Abstract
Background:
There is a lack of evidence that long-term opioid use offers benefit for noncancer pain and an abundance of evidence of harm. Despite clinical guidelines and education, prescribing continues at a higher rate than before the opioids crisis. The objective of trial 1 of the Application of Economics & Social psychology to improve Opioid Prescribing Safety (AESOPS-1) is to discourage unnecessary opioid prescribing in primary care by applying “behavioral insights”—empirically-tested social and psychological interventions that affect choice.
Methods:
AESOPS-1 randomizes primary care clinics in Illinois and California to behavioral intervention or control. Both arms receive opioid guideline education. Clinics randomized to the behavioral intervention arm receive nudges within the electronic health record (EHR) including: 1) an “accountable justification” entered in the chart, 2) a precommitment to address high-risk prescriptions, and 3) a “PainTracker” that broadens discussions about pain. The control arm receives no EHR-based intervention. The primary outcome is the change in weekly milligram morphine equivalents (MME) prescribed. The secondary outcome is the change in the proportion of patients prescribed at least 50 daily MME. To evaluate these outcomes, we will use a difference-in-differences mixed-effects regression model on clinician MME weekly or daily dose. The analysis will be “intent-to-treat.” The intervention period is 18-months, with a 6-month follow-up period to measure persistence of effects.
Discussion:
The AESOPS-1 trial will evaluate the effect of EHR-based interventions in reducing noncancer opioid prescribing in primary care. AESOPS-1 may demonstrate practical and scalable strategies to lower unnecessary population exposure to opioids.
Keywords: opioid use disorder, randomized controlled trial, behavioral economics, nudges
1. Background
In 2018, approximately 19% of adults in the United States filled an opioid prescription [1]. While opioid prescribing in the US has decreased since its peak in 2010, the milligram morphine equivalent (MME) per capita remains approximately two times higher than in 1999 [2, 3]. Despite this high level of prescribing, reports of pain have not decreased [4, 5]. Opioid prescriptions often lead to adverse consequences. In 2017, there were 17,029 prescription opioid overdose deaths in the US [6]. Estimates place aggregate costs for prescription opioid harms at over $78.5 billion (in 2013 US dollars), and 25% of the aggregate economic burden is through public programs (i.e., Medicaid, Medicare, and veterans programs) [7].
Primary care physicians prescribe 45% of all opioids in the US [8]. However, few receive training on managing patients exposed to opioids for prolonged periods [8]. Guidelines now address the high risks of prolonged opioid exposure [9, 10, 11]. In 2016, the Centers for Disease Control and Prevention (CDC) issued the “CDC Guideline for Prescribing Opioids for Chronic Pain” addressing opioid initiation or continuation for chronic pain, opioid selection, quantity, follow-up, tapering, and opioid harm [9]. The Oregon Pain Guidance pain management guidelines incorporate and are compatible with the CDC guidelines and emphasize non-opioid medications as first-line treatment and judicious prescribing of opioids if necessary thereafter. The Oregon Pain Guidance recommends prescribing limits for patients not currently or recently treated with opioids and offers collaborative tapers [10,11].
Together, these guidelines address critical decisions about opioid exposure that patients and clinicians face when initiating, refilling, or discontinuing opioid therapy. Despite these guidelines, primary care clinicians report challenges in following these recommendations [8]. Some of these challenges include the difficulty of preventing the transition from short- to long-term use, tapering patients on long-term opioids, and assessing chronic pain [8, 12]. There is growing interest in studies that use psychological insights to gently persuade clinicians to do things that may improve care. These interventions recognize irrational behaviors by clinicians and patients and attempt to “nudge” their actions through changes to how choices appear in the electronic health record, without providing monetary incentives or by restricting choice [13, 14]. A straightforward example of a nudge is a change from 30 to 10 in the default number of opioid pills appearing on the electronic order entry screen [15]. With this intervention, clinicians prescribed fewer opioid pills simply because they had made a habit of choosing the default quantity. Prior use of behavioral economic interventions for inappropriate antibiotic prescribing was also successful [16]. Together, these studies suggest that adherence to opioid prescribing guidelines may improve with nudges at the time of opioid initiation, opioid refills, and tapering.
In Trial 1 of Application of Economics & Social psychology to improve Opioid Prescribing Safety (AESOPS-1), the main objective is to use behavioral economic interventions to overcome the challenges clinicians face at critical decision points and increase adherence to Oregon Pain Guidance pain management guidelines and CDC guideline recommendations 1, 2, 5, and 7 [9, 10, 11]. We will measure the change in weekly clinician aggregate MME ordered for all patients to evaluate intervention effects. Secondary outcomes include the change in the proportion of patients prescribed receiving at least 50 MM per day.
