Abstract
Aims/Introduction
To identify thresholds for postprandial hyperglycemia and hypertriglyceridemia predictive of all‐cause mortality in patients with type 2 diabetes.
Materials and Methods
A total of 1,928 patients with type 2 diabetes visited our clinic for the first time from 1995 to 1999 and were followed up for ≥1 year. During the first year, 2‐h post‐breakfast blood glucose (2h‐BG) levels were measured in 1,122 patients (BG cohort) and postprandial serum triglyceride (ppTG) levels were measured in 1,826 patients (TG cohort). Patients were retrospectively followed until 2017 and administered questionnaires. Associations between 2h‐BG and ppTG levels and mortality risk were assessed by the multivariate Cox regression analysis.
Results
Over of 17,429 person‐years, 162 deaths occurred in the BG cohort, and over 28,026 person‐years, 253 deaths occurred in the TG cohort. Hazard ratios (HRs) with 95% confidence intervals for all‐cause mortality per 1‐standard deviation increases in 2h‐BG and ppTG were 1.34 (1.08–1.67) and 1.24 (1.06–1.45), respectively. HRs showed increasing trends across quintiles of 2h‐BG (P = 0.034) and ppTG (P = 0.007). The HR was significantly elevated (2.37, 1.26–4.47) in the fifth quintile of 2h‐BG (≥13.8 mmol/L) compared with the first quintile (<7.0 mmol/L; P = 0.008). The HR was also significantly elevated (1.63, 1.03–2.60) in the fifth quintile of ppTG (≥2.30 mmol/L) compared with the first quintile (<0.91 mmol/L; P = 0.038).
Conclusions
Postprandial hyperglycemia and hypertriglyceridemia were associated with all‐cause mortality in patients with type 2 diabetes. We propose thresholds of 13.8 mmol/L 2h‐BG and 2.30 mmol/L ppTG to identify patients at increased risk of mortality.
Keywords: Non‐fasting hypertriglyceridemia, Postprandial hyperglycemia, Type 2 diabetes mortality
Postprandial hyperglycemia and hypertriglyceridemia at clinic visits were associated with an increased risk of all‐cause mortality in real‐world patients with type 2 diabetes. We propose 2‐h post‐breakfast blood glucose levels of 13.8 mmol/L and postprandial serum triglyceride levels of 2.30 mmol/L as estimated thresholds to identify patients at increased risk of all‐cause mortality.2h‐BG, 2‐h post‐breakfast blood glucose; ppTG, postprandial serum triglyceride.

Introduction
Several epidemiological studies have reported associations between postprandial, but not post‐load, hyperglycemia and adverse events in diabetes patients 1 , 2 , 3 , 4 , 5 , 6 , 7 . Our previous studies showed that postprandial hyperglycemia at clinic visits was associated with the incidence of diabetic retinopathy, cardiovascular disease (CVD), all‐cause mortality and cancer mortality 5 , 6 , 7 . Postprandial hyperglycemia has negative impacts on surrogate measures, such as endothelial dysfunction, in diabetes patients. For many non‐pregnant adults with diabetes, glycemic recommendations are a glycated hemoglobin A1c (HbA1c) level of <7% (53 mmol/mol), preprandial capillary plasma glucose levels of 4.4‒7.2 mmol/L and peak postprandial capillary plasma glucose levels of 10.0 mmol/L 8 . Capillary plasma glucose levels that seem to correlate with achieving an HbA1c level of <7% have been determined as the recommended target 8 . However, the threshold for postprandial capillary plasma glucose levels that is predictive of adverse events has not been determined in diabetes patients.
Multiple epidemiological studies have evaluated associations between postprandial hypertriglyceridemia and adverse events in the general population 9 , 10 , 11 , 12 , 13 , 14 , 15 . Various panels have proposed different cut‐offs for non‐fasting hypertriglyceridemia; these include 2.26 mmol/L by the American Heart Association 16 , 2.03 mmol/L by the Athens Expert Panel 17 and 1.98 mmol/L by the European Atherosclerosis Society 18 . A study of participants in the Women’s Health Study also recommended a diagnostic threshold of 1.98 mmol/L for non‐fasting hypertriglyceridemia 19 . However, no studies have assessed the relationship between postprandial hypertriglyceridemia and mortality risk in diabetes patients.
Here, we assessed the relationships between all‐cause mortality and postprandial hyperglycemia and hypertriglyceridemia in patients with type 2 diabetes in a real‐world setting. We estimated thresholds for postprandial glycemia and triglyceridemia that are predictive of all‐cause mortality.
Methods
Definition of postprandial glycemia and triglyceridemia
Capillary blood glucose (BG) levels were measured as previously reported 5 , 6 , 7 . BG levels were presented as plasma equivalents. Laboratory technologists checked when the patients had eaten their last meal, and postprandial time intervals were recorded every 15 min. Postprandial glycemia was defined as 2‐h post‐breakfast BG (2h‐BG) levels, which were measured 2 h ± 30 min after breakfast. Lipid levels were measured every few visits using serum prepared from venous blood regardless of fasting or postprandial conditions. Triglyceride (TG) levels were measured by an enzymatic method. Non‐fasting serum TG levels were defined as postprandial TG (ppTG) levels. The first observations taken in the first year were taken as the baseline 2h‐BG and ppTG levels.
