Abstract
Advancements in pharmaceutical technologies have led to the personalization of therapies over the last decade. Three-dimensional printing (3DP) is an emerging technique in the manufacturing of pharmaceutical dosage forms because of its potential to create complex and customized dosage forms according to the patient’s needs. Among the various 3DP techniques based on different functioning mechanisms, fused deposition modeling (FDM) 3D printing is a versatile and widely used method with advantages such as precision of quantity and the ability to incorporate different fill densities. This method is also economical and easily produces complex designs. Hot-melt extrusion (HME) is an established technique in pharmaceutical manufacturing that is utilized in the development of filaments which are used as “ink roll” or feedstock material in FDM 3D printing. This review discusses the various stages involved in FDM 3D printing, including feedstock filament preparation using HME, digital dosage form designs, filament characterization, and various novel applications, and future perspectives.
Keywords: Fused deposition modeling, 3D printing, customized dosage forms, personalized drug delivery, hot-melt extrusion
Graphical Abstract
1. Introduction:
With the recent FDA-approved 3D printed drug product (Spritam®), research interest in 3D printing technology has been growing. 3D printing is believed to be the most effective way to attain patient-focused pharmaceutical product development. The application of 3D printing in the pharmaceutical field has been an emerging field of research because of its ability to customize therapy to meet the requirements of patients (Jamróz et al., 2018b). Owing to its flexibility and ease of adaptability, 3D printing technology is expanding from the early stage of development to the manufacture of end products with simplified design and production cycles (Zema et al., 2017). Because of its automated process and low operating cost, 3D printing is gaining popularity over traditional manufacturing processes in on-demand production (Norman et al., 2017)(Zhang et al., 2018).
3D printing is the process of creating objects three-dimensionally by depositing material in a layer-by-layer fashion from digital designs. The concept of 3D printing was first developed in the 1980s by Charles Hull for fabricating plastic devices from photopolymers (Pucci et al., 2017). It was later used in various other fields, including automotive, aerospace, robotics, and consumer goods industries for rapid prototyping purposes (Azad et al., 2020). After FDA approval of the first 3D printed pill, Spritam®, in 2015, the application of 3D printing has gained tremendous attention in the pharmaceutical field (Warsi et al., 2018). Although there are various 3D printing approaches such as stereolithography (SLA) and inkjet-powder bed, this review focuses mainly on fused deposition modeling (FDM) 3D printing.
The application of 3D printing techniques might facilitate the generation of precision medicine that aims to tailor therapeutic strategies to meet the unique physiological and lifestyle needs of individual patients (Afsana et al., 2019). Most of the pharmaceutical tablet dosage forms manufactured by the existing large-scale manufacturing processes are produced with one-size-fits-all approaches in which the dosage is based on Phase 3 clinical studies. The disadvantage of this approach is that the administered dose might fall outside the optimum dose for individual patients based on the patient’s clinical requirements and may lead to toxicities, adverse events, or a lack of therapeutic activity (Goyanes et al., 2015b)(Wang et al., 2016). This is usually addressed by tablet splitting or compounded medications. However, tablet splitting can break the integrity of any coating material, which might alter the drug release and lead to ineffective drug administration. Compounded medicines often fail in dose accuracy and may become ineffective when patients do not follow dosing instructions (Shah et al., 2010). Moreover, this will lead to a pill burden for patients. Orphan drugs for treating disease conditions affecting fewer than 200,000 people entail a small market in the pharmaceutical industry. As contemporarary manufacturing equipment are intended for large scale industrial manufacturing, 3D printing is highly adavntageous in development of suitable dosage forms. Recently Saydam et al., developed rufainamide tablets for Lennox-Gastaut Syndrome using FDM 3D printing, which is is a rare form of childhood epilepsy (Saydam and Takka., 2020). The 3D printing technique may offer solutions to all these problems because of its ability to selectively deposit and/or fuse (multiple) materials in a layer-wise manner, offering reproducibility in the creation of personalized pharmaceutical dosage forms by utilizing precise control of factors as well as active pharmaceutical ingredient (API) compartmentalization for combination of drugs (Curti et al., 2020).
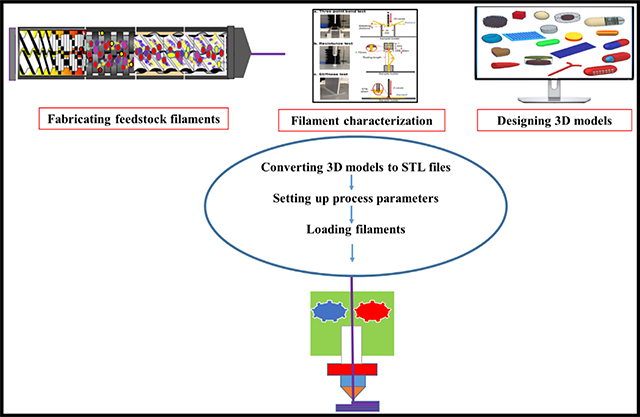
The advantages of 3D printing over conventional tablet manufacturing methods include personalization, improved product complexity, and on-demand manufacturing (Norman et al., 2017). This technology offers tailoring of doses according to the patient’s body mass index, metabolism, genetic variations, and other comorbid conditions, and also enables the production of drug products with tailored release profiles and designs (Trenfield et al., 2019)(Nukala et al., 2019b). It allows the combination of the patient’s daily medications into a single multi-drug dose or polypill. This personalization could improve treatment adherence, especially for pediatric and geriatric patients. Furthermore, 3D printing may be applied in the manufacture of implants such as tracheal splints, bone grafts, and multi-drug implants for chemotherapy (Trenfield et al., 2019); it provides a simple and uniform drug that can easily achieve a targeted release profile. Printing in different shapes attracts the pediatric population where treatment adherence is a major problem. Another major advantage of 3D printing is on-demand manufacturing, which could be useful in time- and resource-constrained settings such as emergency rooms, military operations, and disaster areas, as drug products can be successfully printed according to requirements (Bandari et al., 2021). Personalization and the ability to create complex design dosage forms using the 3D printing technique may not only improve the treatment effectiveness but also minimize wastage of resources because of its unique on-demand manufacturing advantage (Zhang et al., 2018). A graphical representation of the 3D printing ease of use in personalized pharmacotherapy is shown in Fig. 1.
Fig. 1.
Use of 3D printing in the production of dosage forms for personalized pharmacotherapy
Currently available 3D printing techniques mainly rely on three mechanisms: powder solidification, liquid solidification, and extrusion-based systems. A brief introduction to the types of 3D printing techniques and their functioning mechanisms is presented below.
2. Types of 3D printing
2.1. Stereolithography (SLA) 3D printing
SLA is the world’s first and one of the most popular 3D printing technologies (Melchels et al., 2010). This method utilizes liquid solidification via photopolymerization. In this method, a laser is focused on liquid resin, causing polymerization and solidification. This process is repeated in a layer-by-layer manner until a solid object is produced (Melchels et al., 2010)(Lamichhane et al., 2019a). One of main drawbacks of this method is the limited availability of photo-crosslinkable polymers, as most of these polymers are not included on the generally recognized as safe (GRAS) lists. However, it offers advantages such as high resolution and fast 3D printing (Rahman et al., 2018).
2.2. Inkjet 3D printing
This method is also based on liquid solidification. Inkjet printers print free form structures that solidify drop by drop. In this method, the droplets of ink are sprayed from the nozzle, deposited in thin layers, and then cured by cool air or high-energy light (Rahman et al., 2018). Commonly sprayed materials include molten polymers and waxes, 3D curable resins, solutions, and suspensions (Daly et al., 2015). This method offers a higher resolution than powder bed 3D printing. However, it is more challenging to implement because the product geometry depends on the droplet flight path, droplet impact, and surface wetting.
2.3. Binder deposition
This method is based on the powder solidification technique. In this method, a liquid binder is injected into the spreading layer of the powder material in a drop-on-demand manner to selective areas. The unbound powder particles act as a support material for overhanging and porous structures. After the formation of each layer, the formed object is lowered, and a layer of free powder is deposited by a powder jetting system (Goole and Amighi, 2016)(Pardeike et al., 2011). In this method, API and polymer mixtures either dissolve or disperse in the ink or are distributed in the powder bed. After printing, drying or removal of residual solvents and/or unbound powder is carried out. The first FDA approved 3D printed drug product, Spritam®, was based on this technology (Jamróz et al., 2018b)(Norman et al., 2017).
2.4. Powder bed fusion
This method is also based on the powder solidification technique. It involves sintering (partial surface-melting and congealing) or binding of high-melting-point particles with a low-melting-point binder using heat supplied by a laser (Gibson et al., 2010). (In this method, the layers are formed by melting and solidifying the polymeric powder bed using a laser beam. This method is faster but more complex than extrusion-based printing (Norman et al., 2017).
2.5. FDM 3D printing
FDM 3D printing is a type of extrusion-based method where the material is extruded from the nozzle and spread in subsequent layers on the build plate. The material to be fed is in the form of filaments, which are produced by melt extrusion of API and polymer physical mixture (Goyanes et al., 2015a). There are other types of extrusion-based 3D printers that use liquid or semisolid formulations for printing various dosage forms. In FDM 3D printing, filaments fed into the 3D printer are softened, melted, extruded, and deposited layer by layer. Each layer bonds to the layers below and solidifies as it cools down. This process continues until the desired shape is achieved. As the material to be extruded is more viscous, it takes more time to print than most of the other types of 3D printing technologies. The filament qualities such as uniform dimension, elasticity, and stiffness play an important role in printing the dosage form (Bandari et al., 2021) (Lamichhane et al., 2019a). To obtain a printable filament, the selection of suitable polymers and optimization of the HME process parameters are necessary. There are certain disadvantages associated with this method, including the use of high temperatures during the extrusion and printing process, difficulty in producing printable filaments with desired mechanical and viscoelastic properties, and slow printing speed. However, despite these disadvantages, this method is being widely used owing to its low cost, use of non-volatile materials, and simplicity and versatility(Skowyra et al., 2015)(Norman et al., 2017). Although there are various 3D printing approaches such as SLA and inkjet-powder beds being currently investigated for producing 3D printed drug products, the present review focuses mainly on hot-melt extrusion coupled FDM 3D printing.
Hot-melt extrusion (HME) is a continuous manufacturing process that has been used in the plastic and pharmaceutical industries since the 1980s. Over the last two decades, the HME process has been used as a continuous manufacturing technology for the development of various pharmaceutical products such as pellets, granules, films, implants, and ophthalmic and topical delivery systems (Dumpa et al., 2018)(Maniruzzaman et al., 2012)(Sarabu et al., 2019). The application of HME has been demonstrated in the development and manufacture of amorphous solid dispersions, cocrystals, taste masking, modified release dosage forms, floating dosage forms, abuse-deterrent formulations, and patient-centric 3D printed tablets (Butreddy et al., 2020)(Dumpa et al., 2020)(Simöes et al., 2019)(Tan et al., 2020).
HME provides several advantages for the development of pharmaceutical products, such as solvent-free processes without the need for further drying steps, continuous production with fewer processing steps, and highly dense properties of the extrudates and granules produced via HME, which provides an improvement in the compressibility index of APIs with poor flow properties. However, a major drawback of this technique is its limited application in the processing of thermolabile drugs (Jani and Patel, 2014)(Patil et al., 2016).
The HME process involves forcing a blend of API with one or more polymers through the die under specified process conditions such as temperature, screw speed, and feed rate to obtain the desired product with uniform size, shape, and density. The processing steps during HME involve blending the API, polymeric carrier, and other additives such as plasticizers, which are fed through the hopper followed by conveying, melting, mixing, pumping, and pressurization of the physical blend within the extruder (Dumpa et al., 2018)(Maniruzzaman et al., 2012). After the extrusion process, the obtained filaments or extrudates are subjected to further processing steps such as milling, pelletization (tableting), direct shaping (implants, devices), and 3D printing (Kallakunta et al., 2019). Coupling HME with FDM is a new emerging technology for the 3D printing of pharmaceutical products. Filaments, which are used for 3D printing, have been produced by the extrusion of APIs with thermoplastic polymers. The combination of HME and FDM can produce personalized drug products with a structured dosage. Additionally, this combined technology reduces downstream processing associated with conventional dosage forms (Repka et al., 2018). (Repka et al., 2018). A graphical representation of the coupling of HME with FDM for producing pharmaceutical drug products is illustrated in Fig. 2. The current review focuses on the application of HME-based FDM in 3D printing for the development of various dosage forms.
Fig. 2.