2. Materials and Methods
2.1. Overview
AESOPS-1 will take place in three diverse healthcare organizations in two geographically distinct regions of the US: Chicago, Illinois (Northwestern Medicine) and Los Angeles, California (AltaMed Health Services and The Children’s Clinic of Long Beach), representing academic and safety-net health systems, respectively. The target population is primary care clinicians. Within each healthcare organization, by random draw, clinics receive intervention or control. Clinic cluster randomization aims to avoid intra-clinic contamination of the intervention.
All participating clinicians receive guideline education at baseline. The intervention nudges encourage alternatives to opioids for persons naive to opioids and those receiving their first refill. For persons on chronic opioid therapy, the intervention encourages collaborative tapers and broadening assessments to address pain’s effect on the patient’s life. The design is repeated cross-sectional because individual patients are not followed up over time, but clinician orders will be measured repeatedly for outcome assessments [17]. The intervention period is 18 months in length for all participants, with a 6-month follow-up period to measure the interventions’ persistence. All study procedures have been reviewed and approved by the University of Southern California IRB (UP-19–00821).
2.2. Inclusion and Exclusion Criteria
Participants are clinicians who practice ambulatory primary care at the participating clinical sites in Illinois and California. Clinicians are eligible if they treat patients over 18 years of age. A waiver of consent for clinician participation in the trial involving prompts in the electronic health record has been approved by the University of Southern California IRB. In the baseline survey, we will enroll clinicians and obtain informed consent to collect their responses. If a clinician does not consent to participate in the survey, their responses will not be collected. The clinic at which each clinician conducts the majority of visits determines attribution to a clinic. All clinics have electronic health records. Clinics must also be physically separate to limit treatment bleeding.
A visit qualifies for the intervention at the initiation of opioids, initial opioid refill, or when persons receive chronic opioid therapy of 50 or more MMEs per day. Table 1 shows when a visit with a clinician randomized to the intervention group qualifies for the intervention based on the patient’s history of opioid prescriptions in the past 90 days. We define the interventions in greater detail in section 2.3. A visit is eligible for inclusion in outcome measurement if: 1) the patient is 18 years of age or older, 2) the clinician and practice site participate in the study, and 3) the visit occurs during the 18-month intervention period. Visits will be excluded from the primary analysis if the patient has a current diagnosis of cancer. Appendix A lists cancer exclusions (ICD-10 codes).
Table 1:
Visit Inclusion Criteria by Patient Status
| Patient status | Criteria |
|---|---|
| Opioid naïve | Visits where the order is for an included opioid and no prior opioid prescription with a start date of greater than 1 day and less than 91 days |
| At-risk for long-term use | Visit where the order is for an included opioid, there is a prior opioid prescription with a start date greater than 1 day and less than 91 days, and no prior opioid prescription with a start date greater than 90 days |
| Long-term opioid recipient | Total opioid doses are at least 50 MME per day, there are two or more prior opioid prescriptions with two different start dates both greater than 1 day and less than 91 days, and there is a prior opioid prescription with a start date greater than 90 days and less than 181 days |
2.3. Interventions
There is one intervention, and there is a control condition. Clinicians in both conditions receive web-based guideline education consisting of an online educational module at the start of the study period and complete a survey assessment at baseline and following the intervention period. The education module will include content related to the CDC guideline, the Oregon Pain Guidance document, tapering, a treatment physician locator, and the Naloxone Provider Guide (See Education module in Appendix B). The survey will gather information about clinician training, use of the electronic health record, engagement in quality improvement efforts, understanding clinical care guidelines, and empathy and concern measures to determine if these moderate intervention effects [18 – 20].
When clinicians prescribe an opioid, the patient’s status as one of “opioid-naive,” “at-risk for long-term use,” or “long-term opioid recipient” determines the type of nudge that appears. An initial order for an opioid and no opioid prescriptions within the 90 days before this order defines opioid-naive. In our study, opioid naive does not mean the patient has never used an opioid, but rather that the patient has not used an opioid within the past 90 days. Receipt of a second prescription defines at-risk for long-term use. For this, the patient must have a current order for an opioid and both a prior opioid prescription within the 90 days of the current order and no opioid prescriptions written more than 90 days before the current order. The criteria for long-term opioid recipient are: (1) an order for an opioid with a dose greater than 49 MME, (2) two or more opioid prescriptions with two different start dates within the 90 days before this order, and (3) an opioid prescription written more than 90 days before this order. Data from three months in 2020 indicate that the nudges for opioid-naive patients, at-risk for long-term use patients, and long-term opioid recipients would have fired in 9.6%, 6.8%, and 21.9% of all visits, respectively. Based on these findings, we estimate that the nudges for opioid-naive patients, at-risk for long-term use patients, and long-term opioid recipients will appear 61, 44, and 140 times, respectively, within a full-time clinician’s practice.