Study participants
Figure 1 shows a flowchart of the study cohorts. A total of 1,928 patients with type 2 diabetes visited the clinic at the Institute for Adult Diseases, Asahi Life Foundation, Tokyo, Japan, for the first time between 1995 and 1999, and were followed up for ≥1 year. During the first year, 2h‐BG levels were measured in 1,130 patients, and ppTG levels were measured in 1,848 patients. After excluding patients with missing data, 1,122 patients (BG cohort) and 1,826 patients (TG cohort) were retrospectively followed up until 2017, and provided with questionnaires.
Figure 1.
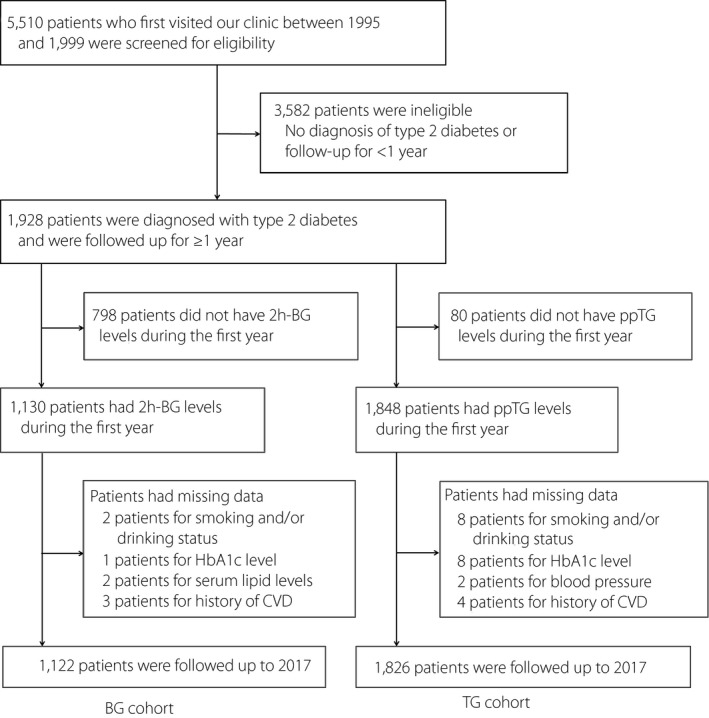
Flowchart of the study cohorts. 2h‐BG, 2‐h post‐breakfast blood glucose; BG, blood glucose; CVD, cardiovascular disease; HbA1c, glycated hemoglobin A1c; ppTG, postprandial serum triglyceride; TG, triglyceride.
The ethics committees of the Institute for Adult Diseases, Asahi Life Foundation approved the study protocol, which followed the Japanese Government’s Ethical Guidelines for Medical and Health Research Involving Human Subjects, and was carried out in accordance with the principles laid out in the Declaration of Helsinki.
End‐point definition
The primary end‐point was all‐cause mortality. The end‐point was determined by a thorough review of medical charts, recorded by attending physicians and from the questionnaire replies 20 . Questionnaires were sent to patients in whom their outcomes could not be confirmed, even after scrutinizing the medical records written by the attending physicians. Patients who did not respond to the first questionnaire were mailed the questionnaire again after about 2 months. The end‐points were then collected as much as possible. Patients lost to follow up were regarded as censored cases on the last day of their clinic visits.
Assessment of covariates
Covariates included HbA1c levels, blood pressure (BP), total cholesterol (TC) levels and high‐density lipoprotein cholesterol (HDL‐C) levels, as previously reported 21 . For data as covariates, values simultaneously measured or most immediately measured as the initial measurement of 2h‐BG and ppTG levels were taken as the baseline. Alcohol drinking was defined as ≥20 g/day.
Statistical analysis
TG levels were analyzed by converting them to a natural logarithm scale. Comparisons of baseline characteristics between the study participants with and without complete follow up were made by the Student’s t‐test, Wilcoxon rank‐sum test, the χ2‐test, and Fisher’s exact test, as appropriate. Baseline characteristics of the BG and TG cohorts stratified by quintiles of 2h‐BG and ppTG levels were compared by analysis of variance, the Kruskal–Wallis test and the Cochran–Armitage trend test.
Multivariate Cox regression analyses were carried out to evaluate the relationships between mortality risk and quintiles of 2h‐BG and ppTG levels. P‐values for linear trends and quadratic trends were calculated by stratification according to 2h‐BG or ppTG quintiles. Covariates included age, sex, diabetes duration, body mass index, systolic BP, HbA1c levels, TC levels, HDL‐C levels, current smoking, alcohol intake, past history of CVD, and past history of cancer. Additionally, changes in mortality risk related to 1‐standard deviation (SD) increments in 2h‐BG and ppTG levels were also assessed. In addition, users of oral antidiabetic drugs, insulin, antihypertensive agents and agents for dyslipidemia were also added as covariates. Kaplan–Meier curves were generated for patients who were stratified using the estimated thresholds for 2h‐BG and ppTG levels derived from analyses of their quintiles. All analyses were carried out using SAS packages version 9.4 (SAS Institute, Cary, NC, USA). Two‐sided values of P < 0.05 were considered statistically significant.
Results
The median (interquartile range) follow‐up periods were 18.7 years (10.1–20.7 years) in the BG cohort, and 18.6 years (9.2–20.7 years) in the TG cohort. The percentages of patients completing follow up were 68.4% (767/1,122) in the BG cohort, and 66.7% (1,218/1,826) in the TG cohort. Table S1 shows differences in the baseline characteristics between patients with and without complete follow up. In the BG cohort, the percentages of men and alcohol consumers were significantly higher in patients who completed (81.9 and 60.5%, respectively) than in those who did not complete (74.4 and 50.4%, respectively) follow up (P = 0.004 and P = 0.002, respectively). Similarly, in the TG cohort, the percentages of men and alcohol consumers were significantly higher in patients who completed (81.9 and 61.7%, respectively) than in those who did not complete (76.6 and 54.0%, respectively) follow up (P = 0.008 and P = 0.001, respectively). No significant differences were observed in any other variables (Table S1).
