Abstract
Autoinflammatory disorders of the innate immune system present with recurrent episodes of inflammation often beginning in early childhood. While there are now more than 30 genetically-defined hereditary fever disorders, many patients lack a clear diagnosis. Many pediatric patients are often grouped with patients with periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome despite failing to meet diagnostic criteria. Here, we categorize these patients as syndrome of undifferentiated recurrent fever (SURF), and identify the unique features which distinguish them from the PFAPA syndrome. SURF patients were more likely to report gastrointestinal symptoms of nausea, vomiting and abdominal pain, and experienced inconsistent responses to on-demand steroid therapy compared to PFAPA patients. For this previously undefined cohort, an optimal course of therapy remains uncertain, with medical and surgical therapies largely driven by parental preference. A subset of patients with SURF underwent tonsillectomy with complete resolution. Flow cytometric evaluation demonstrates leukocytic populations distinct from PFAPA patients, with reduced CD3+ T cell numbers. SURF patient tonsils were predominantly characterized by an IL-1 signature compared to PFAPA, even during the afebrile period. Peripheral blood signatures were similar between groups suggesting that PFAPA and SURF patient tonsils have localized, persistent inflammation, without clinical symptoms. These data suggest that SURF is a heterogenous syndrome on the autoinflammatory disease spectrum.
Keywords: pediatrics, recurrent fever, periodic fever, autoinflammation
INTRODUCTION
Autoinflammatory disorders presenting in childhood are increasingly recognized, via enhanced physician awareness of these rare diseases, and advances and availability in sequencing technologies. However, a concomitant increase in the number of patients with undefined fever disorders has also become apparent, with estimates of 60–80% of recurrent fever patients lacking a molecular or genetic diagnosis.1,2 The most common diagnosis for pediatric patients is Periodic Fever, Aphthous stomatitis, Pharyngitis and Adenitis (PFAPA) syndrome,3 however the lack of a diagnostic test reveals that many patients categorized as having PFAPA syndrome, may be misdiagnosed with PFAPA being used as an umbrella diagnosis.4 In our immunology clinic, we have identified a substantial number of patients with intermittent fevers, similar to the PFAPA phenotype, but with limited associated symptoms of aphthous stomatitis, pharyngitis, and/or adenopathy, and increased reports of gastrointestinal symptoms. These patients similarly have normal growth and development, normal acute phase reactants between febrile episodes, negative microbial cultures, and a lack of response to antibiotics. We previously addressed this subset of patients as syndrome of undifferentiated recurrent fevers (SURF),1,5 but it remained unknown whether they represented an atypical form of the PFAPA syndrome, or an entirely separate clinical entity. Similar patients have been observed in other PFAPA cohorts1,6 and categorized as incomplete PFAPA7 or undifferentiated/undefined systemic autoinflammatory diseases (uSAID),1 however, the most appropriate therapeutic approach was unclear. Here, we report a prospective cohort of SURF patients, to highlight the clinical differences and inflammatory phenotypes that distinguish these patients from typical PFAPA patients, and support tonsillectomy as a potential effective therapy.
METHODS
Human Subjects.
To better distinguish the natural history of recurrent fever vs PFAPA syndrome in pediatric patients, we initiated a prospective study to assess patients evaluated at a tertiary care center in San Diego, California, USA. Any patient 1–17 years of age, seen in consultation in the Rady Children’s Hospital-San Diego Allergy/Immunology clinic for a chief complaint of recurrent fever (at least 6 episodes) was assessed for eligibility to participate. Patients with recurrent infections, primary immunodeficiency, autoimmunity, genetically defined autoinflammatory disorders (familial Mediterranean fever,8 Hyper-IgD syndrome/mevalonic kinase deficiency,9,10 TNF receptor associated periodic syndrome,11 cryopyrin associated periodic syndrome),12–14 or with cyclic neutropenia were excluded.
Patients diagnosed clinically with PFAPA syndrome per modified Marshall’s criteria15, were included as a comparator group:
Inclusion criteria for PFAPA:
Regularly occurring fevers (without evidence of upper respiratory infection).
Onset prior to age 5 years.
At least one of the following symptoms associated with fevers: aphthous stomatitis, pharyngitis, cervical adenitis.
Resolution of symptoms with single, low dose prednisolone (≤1mg/kg po once in the first 24 hours of a febrile episode).
Normal growth as assessed by World Health Organization growth charts
Asymptomatic between episodes
Absence of laboratory evidence of inflammation between episodes (cardio/high sensitivity CRP <0.2 mg/L and ESR <15 mm/h)
Absence of genetically defined autoinflammatory disorders (familial Mediterranean fever, Hyper-IgD syndrome/mevalonic kinase deficiency, TNF receptor associated periodic syndrome, cryopyrin associated periodic syndrome), cyclic neutropenia, immunodeficiency or autoimmunity (excluded clinically).