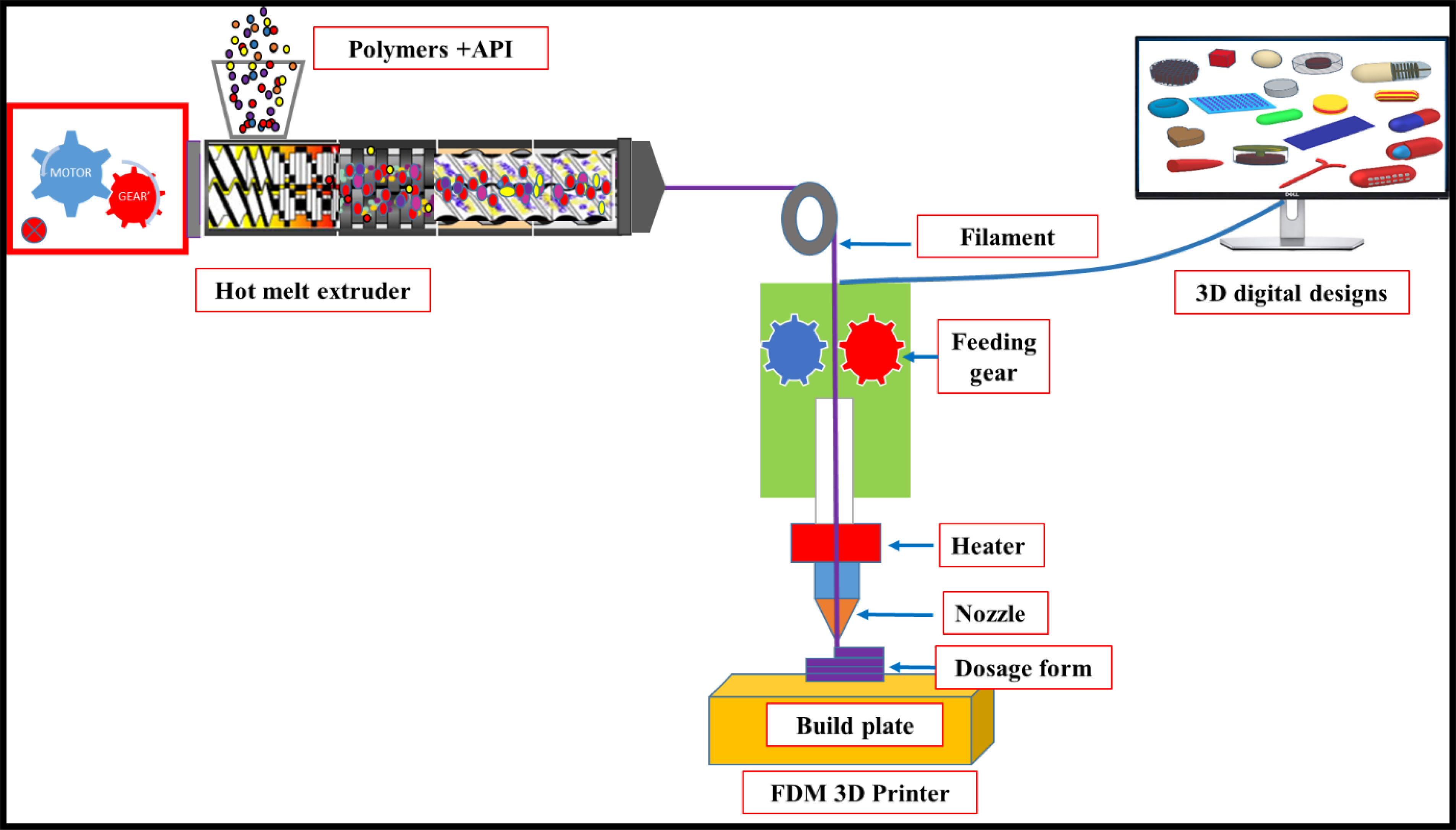
Graphical representation of hot-melt extrusion coupled fused deposition modeling (FDM) 3D printing for the fabrication of pharmaceutical dosage forms. API: active pharmaceutical ingredient.
3. Process steps involved in FDM 3D printing of personalized drug products
The basic procedure involved in FDM 3D printing for the fabrication of personalized drug products from digital designs consists of five common steps: i) designing a 3D digital dosage form, ii) converting the designed 3D models into a printer-readable STL format file, iii) setting up process Parameters and slicing the STL file to a layer-by-layer design, iv) formulation and fabrication of feedstock material, and v) printing and evaluation of dosage forms.
The details involved in FDM 3D printing of pharmaceutical dosage forms are discussed in the following sections (Aho et al., 2019)(Lamichhane et al., 2019b)
3.1. Designing a 3D digital dosage form
The first step in 3D printing of a dosage form is designing the digital structure of the dosage form to meet the requirements of the medication. Dosage forms with different fill levels (porous or dense) or shapes (tablets, capsules, films, implants, donut shapes, etc.) can be designed using computer-aided design (CAD) software. This step of designing the digital dosage form is critical because the structure of the dosage form significantly affects pharmaceutical properties and patient compliance. Simple dosage form designs such as tablets, capsules, donuts, films, and patches can be designed using a Microsoft 3D builder® or Tinkercad® software, which are easy to operate. Designing complex structures such as multiple compartmental tablets or capsules, mouthguards, and implants require more advanced professional CAD software such as Autodesk®.
3.2. Converting the designed 3D models into a printer-readable STL format file
Currently available commercial 3D printers cannot directly read CAD software files. Hence, once the desired 3D model is created, it is converted into an FDM 3D printer-readable STL format file. Most of the commercially available FDM 3D printers work with STL format files.
3.3. Setting up process parameters and slicing the STL file to a layer-by-layer design
The next step is importing STL files into software that controls the FDM 3D printer. This software slices the designed CAD files into layer-by-layer patterns and sends instructions to the 3D printer to print a dosage form. Various parameters such as geometry (length, width, height, and diameter), internal structure (infill density, infill pattern, and wall thickness) of the dosage form, and 3D printing process parameters (nozzle and bed temperature, print speed, layer height, etc.) can be altered and optimized based on the application of dosage form and desired pharmaceutical properties.
3.4. Formulation and fabrication of feedstock material
The filaments that will be used as feedstock material for FDM 3D printing are fabricated with suitable polymeric carriers and APIs using hot-melt extrusion. The manufactured filaments should have desirable mechanical and rheological properties for 3D printing to produce high-quality pharmaceutical dosage forms.
3.5. Printing and evaluation of dosage forms
Dosage forms are printed by combining hot-melt extruded filaments and a digitally controlled FDM 3D printer. The printed dosage forms are further evaluated for suitability to specific applications.
4. Process parameter considerations in FDM 3D printing
Similar to traditional pharmaceutical manufacturing techniques, various process parameters can be altered in FDM 3D printing and can significantly influence many product characteristics as well as dosage form performance. Understanding the effect of each parameter on product quality is necessary for producing a dosage form with desired characteristics for patient-centric pharmacotherapy. An overview of the FDM 3D printer setup and their details are presented in Fig. 3. Below are the various process parameters and their effect on product performance.
Fig. 3.
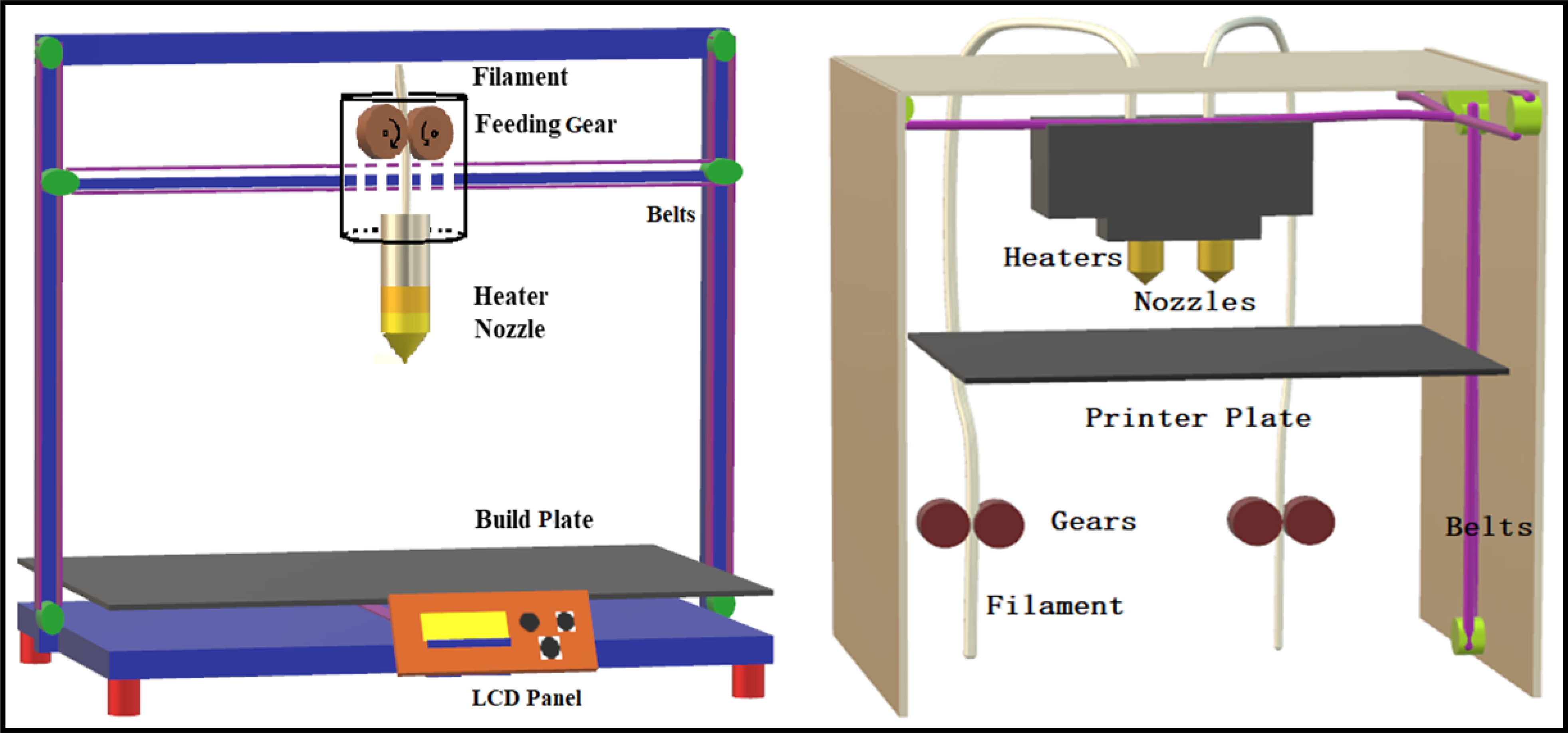
Schematic representation of a single nozzle 3D printer (left) and dual nozzle 3D printer (right).
4.1. Printing temperature
FDM 3D printing requires that the feedstock material is melted or softened and deposited layer by layer to produce dosage forms. The minimum temperature required to achieve melting/deposition needs to be optimized without causing degradation of the polymers and API. Usually, the temperature required is higher than the temperature used in hot-melt extrusion. The viscosity of polymers plays a crucial role in determining the printing temperature because the melted polymers must pass through a narrow nozzle (0.25 – 1.0 mm) of the FDM 3D printer before being deposited on the build plate. In a recent study conducted by Alhijjaj et al. (2019)(Alhijjaj et al., 2019), printed dosage forms were made using polycaprolactone (PCL) at 70 °C, 80 °C, and 90 °C; dosage forms printed at 80 °C and 90 °C were more distorted than those printed at 70 °C. Hence, the authors concluded that there would be a lower limit to the melt flow index at a specific temperature below which quality printing is not possible.
4.2. Build plate temperature and surface
As the printing takes place in a layer-by-layer fashion, the dosage forms need to stay in contact with the printer build plate to produce a finished dosage form. To maintain the adhesiveness of the dosage form with the build plate, the build plate should be heated to a specific temperature based on the type of polymeric carrier and API used in the preparation of feedstock material. Inadequate build plate temperature causes interruption in printing as the dosage forms lose contact with the build plate, resulting in an incomplete dosage form. It is possible that adjusting the build plate temperature is not sufficient to maintain the adhesiveness between the build plate and dosage form; it may be necessary to change the polymers used in the preparation of feedstock filaments.
4.3. Printing speed
The time required to produce a single dosage form depends on the printing speed. This parameter needs to be optimized as high printing speeds result in low-quality dosage forms and low printing speeds require more time to print a single dosage form. The quality of feedstock filaments needs to be considered when choosing print speed since poor quality filaments may break at high speeds. Alhijjaj et al. (2019)(Alhijjaj et al., 2019) investigated the effect of different printing speeds on the quality of dosage forms by printing at three different printing speeds (30 mm/s, 90 mm/s, and 160 mm/s). The results showed that the thickness of dosage forms tends to decrease with increasing temperature at 30 mm/s and 160 mm/s printing speeds, whereas dosage forms printed at 90 mm/s showed consistent thickness independent of temperature, producing the most reproducible results with low standard deviations.