Baseline findings in the BG and TG cohorts
Table 1 summarizes the baseline characteristics of the BG and TG cohorts, as a whole, and stratified by quintiles of 2h‐BG and ppTG levels (natural logarithm). Higher 2h‐BG levels were associated with younger age, longer diabetes duration, higher systolic BP and diastolic BP, higher HbA1c levels, higher TC levels, lower HDL‐C levels, and higher estimated glomerular filtration rate. Higher 2h‐BG levels were also associated with male sex, current smoking, use of oral antidiabetic drugs and use of insulin. Higher ppTG levels were associated with higher body mass index, higher systolic BP and diastolic BP, higher HbA1c levels, higher TC levels, and lower HDL‐C levels. Higher ppTG levels were also associated with male sex, current smoking, history of CVD, use of oral antidiabetic drugs, antihypertensive agents and agents for dyslipidemia (Table 1).
Table 1.
Baseline characteristics of the BG and TG cohorts stratified by quintiles of 2‐h post‐breakfast blood glucose and postprandial serum triglyceride levels
| BG cohort | Quintiles of 2h‐BG levels (mmol/L) | TG cohort total | Quintiles of ppTG † levels (mmol/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Q 1 < 7.0 | Q 2 7.0–8.7 | Q 3 8.7–10.7 | Q 4 10.7–13.8 | Q 5 ≥ 13.8 | P‐value | Q 1 < 0.91 | Q 2 0.91–1.22 | Q 3 1.22–1.61 | Q 4 1.61–2.30 | Q 5 ≥ 2.30 | P‐value | ||
| n | 1,122 | 220 (19.6) | 224 (20.0) | 226 (20.1) | 226 (20.1) | 226 (20.1) | 1,826 | 360 (19.7) | 369 (20.2) | 361 (19.8) | 364 (19.9) | 372 (20.4) | ||
| Men (%) | 892 (79.5) | 162 (73.6) | 175 (78.1) | 179 (79.2) | 188 (83.2) | 188 (83.2) | 0.005 | 1,464 (80.2) | 272 (75.6) | 287 (77.8) | 307 (85.0) | 289 (79.4) | 309 (83.1) | 0.012 |
| Age (years) | 55.9 ± 10.1 | 55.5 ± 11.0 | 57.6 ± 9.6 | 55.3 ± 9.4 | 56.2 ± 10.0 | 54.7 ± 10.5 | 0.034 | 55.6 ± 10.0 | 55.2 ± 9.6 | 57.0 ± 9.5 | 55.3 ± 9.9 | 55.2 ± 10.4 | 55.1 ± 10.3 | 0.058 |
| Duration of diabetes (years) | 6.1 ± 7.0 | 3.9 ± 6.0 | 5.0 ± 7.0 | 6.0 ± 6.6 | 7.5 ± 7.1 | 7.7 ± 7.7 | <0.0001 | 6.1 ± 7.0 | 6.2 ± 7.1 | 6.3 ± 7.4 | 6.0 ± 7.2 | 5.9 ± 6.6 | 6.1 ± 6.9 | 0.98 |
| BMI (kg/m2) | 23.1 ± 3.3 | 22.6 ± 3.5 | 22.9 ± 3.0 | 23.3 ± 3.3 | 23.2 ± 3.3 | 23.4 ± 3.4 | 0.066 | 23.2 ± 3.4 | 21.9 ± 3.2 | 22.8 ± 3.1 | 23.5 ± 3.6 | 23.8 ± 3.3 | 24.3 ± 3.2 | <0.0001 |
| SBP (mmHg) | 127.6 ± 19.3 | 122.2 ± 18.1 | 127.4 ± 18.6 | 129.0 ± 18.2 | 128.4 ± 20.4 | 130.9 ± 19.9 | <0.0001 | 130.1 ± 20.2 | 125.8 ± 19.2 | 129.2 ± 21.2 | 129.7 ± 20.5 | 131.3 ± 20.3 | 134.2 ± 19.0 | <0.0001 |
| DBP (mmHg) | 73.1 ± 11.3 | 70.4 ± 10.7 | 72.8 ± 10.9 | 73.9 ± 10.6 | 73.5 ± 12.4 | 75.1 ± 11.4 | 0.0003 | 75.0 ± 11.6 | 72.2 ± 10.8 | 74.3 ± 11.8 | 75.3 ± 12.1 | 75.3 ± 12.0 | 77.6 ± 10.7 | <0.0001 |
| 2h‐BG (mmol/L) | 10.6 ± 4.4 | 6.0 ± 0.7 | 7.8 ± 0.5 | 9.6 ± 0.6 | 12.1 ± 0.9 | 17.6 ± 3.6 | <0.0001 | – | – | – | – | – | – | – |
| ppTG † (mmol/L) | – | – | – | – | – | – | – | 1.48 ± 1.76 | 0.72 ± 1.21 | 1.06 ± 1.09 | 1.39 ± 1.08 | 1.91 ± 1.10 | 3.38 ± 1.43 | <0.0001 |
| HbA1c (%; mmol/mol) | 7.3 ± 1.4 (57 ± 16) | 6.4 ± 0.8 (47 ± 9) | 6.7 ± 0.8 (50 ± 9) | 7.1 ± 1.0 (54 ± 11) | 7.5 ± 1.0 (58 ± 11) | 9.0 ± 1.6 (75 ± 17) |
