Abstract
Fosphenytoin (FOS) and its active form, phenytoin (PHT), levetiracetam (LEV), and valproic acid (VPA) are commonly used second-line treatments of status epilepticus. However, limited information is available regarding LEV and VPA concentrations following high intravenous doses particularly in young children. The Established Status Epilepticus Treatment Trial (ESETT), a blinded, comparative effectiveness study of FOS, LEV, and VPA for benzodiazepine-refractory status epilepticus provided an opportunity to investigate early drug concentrations. Patients ≥ 2 years who continued to seizure despite receiving adequate doses of benzodiazepines were randomized to FOS, LEV or VPA infused over 10 minutes. A sparse blood sampling approach was used, with up to two samples collected per patient within two hours following drug administration. The objective of this work was to report early drug exposure of PHT, LEV and VPA and plasma protein binding of PHT and VPA. Twenty-seven children with median (interquartile range) age of 4 (2.5, 6.5) years were enrolled. The total plasma concentrations ranged from 69–151.3 μg/mL for LEV, 11.3–26.7 μg/mL for PHT and 126–223 μg/mL for VPA. Free fraction ranged from 4–19% for PHT and 17–51% for VPA. This is the first report in young children of LEV concentrations with convulsive status epilepticus as well as VPA concentrations after a 40 mg/kg dose. Several challenges limited patient enrollment and blood sampling. Additional studies with a larger sample size are required to evaluate the exposure-response relationships in this emergent condition.
Introduction
Convulsive status epilepticus is a medical emergency characterized by abnormally prolonged seizures.1 Benzodiazepines are used as first-line treatment of status epilepticus and have a response rate between 45–70%.2,3 For patients who fail first-line treatment, phenytoin (PHT) and its prodrug fosphenytoin (FOS) are used as the standard of care for second-line treatment.4,5 While levetiracetam (LEV) and valproic acid (VPA) are potentially useful,6–8 until recently no controlled trials have been done to demonstrate their efficacy. Furthermore, limited information is available on their plasma exposures after a high intravenous (IV) dose, particularly in children with status epilepticus.9–12 Most of the available pharmacokinetic information in children comes from the use of these drugs as oral therapy.
The Established Status Epilepticus Treatment Trial (ESETT) was a randomized, blinded, comparative effectiveness study of FOS, LEV, and VPA for the treatment of benzodiazepine-refractory status epilepticus in adults and children.13,14 The primary study outcome was clinical cessation of status epilepticus and improved responsiveness at 60 minutes after the start of study drug infusion without additional antiseizure medication.13,14 We were interested in measuring early drug concentrations as it is the early exposure that will drive the response. ESETT provided us an opportunity to evaluate PHT, LEV and VPA concentrations within two hours after the start of infusion in young children.
Given the challenges in obtaining blood samples in children, a sparse sampling approach was used. Further, the limited sample volume and the blinded exposure required a bioanalytical method capable of simultaneously measuring all three drugs. The objective of this work was to report the concentrations of LEV, PHT and VPA and plasma protein binding of PHT and VPA in young children with benzodiazepine-refractory status epilepticus.
Methods
ESETT was conducted under the exception from informed-consent requirements for emergency research (FDA regulation 21 CFR 50.2412). The institutional review boards for all participating institutions approved the protocol after consultation with local communities and the FDA.13 ESETT was conducted as previously described by Kapur et al.13 This study was performed under an Investigational New Drug application (IND119756, ClinicalTrials.gov NCT01960075) with the Food and Drug Administration.13 Study participants were patients ≥ 2 years, witnessed to have clinically apparent seizures after having received an adequate dose of benzodiazepines. Adequate doses of benzodiazepines were based on international guidelines and depended on patient weight, the specific drug, and the route of administration.2,3,15,16 After randomization, study drug was intravenously infused over 10 minutes. The primary outcome, determined at 60 minutes after the start of study drug infusion, was cessation of clinically apparent seizures, with improved responsiveness and without the use of additional anti-seizure medication.
In order to maintain the same infusion rate and dose volume, the formulations had different concentrations (FOS 16.66 mg/mL of PHT equivalents, VPA 33.33 mg/mL, LEV 50 mg/mL). The doses used for the study were weight-based till 75 kg and capped thereafter as follows: FOS 20 mg/kg of PHT equivalents (max 1500 mg), VPA 40 mg/kg (max 3000 mg) and LEV 60 mg/kg (max 4500 mg).