The remaining patients met the following criteria to be defined as SURF:
Recurrent fevers (without evidence of upper respiratory infection).
Normal growth as assessed by World Health Organization growth charts
Asymptomatic between episodes
Absence of laboratory evidence of inflammation between episodes (cardio/high sensitivity CRP <0.2 mg/L and ESR <15 mm/h)
Absence of genetically defined autoinflammatory disorders (familial Mediterranean fever, Hyper-IgD syndrome/mevalonic kinase deficiency, TNF receptor associated periodic syndrome, cryopyrin associated periodic syndrome) or known low penetrance variants in the autoinflammatory disease genes.
Absence ofyclic neutropenia, immunodeficiency, chronic infection, inflammatory bowel disease or autoimmunity (excluded clinically).
For patients undergoing tonsillectomy, a complete history and physical exam were performed, and all indications (absolute and relative), as well as surgical and post-operative risks discussed with the parents. In all cases, a "shared decision making" approach was utilized, and decision to proceed ultimately made by the parents of the patients. For this surgical subset, patient data was collected using a standardized questionnaire, consisting of demographic data, including age, gender, ethnicity, clinical profiles (presence of symptoms, fever profile, treatments) and detailed family histories. Peripheral blood was collected at the time of tonsillectomy, and monocytes isolated by Percoll gradient per manufacterer’s instruction (Sigma-Aldrich), prior to suspending in Trizol reagent (Life Technologies). Under the same protocol, tonsil tissue from age and sex-matched controls who underwent tonsillectomy for obstructive sleep apnea were obtained as available. Tissue was obtained intra-operatively and sections stained with hematoxylin and eosin for histologic analysis with evaluation by a pediatric pathologist, as per routine standard of care. The remainder of the tissue was mechanically disrupted to create a single cell suspension, and used immediately or frozen in fetal bovine serum (FBS) +10% dimethyl sulfoxide for cryostorage. Written informed consents were obtained from parents or legal guardians for all patients, and written assents were obtained for children (7–12 years) and adolescents (13–17 years) under protocols approved by the UCSD Institutional Review Board and in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Flow cytometry.
Tonsils were mechanically disrupted into single cell suspensions, and applied to a Percoll gradient. 106 cells were stained with conjugated monoclonal antibodies to CD3, CD4, CD8, CD11c, CD14, CD19, CD20, CD27, CD38, CD45RA, CD45RO, CD56 (eBioscience, Inc.) per manufacturer’s instructions. For intracellular staining of IL-1β, cells were permeabilized using the Caltag kit. Initial phenotyping for T, B, NK and dendritic cells was performed on a BD FACSCalibur. All subsequent samples were acquired with a BD Biosciences LSR II cytometer using FACSDiva software. In each case, 100,000 events were collected per sample. Data was analyzed with FlowJo software.
Reverse transcription and quantitative PCR.
RNA was isolated from peripheral blood and tonsil cell suspensions using Trizol reagent (Life Technologies) and cDNA was synthesized using High Capacity cDNA Reverse Transcription reagents (Applied Biosystems), both per manufacturer’s instructions. Relative gene expression was determined using the following primer sets: IL1A (5’-AGTTCTTAGTGCCGTGAGTTTC-3’ and 5’- GTGACTGCCCAAGATGAAGA-3’, IL1B (IDT PrimeTime Assay Hs.PT.58.40959974), IL1RN (5’- TTGTCCTGCTTTCTGTTCTCG-3’ and 5’- CTGTCCTGTGTCAAGTCTGG-3’), IL18 (5’ – GATAGCCAGCCTAGAGGTATGG – 3’ and 5’ – CCTTGATGTTATCAGGAGGATTCA – 3’), IFNB (5’- AGCTGAAGCAGTTCCAGAAG -3’ and 5’- AGTCTCATTCCAGCCAGTGC -3’) and TNF (5’- GGAGAAGGGTGACCGACTCA -3’ and 5’- CTGCCCAGACTCGGCAA -3’) with beta-actin (ACTB; 5′- AAGTCAGTGTACAGGTAAGCC -3′ and 5′-GTCCCCCAACTTGAGATGTATG -3′) as reference gene. Quantitative PCR was performed with a Bio-Rad iCycler using iQ5 software (Bio-Rad). Relative gene expression was determined using the 2ΔΔct method.
Statistical analyses.
Statistical analyses and graphing were performed in Microsoft Excel and Graphpad Prism (version 5.03; Graph Pad, Graph Pad Software Inc., CA) programs with the two-tailed, unpaired Student’s t test, Chi-square test, or Fisher’s exact test. Flow cytometry data were analyzed by FlowJo software. Unless otherwise stated, data are expressed as mean +/− SEM. A p value less than 0.05 was considered statistically significant.
RESULTS
Clinical phenotype of patients with SURF.