<0.0001 |
7.9 ± 1.6 (63 ± 18) | 7.8 ± 1.6 (61 ± 17) | 7.7 ± 1.6 (61 ± 18) | 8.0 ± 1.7 (64 ± 19) | 7.8 ± 1.6 (62 ± 18) | 8.1 ± 1.7 (65 ± 18) | 0.007 |
| TC (mmol/L) | 5.42 ± 0.96 | 5.33 ± 0.91 | 5.47 ± 0.93 | 5.46 ± 1.03 | 5.30 ± 0.93 | 5.53 ± 0.99 | 0.0496 | 5.52 ± 1.01 | 5.07 ± 0.90 | 5.32 ± 0.84 | 5.49 ± 0.99 | 5.73 ± 0.95 | 5.99 ± 1.10 | <0.0001 |
| HDL‐C (mmol/L) | 1.35 ± 0.37 | 1.42 ± 0.38 | 1.40 ± 0.39 | 1.38 ± 0.39 | 1.24 ± 0.31 | 1.29 ± 0.33 | <0.0001 | 1.33 ± 0.36 | 1.54 ± 0.39 | 1.41 ± 0.36 | 1.30 ± 0.32 | 1.24 ± 0.30 | 1.15 ± 0.25 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 78.8 ± 17.9 | 77.1 ± 17.8 | 75.0 ± 17.7 | 79.0 ± 17.9 | 80.2 ± 15.6 | 82.6 ± 19.7 | <0.0001 | 79.1 ± 18.1 | 80.9 ± 17.7 | 78.5 ± 17.4 | 79.1 ± 17.0 | 78.7 ± 18.3 | 78.4 ± 19.8 | 0.35 |
| Current smoker | 469 (41.8) | 84 (38.2) | 84 (37.5) | 79 (35.0) | 108 (47.8) | 114 (50.4) | 0.0008 | 752 (41.2) | 121 (33.6) | 146 (39.6) | 152 (42.1) | 152 (41.8) | 181 (48.7) | <0.0001 |
| Alcohol intake | 643 (57.3) | 112 (50.9) | 133 (59.4) | 131 (58.0) | 138 (61.1) | 129 (57.1) | 0.19 | 1080 (59.2) | 203 (56.4) | 214 (58.0) | 214 (59.3) | 220 (60.4) | 229 (61.6) | 0.12 |
| History of CVD | 7 (0.6) | 1 (0.5) | 3 (1.4) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 0.57 | 11 (0.6) | 0 (0.0) | 1 (0.3) | 3 (0.8) | 2 (0.6) | 5 (1.3) | 0.020 |
| History of cancer | 13 (1.2) | 4 (1.8) | 5 (2.2) | 1 (0.4) | 1 (0.4) | 2 (0.9) | 0.11 | 23 (1.3) | 6 (1.7) | 8 (2.2) | 3 (0.8) | 3 (0.8) | 3 (0.8) | 0.096 |
| Initial therapies | ||||||||||||||
| Oral antidiabetic drugs alone | 456 (40.6) | 67 (30.5) | 75 (33.5) | 85 (37.6) | 108 (47.8) | 121 (53.5) | <0.0001 | 762 (41.7) | 139 (38.6) | 147 (39.8) | 149 (41.3) | 150 (41.2) | 177 (47.6) | 0.017 |
| Insulin | 180 (16.0) | 34 (15.5) | 16 (7.1) | 29 (12.8) | 35 (15.5) | 66 (29.2) | <0.0001 | 256 (14.0) | 51 (14.2) | 51 (13.8) | 47 (13.0) | 46 (12.6) | 61 (16.4) | 0.56 |
| Antihypertensive agents | 237 (21.1) | 33 (15.0) | 55 (24.6) | 50 (22.1) | 43 (19.0) | 56 (24.8) | 0.11 | 378 (20.7) | 50 (13.9) | 67 (18.2) | 72 (19.9) | 86 (23.6) | 103 (27.8) | <0.0001 |
| Lipid‐lowering agents | 138 (12.3) | 29 (13.2) | 33 (14.7) | 27 (12.0) | 19 (8.4) | 30 (13.3) | 0.38 | 224 (12.3) | 19 (5.3) | 40 (10.8) | 36 (10.0) | 58 (15.9) | 71 (19.1) | <0.0001 |
The 2‐h post‐breakfast blood glucose (2h‐BG) and postprandial serum triglyceride (ppTG) levels initially measured during the 1‐year period starting at the first visit were used as the baseline.
Clinical data measured at the same time or most immediately measured as the first measurements of 2h‐BG or ppTG were used as the baseline.
BG, blood glucose; BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Values represent n (%) or means ± standard deviations.
Ln‐transformed.
Associations between 2h‐BG and ppTG levels and risk of all‐cause mortality
Over 17,429 person‐years, 162 deaths occurred in the BG cohort, and over 28,026 person‐years, 253 deaths occurred in the TG cohort. The crude incidence ratios (per 1,000 person‐years) were 9.29 (95% confidence interval [CI] 3.68–14.90) in the BG cohort, and 9.03 (95% CI 4.69–13.37) in the TG cohort.