4.4. Infill density
The porosity of the dosage depends on the percent infill density. Dosage forms with low infill densities are more porous and have a greater surface area and lower hardness. The infill density is a critical parameter that has a profound effect on in vitro drug release profiles and the hardness of the printed dosage form. Many researchers have reported that the infill density and drug release rates are inversely proportional. A study conducted by Goyanes et al. (2017)(Goyanes et al., 2017) reported that tablets printed with 20% infill density dissolved faster than analogous tablets printed with 100% infill density. Some researchers have also developed floating dosage forms (Dumpa et al., 2020) (by reducing the fill density) and orodispersible films with high porous structures that could easily disintegrate (Ehtezazi et al., 2018). demonstrating an advantage of FDM 3D printing.
4.5. Infill pattern
The fill pattern defines how the core structure of the dosage form looks when printed with different fill densities. Similar to infill density, infill patterns may affect the hardness and in vitro release profiles of dosage forms. Commercially available 3D printers have default infill patterns including line, diamond, concentric, hexagonal, shark fill, and diamond shapes. The printing time of the dosage form also varies with different fill patterns. Palekara et al. (2019)(Palekar et al., 2019) observed that printing time varies with infill pattern and was in the following order for 100% infill caplets: diamond > linear > shark fill > hexagonal. The authors reported that the time required for tablet disintegration also varied with infill pattern and was in the order of diamond > hexagonal > linear > shark fill, and concluded that tablets with linear, shark fill, and hexagonal infill patterns had more porous structures than those of the diamond infill pattern, resulting in an easier uptake of the dissolution medium and faster disintegration.
4.6. Shell or wall thickness
The shell or wall can be defined as the structure created by perimeters/outlines in the horizontal plane and has a fill pattern that is different from that of the inner core. The shell has a denser structure than the core due to differences in the fill pattern. This difference in the fill pattern further affects release and hardness profiles. Dosage forms printed without shells or walls have a rough surface compared with that of dosage forms with walls or shells. Zhang et al. (2017a)(Zhang et al., 2017b) printed tablets with 0, 0.4, and 1.6 mm shell thickness and found that tablets with 1.6 mm shell thickness demonstrated extended drug release properties compared to those of tablets with 0 and 0.4 mm shell thickness. Tablets with higher shell thickness have a denser structure, whereas tablets without a shell have a loose structure.
4.7. Top/bottom thickness
Like shell thickness, top/bottom thickness has a separate fill pattern that is different from that of the inner core and significantly affects the physical properties as well as the in vitro drug release characteristics of dosage forms. (Lamichhane et al., 2019b) developed dosage forms with a unique design, keeping one side of the tablet closed and the other side opened to achieve the desired drug release and reported that tablets with top/bottom thickness demonstrated controlled drug release compared to tablets without top/bottom thickness. The authors concluded that tablets without top/bottom thickness had easy access to dissolution media compared to that of the counterpart closed systems.
4.8. Layer height
Layer height will affect the resolution and smoothness of the dosage form. Dosage forms printed with lower layer height will have smoother surfaces but require more time for printing. Dosage forms printed with higher layer heights offer enhanced mechanical properties and higher strength (3D Hubs, 2020)(“The impact of layer height on a 3D print | 3D Hubs,” n.d.).
5. Filament properties and characterization parameters in FDM 3D printing
HME filaments to be used as feedstock material in FDM 3D printing should possess suitable mechanical, rheological, and adhesive properties to produce high-quality dosage forms. The flexibility and brittleness of filaments are two key mechanical properties that are used to assess the suitability of filaments for FDM 3D printing. Currently, polylactic acid (PLA) filaments possess ideal mechanical, rheological, and adhesive properties for FDM 3D printing and are being used as reference material for predicting the suitability of pharmaceutical-grade polymers for printing. A schematic representation of the mechanical properties of filaments used in HME-FDM 3D printing is presented in Fig. 4.
Fig. 4.
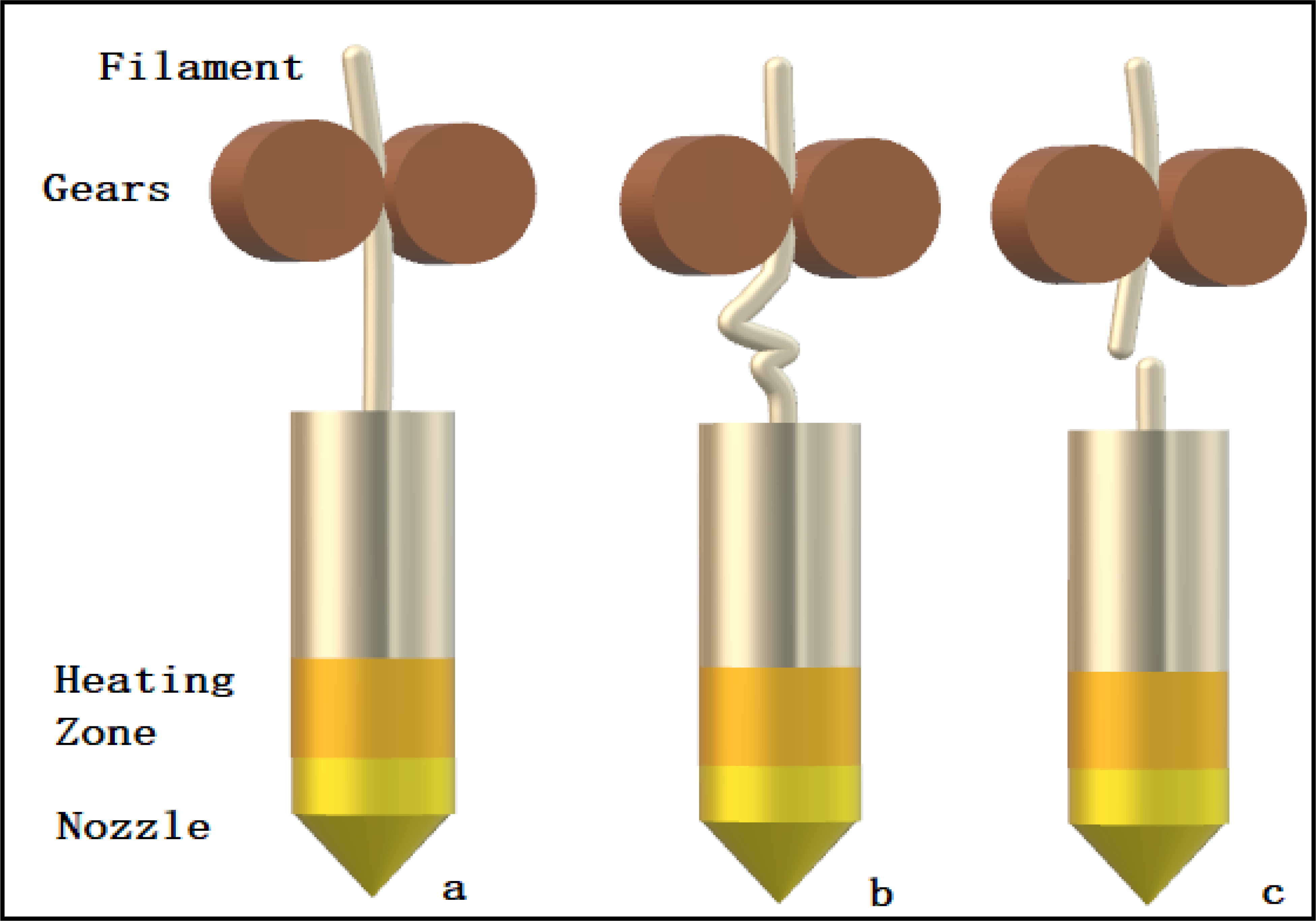
Schematic diagram demonstrating the mechanical behavior of various types of filaments during the 3D printing process: a) optimal filament; b) soft filament; c) brittle filament.
5.1. Brittleness, flexibility, and stiffness
Brittleness of filament is defined as breakage without significant plastic deformation, whereas flexibility is defined as tolerance to bending without breaking (Zhang et al., 2019). Filaments should have a balance between flexibility and brittleness for successful feeding through an FDM 3D printer (Fig. 4a). Brittle filaments are difficult to load into the printer head and can easily break by feeding gears (Fig. 4c) and blocking the nozzle. Filaments that are too flexible will be squeezed between the feeding gears of the printer and will not be pushed through the printer nozzle (Fig. 4b). The tests used to assess the mechanical properties of filaments for FDM 3D printing are discussed below.
Zhang et al. (2017b)(Zhang et al., 2017a) carried out optimization studies on the formulation composition of filaments suitable for 3D printing. The breaking stress (ratio of force to the cross-sectional area) was considered as “load” and the breaking distance was considered as “deformation.” The stiffness of the filament is determined using the following equation, stiffness = load/deformation.
The 3-point bend test was used to assess the breaking force, breaking distance, and stress of a filament. The authors reported that brittle filaments had small breaking distances and that the rough surface of filaments caused high friction during the feeding process; this could be solved by adding suitable polymers to smooth the surface. A printable filament should have high breaking stress, high stiffness, and a long breaking distance. Furthermore, Zhang et al. (2019)(Zhang et al., 2019) described Repka-Zhang tests that included both the flexibility and brittleness/toughness test and the stiffness test. Testing for each single filament formulation was repeated 15 times. In detail, a TA-XT2 analyzer and a TA-95N probe set with a 25 mm supporting gap were used for the flexibility and brittleness tests. The filament samples were cut into rods with a length of 5 cm and then placed on the sample holder (25 mm width gap). The blades moved at a speed of 10 mm/s until they reached a distance of 15 mm below the sample. For the stiffness analysis, the sample holder was a solid flat metal surface, and the filament rods were placed on the surface. The blade was made to penetrate the sample until it created a 35% change in deformation (0.6 mm). The distance of the applied force from the start to the peak point was defined as the breaking distance. The stiffness has been stated to be proportional to the force applied for the deformation and is a constant (Ilyés et al., 2019). Öblom et al. (2019)(Öblom et al., 2019) adopted this method to perform a 3-point bend test. Palekar et al. (2019)(Palekar et al., 2019), Prasad et al. (2019)(Prasad et al., 2019), and Tidau et al. (2019)(Tidau et al., 2019) used different parameters to perform the 3-point bend test.
Aho et al. (2019)(Aho et al., 2019) applied tensile tests to evaluate the mechanical behavior of the filament. The linear part of the stress-strain curves can be used to calculate the elastic modulus that is essentially the measure of stiffness. The yield strength is the strength of the material at the yield point at which the deformation changes from elastic (reversible) to plastic (irreversible), whereas the ultimate strength is the maximum strength. The yield strength here is the same as the stress in the tests conducted by Zhang et al. (2019)(Zhang et al., 2019). The authors reported that filaments containing a high amount of indomethacin (IND) were more brittle than those with low IND content.
Elbadawi (2019)(Elbadawi, 2019) evaluated the effect of different molecular weight polyethylene glycols (PEGs) on PCL-ciprofloxacin (CIP) filaments. The viscosity, solidification properties, and adhesive properties were analyzed. The viscosity and adherence characteristics were measured using the tack function of a rheometer at 40 °C following shear at 170 °C to simulate the process of FDM. The results showed that both CIP and PEG affected all three processing parameters. The ternary blend with 10% w/w PEG 8000 exhibited rheological and adhesive properties ideal for FDM.
Fuenmayor et al. (2018)(Fuenmayor et al., 2018) used dynamic mechanical analysis (DMA) to evaluate the filaments of all formulations using a TA Instruments DMA Q800. The test was performed in single cantilever mode using a frequency of 1 Hz and an amplitude of 15 μm. A temperature range between −80 °C to 150 °C with a 3 °C /min change rate was used to determine the storage modulus, loss modulus, and glass transition temperature. To determine the stiffness, the force that is applied to the filament at any given moment to obtain the desired amplitude expressed in newtons (N) is considered as “load.” The distance of the filament moved from its original position is expressed in meters (m) and is considered as “deformation” To determine the brittleness, the storage modulus (E’) values were determined in single cantilever mode with a frequency of 1 Hz. The filament had a length of 17.5 mm and varying diameters. A quasi-static 3-point bend test of 25 mm filament lengths was performed separately. The force applied to the samples was ramped up at 3 N/min and the test was stopped either when samples broke or when a maximum displacement was achieved. The Brostow-Hagg Lobland-Narkis equation was utilized to obtain brittleness (B) values, and sb and (E’) were calculated based on the room temperature 3-point bend test.