In the Recurrent Fever Disorders Clinic at Rady Children’s Hospital, San Diego, we assessed 200 children with recurrent fevers (at least 6 episodes) including 28 patients with undefined, non-infectious, recurrent fevers who we termed Syndrome of Undifferentiated Recurrent Fever (SURF). Patients reported early onset of fevers (average 3.7 years, range from birth to 14 years, median 3 years), with fevers lasting 3.7–4.8 days (range 2 to 7 days), with a maximum temperature of 40.1°C (range 38.8 – 41.7°C). In contrast to our previously reported PFAPA cohort,16 patients with SURF tended to have less frequent episodes, with symptoms every 42–67.6 days (range 14–180 days). In addition, unlike in PFAPA, parents and patients noted that inflammatory episodes were not similar and difficult to predict.
The need for periodicity in the diagnosis of PFAPA remains controversial among diagnostic and classification criteria.15,17,18 To better delineate the spectrum of symptoms, patients were further sorted into those who had symptoms of the PFAPA triad (aphthous stomatitis, pharyngitis and/or cervical adenitis) and those who did not (Table 1). While a subset of patients experienced aphthous stomatitis or pharyngitis (exudative and/or erythematous) with some episodes, the majority of patients (61%) reported that symptoms of the PFAPA triad were not regularly present during their fever episodes. Only two patients had the complete PFAPA triad of aphthous stomatitis, pharyngitis and adenitis, but episodes were not predictable at any stage of disease and onset occurred after the age of 5 years (10 and 16 years of age). Compared to patients with PFAPA, patients with SURF reported more frequent gastrointestinal symptoms including nausea, vomiting and abdominal pain with episodes, for which no infectious or inflammatory cause was identified (such as viral gastroenteritis or inflammatory bowel disease). Most had a history of infection associated with a portion of episodes, including 10% with streptococcal positive pharyngitis, 21% of patients with at least 2 episodes of otitis media, 33% with sinusitis, and 29% with a history of pneumonia, none of which required hospitalization nor extended courses of antibiotics. A history of sinusitis was twice as common in the group having some PFAPA symptoms compared to those without signs of the PFAPA triad, but both groups report no consistent effect of antibiotics on the majority of fevers. There was no relationship between the timing of the infection and course of other febrile episodes. Half of the SURF cohort had a history of atopy with 42% reporting allergic rhinitis, 21% with asthma and 21% with eczema. Thirty percent had a family member with recurrent fevers of unknown origin in childhood that resolved over time.
Table 1.
Distinguishing clinical characteristics recurrent fever patients
| SURF | SURF (triad) | PFAPA (ref) | p value | |
|---|---|---|---|---|
| Patients, n | 17 | 11 | 94 | |
| Male : Female | 58% male | 63% male | 48% male | n.s. |
| Age of onset (mean ± SD) | 3.58 ± 4.29 years | 2.72 ± 3.19 years | 3.25 ± 2.70 years | n.s. |
| Tmax (°C, mean, range) | 40.2°C (38.8–41.7°C) | 40.0°C (38.8–41.6°C) | 40.1°C (38.8–41.7°C) | n.s. |
| Episode duration (mean range) | 3.2 – 4.9 days | 3.7 – 4.8 days | 3.7 – 5.7 days | n.s. |
| Asymptomatic interval (mean range) | 32.6 –64.5 days | 25.4 – 32.5 days | 27 – 45 days | n.s. |
| Atopy (%) | 41 | 45 | 23 | 0.002 |
| Family history of recurrent fever (%) | 30 | 9 | 43 | <0.0001 |
| Family history of autoimmune disease (%) | 5.8 | 9 | 14 | n.s. |
| Fever only (%) | 30 | 0 | 0 | <0.0001 |
| Pharyngitis (%) | 0 | 72.7 | 64 | <0.0001 |
| Cervical adenitis (%) | 0 | 63.6 | 49 | <0.0001 |
| Aphthous stomatitis (%) | 0 | 45.4 | 51 | <0.0001 |
| Abdominal pain (%) | 41.2 | 63.3 | 26 | <0.0001 |
| Headache (%) | 35.2 | 54.5 | 28 | 0.015 |
| Ocular symptoms (%) | 11.7 | 36.3 | 15 | <0.0001 |
| Nausea / vomiting (%) | 35.2 | 45.4 | 18 | 0.0002 |
| Arthralgia (%) | 17.6 | 27.2 | 26 | n.s. |
| Rash (%) | 5.8 | 18.8 | 7 | 0.004 |
| Infection (%) | 76.4 | 63.6 | 0 | <0.0001 |
| Response to steroid (%) | 5.8 | 18.8 | 100 | <0.0001 |
| Response to colchicine (%) | 42.8 | 16.6 | n/a | <0.0001 |
| Length of follow-up (average, range) | 57.5 months (28–100 months) | 59 months (26–100 months) | 75 months (22–110 months) | 0.02 |
Consistent with the variability in presentation, patients had varied responses to therapy as well. While ibuprofen was effective at reducing fever and associated symptoms during episodes in 93% of patients, prednisolone was not reliably effective at rapidly aborting episodes as often observed in PFAPA patients. Colchicine and montelukast had also been prescribed prophylactically to some patients, with reduced frequency of febrile episodes, and diminished severity of symptoms. Interestingly, the patients without symptoms of the PFAPA triad, and were more likely to have tried and responded to colchicine than those who had any of the PFAPA triad. One patient had complete resolution after 6 months without medical intervention. The majority of parents preferred a “watchful waiting” approach, and continued to treat episodes with on-demand anti-pyretics.