Adjusted hazard ratios (HRs) for all‐cause mortality in each quintile of 2h‐BG and ppTG levels are shown in Table 2. The HRs showed increasing trends across quintiles of 2h‐BG (P for linear trend = 0.034) and ppTG (P for linear trend = 0.007). The HR was significantly elevated (2.37, 95% CI 1.26–4.47) in the fifth quintile (≥13.8 mmol/L) of the 2h‐BG levels compared with the first quintile (<7.0 mmol/L; P = 0.008). The HR was significantly elevated (1.63, 95% CI 1.03–2.60) in the fifth quintile (≥2.30 mmol/L) of the ppTG levels compared with the first quintile (<0.91 mmol/L; P = 0.038; Table 2). The second to fourth quintiles of 2h‐BG and ppTG levels were not significantly different compared with the first quintile. The second quintile of 2h‐BG and ppTG levels had the lowest HRs, but P‐values for the quadratic trend were not significant. Covariates are described in the statistical analysis section. These relationships are plotted in Figure 2.
Table 2.
Adjusted hazard ratios for all‐cause mortality in each quintile of 2‐h post‐breakfast blood glucose and postprandial serum triglyceride levels
| 2h‐BG (mmol/L) Quintiles | Q 1 < 7.0 | Q 2 7.0–8.7 | Q 3 8.7–10.7 | Q 4 10.7–13.8 | Q 5 ≥ 13.8 | P for linear trend | P for quadratic trend |
|---|---|---|---|---|---|---|---|
| No. of events | 26 | 29 | 34 | 28 | 45 | ||
| No. at risk | 220 | 224 | 226 | 226 | 226 | ||
| Person‐years | 3,507.73 | 3,434.87 | 3,665.16 | 3,511.70 | 3,309.48 | ||
| Incidence rate‡ | 7.4 | 8.4 | 9.3 | 8.0 | 13.6 | ||
| HR (95% CI) | 1 (Reference) | 0.97 (0.56–1.68) | 1.28 (0.74–2.21) | 1.01 (0.56–1.80) | 2.37 (1.26–4.47) | 0.034 | 0.067 |
| ppTG † (mmol/L) Quintiles | Q1 < 0.9 | Q2 0.91– 1.22 | Q3 1.22– 1.61 | Q4 1.61– 2.30 | Q5 ≥ 2.30≥ 2.30 | P for linear trend | P for quadratic trend |
|---|---|---|---|---|---|---|---|
| No. of events | 45 | 46 | 44 | 49 | 69 | ||
| No. at risk | 360 | 369 | 361 | 364 | 372 | ||
| Person‐years | 5,645.69 | 5,718.07 | 5,591.42 | 5,484.49 | 5,588.37 | ||
| Incidence rate‡ | 8.0 | 8.0 | 7.9 | 8.9 | 12.3 | ||
| HR (95% CI) | 1 (reference) | 0.88 (0.57–1.35) | 1.01 (0.64–1.59) | 1.24 (0.78–1.98) | 1.63 (1.03–2.60) | 0.007 | 0.10 |
Models were adjusted for age, sex, diabetes duration, body mass index, systolic blood pressure, HbA1c levels, total cholesterol levels, high‐density lipoprotein cholesterol levels, current smoking, alcohol intake, history of cardiovascular disease and history of cancer.
2h‐BG, 2‐h post‐breakfast blood glucose; CI, confidence interval; HR, hazard ratio; ppTG, postprandial serum triglyceride; Q, quintile.
Ln‐transformed. ‡Crude incidence rate per 1,000 person‐years.
Figure 2.
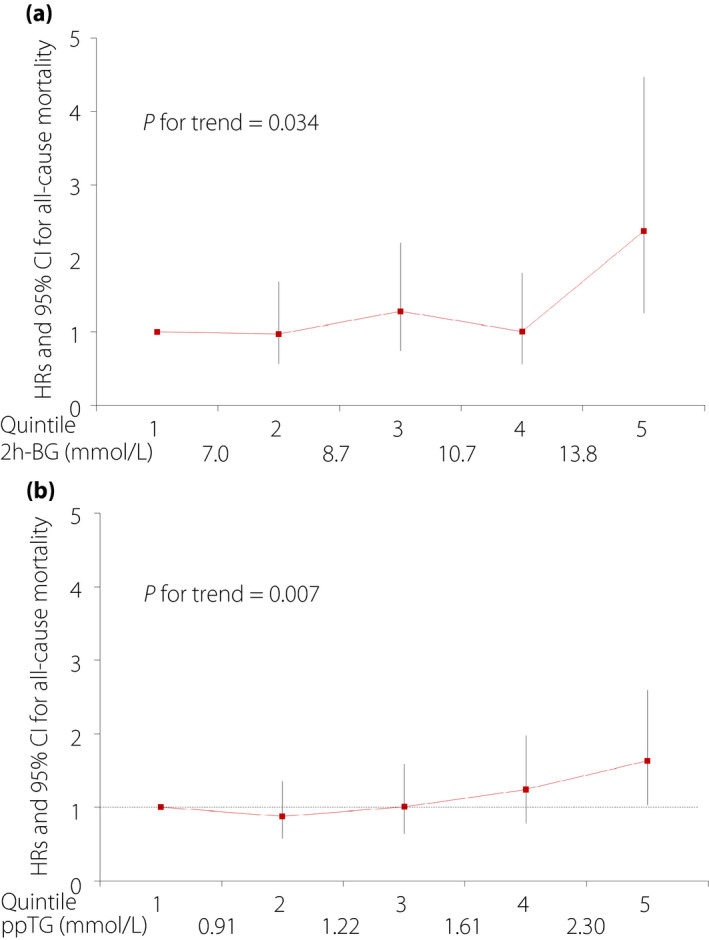
Hazard ratios (HRs) for all‐cause mortality by quintiles of (a) 2‐h post‐breakfast blood glucose (2h‐BG) levels and (b) postprandial serum triglyceride (ppTG) levels, adjusted for age, sex, diabetes duration, body mass index, systolic blood pressure, HbA1c levels,, total cholesterol levels, high‐density lipoprotein cholesterol levels, current smoking, alcohol intake, history of cardiovascular disease and history of cancer.