Gültekin et al. (2019)(Gültekin et al., 2019) used the dry spaghetti fracturability test method to test flexibility using a texture analyzer. Three different filament samples (5 cm length) were used to measure their mechanical strength (flexibility). Isreb et al. (2019)(Isreb et al., 2019a) tested the breaking stress of 10 mm length filaments by measuring tensile strength. The deformation rate was 20 mm/min and data were collected every 50 ms. All samples were measured in triplicate. A stress-strain graph was plotted for each sample. Korte and Quodbach (2018)(Korte and Quodbach, 2018) performed a 3-point bend test using the Repka-Zhang method for breaking distance but used a tensile test to determine the stiffness. All 20-mm-long samples were clamped between two grips with a torque of 1 N. Using the texture analyzer, the elongation and the corresponding forces were measured at a moving speed of 0.01 mm/s until filaments broke. In all cases, for elongations < 0.25%, the linear elastic behavior of the filaments was observed. Therefore, Young’s modulus was calculated using the slope between 0.05% and 0.25% elongation in the stress-strain curves.
Wang et al. (2020)(Wang et al., 2020) claimed that the suitability of filaments for FDM 3D printing depended not only on breaking stress and distance but also on other factors. The authors used Hooke’s law (F = -kx) where “F” is the stress, “x” is the distance the filament moved from the center, and “k” is a constant that can be calculated from the aforementioned equation, to predict the printability of hot-melt extruded filaments, and found that as the value of “k” increased, the suitability of filaments for printing improved, and a minimum “k” value of 40 g/mm3 was required for filament printability.
Recently, Pengchong et al. (2020)(Xu et al., 2020) developed a quantitative model and compared three different texture-analysis methods (three-point bend test (brittleness), stiffness test, and resistance test) (Fig. 5) to study the correlation between the mechanical properties of hot-melt extruded filaments and their suitability for 3D printing. The authors extruded a total of 32 filaments using IND as a model drug and various pharmaceutical polymers. The authors reported that the toughness test showed relatively low variation and high reproducibility compared to those of the brittleness and resistance tests. Filaments with higher brittleness, toughness, and resistance values demonstrated greater printability. The authors concluded that the toughness test differentiated the printable and non-printable filaments more accurately than the brittleness and resistance tests. A threshold toughness of 80 kg/mm2 was determined as the lowest value required for filaments to be successfully used in the FDM 3D printing process.
Fig. 5.
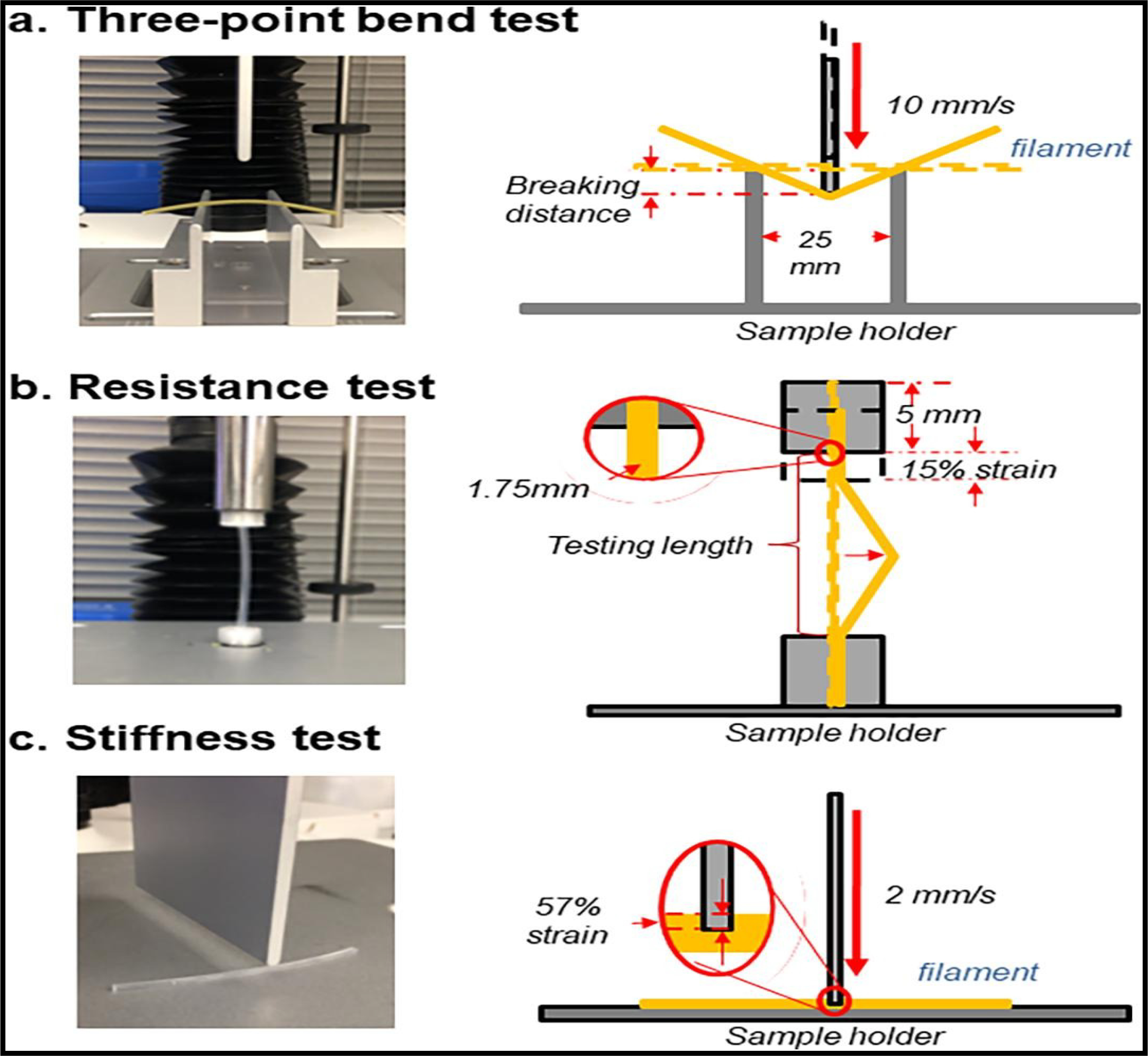
Texture analyzer setup for filament mechanical property test. Illustrations of (a) a three-point bend (3PB) test, (b) resistance test, and (c) stiffness test. (Xu et al., 2020) (permission to reprint the figure has been obtained).
Nasereddin et al. (2018)(Nasereddin et al., 2018) completed feed ability and texture analysis (TA) screening tests. A compression test of the simulated filament through the print head feed process was performed using a TA.XT2 plus texture analyzer equipped with an internal unit and a 5-kg load cell. The filaments were axially compressed at a compression rate of 3.15 mm/s, which corresponds to the drum moving speed of the MakerBot® Replicator 2X. The 5-cm-long filament sheet had a compression distance of 15 mm and a trigger force of 0.05 N, collecting data during both compression and release.
The above-presented case studies show that various researchers have developed and used different approaches to assess the suitability of hot-melt extruded feedstock filaments for the FDM 3D printing process. However, due to variations in the specifications and settings of each 3D printer, it is a challenging task to derive a universal characterization test that perfectly determines the suitability of hot-melt extruded Filaments for FDM 3D printing. Among all the developed characterization tests, the toughness test developed by Pengchong et al. (2020)(Xu et al., 2020) could detect the printability of filaments without the need for testing in a real 3D printer.
6. Applications of FDM 3D printing for drug delivery
Based on the advantages of HME coupled FDM 3D printing, several research groups have developed and evaluated both traditional and novel dosage forms, including conventional immediate-release tablets and capsules, controlled- and modified-release dosage forms, novel floating systems for increased gastric retention, pulsatile release systems, transdermal and transmucosal delivery systems, and personalized implants and devices. Fig. 6 shows various dosage forms that have been designed in recent years using FDM 3D printing. The case studies related to various dosage forms produced by HME-based FDM 3D printing are discussed below.
Fig. 6.
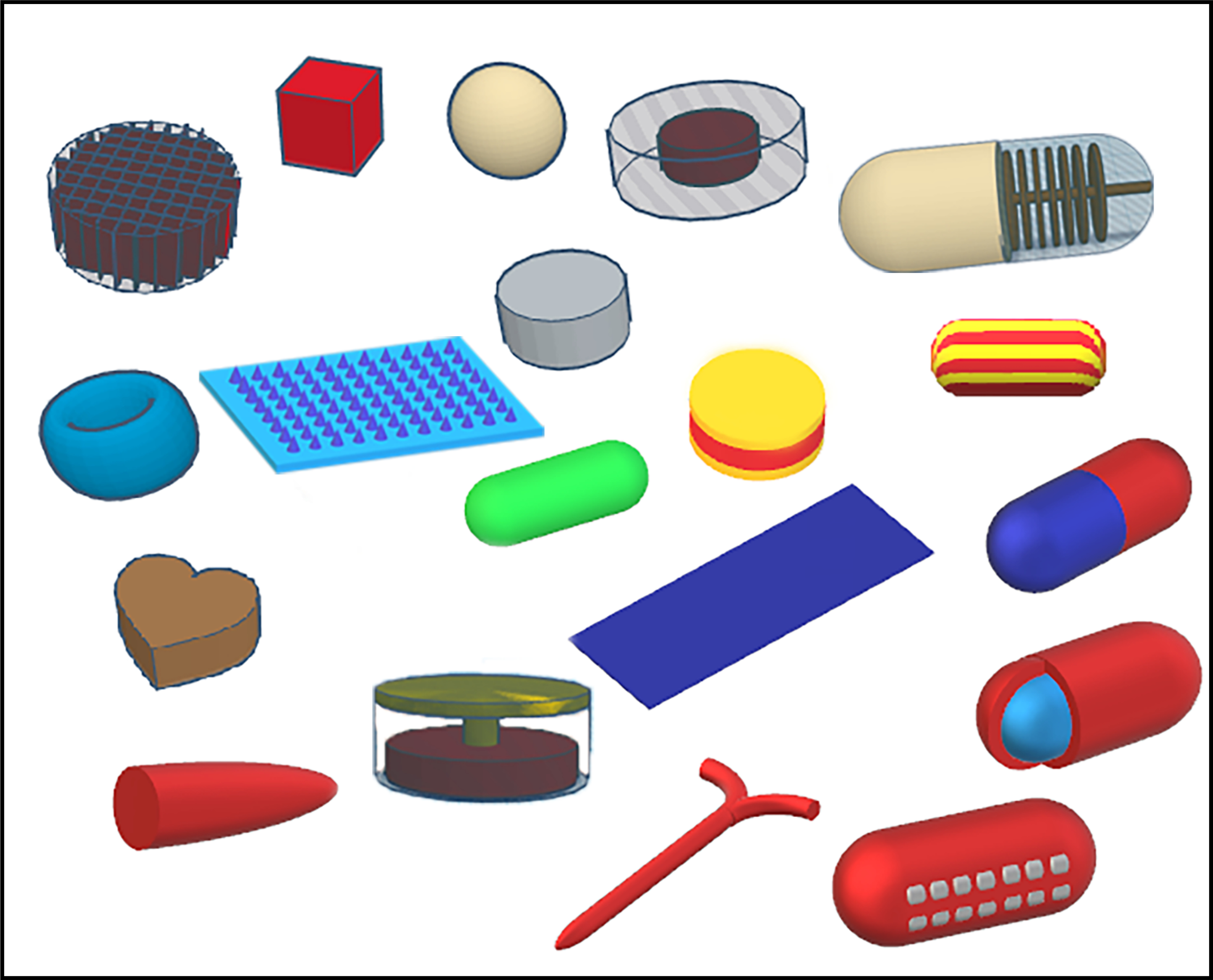
Examples of various complex dosage forms that have been produced with fused deposition modeling 3D printing.
6.1. Immediate-release tablets and capsules
Several immediate-release dosage forms have been developed using 3D printing. Kempin et al. (2018)(Kempin et al., 2018) used a combination of PEGs (molecular weight 6000 and 2000), kollidon VA64, polyvinylpyrrolidine (PVP K12), and poloxamer 407 to produce immediate-release tablets of the thermo-sensitive drug pantoprazole sodium. The printing temperature used in this study was less than 90 °C. Tablets printed with PVP K12 showed complete drug release in 10 min and met the target time of 15 min for complete dissolution. The authors also investigated the effect of fill density on the drug-release profile and reported that tablets printed with lower fill densities had higher dissolution rates compared with those with higher fill densities due to the increased porosity and surface area of tablets with lower fill density.