The protocol target was to collect two blood samples, one between 20–50 minutes and the second between 60–120 minutes after the start of study drug infusion. ESETT enrolled patients from November 2015 to December 2018. The ancillary pharmacokinetic study began enrollment in November 2017. The adult arm of ESETT was terminated for futility prior to initiation of the pharmacokinetic study;13 hence, blood samples were only collected from children (2 to < 18 years).
Approximately 2–3 mL of blood was collected using EDTA vacutainer tubes. Tubes were inverted several times to ensure mixing, and centrifuged for 10 minutes at 2000 x g. The plasma samples were transferred into labeled cryogenic vials and stored at −80 0C prior to and after shipment to the Center for Orphan Drug Research for analysis.
Sample analysis
Calibration standard concentrations ranged from 3.1 to 600 μg/mL for LEV and VPA and 0.625 to 100 μg/mL for PHT. Three levels of QC samples (low, mid and high) were used with concentrations of 60 μg/mL, 125 μg/mL and 250 μg/mL for LEV, 75 μg/mL,150 μg/mL and 300 μg/mL for VPA, and 10 μg/mL, 25 μg/mL and 50 μg/mL for PHT. VPA-d6, LEV-d6 and PHT-d10 were used as internal standards.
For the preparation of unbound samples, Centrifree® ultrafiltration (Merck Millipore, Ireland) devices were used to separate proteins from the plasma. The filtrate was then treated in the same manner as other plasma samples. Acetonitrile was used for protein precipitation. The samples were then analyzed using a TSQ Quantum Access triple quad mass spectrometer (Thermo Scientific, CA, USA) with electrospray ionization and a Dionex Ultimate 3000 HPLC system (California, USA). Reverse-phase chromatographic separation was performed using an InfinityLab Poroshell 120 EC-C18 (Agilent, California, USA) column (2.1×100 mm, 2.7 μm). The analytes were separated using isocratic mobile phase with a composition of 25% 10 mM ammonium acetate and 75% acetonitrile at a flow rate of 0.15 mL/min and run time of five minutes. The conditions for liquid chromatography-tandem mass spectrometry (LC-MS/MS) included heated electrospray ionization and with multiple reaction monitoring (MRM) using negative polarity for VPA and PHT and positive polarity for LEV. The m/z ratios for parent and product ions used for the MRM method were 171 and 126 for LEV, 251 and 102 for PHT, and 143 and 143 for VPA.
Drug concentrations were calculated using a linear equation with (1/x) weighting for LEV and VPA and uniform weighting for PHT. The lower limit of quantitation was 3 μg/mL for LEV and VPA and 0.6 μg/mL for PHT. The limit of detection for LEV, PHT and VPA was 2, 0.5, and 3 ng/mL, respectively. The % coefficient of variation (CV) for the calibration standards and quality control samples was < 15%. The intra- and inter-day accuracy for calibration and quality control samples were within ± 15% of the target concentration.
Results
Blood samples were collected from 27 children, with median (interquartile range) age of 4 (2.5, 6.5) years and weight of 17 (15.7, 20.9) kg. Eighteen patients were primary outcome successes. Twenty-one of the 27 children had epilepsy; while six didn’t have a prior epilepsy diagnosis nor were taking anti-seizure medications. Of the 21 epilepsy patients, 5 were taking one and 16 were taking two or more chronic anti-seizure medications. Maintenance doses ranged from 400–2250 mg/day for LEV and 200–1050 mg/day for VPA. Other co-administered drugs that may have potential drug-drug interactions with one or more of the study drugs included oxcarbazepine, diazepam, ibuprofen, zonisamide, clonazepam, gabapentin, carbamazepine, phenobarbital and clobazam.
A total of 44 blood samples were collected (two samples each from 17 patients and one sample each from 10 patients, of which five were in the first sampling window). Of these, 11 patients (40.7%) were randomized to FOS, nine (33.3%) to VPA and seven (25.9%) to LEV. Fourteen patients were taking one or two of the ESETT study drugs as chronic therapy prior to enrollment. Nine patients had measurable concentrations of two of these drugs in their plasma (15 samples), and one patient had measurable concentrations of all three drugs. The remaining four patients were randomized to the same drug that they were taking on a chronic basis. Protein binding could not be measured in three plasma samples due to a limited volume. Unbound VPA concentrations were below the limit of detection for two samples.