Interventions include Accountable Justification (AJ), Precommitments, and PainTracker. All nudges appear within the Epic electronic health record system. Clinicians in the intervention arm prescribing opioids to opioid-naive or at-risk for long-term use patients must justify an order. An opioid order directed to a patient that meets the criteria for long-term opioid recipient prompts a request for a precommitment to a taper-discussion and initiation of PainTracker assessments during collaborative tapers. Failure to initiate a taper prompts an accountable justification alert.
Accountable Justifications elicit a free text response, explaining the reason for prescribing an opioid. The prompt also informs the clinician that her response to the justification will appear in the health record as a “high-risk prescribing justification” note. If the clinician fails to enter a justification, the phrase “no justification given” appears in the medical record. The justification asks the clinician to explain her clinical reasoning for an opioid order. It may convey that such actions are not always justified. It provides social accountability in the form of a publicly visible response. It also implicitly designates norm-congruent behavior as the default behavior, departures from which require additional effort [21 – 23]. Accountable justifications reduced unnecessary prescriptions in a previous trial set in different prescribing contexts [16]. Figure 1 displays the three types of accountable justifications.
Figure 1: EHR Justification Warnings and Alerts.
Figure 1: A) An accountable justification for opioid-naive prescribing, B) An accountable justification for persons with opioid prescriptions in the past 30 days and at-risk for long-term use, C) An accountable justification for persons with opioid prescriptions in the past 90 days and at-risk for long-term use, D) Precommitment and accountable justification for failure to initiate an opioid taper.
As mentioned, precommitments ask the clinician to commit to having a discussion with the patient about trying to taper opioids at a future visit (Figure 1D). Precommitment to having a taper discussion may appear easier to tackle at a future visit due to present-bias [24]. Coupled with our need for consistency, precomittments may effectively set a course for a taper discussion since people place a high value on being congruent with their prior commitments [25, 26]. Public physician commitments have previously reduced inappropriate antibiotic prescribing in a randomized trial [27].
PainTracker is a patient-reported outcome instrument that assesses chronic pain and its multiple effects on a person’s life [12]. In AESOPS-1, long-term opioid recipients complete an abridged version of the PainTracker to reframe a patient’s visit around reaching functional goals instead of driving down pain intensity scores. The patient receives a PainTracker assessment three days after their initial opioid taper and every 30 days after that. Patients complete the assessment via the patient portal or at the time of the clinic appointment (see PainTracker Appendix B). Clinicians then use the results to monitor the patient’s symptoms and expand conversations with patients about broader life problems that impact and are impacted by pain. We expect a response rate of approximately 77% based on the PainTracker completion rate in a previous study involving patients with chronic pain [28].
2.4. Workflow for Delivery of Accountable Justification Nudges
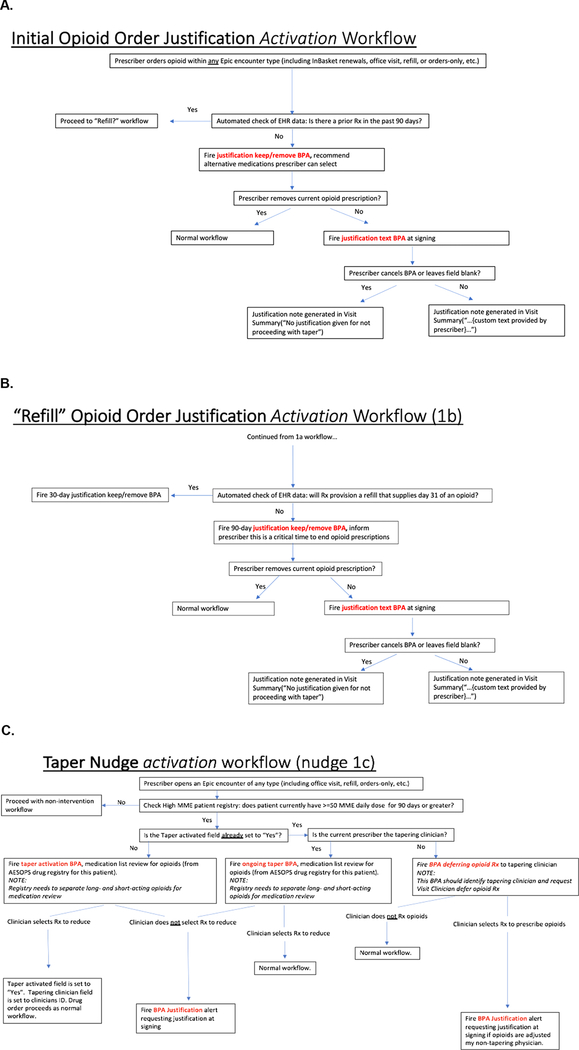
When a clinician places an opioid order, the electronic health record checks the patient’s recent prescribing history and assigns the right prompt. Suppose the patient receiving the order is opioid-naive. In this case, the prompt explains that guidelines discourage initiating opioids. It provides a button that, if clicked, removes the order and offers a choice among non-opioid substitute medications and physical therapy. If the clinician does not remove the opioid order, then a justification prompt appears (Figure 1A). Suppose the patient is at-risk for long-term opioid use. Much like the previous case, the prompt indicates that the patient received an opioid prescription within the past 30 or 90 days and explains that guidelines discourage initiating refills for opioids. It provides a button that, if clicked, removes the order and offers