Arafat et al. (2018)(Arafat et al., 2018) printed oral theophylline dosage in a radiator-like design for immediate release profiles. The authors used a blend of polyethylene oxide (PEO) of different molecular weight with theophylline to fabrícate filaments for 3D printing. PEG 6000 was used as the plasticizer. Radiator-like structures were designed with varying inter-plate spacings of 5, 10, 15, or 20 mm. From the drug release profiles of printed dosage forms, the authors concluded that a minimum of 1 mm inter-plate spacing is essential to increase drug release from the structure and meet USP criteria for immediate-release products. Drug release from radiator designs with 0.5 mm inter-plate spacing was slower compared to that of other structures despite a similar surface to mass ratio. This may be due to the inter-plate adhesion caused by the swelling nature of PEO, which in turn reduced the contact surface area with the dissolution media and consequently the drug release. Filaments prepared from PEO with molecular weights of 300K and 600K demonstrated desirable mechanical and rheological properties for FDM 3D printing.
Sadia et al. (2018a)(Sadia et al., 2018a) developed channeled tablets using FDM 3D printing to accelerate drug release from 3D printed dosage forms with a basic caplet-shaped design measuring 17.2 × 6.8 × 6.25 mm. The caplet was perforated with channels of different widths ranging from 0.2 mm to 1.0 mm. Eudragit® E was used as a matrix polymer with hydrochlorothiazide as the model drug. Different disintegrants, including Ac-Di-Sol®, Primellose®, Primojel®, Polyplasdone-XL®, and Explotab®, were studied. Tablets were printed with filaments containing disintegrants and compared with printed tablets (control) without disintegrants. The authors reported that there was no significant increase in drug release in tablets containing disintegrants. However, channeled tablets exhibited faster drug release compared to that of the control without channels. Tablets with short channels (0.2 – 0.4 mm) showed little improvement, whereas channels with widths greater than 0.8 mm showed a significant increase in drug release. A cumulative drug release from tablets of 88.5% was seen at 30 min with a channel width of 1.0 mm. The study reported that a channel size of ≥ 0.6 mm was necessary to accelerate drug release from the dosage form to meet drug-release criteria according to pharmacopeia.
Although various immediate-release dosage forms have been developed using FDM 3D printing technology employing a layer-by-layer deposition approach, it is challenging to achieve immediate-release profiles using this technique. More research is needed to address the remaining challenges.
6.2. Controlled- and delayed-release dosage forms
Controlled-release dosage forms maintain constant plasma drug concentrations for a longer time and reduce the frequency of medication compared to that of conventional administration. This enhances patient compliance and improves pharmacotherapy. Yang et al. (2018)(Yang et al., 2018) developed 3D printed ibuprofen tablets with an internal scaffold structure to provide controlled drug release. Ethylcellulose (EC) was used as the matrix polymer and HPMC (K100-LV), polyvinyl alcohol (PVA), sodium alginate, and xanthan gum were evaluated as release modifiers. The amount of ibuprofen in the formulation was varied between 16% to 24% (w/w) to determine the effect of drug loading on 3D printability and in vitro release profiles. Tablets with different shell thicknesses (0.4, 0.8, and 1.2 mm) and infill densities (15%, 20%, and 25%) were printed to determine the effect of internal structure on drug release rate. Formulation with a 20% w/w drug load produced optimal filaments for 3D printing. Drug release profiles showed that tablets with 25% w/w HPMC as a release modifier showed 95% drug release in 24 h compared to 17.8% without HPMC. By increasing the drug concentration in the formulation, the cumulative drug release at 24 h increased but the quality of the printed tablets decreased. The fill density and shell thickness of the tablets had a significant inversely proportional effect on release performance. The authors concluded that changing both printing parameters and formulation composition resulted in a 24-h controlled release formulation.
Tan et al. (2019)(Tan et al., 2019) optimized various polymeric compositions for FDM 3D printing of sustained-release theophylline caplets. The study used hot-melt extrusion to disperse theophylline in the polymer matrix and fabricated the feedstock filaments for 3D printing. Hydroxypropyl cellulose (HPC), Eudragit RL PO, PEG, and theophylline were combined in different ratios to produce filaments with suitable mechanical properties for FDM 3D printing. Caplet-shaped dosage forms were designed as CAD models (length 20 mm, width 10 mm, and height 5 mm) and combined with previously extruded filaments to print the dosage forms. The printed caplets were tested for in vitro dissolution profiles; the drug release was sustained for more than 10 h in an optimized formulation. The tablets containing lower amounts of Eudrgit RL PO exhibited faster drug release compared to that of the other formulations. The authors concluded that FDM 3D printing is a cost-effective and convenient manufacturing technique to produce personalized medicines.
Borujeni et al. (2020)(Homaee Borujeni et al., 2020) used FDM 3D printing to obtain dosage forms with a zero-order drug release. Filaments extruded using hot-melt extruder contained carbamazepine, EC, and HPC (3%, 64.7%, and 32.3% w/w, respectively) and 20% w/w of triethyl citrate. Cylindrical tablets with 13 mm diameter and 3.5 mm thickness were designed and printed with the fabricated filaments. The tablets showed excellent uniformity in weight, drug content, and physical dimensions and possessed desirable mechanical properties for packing and handling purposes. Both filaments used for 3D printing and printed tablets were subjected to in vitro dissolution studies. Drug release from the filaments followed first-order drug release whereas a zero-order drug release pattern was observed from the 3D printed tablets. This study concluded that the methodology for making HPC/EC 3D printed tablets could be used to manufacture dosage forms with zero-order drug-release profiles.
Delayed-release dosage forms are beneficial for drugs that are not stable in the gastric region or are only absorbed in the lower parts of the gastrointestinal tract. Okwuosa et al. (2017)(Okwuosa et al., 2017) fabricated gastro-resistant 3D printed tablets using a dual nozzle FDM 3D printer. The authors printed the inner drug core with one nozzle and the enteric outer core that provided gastric resistance with the second nozzle. PVP was used as the matrix polymer for the inner core and methacrylic acid co-polymer for the outer enteric coat. Budesonide, theophylline, and diclofenac sodium were used as model drugs. Capsules were printed with different enteric-coat shell thicknesses (0.17, 0.35, 0.52, 0.70, or 0.87 mm). Tablets with an enteric-coat shell thickness of less than 0.35 mm did not provide any gastric resistance; drug release was observed in less than one hour. With an enteric shell thickness of 0.52 mm and above, less than 3% drug release was seen in the gastric medium at 2 h. All three model drugs demonstrated similar pH-dependent drug release. From the above observations, the authors concluded that a minimum enteric shell thickness of 0.52 mm was necessary to provide gastric resistance.
Goyanes et al. (2017)(Goyanes et al., 2017) used HPMC AS as an enteric polymer for the development of modified release “printlets” Three grades of HPMC AS were evaluated for gastric protection and paracetamol was used as a model drug. Tablets with two different drug loadings (5% and 50%) and infill densities (20% and 100%) were tested for in vitro release performance. The authors reported that drug release was faster in tablets printed with LG grade, followed by MG and then HG grade. All the tablets showed less than 10% drug release in the first two hours, in accordance with USP criteria for delayed-release dosage forms. The authors highlighted that even with 50% drug loading, less than 10% drug release was observed in the first two hours, indicating the potential of the 3D printing technique in manufacturing delayed-release dosage forms with higher drug load. The authors stated that tablets with 20% infill showed hollow spaces in the inner part of their structures and faster drug release compared to that of tablets with 100% infill. Drug release from these 3D printed tablets was governed by both drug diffusion and polymer surface erosion mechanisms.
The above case studies demonstrate the potential of FDM 3D printing for producing various modified-release dosage forms. Dual-nozzle 3D printers offer special advantages for printing outer enteric coats and inner drug coats for preparing delayed-release dosage forms.
6.3. Novel floating drug delivery systems
Floating drug delivery dosage forms are low-density systems that can float on gastric contents for predictable and prolonged periods and deliver medication in the upper part of the gastrointestinal tract. They are advantageous for drugs such as weak acids that are soluble in acidic environments, or drugs that are degraded by enzymes or in alkaline pH (Streubel et al., 2OO6)(WorsØe et al., 2011). Using the capability of FDM 3D printing in the design and printing of hollow or porous dosage forms, several studies have demonstrated the potential of this technique for developing gastro-retentive floating drug delivery systems without adding any gas-forming agents (WorsØe et al., 2011)(Dumpa et al., 2020). Chai et al. (2017)(Chai et al., 2017) were the first to demonstrate the feasibility of FDM 3D printing for developing floating dosage forms. HPC EXF and domperidone were blended in a 90:10 w/w ratio to prepare filaments for 3D printing. The extrusion and printing temperatures were 145 °C and 210 °C, respectively. The extruded filaments were then printed into hollow-structured cylindrical tablets with a 10 mm diameter and 3.2 mm height. The floating capability of the printed tablets was strongly related to density; tablets with a density greater than 0.9 g/cm3 tended to sink to the bottom of the dissolution vessels. Optimized floating tablets remained afloat in dissolution media for 10 h and demonstrated sustained drug release; when evaluated in vivo in New Zealand rabbits, tablets remained buoyant in the rabbits’ stomachs for 8 h and disappeared at the end of 12 h. In vivo pharmacokinetics in rabbits showed a tmax of 1.5 h and 5 h for commercial and 3D-printed floating domperidone tablets, respectively, demonstrating the success of FDM 3D printing dosage forms in vivo.
Dumpa et al. (2020)(Dumpa et al., 2020) used FDM 3D printing to fabricate gastro-retentive floating pulsatile tablets. HPC and EC polymers were used to print hollow structured shells with different geometries (shell thickness, wall thickness, and infill density) and compressed immediate-release tablets into the previously printed hollow structures. The authors reported that all the prepared tablets floated immediately without lag time. The lag time for the pulse release of medication varied from 30 min to 6 h based on the geometrical properties and concentration of EC. The tablets with 2 mm shell thickness, 1.6 mm wall thickness, 100% infill density, and 0.5% EC demonstrated the desired lag time of 6 h. This study demonstrated the potential of FDM 3D printing in the development of complex customized drug delivery systems for personalized pharmacotherapy.
(Lamichhane et al., 2019b) developed customized 3D printed tablets for intra-gastric floating delivery of pregabalin. Tablets with three different infill densities (25%, 50%, and 75%) were printed either as open or closed systems. The open-system tablets did not have any top or bottom, whereas the closed-system tabletswere printed with a top and bottom thickness of 0.4 mm. Open systems failed to show floating behavior because dissolution media entered the void spaces and increased the density of tablets. The presence of top and bottom layers in the closed system prevented the entry of media into the void spaces and tablets were able to remain buoyant. Based on these results, the authors designed a novel customized tablet with a closed bottom and partially opened top, and demonstrated their good floating behavior and zero-order drug release.
Kimura et al. (2019)(Kimura et al., 2019) similarly fabricated floating dosage forms with zero-order drug release profiles using itraconazole, which is a poorly water-soluble weak base, as a model drug. HPC and PVP were used as matrix polymers for filament preparation. Hollow structured tablets with different shell thicknesses (0.5 – 1.5 mm) and different top/bottom thicknesses (0.3 – 0.5 mm) were printed and evaluated for drug release in vitro. Tablets with 1.5 mm sidewall thickness and 0.5 mm top/bottom thickness demonstrated zero-order drug release for 12 h. The authors concluded that dosage forms with desired floating time and drug release rate can be developed using FDM 3D printing.
6.4. Orodispersible and buccal films
Orodispersible films (ODFs) have been reported to improve patient compliance and ease of swallowing in patients suffering from dysphagia. They are also considered to be age-appropriate dosage forms because of their disintegrating nature in the mouth without the need for water. Drugs delivered by ODFs also avoid first-pass metabolism.
Jamróz et al. (2017)(Jamróz et al., 2017) developed aripiprazole orodispersible films using FDM 3D printing and compared their physicochemical and mechanical properties with cast films. The drug was mixed with PVA and filaments were fabricated using HME at 172 °C. Films with dimensions of 20 × 30 × 0.15 mm were designed and printed; upon visual inspection, the 3D printed films appeared homogeneous with no evidence of drug crystals, whereas crystals of aripiprazole appeared in the cast films. Although the thickness of the 3D printed films was two times higher than that of the cast films, their disintegration time was similar (43 s for the 3D printed films and 38 s for the cast films). The 3D printed films had higher flexibility and lower durability than those of the cast films. DSC thermograms showed that the drug was completely amorphous in the 3D printed films and remained crystalline in the cast films. In vitro dissolution studies demonstrated that 95% of the drug was released at 15 min in the 3D printed films compared to 75% in the cast films due to the amorphous form of the API in the 3D printed films. The standard deviations were reported to be smaller in the dissolution profile of the 3D printed films compared to those of the cast films, indicating the potential of FDM 3D printing for manufacturing uniform ODFs.