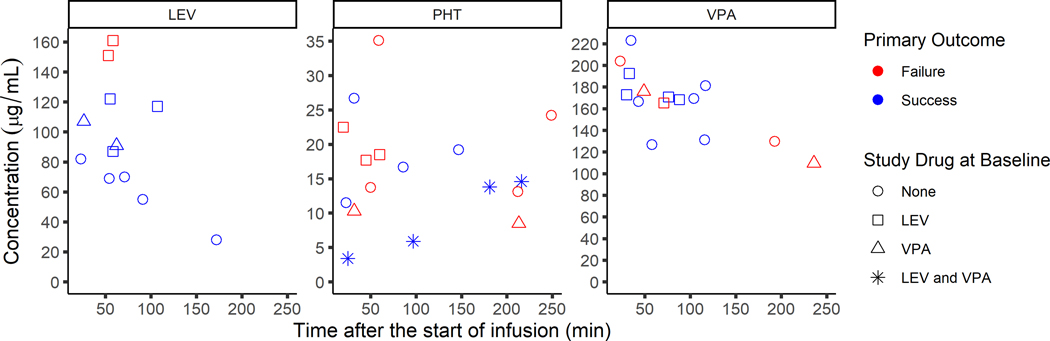
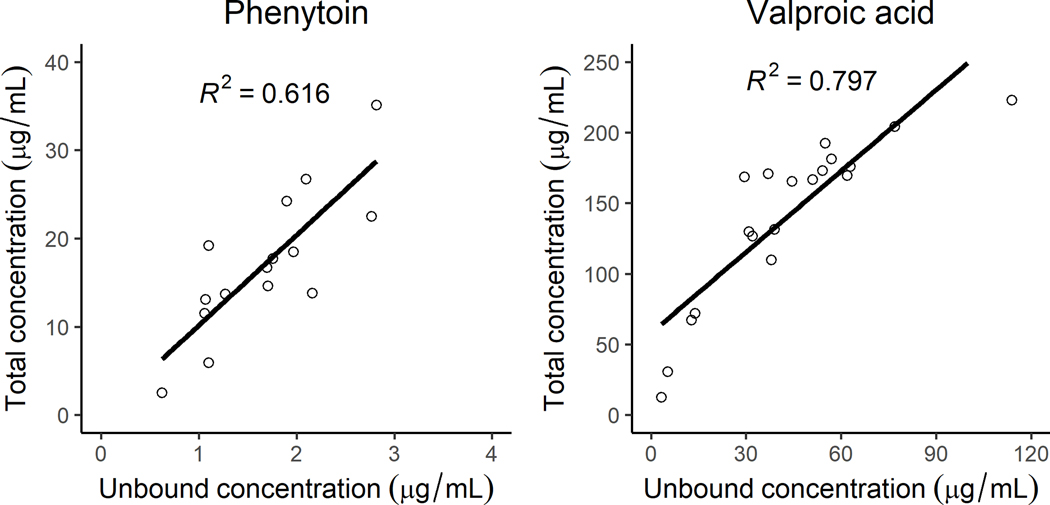
The total concentrations ranged from 69–151.3 μg/mL for LEV, 11.3–26.7 μg/mL for PHT and 126–223 μg/mL for VPA. Figure 1 shows concentrations and their corresponding treatment response. Unblinding confirmed that the measured concentrations corresponded to the intended randomized study drug. The unbound concentrations ranged from 1–2.8 μg/mL for PHT and 31–114 μg/mL for VPA (Figure 2). The free fraction ranged from 4–19% for PHT and 17–51% for VPA. Correlation between the unbound and total concentrations for PHT and VPA measured using spearman’s rho was R2=0.616 and R2=0.797, respectively. As expected, VPA shows a trend towards non-linear binding with increasing concentrations. Two patients with measurable VPA concentrations had the highest free fraction of PHT following IV infusion.
Figure 1:
Total concentrations (μg/mL) of levetiracetam (left), phenytoin (middle) and valproic acid (right) versus the time of blood collection after the start of study drug infusion (minutes) overlaid with primary outcome results (red= failure, blue= success)
Figure 2:
Total vs. unbound concentration (μg/mL) for phenytoin (left panel) and valproic acid (right panel)
Discussion
This report presents the plasma concentrations and protein binding of three commonly used drugs for second-line treatment in children with convulsive status epilepticus. Additional anti-seizure drugs used prior to or after the randomized therapy were also measurable in the plasma samples. While drug concentrations were generally in the therapeutic ranges (PHT 10–20 μg/ml, LEV 12–46 μg/ml, and VPA 50–150 μg/ml),17,18 concentrations were widely variable despite mg/kg dosing.