Tonsillectomy leads to a rapid resolution of symptomatic episodes in SURF.
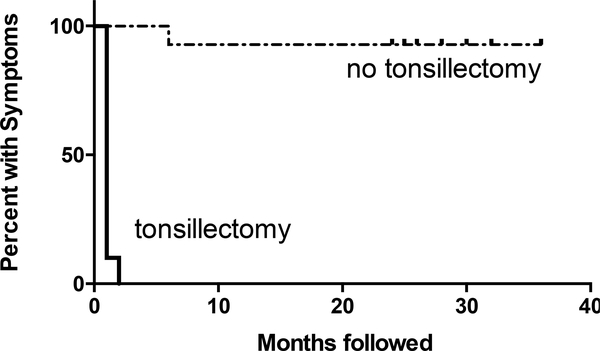
Previous studies have shown that tonsillectomy can be a therapeutic option with improvement in symptoms and quality of life in many patients with PFAPA.16,19 An independent cohort of patients with SURF elected to undergo tonsillectomy in hopes of achieving a similar response. Older patient age and a parental history of tonsillectomy were the primary factors for parents to opt for tonsillectomy rather than daily medications (Table 2). Compared to the PFAPA cohort, undefined recurrent fever patients whose families opted for tonsillectomy were predominantly male, older at the age of symptoms onset, and older at the time of surgery, though their febrile cycles were similar (Table 3). Fifteen patients had had a tonsillectomy with or without adenoidectomy with complete resolution of symptoms within 2 months post-surgery, with the average time to resolution of symptoms at 1.13 months. The average length of follow up post-tonsillectomy is 70 months (range 27–96 months), with no patients reporting recurrence of symptoms (Figure 1). There was no difference in response rate between patients that had any symptoms of the PFAPA triad compared to those that did not. This modestly sized cohort suggests that tonsillectomy may be an effective surgical therapy for management of pediatric patients with SURF.
Table 2.
Patients undergoing tonsillectomy tend to be older than those opting for medical management
| SURF Patients | Tonsillectomy (n = 15) | Medical therapy (n = 13) | p value |
|---|---|---|---|
| Male : Female | 66% male | 53% male | 0.06* |
| Age of onset, mean (range) | 6.0 years (1 month to 15 years) | 1.24 years (1 month to 6 years) | 0.0007 |
| Age at Surgery, mean, (range) | 7.06 years (3 – 16 years) | n.a. | n.a. |
| Febrile episodes, mean (range) | 3.2 – 4.6 days (3 – 5 days) | 3.8 – 4.9 days (2 – 7 days) | 0.52 |
| Asymptomatic Intervals, mean (range) | 24.3 – 41 days (14 – 90 days) | 52.6 – 83.7 days (14 – 90 days) | 0.29 |
| Tmax (°C, mean, range) | 40.1°C (39.4 – 41.6°C) | 40.0°C (38.8 – 41.7°C) | 0.94 |
| Parental history of tonsillectomy (%) | 33% | 7% | 0.001* |
by Fisher’s exact test, all others by unpaired Student’s t test
n.a., not applicable
Table 3.
Clinical Characteristics of patients undergoing tonsillectomy for SURF compared to PFAPA
| Patients | SURF Patients (n=15) | PFAPA cohort16 | p value |
|---|---|---|---|
| Male : Female | 66% male | 48.3% male | 0.009* |
| Age of onset, mean ± SD | 6.0 ± 4.16 years | 2.78 ± 2.39 years | 0.0001 |
| Age at Surgery, mean ± SD | 7.06 ± 4.09 years | 5.56 ± 3.35 years | 0.009 |
| Febrile episodes, mean | 3.2 – 4.6 days | 3.3 – 4.7 days | 0.88 |
| Asymptomatic Intervals, mean | 24.3 – 41 days | 23 – 38 days | 0.95 |
| Tmax (°C, mean, range) | 40.1°C (39.4 – 41.6°C) | 40.2°C (38.8 – 41.7°C) | 0.80 |
by Fisher’s exact test, all others by unpaired Student’s t test
n.a., not applicable
Figure 1. Tonsillectomy leads to rapid resolution of febrile episodes in SURF patients.
Time to resolution of febrile symptoms post-tonsillectomy (n=15), compared to patients who did not undergo tonsillectomy (n=13).
Tonsillar cells from SURF patients are distinct from PFAPA during afebrile periods.
