with diabetes, compared with self‐monitoring of blood glucose 30 . Another multicenter, randomized controlled trial also showed that using the flash glucose‐sensing technology among type 2 diabetes mellitus patients on intensive insulin therapy does not cause significant changes in HbA1c level or lower risk of hypoglycemia, thus offering a safe and effective alternative for self‐monitoring of blood glucose 31 . A retrospective pilot study also showed that the flash glucose‐sensing system accurately reflected an improvement in TIR in type 1 diabetes mellitus patients on sodium–glucose cotransporter 2 inhibitors 16 . The results of these studies support that the flash glucose‐sensing system provides comprehensive and accurate data.
The present study had several limitations. First, the cross‐sectional design did not allow us to explore the temporal relationship between the TIR level and the development of PDN; therefore, further longitudinal studies are required. Second, there were unavoidable biases associated with patient selection, the information obtained and the confounding variables, as our participants were enrolled from a single center.
In conclusion, we found that TIR is correlated with PDN independent of the HbA1c level, other GV metrics and risk factors in diabetes patients. The current study also emphasized that TIR was a valuable clinical evaluation indicator for patients with diabetes.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81970705); Central Plains Thousand Talents Plan (204200510026); the Overseas Research and Study Program for Talents in Health Science and Technology of Henan Province (2018078, 2018098).
J Diabetes Investig 2021; 12: 828–836
References
- 1. Atlas ID: International Diabetes Federation, 2017. [DOI] [PubMed]
- 2. Pop‐Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hebert HL, Veluchamy A, Torrance N, et al. Risk factors for neuropathic pain in diabetes mellitus. Pain 2017; 158: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang J, Zhao Z, Yuan H, et al. The mechanisms of glycemic variability accelerate diabetic central neuropathy and diabetic peripheral neuropathy in diabetic rats. Biochem Biophys Res Commun 2019; 510: 35–41. [DOI] [PubMed] [Google Scholar]
- 5. Alkhatatbeh M, Abdul‐Razzak KK. Neuropathic pain is not associated with serum vitamin D but is associated with female gender in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care 2019; 7: e000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abbott CA, Malik RA, van Ross ER, et al. Prevalence and characteristics of painful diabetic neuropathy in a large community‐based diabetic population in the U.K. Diabetes Care 2011; 34: 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464–1476. [DOI] [PubMed] [Google Scholar]
- 9. Jung HS. Clinical implications of glucose variability: chronic complications of diabetes. Endocrinol Metab 2015; 30: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pai YW, Lin CH, Lee IT, et al. Variability of fasting plasma glucose and the risk of painful diabetic peripheral neuropathy in patients with type 2 diabetes. Diabetes Metab 2018; 44: 129–134. [DOI] [PubMed] [Google Scholar]
- 11. Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta‐analysis of randomized controlled trials. Diabetes Care 2020; 43: 1146–1156. [DOI] [PubMed] [Google Scholar]
- 12. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017; 40: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018; 41: 2370–2376. [DOI] [PubMed] [Google Scholar]
- 14. Guo Q, Zang P, Xu S, et al. Time in range, as a novel metric of glycemic control, is reversely associated with presence of diabetic cardiovascular autonomic neuropathy independent of HbA1c in Chinese type 2 diabetes. J Diabetes Res 2020; 2020: 5817074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arumugam T, Razali SNO, Vethakkan SR, et al. Relationship between ultrasonographic nerve morphology and severity of diabetic sensorimotor polyneuropathy. Eur J Neurol 2016; 23: 354–360. [DOI] [PubMed] [Google Scholar]
- 16. Themistocleous AC, Ramirez JD, Shillo PR, et al. The Pain in Neuropathy Study (PiNS): a cross‐sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain 2016; 157: 1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki D, Yamada H, Yoshida M, et al. Sodium‐glucose cotransporter 2 inhibitors improved time‐in‐range without increasing hypoglycemia in Japanese patients with type 1 diabetes: a retrospective, single‐center, pilot study. J Diabetes Investig 2020; 11: 1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang L, Lin P, Ma A, et al. C‐peptide is independently associated with an increased risk of coronary artery disease in T2DM subjects: a cross‐sectional study. PLoS One 2015; 10: e0127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Javed S, Hayat T, Menon L, et al. Diabetic peripheral neuropathy in people with type 2 diabetes: too little too late. Diabetic Med 2020; 37: 573–579. [DOI] [PubMed] [Google Scholar]
- 20. Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diabetes Rep 2019; 19: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pai YW, Tang CL, Lin CH, et al. Glycaemic control for painful diabetic peripheral neuropathy is more than fasting plasma glucose and glycated haemoglobin. Diabetes Metab 2020. 10.1016/j.diabet.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 22. Algeffari MA. Painful diabetic peripheral neuropathy among Saudi diabetic patients is common but under‐recognized: multicenter cross‐sectional study at primary health care setting. J Family Community Med 2018; 25: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009; 35: 206–213. [DOI] [PubMed] [Google Scholar]
- 24. Agiostratidou G, Anhalt H, Ball D, et al. Standardizing Clinically Meaningful Outcome Measures Beyond HbA1c for Type 1 Diabetes: A Consensus Report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017; 40:1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Runge AS, Kennedy L, Brown AS, et al. Does time‐in‐range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes 2018; 36: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima‐media thickness in type 2 diabetes. Diabetes Technol Ther 2020; 22: 72–78. [DOI] [PubMed] [Google Scholar]
- 27. Hu YM, Zhao LH, Zhang XL, et al. Association of glycaemic variability evaluated by continuous glucose monitoring with diabetic peripheral neuropathy in type 2 diabetic patients. Endocrine 2018; 60: 292–300. [DOI] [PubMed] [Google Scholar]
- 28. Oyibo SO, Prasad YD, Jackson NJ, et al. The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: a pilot study. Diabetic Med 2002; 19: 870–873. [DOI] [PubMed] [Google Scholar]
- 29. Zhang C, Tang M, Lu X, et al. Relationship of ankle‐brachial index, vibration perception threshold, and current perception threshold to glycemic variability in type 2 diabetes. Medicine 2020; 99: e19374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scott EM, Bilous RW, Kautzky‐Willer A. Accuracy, user acceptability, and safety evaluation for the FreeStyle libre flash glucose monitoring system when used by pregnant women with diabetes. Diabetes Technol Ther 2018; 20: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haak T, Hanaire H, Ajjan R, et al. Flash glucose‐sensing technology as a replacement for blood glucose monitoring for the management of insulin‐treated type 2 diabetes: a multicenter, open‐label randomized controlled trial. Diabetes Ther 2017; 8: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