This is the first report of LEV concentrations in young children with generalized convulsive status epilepticus. This is important because we do not understand the pharmacokinetics of many drugs during the course of treatment of convulsive status epilepticus, a condition in which severe metabolic derangements are common which may alter pharmacokinetics of anti-seizure medications. Two previous studies reported LEV concentrations in older children, although neither included patients with convulsive status epilepticus.9,10 When dose-normalized, LEV concentrations in these studies were similar to those we observed.9,10 VPA concentrations in children with status epilepticus have been reported previously in a case report11 and a study in 11 children with status epilepticus or acute repetitive seizures dosed at 15–20 mg/kg.12 When the concentrations (median concentration at 30 minutes 99 μg/mL, range 67–161 μg/mL) are dose-normalized, they also agreed with our results following 40 mg/kg doses.12 Similarly, PHT concentrations, both unbound and total, were consistent with previously published reports.19–23 It is worth noting that in our study the concentrations in three patients (five samples) randomized to LEV and one patient (two samples) randomized to VPA may have been affected by the use of the respective randomized drug as chronic oral therapy.
While we have limited information, this still represents the largest cohort of pediatric status epilepticus patients with drug concentrations and treatment response. Based on visual observation, there was no apparent signal that drug concentration was a driver of responsiveness. This observation was consistent with a previous report of 29 status epilepticus patients treated with LEV that found no significant difference in LEV exposure between responders and non-responders after a median loading dose of 28 mg/kg.24 While pharmacokinetic samples were not collected in ESETT adult patients, we found no significant association of weight-normalized dose and treatment response,25 which supports our observations in the pediatric arm.
VPA plasma protein binding appeared non-linear, which agrees with published report.26 The unbound VPA fraction was higher than what has been reported following oral administration,26,27 most likely due to higher VPA concentrations in our study. Our results are in agreement with the fraction unbound reported after IV administration in adult epilepsy patients taking antiseizure medications including oxcarbazepine, carbamazepine, and phenobarbital.28 In two patients on chronic oral VPA therapy, we found increased PHT free fraction as suggested by prior reports.29,30 This interaction can result in higher unbound PHT concentrations, while the total concentrations are unchanged, leading to a misinterpretation of the total concentration.
These results are limited by the number of patients from whom plasma samples could be collected. This was mainly due to a delay in the start of the ancillary pharmacokinetic study as well as the early termination of the adult arm of the ESETT study for futility. We found that obtaining plasma samples in young children within ESETT was challenging as a second IV line or multiple venous punctures were required.31
We utilized blood sampling windows so as to improve the likelihood of collecting requisite number of blood samples. Even so, we had instances of blood collection beyond the sampling window and, in approximately one third of the patients, only one sample could be collected. Other strategies that allow blood collection from the same IV line used for drug infusion (e.g., PIVO™), may increase the number of samples collected in future studies.32,33
Conclusions
This is the first report of LEV concentrations in young children with convulsive status epilepticus and of VPA concentrations after a 40 mg/kg dose. The results of this study provide clinicians with new information about treating status epilepticus in very young children. As previously reported,14 the safety of administering large loading doses of all three drugs, particularly LEV at 60 mg/kg, was confirmed. This will likely support more aggressive treatment of this life-threatening condition. The small number of patients precluded analysis of exposure-response, which warrants a further study with a larger sample size.
Acknowledgements:
We would like to acknowledge the ESETT Data and Safety Monitoring Board. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, or the United States Government.