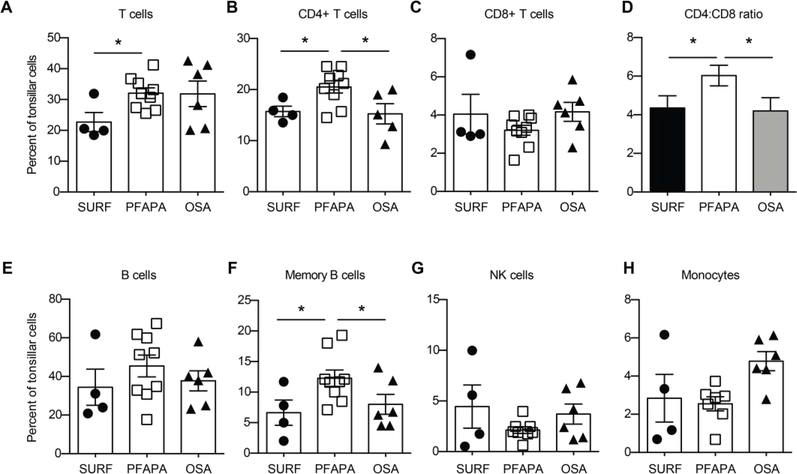
Tonsillectomy has been shown to be a successful therapy for PFAPA, a response postulated to be due to altered lymphocyte composition/recruitment,16,20 localized, persistent inflammation,16,21 or altered microbiota,16,20,22 compared to recurrent pharyngitis or obstructive sleep apnea controls. To determine if the improvement in febrile episodes observed in SURF patients was due to similar cellular constituents, we phenotypically characterized post-operative palatine tonsillar samples by flow cytometry and gene expression. Flow cytometry of isolated cellular constituents reveals that tonsils from patients with SURF have reduced CD3+ T cell infiltration, especially of the CD4+ subset, with a trend towards increased NK cells compared to PFAPA patient tonsils. We again observed that tonsils from PFAPA patients have a significantly larger memory B cell population, defined as CD27+, CD19+, CD38 negative cells compared to SURF patients, consistent with our prior report studying a different group of age matched PFAPA patients.16 In contrast, CD8+ T cell subsets, monocyte/macrophage and dendritic cell populations were similar among all groups (Figure 2 and data not shown). Interestingly, the SURF tonsil cellular components were more consistent with those of patients with OSA. These differences suggest that SURF is likely a pathologically independent entity from PFAPA and not simply atypical PFAPA.
Figure 2. SURF tonsils have an inflammatory cell infiltrate distinct from PFAPA tonsils.
A-C, CD3+ T cells, and CD3+ CD4+ T cells from tonsils of patients with SURF are decreased, with similar percentages of CD8+ T cells, and subsequently a reduced CD4:CD8 ratio (D). E-F, Total CD19+ B cells are similar between groups, but CD19+CD20+CD27+CD38- memory B cells are reduced in SURF tonsils compared to PFAPA tonsils. G,H, CD56+ NK cells (G) and CD11c+CD14+ monocytes (H) are similar between the two groups. Data shown as mean ± SEM, with *, p<0.05, by Student’s t test.
SURF patient tonsils have an IL-1 signature
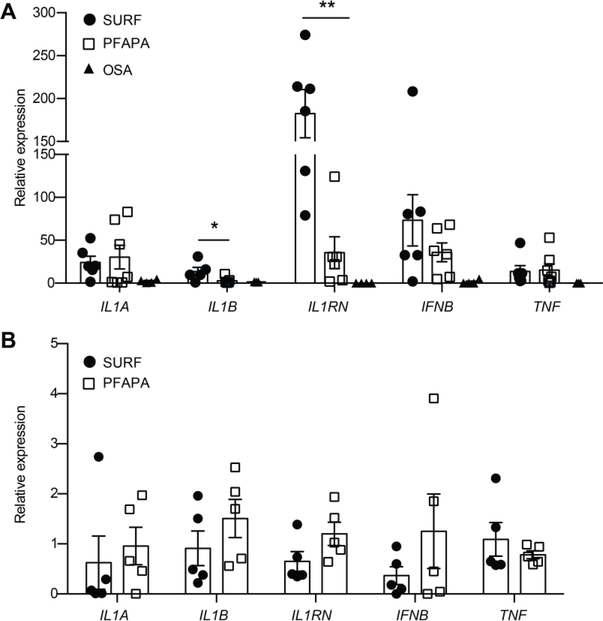
Prior work by our group demonstrated tonsillar tissue had an inflammatory signature that was present during afebrile episodes.16 To determine if SURF tonsils had a similar inflammatory signature, RNA was extracted from whole tonsils and evaluated for expression of proinflammatory cytokines, including IL1A, IL1B, IL1RN, IL18, TNF and IFNB. Notably, transcripts for IL1B and IL1RN were significantly upregulated in SURF tonsils compared to PFAPA, while IL1A, IL18, IFNB and TNF expression was similar between groups (Figure 3A and data not shown). Both PFAPA and SURF tonsils had elevated expression of all 5 cytokine transcripts compared to OSA tonsils.