HRs for all‐cause mortality per 1‐SD increases in 2h‐BG and ppTG levels were 1.34 (95% CI 1.08–1.67) and 1.24 (95% CI 1.06–1.45), respectively, after adjusting for the same covariates (models 1 and 2 in Table 3). After further adjusting for users of oral antidiabetic drugs, insulin, antihypertensive agents and agents for dyslipidemia, HRs per 1‐SD increases in 2h‐BG and ppTG levels were 1.34 (95% CI 1.07–1.68) and 1.18 (95% CI 1.00–1.39), respectively (models 3 and 4 in Table 3). Therefore, similar results were obtained when the effects of drug administration were further adjusted. Consequently, the present results were independent of drug administration. Additionally, no interaction was observed between 2h‐BG and HbA1c levels (P = 0.69).
Table 3.
Multivariate Cox proportional hazards models of the associations between all‐cause mortality and 2‐h post‐breakfast blood glucose and postprandial serum triglyceride levels
| BG cohort (events/patients 162/1,122) | TG cohort (events/patients 253/1,826) | ||||
|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | ||
| Model 1 | Model 2 | ||||
| 2h‐BG (1 SD; mmol/L) | 1.34 (1.08–1.67) | 0.009 | ppTG † (1 SD; mmol/L) | 1.24 (1.06–1.45) | 0.008 |
| Model 3 | Model 4 | ||||
| 2h‐BG (1 SD; mmol/L) | 1.34 (1.07–1.68) | 0.010 | ppTG † (1 SD; mmol/L) | 1.18 (1.00–1.39) | 0.045 |
Models 1 and 2 were adjusted for age, sex, diabetes duration, body mass index, systolic blood pressure, HbA1c levels, total cholesterol levels, high‐density lipoprotein cholesterol levels, current smoking, alcohol intake, history of cardiovascular disease and history of cancer.
Models 3 and 4 were adjusted for the use of oral antidiabetic drugs, insulin, antihypertensive agents and agents for dyslipidemia in addition to the covariates included in models 1 and 2.
Ln‐transformed. 2h‐BG, 2‐h post‐breakfast blood glucose; ppTG, postprandial serum triglyceride; SD, standard deviation.
Kaplan–Meier curves in patients stratified using the estimated thresholds for 2h‐BG and ppTG levels
The BG and TG cohorts were stratified using the estimated 2h‐BG threshold of 13.8 mmol/L and the estimated ppTG threshold of 2.30 mmol/L, respectively. Kaplan–Meier curves in patients who were stratified using these 2h‐BG and ppTG thresholds showed increasing risk separation from approximately 2 and 5 years, respectively (Figure 3).
Figure 3.
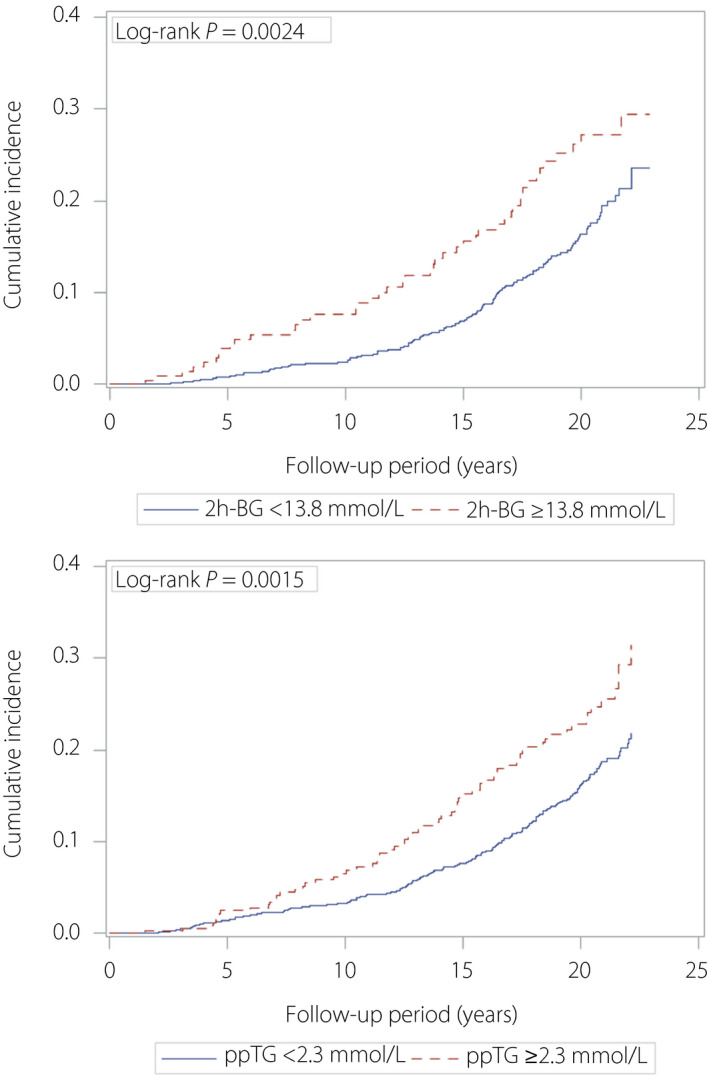
Kaplan–Meier curves in patients stratified using the estimated thresholds for 2‐h post‐breakfast blood glucose (2h‐BG) and postprandial serum triglyceride (ppTG) levels.