a choice among non-opioid substitute medications and physical therapy. If the clinician does not remove the opioid order, then a justification prompt appears (Figures 1B and 1C). Finally, suppose the patient receiving the opioid order is a long-term opioid recipient. Here, there are several cases. In the first case, no clinician treating this patient has ever encountered the intervention at the patient’s visit. A prompt asks the clinician if she is the primary clinician responsible for this patient’s long-term opioid therapy. Answering ‘Yes’ prompts a request for a commitment to a future taper discussion with the patient; answering ‘No’ dismisses the screen. In the second case, the ordering clinician has, at a previous visit, identified herself as the primary clinician responsible for this patient’s long-term opioid therapy. If she has committed to a taper discussion at that prior visit and the order exceeds the dose at the last visit, then a prompt asks the clinician to justify her failure to taper (Figure 1D). In the third case, the ordering clinician has not, at a previous visit, identified herself as the primary clinician responsible for this patient’s long-term opioid therapy. However, another clinician has done so, and the patient and her primary opioid prescriber have initiated a collaborative taper. Here, a prompt indicates the patient is on a taper and asks the clinician to defer prescribing opioids to the primary prescriber named in the prompt. Figure 2 displays the workflows illustrating the three nudges.
Figure 2: EHR Workflow Schemas for Accountable Justification Prompts.
Figure 2: A) Workflow for opioid-naive AJ alert B) Workflow for initial opioid refill AJ prompt C) Workflow for tapering AJ alert
2.5. Randomization procedures
To increase the likelihood that the control and intervention arms are well-balanced, clinic randomization will be stratified by a median split of clinic average daily MME (high vs. low) nested inside clinic organization. We will conduct the stratified randomization of clinics by clinic organization (i.e., Northwestern Medicine, AltaMed Health Services, and The Children’s Clinic) using the statistical computing language R and true random numbers generated through atmospheric noise and collected at random.org. For each clinic organization, we will construct ordered collections of clinics. We will then employ the random package in R to return a random set of numbers from random.org that will permute numbered clinics by their random position in the set. We will then assign the clinics with a (random) rank below the median to the intervention group. Since clinic daily MME stratifies randomization within each healthcare system, each of the high vs. low average daily MME groups receive this assignment. Because the number of clinics at each organization is not always divisible by two, these remainder clinics will be randomized to conditions separately. Study initiation reveals treatment and control allocation.
3. Measures
The primary outcome is the change in clinician-level weekly milligram morphine equivalents (MMEs) before and after the intervention (or control) started, adjusting for concurrent temporal trends in prescribing. The number of prescriptions written per clinician per month will act to control for factors external to the study impacting their prescribing rate over time. The preintervention measure is the average weekly MME ordered in the 6-months before the intervention. The post-intervention measure is the average weekly MME ordered in the 6-months following the 18-month intervention period. The difference in weekly MMEs ordered will be evaluated separately for two groups: (i) 50 MME and above, and (ii) below 50 MME daily dose orders. For an observation to qualify for (i), a clinician must have treated a patient with at least one visit where 50 MME or more daily dose triggered high-risk decision support. Otherwise, observations are part of the less than 50 MME analysis group. The daily dose threshold of 50 MME was chosen to reflect the CDC guideline recommending that clinicians prescribe the lowest effective dosage and carefully evaluate the benefits and risks of prescriptions of 50 MME or greater per day. These guidelines are based on evidence of increased risk of fatal and nonfatal overdose for individuals on 50–99 MME per day compared to individuals on lower daily doses [9].