Ehtezazi et al. (2018)(Ehtezazi et al., 2018) similarly demonstrated the application of FDM 3D printing in manufacturing multilayered fast-dissolving films. Feedstock filaments were prepared either with PVA or PEO as the matrix polymer and paracetamol as a model drug. Sodium starch glycolate and croscarmellose sodium were added to the formulation to aid in the disintegration of films and sodium lauryl sulfate to increase the dissolution rate. The authors printed either single-layered or multilayered films with additional taste-masking layers separated from the drug layers. The printed films demonstrated suitable uniform weight and thickness. Films printed with mesh design had appropriate flexibility and showed faster drug release. This property of films is highly desirable during the handling and packing process. Films printed with PEO became brittle upon storage, while the PVA films maintained the same mechanical properties. The addition of taste-masking layers delayed drug release from the films. This work demonstrated the proof-of-concept in preparing fast-dissolving films by FDM 3D printing.
Eleftheriadis et al. (2019)(Eleftheriadis et al., 2019) employed FDM 3D printing for manufacturing diclofenac sodium mucoadhesive films that enabled unidirectional drug release. The films were made of PVA with chitosan added as a permeation enhancer. Ethyl cellulose and commercial wafer sheets were used as the backing layer. The films were evaluated for structural integrity and dose uniformity. Solid-state characterization of the films showed that the drug was converted to an amorphous form. The backing layers EC and wafer sheets were evaluated by determining the diffusion profile of the drug through these layers and water sorption. They found that EC acted as a more effective backing membrane than the commercial wafer layers. The presence of chitosan enhanced the mucoadhesion and permeation properties as demonstrated by ex vivo studies. The in vitro studies showed that the drug release followed first-order kinetics. This work demonstrated that FDM 3D printing is a useful approach for manufacturing multi-layered mucoadhesive buccal films having unidirectional release properties.
6.5. Pediatric dosage forms
Formulations that are acceptable and appropriate for end-users can enhance patient compliance and thereby therapeutic outcomes. Owing to the bitter taste of APIs and the unavailability of suitable doses, it is a challenge to administer drugs to pediatric populations. With the inherent advantage of FDM 3D printing to produce dosage forms with the required dose and shape, this technology offers additional advantages in manufacturing dosage forms for pediatric populations. Below are examples of dosage forms developed using FDM 3D printing that are suitable, attractive, and palatable to pediatric populations.
Scoutaris et al. (2018)(Scoutaris et al., 2018) developed candy-like formulations for pediatric medicines with enhanced palatability using FDM 3D printing by extruding HPMCAS- and PEG-based filaments loaded with indomethacin to obtain formulations of different shapes, such as a heart, ring, bottle, bear, and lion. After printing dosage forms, they performed physicochemical characterization using DSC, FTIR, XRD, and Raman analysis, which showed that the drug was molecularly dispersed in the formulations. The in vivo taste-masking evaluation in 10 healthy human volunteers demonstrated excellent concealing of the drug bitterness. The in vitro drug dissolution studies showed that the drug release was independent of the formulation shape and was completed in 60 min, demonstrating that FDM 3D printing could be employed for the production of pediatric dosage forms with desired shapes and taste-masking.
Wang et al. (2020)(Wang et al., 2020) developed taste-masked donut-shaped tablets for pediatric applications. Caffeine citrate (CC) was chosen as the model drug and HPC and Eudragit® EPO were used as the matrix polymers for filament fabrication. A three-point bend test was used to calculate the stiffness constant “k” to assess the printability of the filaments. The donuts were printed with three different infill densities (10%, 50%, and 100%). The in vitro dissolution studies conducted in simulated salivary fluid (pH 6.8, artificial saliva) demonstrated that concentrations of CC observed in the dissolution media from all the printed donuts were less than the bitter threshold of CC (0.25 mg/mL). The authors reported that the addition of Eudragit EPO to the formulation enhanced the taste-masking efficiency of printed donuts. The optimized formulation exhibited immediate release profiles in the gastric medium. The authors concluded that taste-masked dosage forms with enhanced pediatric appeal could be produced using FDM 3D printing technology.
Thus, FDM 3D printing can offer additional advantages for manufacturing pediatric dosage forms compared to traditional manufacturing techniques. From the above case studies, it can be concluded that dosage forms with the required dose, shape, and size can be produced that could enhance medication adherence, therapeutic outcomes, and safety in pediatric populations.
6.6. Abuse-deterrent dosage forms
Abuse-deterrent formulations (ADFs) are reformulations of controlled drug substances that hinder or reduce the extraction of drugs from the dosage form, making it unattractive for drug abusers. These ADFs also prevent administration of dosage form through alternative routes, making the dosage forms less rewarding. Turk et al. (2012)(Turk et al., 2012) studied 3D printing technology in the development of ADFs. Nukala et al. (2019)(Nukala et al., 2019a) developed egg-shaped ADFs with immediate release properties. The authors prepared placebo filaments using various hydrophilic polymers such as PVA, HPC, Kollidon® VA64, Affinisol™ 15LV, and Kollicoat® IR. The prepared filaments were evaluated for printability and crush resistance. Based on these criteria, PVA was found to be a suitable filament for the printing of ADF. Metformin HCl was loaded into PVA and the filaments were used for printing the “egglets” The size, infill density, and drug load of the egglets were optimized using a multifactorial design with solvent extraction and drug release as responses. The optimized formulation demonstrated a hardness of > 500 N; 80% of particles were more than 1 mm in size and resistant to snorting and intravenous abuse. Drug extraction studies conducted in level 2 and level 3 solvents showed less than 15% drug release. The optimized formulation also met the FDA requirement of < 85% drug release in 30 min. This showed that HME coupled with FDM 3D printing could be a promising tool for the preparation of patient-tailored immediate release ADFs.
6.7. Personalized implants
FDM 3D printing can produce dosage forms with complex structures and customized implants according to the patient’s needs and has gained significant importance in this area of research. In a study reported by Fu et al. (2018)(Fu et al., 2018), vaginal rings were prepared by FDM 3D printing using PLA and PCL as carriers. A solid dispersion was prepared by mixing progesterone and PEG 4000, blended with a mixture of PLA, PCL, and Tween 80, and extruded to form filaments. The extruded filaments were used as feedstock material to print O-, Y-, and M-shaped vaginal rings that were characterized using XRD, DSC, TGA, and in vitro dissolution. The studies showed that progesterone was present in the implants in a stable and amorphous form and that the drug release was sustained for more than seven days. The authors concluded that FDM 3D printing is an effective strategy to produce personalized implants.
Liang et al. (2018)(Liang et al., 2018) developed a wearable personalized oral device in the form of a mouth guard; this was the first study to evaluate the performance of FDM 3D printed implants in humans. The authors used clobetasol propionate (CBS), which is widely used in the treatment of oral inflammatory conditions, as a model drug. Clobetasol propionate was replaced with food-grade vanillic acid for the human release study. PVA and PLA were employed as matrix polymers at two different ratios with 10% (w/w) CBS or 2.5% (w/w) vanillic acid drug loading. The fabricated filaments exhibited desirable mechanical properties suitable for 3D printing; the APIs were converted to an amorphous form in the fabricated filaments. In vitro release studies were conducted in simulated saliva. Mouthguards printed with filaments having a PVA/PLA ratio of 6:3 showed a cumulative release of 39% in 2 weeks, whereas mouthguards printed with a PVA/PLA ratio of 5:4 demonstrated a cumulative drug release of 19%. To evaluate the tailor-fitting mouth guards in humans, the authors scanned the maxillary anatomical imprints using an intraoral scanner, and used the imprints as templates for 3D printing of customizable mouth guards. The printed mouth guards were successfully fitted to each individual’s anatomy. The entire process from the intraoral scan to the final wearing of the customized VA-loaded mouthguard took less than two hours; this technology confers advantages in terms of speed and efficiency compared to conventional manufacturing techniques such as casting and molding. Examples of various polymers, APIs, and other excipients used in HME-based FDM 3D printing are presented in Table 1.
Table 1.
Details of active pharmaceutical ingredients (APIs), polymers, and other excipients used in hot-melt extrustion-fused deposition modeling 3D printing.
| Polymers | API | Other additives used | Extrusion Temperature (°C) | 3D printing Temperature (°C) | Application | Ref. |
|---|---|---|---|---|---|---|
| Hydroxypropyl cellulose (HPC) | Domperidone | NA | 145 | 210 | Sustaineed-release (SR) floating tablets | (Chai et al., 2017) |
| HPC | Acetaminophen | Polyethylene glycol (PEG) as plasticizer | 165 | 180 | Capsular oral device for pulse release | (Melocchi et al., 2015) |
| Eudragit E | Hydrochlorothiazide | Triethyl citrate (TEC) as a plasticizer, Tricalcium phosphate and sodium CMC as disintegrating agents. | 100 | 135 | Immediate-release (IR) channeled tablets | (Sadia et al., 2018a) |
| Polyvinyl alcohol (PVA) | Glipizide | NA | 180 | 195 | Controlled-release (CR) oral device | (Li et al., 2017) |
| HPC-SSL | Theophylline | Triacetin as a plasticizer, Ac-di-sol, Explotab, Polyplasdone-XL, Primojel as disintegrating agents | 120 | 190 | Gaplets for accelerated drug release | (Arafat et al., 2018) |
| HPMC-AS LG, MG, HG | Paracetamol | Mg stearate as a lubricant, Methyl paraben as a plasticizer | 80–110 | 180–190 | Modified release tablets | (Goyanes et al., 2015a) |
| Ethyl cellulose | Ibuprofen | HPMC, Sodium alginate, PVA, Xanthan gum as release modifiers | 100–120 | 170–178 | SR tablets | (Yang et al., 2018) |
| Polyvinyl pyrrolidone | Theophylline or Dipyridamole | TEC as plasticizer and TALC as filler | 90 | 110 | IR tablets | (Okwuosa et al., 2016) |
| HPMC 15 LV | Diltiazem HCl and Diazepam | NA | 135 | 180 | Tailored drug release tablets | (Kadry et al., 2018) |
| PVA | Metformin | Sorbitol as thermal lubricant | 170 | 200 | Abuse-deterrent IR egglets | (Nukala et al., 2019a) |
| PEO 200K, 300K, 600K | Theophylline | PEG 6K as plasticizer | 65, 70, 80 | 110, 145 | Radiator like IR dosage form | (Isreb et al., 2019b) |
| Kollicoat IR | Aripiprazole | NA | 150 | z210 | Amorphous aripiprazole tablets | (Jamróz et al., 2018a) |
| PEG 6K, 20K, PVP, Kollidon VA 64, Poloxomer-407 | Pantoprazole | TEC as plasticizer | 49 | 55 | IR Tablets of thermosensiti ve drug | (Kempin et al., 2018) |
| PVA, PLA | Clobetasol, Vanillic acid | NA | 150 | 180 | Personalized oral device | (Kempin et al., 2018) |
| PEO, PVA | Ibuprofen | PEG 4K as plasticizer, SSG, starch, sodium CMC as disintegrants | 60, 90 | 165, 190 | Fast dissolving oral films | (Ehtezazi et al., 2018) |
7. Advantages of FDM 3D printing in individualized pharmacotherapy
To achieve greater success in customized drug delivery and personalized pharmacotherapy, various challenges need to be addressed. Due to variations in genetic conditions, metabolism, weight, height, body surface area, and disease conditions from patient to patient, there is a need for a wide range of dosage forms and strengths to meet the requirements of each individual. FDM 3D printing offers solutions to various challenges in individualized pharmacotherapy because of its inherent advantages shown in Table 2.
Table 2.
Solutions offered by FDM 3D printing for various challenges in individualized pharmacotherapy.
| Challenges | 3D printing approach | Benefits | References |
|---|---|---|---|
| Patients taking multiple medications/polypills |
Multiple layered tablets; each layer can be printed with a different drug | Reduced chance of missing dose | (Sadia et al., 2018b) |
| Personalized dosing | Definite amount of dose can be dispensed by varying the size and shape of the dosage form | Delivering the desired dose | (Pietrzak et al., 2015) |
| Pediatric dosage forms | Dosage forms can be designed in attractive shapes/suitable sizes | Enhanced patient compliance | (Scoutaris et al., 2018) |
| Patients with difficulty in swallowing | Porous structures which dissolve or disintegrate easily in the mouth can be designed as fast dissolving films | Ease of administration | (Ehtezazi et al., 2018) |
| Variation in anatomical body parts | Customized implants can be fabricated | Increased patient comfort and thereby compliance | (Pietrzak et al., 2015) |
| On-demand manufacturing | Tablets can be printed at clinics or pharmacies upon request | Increased availability of medicines | (Trenfield et al., 2018) |
| Conditions that require complex release profiles | Dosage forms like pulsatile delivery systems can be designed | Enhanced patient compliance and thereby a therapeutic effect | (Trenfield et al., 2018) |
8. Future Perspectives
The proposed prospects of FDM 3D printing in pharmaceutical product development are shown in Fig. 7. Due to the non-availability of all the required dosage regimens for individualized pharmacotherapy, pharmacy-based dosage printing is an alternative to conventional industrial manufacturing. Furthermore, 3D printing of pharmaceuticals is also a viable solution in situations such as on-demand manufacturing of required medications at clinics, under disaster relief conditions, and in operating theaters and military emergencies (Khaled et al., 2014). With the increased use of innovative technologies in health care, diagnosis of disease conditions and production of medications at patients’ home facilities with online approval from physicians will soon become a reality. Continued research in this field and strict regulatory guidelines will bring drastic changes in drug dispensing and patient-centric drug delivery.