Discussion
Here, we showed that postprandial hyperglycemia and hypertriglyceridemia at clinic visits were associated with an increased risk of all‐cause mortality in real‐world patients with type 2 diabetes. We propose approximate thresholds of 13.8 mmol/L for 2h‐BG and 2.30 mmol/L for ppTG to identify patients at high risk of mortality.
Our previous study showed that postprandial hyperglycemia at clinic visits was associated with all‐cause mortality in patients with type 2 diabetes 6 , 7 . However, there is no evidence for a threshold of postprandial glycemia predictive of mortality in patients with type 2 diabetes. In this real‐world study, an estimated threshold for postprandial glycemia was identified.
Time in range (3.9–10.0 mmol/L), that is a new metric derived from continuous glucose monitoring, is associated with the risk of microvascular complications 22 , 23 . A strong correlation between time in range and HbA1c levels was observed, with a time in range of 70% equating to a HbA1c level of 7% 8 , 24 , 25 . Additionally, as targets for achieving control, time above range of >10.0 mmol/L was proposed for <25% of readings, and time above range of >13.9 mmol/L was proposed for <5% of readings. In the present study, the threshold for 2h‐BG predictive of mortality may correspond to a target of time above range for <5% of readings.
In a prospective study from the Danish general population, non‐fasting hypertriglyceridemia was associated with the incidence of myocardial infarction, ischemic heart disease and all‐cause mortality 9 . Later, after 31 years of follow up, the same study reported that non‐fasting hypertriglyceridemia was associated with mortality from CVD, cancer and other causes 10 . Non‐fasting hypertriglyceridemia was also associated with a 25‐year mortality risk from coronary heart disease in the Multiple Risk Factor Intervention Trial 11 . The Women’s Health Study showed that non‐fasting hypertriglyceridemia was associated with the incidence of CVD independent of classical risk factors, but fasting hypertriglyceridemia showed little independent association 12 . In a study of residents in four Japanese communities, non‐fasting triglyceride levels predicted the incidence of ischemic CVD 13 , 14 . The results of these studies support our results, but no previous study assessed the mortality risk related to non‐fasting hypertriglyceridemia in diabetes patients. To our knowledge, ours is the first study to show that non‐fasting hypertriglyceridemia was associated with all‐cause mortality in patients with type 2 diabetes.
Postprandial hypertriglyceridemia is associated with CVD risk, and is relevant to an increase in chylomicron remnant lipoproteins derived from the intestine 26 . In diabetes patients, remnant lipoprotein cholesterol levels persist high all day long, with the exception of a few hours before breakfast 27 . Insulin resistance accelerates the postprandial accumulation of remnant lipoproteins 28 , 29 . Therefore, the characterization of postprandial lipid profiles is important for patients with diabetes. Various cut‐offs for postprandial hypertriglyceridemia for prediction of adverse events were proposed in general populations 16 , 17 , 18 , 19 . However, there is no evidence for a threshold of postprandial hypertriglyceridemia predictive of mortality in diabetes patients. In this real‐world study, we identified an estimated threshold for postprandial triglyceridemia predictive of mortality in patients with type 2 diabetes. Further research is required to validate our results.
The present study’s strengths included the analysis of postprandial glycemia and triglyceridemia, as measured by monitoring in the outpatient clinic in a real‐life condition, and a long‐term follow up. Several potential limitations deserve consideration. First, this was a historical cohort study. Changes in measurement methods and self‐reported postprandial time intervals may have led to information bias. However, using linear regression equations from duplicate assays, data obtained using different measurement methods could be converted. The time when patients started eating their last meal was thoroughly assessed by a laboratory technician and postprandial time intervals were determined. Data were excluded if snacks between meals were consumed and if meals were consumed following hypoglycemia. Second, bias may have occurred because of relatively high losses to follow up. Significant differences in baseline characteristics between patients with and without complete follow up were identified for sex and alcohol consumption (Table S1). Third, almost half of the events were found in the questionnaire replies. In the BG and TG cohorts, a thorough review of medical charts, recorded by the attending physician, documented 82 (51%) and 124 (49%) deaths, respectively, and replies to questionnaires by family members confirmed 80 (49%) and 129 (51%) deaths, respectively. Fourth, 2h‐BG and ppTG levels were measured after an ordinary meal, without standardization of dietary intake. However, the present study aimed to evaluate the effects of postprandial hyperglycemia and hypertriglyceridemia at clinic visits on mortality using real‐world data. Therefore, standardization of dietary intake was not considered. Finally, the study participants were enrolled in a single Japanese clinic. Therefore, generalizability to other ethnicities may be limited.
In conclusion, postprandial hyperglycemia and hypertriglyceridemia at clinic visits were associated with an increased risk of all‐cause mortality in real‐world patients with type 2 diabetes. We propose 2h‐BG levels of 13.8 mmol/L and ppTG levels of 2.30 mmol/L as estimated thresholds to identify patients at increased risk of all‐cause mortality.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Comparison of baseline characteristics between patients who did and did not complete follow‐up in the BG and TG cohorts.
Acknowledgments
The authors thank all study participants for their cooperation. We also thank the staff of the General Affairs Department of the Institute for Adult Diseases, Asahi Life Foundation, for their support with the questionnaire surveys. No specific funding or grants were received for this work.