Figure 3. SURF and PFAPA patient tonsils have a localize inflammatory signature.
A, Gene expression analysis from whole tonsillar tissue from PFAPA (n=7) and SURF tonsils (n=6), demonstrates significant increases in IL1B and IL1RN gene expression in SURF tonsils compared to PFAPA tonsils from patients of similar age. Expression of IL1A, IFNB and TNF are similar between fever disorders. OSA, obstructive sleep apnea. B. Pro-inflammatory gene expression in peripheral blood mononuclear cells does not differ between SURF and PFAPA patients (n=5 per group). Each symbol represents a different patient, average of technical triplicates, shown as the mean ± SEM. *, p<0.05, **, p<0.01 by Student’s two-tailed t-test.
Others have show that asymptomatic PFAPA patients have peripheral blood cell gene expression levels indistinguishable from healthy controls.21 To determine if these elevated inflammatory cytokine levels we observed in SURF patients were unique to the tonsillar microenvironment, peripheral blood monocytes from patients with PFAPA and SURF were similarly assessed for transcript expression at the time of tonsillectomy. Expression of IL1A, IL1B, IL1RN, TNF and IFNB did not differ between the two patient groups (Figure 3B). These data suggest that similar to PFAPA patients, SURF patients may have localized, subclinical inflammation between episodes, despite an absence of serologic inflammation.
DISCUSSION
Autoinflammatory diseases result from inborn errors in the innate immune system leading to dysregulation of myeloid cells, IL-1β, type I interferon and NF-κB, and episodic systemic inflammation.23,24 To date, more than 30 monogenic disorders have been described and increasing numbers of patients identified, using a translational bench to bedside approach.25 Relatedly, immunologists are frequently tasked with evaluating pediatric patients with recurrent fever. While the majority of these patients have recurrent infections, there is an increasing recognition of a subset of patients with recurrent fever and systemic inflammation with no evidence of pathogenic microbial involvement, who are now classified as having an autoinflammatory disease. In the pediatric population, PFAPA syndrome is a common and well recognized clinical entity, while hereditary fever disorders are much less frequently diagnosed in the United States and Western Europe. The lack of genetic or biologic diagnostic test for PFAPA syndrome, however, may lead to erroneous diagnoses.3 Thus, it is now recognized that many children with noninfectious recurrent fever do not fit into either of these diagnostic categories and have been referred to by our group and others as patients with SURF.
An ongoing challenge for these patients specifically, is the changing definition of PFAPA syndrome. Since its initial description in 1987, several modifications of the necessary criteria for PFAPA have been published, including two in the last year.17,18 As the defining characteristics differ with respect to the requirement of periodicity, steroid responsiveness, and associated symptoms, it is possible that some of the patients we have included as SURF, would fall into a PFAPA category if classification rather than diagnostic criteria are used. While dividing the SURF patients further into these subsets generates smaller cohorts, the differences observed between SURF patients without any of the PFAPA triad symptoms, compared to those with pharyngitis, aphthous stomatitis, or cervical lymphadenitis during episodes, and compared to our prior PFAPA cohort suggests that the SURF patients overall are a phenotypically heterogeneous group. Most notable in the SURF patients is the higher incidence of abdominal pain, nausea and vomiting during febrile episodes, which may be features which more easily distinguish them from PFAPA patients. These symptoms are reminiscent of recently described patients with low-penetrance NLRP3 mutations,26 a population which has been shown to have an intermediate biologic phenotype, yet responded to IL-1 blockade albeit at higher doses.
Given the recognition that novel disorders continue to be genetically identified, many patients will fall into subcategories of recurrent fever with features of autoinflammation and/or immunodeficiency.2,25,27 It remains to be seen whether these patients represent autoinflammatory gene variants of known syndromes, complex genetic autoinflammatory disorders, or independent entities.1,28 In contrast to a recently described European cohort,29 we found that patients with PFAPA syndrome and SURF, were as likely to report a history of affected relatives as those with identified genetic variants. We were surprised to find that the majority of affected relatives were in SURF patients who did not have features of the PFAPA triad, and who were more likely to trial and respond to colchicine. These disease features may indicate oligogenic inheritance or a contribution of environmental or epigenetic factors.2 Consequently, the precise etiology underlying the recurrent fevers in SURF remains unknown. Thus, the diagnostic approach and the most effective therapy for these patients remains unclear, and best addressed on an individual basis.