Funding Sources:
Research reported in this publication was supported by National Institutes of Health, National Institutes of Neurological Disorders and Stroke under Awards U01NS088034, U01NS088023, U01NS056975, U01NS059041, and R01NS099653. (Clinicaltrials.gov identifier NCT01960075)
Conflicts of Interest
Dr. Sathe, Ms. Mishra, Dr. Ivaturi, Dr. Brundage, Dr. Lowenstein, Dr. Babcock and Dr. Coles have nothing to disclose. Dr. Cloyd reports licensing fees from Ligand, personal fees from UCB, grants and personal fees from Neurelis, that are outside the submitted work. In addition, Dr. Cloyd has an issued patent for IV carbamazepine and a pending patent on IV topiramate with royalties paid by Ligand. Dr. Elm reports grants from NIH/NINDS, during the conduct of the study. Dr. Chamberlain reports grants from NIH, during the conduct of the study. Dr. Silbergleit reports grants from NIH, during the conduct of the study. Dr. Kapur reports grants from NIH/NINDS, during the conduct of the study. Dr. Shinnar reports grants from NINDS, during the conduct of the study; personal fees from UCB Pharma, personal fees from Eisai, outside the submitted work. Dr. Cock reports personal fees from Sage Pharmaceuticals Ltd, personal fees from Bial Pharma UK, Eisai Europe Ltd, personal fees from UCB Pharma Ltd, non-financial support from Special Products Ltd, other from Novartis, other from GW Pharma, outside the submitted work. Dr. Fountain reports grants from NIH, during the conduct of the study; grants from UCB, grants from SK Life Sciences, grants from Xenon, grants from GW Pharma, and grants from DSLP, outside the submitted work.
Footnotes
Data Sharing
ESETT data will be available through the NINDS repository of Archived Clinical Research Datasets which is found at https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/ClinicalResearch/Archived-Clinical-Research-Datasets Trial results will also be posted to clinicaltrials.gov. NINDS requires all investigators seeking access to data from archived NINDS-supported trials to agree to certain terms and conditions. To request a dataset, please complete the NINDS Data Request Form and send it to the NINDS Clinical Research Liaison at CRLiaison@ninds.nih.gov
REFERENCES
- 1.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus - Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56(10):1515–23. [DOI] [PubMed] [Google Scholar]
- 2.Glauser T, Shinnar S, Gloss D, et al. Evidence-Based Guideline : Treatment of Convulsive Status Epilepticus in Children and Adults : Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr 2016;16(1):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17(1):3–23. [DOI] [PubMed] [Google Scholar]
- 4.Cook AM, Castle A, Green A, et al. Practice variations in the management of status epilepticus. Neurocrit Care 2012;17(1):24–30. [DOI] [PubMed] [Google Scholar]
- 5.Riviello JJ, Claassen J, Laroche SM, et al. Treatment of status epilepticus: An international survey of experts. Neurocrit Care 2013;18(2):193–200. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez V, Januel JM, Burnand B, Rossetti AO. Second-line status epilepticus treatment: Comparison of phenytoin, valproate, and levetiracetam. Epilepsia 2011;52(7):1292–6. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal P, Kumar N, Chandra R, Gupta G, Antony AR, Garg N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure 2007;16(6):527–32. [DOI] [PubMed] [Google Scholar]
- 8.Tripathi M, Vibha D, Choudhary N, et al. Management of refractory status epilepticus at a tertiary care centre in a developing country. Seizure 2010;19(2):109–11. [DOI] [PubMed] [Google Scholar]
- 9.Wheless JW, Clarke D, Hovinga CA, et al. Rapid infusion of a loading dose of intravenous levetiracetam with minimal dilution: a safety study. J Child Neurol 2009;24(8):946–51. [DOI] [PubMed] [Google Scholar]
- 10.Abend NS, Monk HM, Licht DJ, Dlugos DJ. Intravenous levetiracetam in critically ill children with status epilepticus or acute repetitive seizures. Pediatr Crit Care Med 2009;10(4):505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovinga CA, Chicella MF, Rose DF, Eades SK, Dalton JT, Phelps SJ. Use of intravenous valproate in three pediatric patients with nonconvulsive or convulsive status epilepticus. Ann Pharmacother 1999;33(5):579–84. [DOI] [PubMed] [Google Scholar]
- 12.Visudtibhan A, Bhudhisawadi K, Vaewpanich J, Chulavatnatol S, Kaojareon S. Pharmacokinetics and clinical application of intravenous valproate in Thai epileptic children. Brain Dev 2011;33(3):189–94. [DOI] [PubMed] [Google Scholar]