J Diabetes Investig 2021; 12: 886–893
References
- 1. Raz I, Ceriello A, Wilson PW, et al. Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial versus fasting/premeal glycemia. Diabetes Care 2011; 34: 1511–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: The diabetes intervention study, 11‐year follow‐up. Diabetologia 1996; 39: 1577–1583. [DOI] [PubMed] [Google Scholar]
- 3. Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: Lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006; 91: 813–819. [DOI] [PubMed] [Google Scholar]
- 4. Cavalot F, Pagliarino A, Valle M, et al. Postprandial blood glucose predicts cardiovascular events and all‐cause mortality in type 2 diabetes in a 14‐year follow‐up: Lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011; 34: 2237–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takao T, Takahashi K, Yoshida Y, et al. Effect of postprandial hyperglycemia at clinic visits on the incidence of retinopathy in patients with type 2 diabetes: An analysis using real‐world long‐term follow‐up data. J Diabetes Investig 2020; 11: 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takao T, Suka M, Yanagisawa H, et al. Impact of postprandial hyperglycemia at clinic visits on the incidence of cardiovascular events and all‐cause mortality in patients with type 2 diabetes. J Diabetes Investig 2017; 8: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takao T, Takahashi K, Suka M, et al. Association between postprandial hyperglycemia at clinic visits and all‐cause and cancer mortality in patients with type 2 diabetes: A long‐term historical cohort study in Japan. Diabetes Res Clin Pract 2019; 148: 152–159. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association . 6. Glycemic targets: Standards of medical care in diabetes‐2020. Diabetes Care 2020; 43(Suppl. 1): S66–S76. [DOI] [PubMed] [Google Scholar]
- 9. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007; 298: 299–308. [DOI] [PubMed] [Google Scholar]
- 10. Langsted A, Freiberg JJ, Tybjærg‐Hansen A, et al. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: The Copenhagen City Heart Study with 31 years of follow‐up. J Intern Med 2010; 270: 65–75. [DOI] [PubMed] [Google Scholar]
- 11. Eberly LE, Stamler J, Neaton JD, et al. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med 2003; 163: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 12. Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007; 298: 309–316. [DOI] [PubMed] [Google Scholar]
- 13. Iso H, Naito Y, Sato S, et al. Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am J Epidemiol 2001; 153: 490–499. [DOI] [PubMed] [Google Scholar]
- 14. Iso H, Imano H, Yamagishi K, et al. Fasting and non‐fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: The Circulatory Risk in Communities Study (CIRCS). Atherosclerosis 2014; 237: 361–368. [DOI] [PubMed] [Google Scholar]
- 15. Tada H, Nomura A, Yoshimura K, et al. Fasting and non‐fasting triglycerides and risk of cardiovascular events in diabetic patients under statin therapy. Circ J 2020; 84: 509–515. [DOI] [PubMed] [Google Scholar]
- 16. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: A scientific statement from the American heart association. Circulation 2011; 123: 2292–2333. [DOI] [PubMed] [Google Scholar]
- 17. Kolovou GD, Mikhailidis DP, Kovar J, et al. Assessment and clinical relevance of non‐fasting and postprandial triglycerides: An expert panel statement. Curr Vasc Pharmacol 2011; 9: 258–270. [DOI] [PubMed] [Google Scholar]
- 18. Hegele RA, Ginsberg HN, Chapman JM, et al. The polygenic nature of hypertriglyceridaemia: Implications for definition, diagnosis, and management. Lancet Diabetes Endocrin 2013; 13: 70191–70198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White KT, Moorthy MV, Akinkuolie AO, et al. Identifying an optimal cutpoint value for the diagnosis of hypertriglyceridemia in the nonfasting state. Clin Chem 2015; 61: 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takao T, Suka M, Yanagisawa H, et al. Combined effect of diabetic retinopathy and diabetic kidney disease on all‐cause, cancer, vascular, and non‐cancer non‐vascular mortality in patients with type 2 diabetes: A real‐world longitudinal study. J Diabetes Investig 2020;11: 1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takao T, Suka M, Yanagisawa H, et al. Predictive ability of visit‐to‐visit variability in HbA1c and systolic blood pressure for the development of microalbuminuria and retinopathy in people with type 2 diabetes. Diabetes Res Clin Pract 2017; 128: 15–23. [DOI] [PubMed] [Google Scholar]
- 22. Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018; 41: 2370–2376. [DOI] [PubMed] [Google Scholar]
- 23. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019; 42: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol 2019; 13: 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time‐in‐range in patients with diabetes. Diabetes Technol Ther 2019; 21: 81–85. [DOI] [PubMed] [Google Scholar]
- 26. Masuda D, Yamashita S. Postprandial hyperlipidemia and remnant lipoproteins. J Atheroscler Thromb 2017; 24: 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka A. Postprandial hyperlipidemia and atherosclerosis. J Atheroscler Thromb 2004; 11: 322–329. [DOI] [PubMed] [Google Scholar]
- 28. Ai M, Tanaka A, Ogita K, et al. Relationship between plasma insulin concentration and plasma remnant lipoprotein response to an oral fat load in patients with type 2 diabetes. J Am Coll Cardiol 2001; 15: 1628–1632. [DOI] [PubMed] [Google Scholar]
- 29. Kim HS, Abbasi F, Lamendola C, et al. Effect of insulin resistance on postprandial elevations of remnant lipoprotein concentrations in postmenopausal women. Am J Clin Nutr 2001; 74: 592–595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Comparison of baseline characteristics between patients who did and did not complete follow‐up in the BG and TG cohorts.