For patients with PFAPA, randomized studies of tonsillectomy with or with out adenoidectomy have indicated that the palatine tonsils are linked to disease pathology.19,30 We previously showed tonsils from pediatric PFAPA patients have a distinct pro-inflammatory signature compared to tonsils from patients with recurrent streptococcal pharyngitis.16 In addition, we observed that despite tonsillectomy being performed during the asymptomatic interfebrile period, PFAPA patient tonsil-derived cells maintained a unique inflammatory signature with increased IL1RN and TNF expression.16 In SURF patients, we found a persistent IL1B and IL1RN signature. Despite the difference in gene expression, the benefit of tonsillectomy between the groups is similar, as we saw no recurrence of febrile episodes up to 8 years after the surgery.
In our cohort, which was not a randomized trial, therapeutic decisions, whether medical or surgical, were largely driven by parental preference, with parental history of tonsillectomy and patient age as the primary determinants of pursuing medical therapy vs tonsillectomy. As a result, we have no families that first received daily therapy with colchicine, cimetidine, or montelukast, and then opted for tonsillectomy. Parents of children approaching school age were more likely to pursue tonsillectomy, whereas parents of younger patients were more frequently in favor of a trial of daily medications. The individual response to on-demand prednisolone therapy may have influenced the parental decision-making process. Unlike patients with PFAPA syndrome, patients with SURF were less likely to respond to on-demand steroid therapy with only 10% of the total cohort reporting improvement in fevers, while the majority noted that it did not help completely resolve the episode, and sometimes did not work at all. Only 1 patient in the tonsillectomy group experienced resolution of febrile episodes with on-demand prednisone use. While our approach is to use the lower median dose of steroid (≤1mg/kg po once in the first 24 hours of a febrile episode) as described by Tasher et al.31 and defined response as resolution of symptoms with this single dose, it is possible that increased or repetitive dosing would have led to improved symptoms. Similar to Gattorno et al,1 however, parents reported colchicine to be effective in reducing the severity and frequency of febrile episodes. Colchicine has previously been described to be efficacious in PFAPA patients, notably those carrying a low-penetrance variant in MEFV or from ethnicities with high carriage rates.32,33 While the classification of such patients as FMF has been a source of debate,1 the phenotypic differences between FMF, compared to PFAPA and SURF patients suggest that the latter may be considered part of a recurrent fever spectrum, likely with multifactorial influences resulting in their inflammatory disorder.
The role of IL-1 in PFAPA has been suggested previously, but IL-1 targeted therapy with either anakinra or canakinumab has been met with limited success.21,34 We observed that in the tonsils, transcripts for all pro-inflammatory genes measured were greater in those from PFAPA and SURF patients, compared to tonsils from children with OSA. However, gene expression for IL1B and IL1RN were significantly upregulated in SURF tonsils compared to PFAPA, suggesting that targeting this pathway may be a therapeutic option for patients with SURF.
Our study has several limitations. All patients were recruited from the greater San Diego area, where a varied ancestry may influence the underlying genetic background with impacts on genetic modifiers of disease. Notably, others have shown that the presence of a single variant may impact the efficacy of tonsillectomy.35 It is possible that in a more genetically homogeneous population, or in populations with higher frequency low-penetrance variants, that tonsillectomy for SURF will be less effective. Due to limited pediatric tissue samples, we compared tonsillar samples from age-matched patients with obstructive sleep apnea, in contrast to our prior study which used culture-positive recurrent streptococcal pharyngitis. Cadaveric samples from healthy children were not available. While both studies show proinflammatory markers in the tonsils which are unique to PFAPA and SURF, it is possible that some of the differences we observed are due to underlying pathology of OSA or recurrent pharyngitis. Furthermore, tonsillectomy was performed during afebrile periods. Given that both SURF and PFAPA patients have periodic fevers, some variability may be related to the timing of the surgery within the disease cycle. Finally, this study was designed to examine the tonsillar microenvironment, and was not meant to be a treatment study. The heterogeneous nature of the SURF cohort suggests that several etiologies culminating in an IL-1-driven, inflammatory pathway may be contributing, including genetic, epigenetic and environmental factors. Until larger studies with multi-center cohorts are performed to understand the role of these contributors in recurrent fever pathogenesis, patients and families must continue to be evaluated and counseled on a case by case basis, taking into account severity of illness, quality of life and any therapeutic potential benefits balanced against medicosurgical risks.
Highlights.
Undefined recurrent fevers comprise a large subset of autoinflammatory patients.
Tonsils from undefined recurrent fever patients are distinct from PFAPA syndrome.
Undefined recurrent fever tonsils have an IL-1 signature.
Acknowledgements
This work was supported by NIH 1K08HD075830–01A1 (L.B.), Thrasher Research Fund (L.B.), The Hartwell Foundation (L.B), and A.P. Giannini (L.B.). The authors would like to acknowledge Dr. Hal M. Hoffman (University of California, San Diego) for helpful discussion, and the patients and families who participated.