Secondary outcomes include the change in the proportion of a clinician’s patients prescribed opioids of at least 50 MME per day between the pre- and post-intervention periods, and the rate of patients at-risk for long-term use transitioning to chronic opioid use. Daily MME for each clinician will be estimated as the average of the sum of milligram morphine equivalents written within a weekly observation period divided by 7 days in the pre- and post-intervention periods. The proportion of a clinician’s patients prescribed 50 MME or more will be measured as the number of patients prescribed 50 MME or more per day by the clinician in the numerator and the number of patient visits where an opioid was prescribed by the clinician in the denominator for each 6-month time period. The difference between these proportions from the pre- to post-period will be quantified. The rate of patients at-risk for long-term use transitioning to chronic use will be measured separately for each clinician as the number of their patients meeting the criteria for a long-term opioid recipient in the numerator (Table 1) and the number of patient visits at which the opioid refill nudge fired in the denominator over time. Appendix A lists opioid prescribing diagnosis exclusions and medications for outcome assessments.
A baseline survey will capture clinician characteristics for statistical reporting, job satisfaction, knowledge of guidelines, satisfaction with the electronic health record, empathy and concern (see Table 2). The empathy and perspective taking subscales of the Empathy Index and Interpersonal Reactivity Index measure two separate concepts: clinician empathy and concern [19, 20]. We hypothesize that clinicians with greater empathy, defined as “feeling what others feel,” will prescribe more opioids than clinicians with greater concern due to vicarious suffering. Table 2 lists study measures.
Table 2:
AESOPS-1 Measures and Timepoints
| Measure | Source | Timepoint |
|---|---|---|
| Clinician’s empathy and concern | [19, 20] | Baseline |
| Clinician’s job satisfaction | [18] | Baseline |
| Clinician’s participation in past quality improvement efforts | [18] | Baseline |
| Clinician’s specialty | [18] | Baseline |
| Clinician’s understanding of clinical care guidelines | [18] | Baseline |
| Clinician’s use of and satisfaction with the EHR | [18] | Baseline |
| Clinician’s years of working as a physician | [18] | Baseline |
| Weekly MMEs ordered | EHR | Throughout the pre-intervention, intervention, and post-intervention periods |
3.1. Data Collection and Management
Primary and secondary clinical outcomes will be captured from electronic health records, and secondary outcomes will be gathered through clinician surveys at baseline.
3.2. Statistical Analysis Plan
To describe the data, we will use means and medians for continuous measures, frequencies for count data, standard deviations, and interquartile ranges for the variance. We will evaluate changes in opioid prescribing rates by group using an intent-to-treat difference-in-differences framework in a censored mixed-effects regression model on weekly MMEs prescribed. With the intent-to-treat approach, we analyze clinician data according to their random assignment at baseline. We will estimate the fixed effects of the intervention, time, and interventions over time (i.e., interactions between randomization assignment and time), using the 6-months before the intervention baseline period. The use of a mixed-effects hierarchical knotted spline regression model offers a flexible way to accommodate non-linear trends before and after introducing the intervention. This model places a knot at the intervention start date allowing slopes before and during treatment to vary for each treatment arm. Log-transformed MMEs is the dependent variable. Typically, a log-transformation ensures a Gaussian outcome and allows for the extraction of percent-change in prescribing.
To account for data clustering at zero, indicating weeks in which a physician wrote no opioid prescriptions, we will use a censored regression. Censored regression has a continuous component and a discrete component. The continuous part includes the natural log MME doses estimated over weeks, where the physician prescribed an opioid. The discrete component includes weeks in which the physician wrote no qualifying prescriptions. A censored regression yields treatment estimates that decompose into the treatment’s effect on the probability of a clinician prescribing an opioid within the week and its effect on the quantity of MME written conditional on that prescriber writing an opioid prescription [29]. We will estimate the association of intervention assignment and weekly MME prescribed using equation [1]:
| [1] |
where log(MME)* denotes the censored dependent variable, time is dichotomous for the intervention period, t* is the spline knot for the start of the intervention, group is dichotomous for the EHR intervention group, trialit is dichotomous and denotes if a clinician at time t was randomized to the intervention group in a simultaneous trial of AESOPS (AESOPS-2), i is the clinician level, ζ is the clinician random effect and η is the clinic random effect. Hypotheses regarding the relationship between the independent variables and the censored outcome will be tested by the likelihood-ratio method with a 0.05 level of significance. The resulting likelihood-ratio test statistic follows a chi-square distribution with n-1 degrees of freedom in large samples [30].