- 13.Kapur J, Elm J, Chamberlain JM, et al. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N Engl J Med 2019;381(22):2103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamberlain JM, Kapur J, Shinnar S, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet 2020;1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meierkord H, Boon P, Engelsen B, et al. EFNS guideline on the management of status epilepticus. Eur J Neurol 2006;13(5):445–50. [DOI] [PubMed] [Google Scholar]
- 16.Sathe AG, Tillman H, Coles LD, et al. Underdosing of Benzodiazepines in Patients with Status Epilepticus Enrolled in Established Status Epilepticus Treatment Trial. Acad Emerg Med 2019;0(8):940–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patsalos PN, Berry DJ, Bourgeois BFD, et al. Antiepileptic drugs - Best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008;49(7):1239–76. [DOI] [PubMed] [Google Scholar]
- 18.Limdi NA, Knowlton RK, Cofield SS, et al. Safety of rapid intravenous loading of valproate. Epilepsia 2007;48(3):478–83. [DOI] [PubMed] [Google Scholar]
- 19.Ogutu BR, Newton CRJC, Muchohi SN, et al. Pharmacokinetics and clinical effects of phenytoin and fosphenytoin in children with severe malaria and status epilepticus. Br J Clin Pharmacol 2003;56(1):112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piper JD, Hawcutt DB, Verghese GK, Spinty S, Newland P, Appleton R. Phenytoin dosing and serum concentrations in paediatric patients requiring 20 mg/kg intravenous loading. Arch Dis Child 2014;99(6):585–6. [DOI] [PubMed] [Google Scholar]
- 21.Prusakov AB, Patel AD, Cole JW. Impact of Obesity on Fosphenytoin Volume of Distribution in Pediatric Patients. J Child Neurol 2018;33(8):534–6. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka J, Kasai H, Shimizu K, Shimasaki S, Kumagai Y. Population pharmacokinetics of phenytoin after intravenous administration of fosphenytoin sodium in pediatric patients, adult patients, and healthy volunteers. Eur J Clin Pharmacol 2013;69(3):489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selioutski O, Grzesik K, Vasilyeva ON, et al. Evaluation of phenytoin serum levels following a loading dose in the acute hospital setting. Seizure 2017;52(2017):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrenoud M, André P, Buclin T, Decosterd LA, Rossetti AO, Novy J. Levetiracetam circulating concentrations and response in status epilepticus. Epilepsy Behav 2018;88(2018):61–5. [DOI] [PubMed] [Google Scholar]
- 25.Sathe AG, Elm JJ, Cloyd JC, et al. The association of patient weight and dose of fosphenytoin, levetiracetam and valproic acid with treatment success in status epilepticus. Epilepsia 2020;61:e66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cloyd JC, Dutta S, Cao G, Walch JK, Collins SD, Granneman GR. Valproate unbound fraction and distribution volume following rapid infusions in patients with epilepsy. Epilepsy Res 2003;53(1–2):19–27. [DOI] [PubMed] [Google Scholar]
- 27.Scheyer RD, Cramer JA, Toftness BR, Hochholzer JM, Mattson RH. In vivo determination of valproate binding constants during sole and multi-drug therapy. Ther Drug Monit 1990;12(2):117–23. [PubMed] [Google Scholar]
- 28.Dutta S, Faught E, Limdi NA. Valproate protein binding following rapid intravenous administration of high doses of valproic acid in patients with epilepsy. J Clin Pharm Ther 2007;32(4):365–71. [DOI] [PubMed] [Google Scholar]
- 29.Dahlqvist R, Borga O, Rane A, Walsh Z, Sjoqvist F. Decreased plasma protein binding of phenytoin in patients on valproic acid. Br J Clin Pharmacol 1979;8(6):547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perucca E, Hebdige S, Frigo GM, Gatti G, Lecchini S, Crema A. Interaction between phenytoin and valproic acid: Plasma protein binding and metabolic effects. Clin Pharmacol Ther 1980;28(6):779–89. [DOI] [PubMed] [Google Scholar]
- 31.Cock HR, Coles LD, Elm J, et al. Lessons from the Established Status Epilepticus Treatment Trial. Epilepsy Behav 2019;101(2019):106296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulloy DF, Lee SM, Gregas M, Hoffman KE, Ashley SW. Effect of peripheral IV based blood collection on catheter dwell time, blood collection, and patient response. Appl Nurs Res 2018;40(November 2017):76–9. [DOI] [PubMed] [Google Scholar]
- 33.Adams S, Toroni B, Lele M. Effect of the PIVO Device on the Procedure of Phlebotomy from Peripheral IV Catheters. Nurs Res Pract 2018;2018:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]