Fig. 7.
Proposed future potential of integrating fused deposition modeling 3D printing with the health care system to provide improved individualized pharmacotherapy.
9. Conclusions
Pharmaceutical research has paid increased attention to the capability of 3D printing to personalize and customize dosage forms. The FDA approval of the first 3D printed drug showed pharmaceutical companies the industrial feasibility of this technique. To make this manufacturing technique a reality in the pharmaceutical field, a deeper understanding of the various process and machine-related parameters that can affect critical quality attributes of final dosage forms is essential. Creating a database of all pharmaceutical polymers with their specific applications that could be used in the 3D printing process would save research time and effort. Although efforts are underway to further advance and overcome limitations associated with this methodology, there are still many challenges that need to be addressed such as poor resolution, low production capabilities, high thermal exposure, safety, and regulatory concerns. Solving these limitations and putting regulations in place would likely revolutionize the process of personalizing dosage forms along with safety, efficacy, and adherence.
Acknowledgments
Funding
This project was also partially supported by Grant Number P30GM122733–01A1, funded by the National Institute of General Medical Sciences (NIGMS) a component of the National Institutes of Health (NIH) as one of its Centers of Biomedical Research Excellence (COBRE).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afsana, Jain V, Haider N, Jain K, 2019. 3D Printing in Personalized Drug Delivery. Curr. Pharm. Des. 24(42), 5062–5071. 10.2174/1381612825666190215122208 [DOI] [PubMed] [Google Scholar]
- Aho J, Bøtker JP, Genina N, Edinger M, Arnfast L, Rantanen J, 2019. Roadmap to 3D-Printed Oral Pharmaceutical Dosage Forms: Feedstock Filament Properties and Characterization for Fused Deposition Modeling. J. Pharm. Sci. 108, 26–35. 10.1016/j.xphs.2018.11.012 [DOI] [PubMed] [Google Scholar]
- Alhijjaj M, Nasereddin J, Belton P, Qi S, 2019. Impact of processing parameters on the quality of pharmaceutical solid dosage forms produced by fused deposition modeling (FDM). Pharmaceutics. 11(12), 633. 10.3390/pharmaceutics11120633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafat B, Wojsz M, Isreb A, Forbes RT, Isreb M, Ahmed W, Arafat T, Alhnan MA, 2018. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 118, 191–199. 10.1016/j.ejps.2018.03.019 [DOI] [PubMed] [Google Scholar]
- Azad MA, Olawuni D, Kimbell G, Badruddoza AZM, Hossain MS, Sultana T, 2020. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials-process perspective. Pharmaceutics. 12(2), 124. 10.3390/pharmaceutics12020124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandari S, Nyavanandi D, Dumpa N, Repka MA, 2021. Coupling Hot Melt Extrusion and Fused Deposition Modeling: Critical Properties for Successful Performance. Adv. Drug Deliv. Rev. S0169–409X(21)00040–5. 10.1016/j.addr.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butreddy A, Sarabu S, Bandari S, Dumpa N, Zhang F, Repka MA, Repka MA, 2020. Polymer-Assisted Aripiprazole-Adipic Acid Cocrystals Produced by Hot Melt Extrusion Techniques. Cryst. Growth Des. 20 (7), 4335–4345. 10.1021/acs.cgd.0c00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Chai H, Wang X, Yang J, Li J, Zhao Y, Cai W, Tao T, Xiang X, 2017. Fused deposition modeling (FDM) 3D printed tablets for intragastric floating delivery of domperidone. Sci. Rep. 7, 1–9. 10.1038/s41598-017-03097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti C, Kirby DJ, Russell CA, 2020. Current formulation approaches in design and development of solid oral dosage forms through three-dimensional printing. Prog. Addit. Manuf. 550(1–2), 418–428. 10.1007/s40964-020-00127-5 [DOI] [Google Scholar]
- Daly R, Harrington TS, Martin GD, Hutchings IM, 2015. Inkjet printing for pharmaceutics - A review of research and manufacturing. Int. J. Pharm. 494(2), 554–567. 10.1016/j.ijpharm.2015.03.017 [DOI] [PubMed] [Google Scholar]
- Dumpa NR, Bandari S, Repka MA, 2020. Novel gastroretentive floating pulsatile drug delivery system produced via hot-melt extrusion and fused deposition modeling 3D printing. Pharmaceutics. 12(1), 52. 10.3390/pharmaceutics12010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumpa NR, Sarabu S, Bandari S, Zhang F, Repka MA, 2018. Chronotherapeutic Drug Delivery of Ketoprofen and Ibuprofen for Improved Treatment of Early Morning Stiffness in Arthritis Using Hot-Melt Extrusion Technology. AAPS PharmSciTech. 19(6), 2700–2709. 10.1208/s12249-018-1095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehtezazi T, Algellay M, Islam Y, Roberts M, Dempster NM, Sarker SD, 2018. The Application of 3D Printing in the Formulation of Multilayered Fast Dissolving Oral Films. J. Pharm. Sci. 107, 1076–1085. 10.1016/j.xphs.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Elbadawi M, 2019. Rheological and Mechanical Investigation into the Effect of Different Molecular Weight Poly(ethylene glycol)s on Polycaprolactone-Ciprofloxacin Filaments. ACS Omega. 4(3), 5412–5423. 10.1021/acsomega.8b03057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriadis GK, Ritzoulis C, Bouropoulos N, Tzetzis D, Andreadis DA, Boetker J, Rantanen J, Fatouros DG, 2019. Unidirectional drug release from 3D printed mucoadhesive buccal films using FDM technology: In vitro and ex vivo evaluation. Eur. J. Pharm. Biopharm. 144, 180–192. 10.1016/j.ejpb.2019.09.018 [DOI] [PubMed] [Google Scholar]
- Fu J, Yu X, Jin Y, 2018. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 539, 75–82. 10.1016/j.ijpharm.2018.01.036 [DOI] [PubMed] [Google Scholar]
- Fuenmayor E, Forde M, Healy AV, Devine DM, Lyons JG, McConville C, Major I, 2018. Material considerations for fused-filament fabrication of solid dosage forms. Pharmaceutics. 10(2), 44. 10.3390/pharmaceutics10020044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson I, Rosen DW, Stucker B, 2010. Additive manufacturing technologies: Rapid prototyping to direct digital manufacturing, Additive Manufacturing Technologies: Rapid Prototyping to Direct Digital Manufacturing. XXII, 459 p. 10.1007/978-1-4419-1120-9 [DOI] [Google Scholar]
- Goole J, Amighi K, 2016. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 499, 376–394. 10.1016/j.ijpharm.2015.12.071 [DOI] [PubMed] [Google Scholar]
- Goyanes A, Buanz ABM, Hatton GB, Gaisford S, Basit AW, 2015a. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 89, 157–162. 10.1016/j.ejpb.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Goyanes A, Chang H, Sedough D, Hatton GB, Wang J, Buanz A, Gaisford S, Basit AW, 2015b. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int. J. Pharm. 496, 414–420. 10.1016/j.ijpharm.2015.10.039 [DOI] [PubMed] [Google Scholar]
- Goyanes A, Fina F, Martorana A, Sedough D, Gaisford S, Basit AW, 2017. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 527, 21–30. 10.1016/j.ijpharm.2017.05.021 [DOI] [PubMed] [Google Scholar]
- Gültekin HE, Tort S, Acartürk F, 2019. An Effective Technology for the Development of Immediate Release Solid Dosage Forms Containing Low-Dose Drug: Fused Deposition Modeling 3D Printing. Pharm. Res. 36(9), 128. 10.1007/s11095-019-2655-y [DOI] [PubMed] [Google Scholar]
- Homaee Borujeni S, Mirdamadian SZ, Varshosaz J, Taheri A, 2020. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose. 27, 1573–1589. 10.1007/s10570-019-02881-4 [DOI] [Google Scholar]
- Ilyés K, Kovács NK, Balogh A, Borbás E, Farkas B, Casian T, Marosi G, Tomuţă I, Nagy ZK, 2019. The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique: Material considerations–printability–process modulation, with consecutive effects on in vitro release, stability and degradation. Eur. J. Pharm. Sci. 129, 110–123. 10.1016/j.ejps.2018.12.019 [DOI] [PubMed] [Google Scholar]
- Isreb A, Baj K, Wojsz M, Isreb M, Peak M, Alhnan MA, 2019a. 3D printed oral theophylline doses with innovative ‘radiator-like’ design: Impact of polyethylene oxide (PEO) molecular weight. Int. J. Pharm. 564, 98–105. 10.1016/j.ijpharm.2019.04.017 [DOI] [PubMed] [Google Scholar]
- Isreb A, Baj K, Wojsz M, Isreb M, Peak M, Alhnan MA, 2019b. 3D printed oral theophylline doses with innovative ‘radiator-like’ design: Impact of polyethylene oxide (PEO) molecular weight. Int. J. Pharm. 564, 98–105. 10.1016/j.ijpharm.2019.04.017 [DOI] [PubMed] [Google Scholar]
- Jamróz W, Kurek M, Czech A, Szafraniec J, Gawlak K, Jachowicz R, 2018a. 3D printing of tablets containing amorphous aripiprazole by filaments co-extrusion. Eur. J. Pharm. Biopharm. 131, 44–47. 10.1016/j.ejpb.2018.07.017 [DOI] [PubMed] [Google Scholar]
- Jamróz W, Kurek M, tyszczarz E, Szafraniec J, Knapik-Kowalczuk J, Syrek K, Paluch M, Jachowicz R, 2017. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 533, 413–420. 10.1016/j.ijpharm.2017.05.052 [DOI] [PubMed] [Google Scholar]
- Jamróz W, Szafraniec J, Kurek M, Jachowicz R, 2018b. 3D Printing in Pharmaceutical and Medical Applications – Recent Achievements and Challenges. Pharm. Res. 35(9), 176. 10.1007/s11095-018-2454-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani R, Patel D, 2014. Hot melt extrusion: An industrially feasible approach for casting orodispersible film. 10(4), 292–305. Asian J. Pharm. Sci. 10.1016/j.ajps.2015.03.002 [DOI] [Google Scholar]
- Kadry H, Al-Hilal TA, Keshavarz A, Alam F, Xu C, Joy A, Ahsan F, 2018. Multi-purposable filaments of HPMC for 3D printing of medications with tailored drug release and timed-absorption. Int. J. Pharm. 544, 285–296. 10.1016/j.ijpharm.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Kallakunta VR, Sarabu S, Bandari S, Tiwari R, Patil H, Repka MA, 2019. An update on the contribution of hot-melt extrusion technology to novel drug delivery in the twenty-first century: part I. Expert Opin. Drug Deliv. 16(5), 539–550. 10.1080/17425247.2019.1609448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempin W, Domsta V, Grathoff G, Brecht I, Semmling B, Tillmann S, Weitschies W, Seidlitz A, 2018. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm. Res. 35(6), 124. 10.1007/s11095-018-2405-6 [DOI] [PubMed] [Google Scholar]
- Khaled SA, Burley JC, Alexander MR, Roberts CJ, 2014. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 461(1–2), 105–11. 10.1016/j.ijpharm.2013.11.021 [DOI] [PubMed] [Google Scholar]
- Kimura S. ichiro, Ishikawa T, Iwao Y, Itai S, Kondo H, 2019. Fabrication of zero-order sustained-release floating tablets via fused depositing modeling 3D printer. Chem. Pharm. Bull. 67(9), 992–999. 10.1248/cpb.c19-00290 [DOI] [PubMed] [Google Scholar]
- Korte C, Quodbach J, 2018. Formulation development and process analysis of drug-loaded filaments manufactured via hot-melt extrusion for 3D-printing of medicines. Pharm. Dev. Technol. 23(10), 1117–1127. 10.1080/10837450.2018.1433208 [DOI] [PubMed] [Google Scholar]
- Lamichhane S, Bashyal S, Keum T, Noh G, Seo JE, Bastola R, Choi J, Sohn DH, Lee S, 2019a. Complex formulations, simple techniques: Can 3D printing technology be the Midas touch in pharmaceutical industry? Asian J. Pharm. Sci. 14, 465–479. 10.1016/j.ajps.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane S, Park J, Sohn DH, Lee S, 2019b. lamichhane GRDDS of pregabalin.pdf.