Equation [2] shows a simplified version of the model we will use to analyze the secondary outcomes:
| [2] |
where , time is dichotomous for the intervention period, t* is the start of the intervention, “Group” identifies intervention or control, “Trialit” is dichotomous and identifies if a clinician at time t was randomized to the intervention group in a simultaneous trial called “AESOPS-2”, i is the clinician level, ζ is the clinician random effect and η is the clinic random effect. For the analysis of the change in the proportion of a clinician’s patients prescribed 50 MME or more, pit in Equation [2] is equal to 1 if an order of 50 MME or more per day is ordered, conditional on an opioid prescription being written for a patient by provider i at a visit at time t, and 0 otherwise. For the analysis of the rate of patients at-risk for long-term use transitioning to chronic use, pit in Equation [2] is equal to 1 if a patient meets the criteria for a long-term opioid recipient (defined in Table 1), conditional on a previous appointment at which the opioid refill nudge appeared for provider i, and 0 otherwise.
3.3. Sample Size and Power Calculations
The following health systems constitute the sample. Northwestern Medicine includes 60 primary care clinics that contain 387 primary care clinicians with 12,552 adult patients on chronic opioid therapy for noncancer pain—on opioids for greater than three months. AltaMed Health Services has 35 clinics with 134 clinicians with 17,674 such patients. The Children’s Clinic has 6 to 9 clinics containing 26 clinicians with 3,307 such patients. The number of noncancer chronic pain outpatient visits with opioid prescriptions in all the clinics included in the study exceeds 300,000 annually.
Silent tracking of qualifying visits (see Section 2.3) for prompts found that long-term high-risk opioid recipients attended nearly 60% of the time. Power calculations assume prescriptions for high-risk opioid recipients weigh heavily in daily MME calculations. We assume an average of 5.5 clinicians participating per clinic based on previous work [31]. Assuming a clinic-level intraclass correlation coefficient of 0.01 for clinics, alpha of 0.025, we calculate the following number of clinics needed to achieve 0.8 and 0.85 statistical power for a one-tailed test for each of 3-, 5-, 7- and 12-percentage point reductions in the primary outcome [32]. Based on Weimer et al., we assume the baseline dose for the long-term high-risk patients is approximately 263 (± 35) MMEs per day per high-risk patient [33]. For the primary analysis of clinicians who prescribe less than 50 MMEs per day per patient, we assume the baseline dose is a tenth of the dose assumed for long-term high-risk patients (26.3 (±3.5) MMEs per day). With at least 101 clinics participating in AESOPS-1, we will exceed the number of clinics needed to have an 80% chance to detect a 7% change in opioid dosage for the 50 daily MMEs and above analysis as well as the below 50 daily MMEs analysis. Table 3 depicts the number of total clinics needed to detect a 3%, 5%, 7%, and 12% reduction in weekly MME with .80 and .85 statistical power.
Table 3.
Power calculations
| MME Reduction | ||||
|---|---|---|---|---|
| Statistical Power | 3% | 5% | 7% | 12% |
| 0.80 | 509 clinics | 183 clinics | 93 clinics | 32 clinics |
| 0.85 | 591 clinics | 213 clinics | 109 clinics | 37 clinics |
3.4. Safety Assessment Plan
The National Institutes of Health established a data safety and monitoring board. The Board includes experts in opioids, overprescribing, biostatistics, or research methods. When notified of an unanticipated event, the data safety and monitoring board will convene and decide whether the study should continue. Data for patients who abruptly stopped an opioid prescription, had an emergency room eligible visit with a diagnosis that could represent a severe complication of untreated pain, died, or left the health system will be extracted from study site EHRs and reported to the Board. Per CDC guideline clarification, abrupt discontinuation of opioids for persons whose most recent prescription exceeds 49-milligram morphine equivalent daily dose is considered unsafe; or as reported to study staff [34]. The Board receives safety measures from study personnel six- and 12-months into the study period.
4. Discussion
This two-group (intervention and control arms) multisite cluster-randomized trial will evaluate the effects of three behavioral EHR interventions on guideline discordant opioid prescribing in primary care. The intervention prompts clinicians through the electronic health record for three categories of patient visits: opioid-naive, at-risk for long-term use, and long-term opioid recipient. The behavioral science principles underlying these nudges include injunctive social norms, social accountability, resetting the default action, and reducing present-biased preferences. We hypothesize that clinics randomized to the intervention group will have lower amounts of opioids prescribed compared to control clinics as measured by weekly MMEs prescribed.
A possible limitation of this study is its difficulty in nudging clinicians during “call-in” opioid refills. Failure to intervene at all encounters may diminish the intervention’s effect since the study captures only in-person visits. However, call-ins for refills are fundamentally different. They often do not directly involve a prescribing clinician discussion and are often brokered by a medical assistant or a nurse. Additionally, patient insurance differs, so coverage for non-opioid substitute medications or services varies by patient. To address this limitation, we will evaluate if patient insurance type is significantly associated with our primary outcome. Because our outcome measure averages over prescriptions, it will not identify the precise behaviors that led to the reduction in MME (e.g., fewer new starts, fewer refills, lowering chronic doses). However, we will address this limitation by conducting sensitivity analyses to evaluate the rates of these behaviors.
Through AESOPS-1, we will test the impact of novel behavioral nudges on clinicians to reduce opioid orders. This study will be pivotal in understanding how to increase adherence to the CDC guideline and Oregon Pain Guidance pain management guidelines. These findings will also contribute to a better understanding of how to reduce opioid exposure in clinical populations.
Acknowledgements
The National Institutes of Health (R33AG057395; PI: Jason Doctor) supports this work. The authors would like to thank Sohini Deva, Tiffany Brown, Ji Young, Michael Schachter, and Michael Suna for their contributions to this study.
Funding: The National Institutes of Health supports this work (R33AG057395; PI: Jason Doctor)
Abbreviations:
- AJ.
Accountable Justification
- AESOPS
Application of Economic and Social psychology to improve Opioid Prescribing Safety
- CDC
Centers for Disease Control and Prevention
- EHR
Electronic Health Record
- MME
Milligram Morphine Equivalent
Appendix
Appendix A:
Opioid Prescribing Diagnoses Exclusions and Medications for Outcome Assessments
| Opioids Trigger List | Link | (“Outcome” Tab) |
| Opioids Outcome List | Link | (“Grouper” Tab) |
| Excluded Cancer Diagnoses | Link | |
| Non-Opioid Dosing for Alternatives | Link |
Appendix B:
Education Module and PainTracker Survey
| Education Module | Link |
| PainTracker Assessment | Link |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Scheiber LZ, Guy GP, Seth P, and Losby JL, “Variation in adult outpatient opioid prescription dispensing by age and sex - United States, 2008–2018,” MMWR. Morbidity and Mortality Weekly Report, vol. 69, no. 11, pp. 298–302, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guy GP Jr et al. , “Vital signs: Changes in opioid prescribing in the United States, 2006–2015,” MMWR Morbidity and Mortality Weekly Report, vol. 66, no. 26, pp. 697–704, July. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Centers for Disease Control and Prevention, 2019. Annual Surveillance Report of Drug-Related Risks and Outcomes — United States Surveillance Special Report, Centers for Disease Control and Prevention, US Department of Health and Human Services, Nov. 1, 2019. Accessed on: Aug. 18, 2020. [Online]. Available: https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillancereport.pdf. [Google Scholar]
- [4].Chang H-Y, Daubresse M, Kruszewski SP, and Alexander GC, “Prevalence and treatment of pain in EDs in the United States, 2000 to 2010,” Am. J. Emerg. Med, vol. 32, no. 5, pp. 421–431, May 2014. [DOI] [PubMed] [Google Scholar]
- [5].Daubresse M et al. , “Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000–2010,” Med. Care, vol. 51, no. 10, pp. 870–878, October. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Scholl L, “Drug and opioid-involved overdose deaths — United States, 2013–2017,” MMWR Morb. Mortal. Wkly. Rep, vol. 67, 2019, doi: 10.15585/mmwr.mm6751521e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Florence CS, Zhou C, Luo F, and Xu L, “The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013,” Med. Care, vol. 54, no. 10, pp. 901–906, October. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tong ST et al. , “Chronic opioid prescribing in primary care: Factors and perspectives,” Ann. Fam. Med, vol. 17, no. 3, pp. 200–206, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dowell D, Haegerich TM, and Chou R, “CDC guideline for prescribing opioids for chronic pain - United States, 2016,” MMWR Recomm. Rep, vol. 65, no. 1, pp. 1–49, March. 2016. [DOI] [PubMed] [Google Scholar]
- [10].Oregon Pain Guidance Group, Opioid and Benzodiazepine Tapering Flow Sheets, 2016. Accessed on: Aug. 18, 2020. [Online]. Available: https://www.oregonpainguidance.org/app/content/uploads/2016/05/Opioid-and-Benzodiazepine-Tapering-flow-sheets.pdf?x91687
- [11].Oregon Pain Guidance Group, Pain Treatment Guidelines, 2020. Accessed on: Aug. 18, 2020. [Online]. Available: https://www.oregonpainguidance.org/pain-treatment-guidelines/
- [12].Langford DJ, Tauben DJ, Sturgeon JA, Godfrey DS, Sullivan MD, and Doorenbos AZ, “Treat the patient, not the pain: Using a multidimensional assessment tool to facilitate patient-centered chronic pain care,” J. Gen. Intern. Med, vol. 33, no. 8, pp. 1235–1238, August. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fox CR, Doctor JN, Goldstein NJ, Meeker D, Persell SD, Linder JA, “Details matter: predicting when nudging clinicians will succeed or fail,” BMJ, vol. 370, no. m3256, September. 2020. [DOI] [PubMed] [Google Scholar]