- Li Q, Wen H, Jia D, Guan X, Pan H, Yang Y, Yu S, Zhu Z, Xiang R, Pan W, 2017. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int J Pharm. 525(1), 5–11.. 10.1016/j.ijpharm.2017.03.066 [DOI] [PubMed] [Google Scholar]
- Liang K, Carmone S, Brambilla D, Leroux JC, 2018. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci. Adv. 4, 1–12. 10.1126/sciadv.aat2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniruzzaman M, Boateng JS, Snowden MJ, Douroumis D, 2012. A Review of Hot-Melt Extrusion: Process Technology to Pharmaceutical Products. ISRN Pharm. 2012, 9. 10.5402/2012/436763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchels FPW, Feijen J, Grijpma DW, 2010. A review on stereolithography and its applications in biomedical engineering. 31(24), 6121–30. Biomaterials. 10.1016/j.biomaterials.2010.04.050 [DOI] [PubMed] [Google Scholar]
- Melocchi A, Parietti F, Loreti G, Maroni A, Gazzaniga A, Zema L, 2015. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 30, 360–367. 10.1016/j.jddst.2015.07.016 [DOI] [Google Scholar]
- Nasereddin JM, Wellner N, Alhijjaj M, Belton P, Qi S, 2018. Development of a Simple Mechanical Screening Method for Predicting the Feedability of a Pharmaceutical FDM 3D Printing Filament. Pharm. Res. 35(8), 151. 10.1007/s11095-018-2432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J, Madurawe RD, Moore CMV, Khan MA, Khairuzzaman A, 2017. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 108, 39–50. 10.1016/j.addr.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Nukala PK, Palekar S, Patki M, Patel K, 2019a. Abuse Deterrent Immediate Release Egg-Shaped Tablet (Egglets) Using 3D Printing Technology: Quality by Design to Optimize Drug Release and Extraction. AAPS PharmSciTech. 20(2), 80. 10.1208/s12249-019-1298-y [DOI] [PubMed] [Google Scholar]
- Nukala PK, Palekar S, Solanki N, Fu Y, Patki M, Sohatee AA, Trombetta L, Patel K, 2019b. Investigating the application of FDM 3D printing pattern in preparation of patient-tailored dosage forms. J. 3D Print. Med. 3, 23–37. 10.2217/3dp-2018-0028 [DOI] [Google Scholar]
- Öblom H, Zhang J, Pimparade M, Speer I, Preis M, Repka M, Sandler N, 2019. 3D-Printed Isoniazid Tablets for the Treatment and Prevention of Tuberculosis—Personalized Dosing and Drug Release. AAPS PharmSciTech. 20, 1–13. 10.1208/s12249-018-1233-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwuosa TC, Pereira BC, Arafat B, Cieszynska M, Isreb A, Alhnan MA, 2017. Fabricating a Shell-Core Delayed Release Tablet Using Dual FDM 3D Printing for Patient-Centred Therapy. Pharm. Res. 34, 427–437. 10.1007/s11095-016-2073-3 [DOI] [PubMed] [Google Scholar]
- Okwuosa TC, Stefaniak D, Arafat B, Isreb A, Wan KW, Alhnan MA, 2016. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets. Pharm. Res. 33, 2704–2712. 10.1007/s11095-016-1995-0 [DOI] [PubMed] [Google Scholar]
- Palekar S, Nukala PK, Mishra SM, Kipping T, Patel K, 2019. Application of 3D printing technology and quality by design approach for development of age-appropriate pediatric formulation of baclofen. Int. J. Pharm. 556, 106–116. 10.1016/j.ijpharm.2018.11.062 [DOI] [PubMed] [Google Scholar]
- Pardeike J, Strohmeier DM, Schrödl N, Voura C, Gruber M, Khinast JG, Zimmer A, 2011. Nanosuspensions as advanced printing ink for accurate dosing of poorly soluble drugs in personalized medicines. Int. J. Pharm. 420(1):93–100. 10.1016/j.ijpharm.2011.08.033 [DOI] [PubMed] [Google Scholar]
- Patil H, Tiwari RV, Repka MA, 2016. Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech. 17, 20–42. 10.1208/s12249-015-0360-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak K, Isreb A, Alhnan MA, 2015. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur. J. Pharm. Biopharm. 96, 380–387. 10.1016/j.ejpb.2015.07.027 [DOI] [PubMed] [Google Scholar]
- Prasad E, Islam MT, Goodwin DJ, Megarry AJ, Halbert GW, Florence AJ, Robertson J, 2019. Development of a hot-melt extrusion (HME) process to produce drug loaded Affinisol™ 15LV filaments for fused filament fabrication (FFF) 3D printing. Addit. Manuf. 29, 16. 10.1016/j.addma.2019.06.027 [DOI] [Google Scholar]
- Pucci JU, Christophe BR, Sisti JA, Connolly ES, 2017. Three-dimensional printing: technologies, applications, and limitations in neurosurgery. Biotechnol. Adv. 35(5), 521–529. 10.1016/j.biotechadv.2017.05.007 [DOI] [PubMed] [Google Scholar]
- Rahman Z, Barakh Ali SF, Ozkan T, Charoo NA, Reddy IK, Khan MA, 2018. Additive Manufacturing with 3D Printing: Progress from Bench to Bedside. AAPS J. 20(6), 101. 10.1208/s12248-018-0225-6 [DOI] [PubMed] [Google Scholar]
- Repka MA, Bandari S, Kallakunta VR, Vo AQ, McFall H, Pimparade MB, Bhagurkar AM, 2018. Melt extrusion with poorly soluble drugs – An integrated review. Int. J. Pharm. 535(1–2), 68–85. 10.1016/j.ijpharm.2017.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadia M, Arafat B, Ahmed W, Forbes RT, Alhnan MA, 2018a. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 269, 355–363. 10.1016/j.jconrel.2017.11.022 [DOI] [PubMed] [Google Scholar]
- Sadia M, Isreb A, Abbadi I, Isreb M, Aziz D, Selo A, Timmins P, Alhnan MA, 2018b. From ‘fixed dose combinations’ to ‘a dynamic dose combiner’: 3D printed bi-layer antihypertensive tablets. Eur. J. Pharm. Sci. 123, 484–494. 10.1016/j.ejps.2018.07.045 [DOI] [PubMed] [Google Scholar]
- Sarabu S, Bandari S, Kallakunta VR, Tiwari R, Patil H, Repka MA, 2019. An update on the contribution of hot-melt extrusion technology to novel drug delivery in the twenty-first century: part II. Expert Opin. Drug Deliv. 16(6), 567–582. 10.1080/17425247.2019.1614912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydam M, Takka S, 2020. Improving the dissolution of a water-insoluble orphan drug through a fused deposition modelling 3-Dimensional printing technology approach. Eur. J. Pharm. Sci. 152, 105426. 10.1016/j.ejps.2020.105426 [DOI] [PubMed] [Google Scholar]
- Scoutaris N, Ross SA, Douroumis D, 2018. 3D Printed “Starmix“ Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 35, 1–11. 10.1007/s11095-017-2284-2 [DOI] [PubMed] [Google Scholar]
- Shah RB, Collier JS, Sayeed VA, Bryant A, Habib MJ, Khan MA, 2010. Tablet splitting of a narrow therapeutic index drug: A case with levothyroxine sodium. AAPS PharmSciTech. 11(3):1359–67. 10.1208/s12249-010-9515-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões MF, Pinto RMA, Simões S, 2019. Hot-melt extrusión in the pharmaceutical industry: toward filing a new drug application. Drug Discov. Today. 24(9):1749–1768. 10.1016/j.drudis.2019.05.013 [DOI] [PubMed] [Google Scholar]
- Skowyra J, Pietrzak K, Alhnan MA, 2015. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 68, 11–17. 10.1016/j.ejps.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Streubel A, Siepmann J, Bodmeier R, 2006. Gastroretentive drug delivery systems. Expert Opin. Drug Deliv. 3(2), 217–33. 10.1517/17425247.3.2.217 [DOI] [PubMed] [Google Scholar]
- Tan DK, Maniruzzaman M, Nokhodchi A, 2020. Development and optimisation of novel polymeric compositions for sustained release theophylline caplets (PrintCap) via FDM 3D printing. Polymers (Basel). 12(1), 27. 10.3390/polym12010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DK, Maniruzzaman M, Nokhodchi A, 2019. Development and Optimisation of Novel Polymeric Compositions for Sustained Release Theophylline. Polymers (Basel). 12, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The impact of layer height on a 3D print | 3D Hubs [WWW Document], n.d. URL https://www.3dhubs.com/knowledge-base/impact-layer-height-3d-print/#why (accessed 8.5.20).
- Tidau M, Kwade A, Finke JH, 2019. Influence of high, disperse api load on properties along the fused-layer modeling process chain of solid dosage forms. Pharmaceutics. 11(4):194. 10.3390/pharmaceutics11040194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenfield SJ, Awad A, Goyanes A, Gaisford S, Basit AW, 2018. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 39, 440–451. 10.1016/j.tips.2018.02.006 [DOI] [PubMed] [Google Scholar]
- Trenfield SJ, Awad A, Madla CM, Hatton GB, Firth J, Goyanes A, Gaisford S, Basit AW, 2019. Shaping the future: recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 16(10), 1081–1094. 10.1080/17425247.2019.1660318 [DOI] [PubMed] [Google Scholar]
- Turk DC, O’Connor AB, Dworkin RH, Chaudhry A, Katz NP, Adams EH, Brownstein JS, Comer SD, Dart R, Dasgupta N, Denisco RA, Klein M, Leiderman DB, Lubran R, Rappaport BA, Zacny JP, Ahdieh H, Burke LB, Cowan P, Jacobs P, Malamut R, Markman J, Michna E, Palmer P, Peirce-Sandner S, Potter JS, Raja SN, Rauschkolb C, Roland CL, Webster LR, Weiss RD, Wolf K, 2012. Research design considerations for clinical studies of abuse-deterrent opioid analgesics: IMMPACT recommendations. Pain. 153(10), 1997–2008. 10.1016/j.pain.2012.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dumpa N, Bandari S, Durig T, Repka MA, 2020. Fabrication of Taste-Masked Donut-Shaped Tablets Via Fused Filament Fabrication 3D Printing Paired with Hot-Melt Extrusion Techniques. AAPS PharmSciTech. 21(7):243. 10.1208/s12249-020-01783-0 [DOI] [PubMed] [Google Scholar]
- Wang J, Goyanes A, Gaisford S, Basit AW, 2016. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 503, 207–212. 10.1016/jjjpharm.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Warsi MH, Yusuf M, Al Robaian M, Khan M, Muheem A, Khan S, 2018. 3D Printing Methods for Pharmaceutical Manufacturing: Opportunity and Challenges. Curr. Pharm. Des. 24(42), 4949–4956. 10.2174/1381612825666181206121701 [DOI] [PubMed] [Google Scholar]
- WorsØe J, Fynne L, Gregersen T, Schlageter V, Christensen LA, Dahlerup JF, Rijkhoff NJM, Laurberg S, Krogh K, 2011. Gastric transit and small intestinal transit time and motility assessed by a magnet tracking system. BMC Gastroenterol. 11, 145. 10.1186/1471-230X-11-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Li J, Meda A, Osei-Yeboah F, Peterson ML, Repka M, Zhan X, 2020. Development of a quantitative method to evaluate the printability of filaments for fused deposition modeling 3D printing. Int. J. Pharm. 588, 119760. 10.1016/jjjpharm.2020.119760 [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang H, Li H, Ou Z, Yang G, 2018. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 115, 11–18. 10.1016/j.ejps.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Zema L, Melocchi A, Maroni A, Gazzaniga A, 2017. Three-Dimensional Printing of Medicinal Products and the Challenge of Personalized Therapy. J. Pharm. Sci. 106(7), 1697–1705. 10.1016/j.xphs.2017.03.021 [DOI] [PubMed] [Google Scholar]
- Zhang J, Feng X, Patil H, Tiwari RV, Repka MA, 2017a. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 519(1–2), 186–197. 10.1016/jjjpharm.2016.12.049 [DOI] [PubMed] [Google Scholar]
- Zhang J, Vo AQ, Feng X, Bandari S, Repka MA, 2018. Pharmaceutical Additive Manufacturing: a Novel Tool for Complex and Personalized Drug Delivery Systems. AAPS PharmSciTech. 19, 3388–3402. 10.1208/s12249-018-1097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xu P, Vo AQ, Bandari S, Yang F, Durig T, Repka MA, 2019. Development and evaluation of pharmaceutical 3D printability for hot melt extruded cellulose-based filaments. J. Drug Deliv. Sci. Technol. 52, 292–302. 10.1016/j.jddst.2019.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang W, Vo AQ, Feng X, Ye X, Kim DW, Repka MA, 2017b. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation. Carbohydr. Polym. 177, 49–57. 10.1016/j.carbpol.2017.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]