- [14].Thaler RH, Sunstein CR, Nudge: Improving Decisions About Health, Wealth, and Happiness. Penguin Group, 2009. [Google Scholar]
- [15].Delgado MK et al. , “Association between electronic medical record implementation of default opioid prescription quantities and prescribing behavior in two emergency departments,” J. Gen. Intern. Med, vol. 33, no. 4, pp. 409–411, April. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Meeker D et al. , “Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial,” JAMA, vol. 315, no. 6, pp. 562–570, February. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gerber JS et al. , “Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial,” JAMA, vol. 309, no. 22, pp. 2345–2352, 2013. [DOI] [PubMed] [Google Scholar]
- [18].Persell SD et al. , “Use of behavioral economics and social psychology to improve treatment of acute respiratory infections (BEARI): rationale and design of a cluster randomized controlled trial [1RC4AG039115–01] - study protocol and baseline practice and provider characteristics,” BMC Infect. Dis, vol. 13, p. 290–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jordan MR, Amir D, and Bloom P, “Are empathy and concern psychologically distinct?,” Emotion, vol. 16, no. 8, pp. 1107–1116, September. 2016. [DOI] [PubMed] [Google Scholar]
- [20].Davis MH, “Measuring individual differences in empathy: Evidence for a multidimensional approach,” J. Pers. Soc. Psychol, vol. 44, pp. 113–126, 1983. [Google Scholar]
- [21].Schultz PW, Nolan JM, Cialdini RB, Goldstein NJ, and Griskevicius V, “The constructive, destructive, and reconstructive power of social norms,” Psychol. Sci, vol. 18, no. 5, pp. 429–434, May 2007. [DOI] [PubMed] [Google Scholar]
- [22].Lerner JS and Tetlock PE, “Accounting for the effects of accountability,” Psychol. Bull, vol. 125, no. 2, pp. 255–275, March. 1999. [DOI] [PubMed] [Google Scholar]
- [23].Dinner I, Johnson EJ, Goldstein DG, and Liu K, ❛❛❛Partitioning default effects: Why people choose not to choose’: Correction to Dinner et al. (2011),” J. Exp. Psychol. Appl, vol. 17, no. 4, p. 432, December. 2011. [DOI] [PubMed] [Google Scholar]
- [24].O’Donoghue T and Rabin M, “Doing it now or later,” Am. Econ. Rev, vol. 89, no. 1, pp. 103–124, March. 1999, Accessed: Aug. 21, 2020. [Online]. [Google Scholar]
- [25].Cialdini RB, Cacioppo JT, Bassett R, and Miller JA, “Low-ball procedure for producing compliance: Commitment then cost,” J. Pers. Soc. Psychol, vol. 36, no. 5, pp. 463–476, May 1978. [Google Scholar]
- [26].Aronson E, “The return of the repressed: Dissonance theory makes a comeback,” Psychol. Inq, vol. 3, no. 4, pp. 303–311, 1992. [Google Scholar]
- [27].Meeker D et al. , “Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial,” JAMA Intern. Med, vol. 174, no. 3, pp. 425–431, March. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sullivan M et al. , “A controlled pilot trial of PainTracker Self-Manager, a web-based platform combined with patient coaching, to support patients’ self-management of chronic pain,” The Journal of Pain, vol. 19, no. 9, pp. 996–1005, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McDonald JF and Moffitt RA, “The uses of Tobit analysis,” Rev. Econ. Stat, vol. 62, no. 2, pp. 318–321, 1980. [Google Scholar]
- [30].Tobin J, “Estimation of relationships for limited dependent variables,” Econometrica, vol. 26, no. 1, pp. 24–36, 1958. [Google Scholar]
- [31].Doctor JN et al. , “Opioid prescribing decreases after learning of a patient’s fatal overdose,” Science, vol. 361, no. 6402, pp. 588–590, August. 2018. [DOI] [PubMed] [Google Scholar]
- [32].Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, and Campbell MJ, “Patterns of intra-cluster correlation from primary care research to inform study design and analysis,” J. Clin. Epidemiol, vol. 57, no. 8, pp. 785–794, August. 2004. [DOI] [PubMed] [Google Scholar]
- [33].Weimer MB, Hartung DM, Ahmed S, and Nicolaidis C, “A chronic opioid therapy dose reduction policy in primary care,” Subst. Abus, vol. 37, no. 1, pp. 141–147, 2016. [DOI] [PubMed] [Google Scholar]
- [34].Dowell D, Haegerich T, and Chou R, “No shortcuts to safer opioid prescribing,” N. Engl. J. Med, vol. 380, no. 24, pp. 2285–2287, June. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]