Abbreviations
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IFN
interferon
- lb-1
interleukin-1
- IL-1Ra
interleukin-1 receptor antagonist (protein)
- IL1RN
interleukin-1 receptor antagonist (gene)
- NK
natural killer
- OSA
obstructive sleep apnea
- PFAPA
periodic fever, aphthous stomatitis, pharyngitis, and adenitis
- RT-qPCR
reverse transcription quantitative polymerase chain reaction
- SEM
standard error of mean
- SURF
syndrome of undifferentiated recurrent fever
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures of Conflicts of Interest
The authors declare no conflicts of interest.
Ethical Approval.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the UCSD Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
References
- 1.Papa R et al. Next generation sequencing panel in undifferentiated autoinflammatory diseases identify patients with colchicine-responder recurrent fevers. Rheumatology (Oxford) (2019). [DOI] [PubMed]
- 2.Schnappauf O & Aksentijevich I Current and future advances in genetic testing in systemic autoinflammatory diseases. Rheumatology (Oxford) 58, vi44–vi55 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harel L et al. The First International Conference on Periodic Fever, Aphthous Stomatitis, Pharyngitis, Adenitis Syndrome. J Pediatr 193, 265–274 e3 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Federici L et al. A decision tree for genetic diagnosis of hereditary periodic fever in unselected patients. Ann Rheum Dis 65, 1427–32 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broderick L, Kastner DL & Hoffman HM Recurrent Fever Syndromes. in Primary Immunodeficiency Diseases: A Molecular and Genetic Approach (eds. Ochs HD, Smith CIE & Puck JM) (2013).
- 6.Hofer M et al. International periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis syndrome cohort: description of distinct phenotypes in 301 patients. Rheumatology (Oxford) 53, 1125–9 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Lantto U, Koivunen P, Tapiainen T & Renko M Long-Term Outcome of Classic and Incomplete PFAPA (Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Adenitis) Syndrome after Tonsillectomy. J Pediatr 179, 172–177 e1 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell 90, 797–807 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Drenth JP et al. Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat Genet 22, 178–81 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Houten SM et al. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat Genet 22, 175–7 (1999). [DOI] [PubMed] [Google Scholar]
- 11.McDermott MF et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97, 133–44 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Aksentijevich I et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum 46, 3340–8 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dode C et al. New mutations of CIAS1 that are responsible for Muckle-Wells syndrome and familial cold urticaria: a novel mutation underlies both syndromes. Am J Hum Genet 70, 1498–506 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman HM, Mueller JL, Broide DH, Wanderer AA & Kolodner RD Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet 29, 301–5 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas KT, Feder HM Jr., Lawton AR & Edwards KM Periodic fever syndrome in children. J Pediatr 135, 15–21 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Luu I et al. Immune Dysregulation in the Tonsillar Microenvironment of Periodic Fever, Aphthous Stomatitis, Pharyngitis, Adenitis (PFAPA) Syndrome. J Clin Immunol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amarilyo G et al. Consensus treatment plans for periodic fever, aphthous stomatitis, pharyngitis and adenitis syndrome (PFAPA): a framework to evaluate treatment responses from the childhood arthritis and rheumatology research alliance (CARRA) PFAPA work group. Pediatr Rheumatol Online J 18, 31 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gattorno M et al. Classification criteria for autoinflammatory recurrent fevers. Ann Rheum Dis 78, 1025–1032 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Forsvoll J & Oymar K The role of tonsillectomy in the Periodic Fever, Aphthous stomatitis, Pharyngitis and cervical Adenitis syndrome; a literature review. BMC Ear Nose Throat Disord 18, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dytrych P et al. Polyclonal, newly derived T cells with low expression of inhibitory molecule PD-1 in tonsils define the phenotype of lymphocytes in children with Periodic Fever, Aphtous Stomatitis, Pharyngitis and Adenitis (PFAPA) syndrome. Mol Immunol 65, 139–47 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Stojanov S et al. Periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) is a disorder of innate immunity and Th1 activation responsive to IL-1 blockade. Proc Natl Acad Sci U S A 108, 7148–53 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tejesvi MV et al. Tonsillar microbiota in children with PFAPA (periodic fever, aphthous stomatitis, pharyngitis, and adenitis) syndrome. Eur J Clin Microbiol Infect Dis 35, 963–70 (2016). [DOI] [PubMed] [Google Scholar]
- 23.de Jesus AA, Canna SW, Liu Y & Goldbach-Mansky R Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol 33, 823–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manthiram K, Zhou Q, Aksentijevich I & Kastner DL The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat Immunol 18, 832–842 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Holzinger D, Kessel C, Omenetti A & Gattorno M From bench to bedside and back again: translational research in autoinflammation. Nat Rev Rheumatol 11, 573–85 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Kuemmerle-Deschner JB et al. Clinical and Molecular Phenotypes of Low-Penetrance Variants of NLRP3: Diagnostic and Therapeutic Challenges. Arthritis Rheumatol 69, 2233–2240 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Broderick L Recurrent Fevers for the Pediatric Immunologist: It’s Not All Immunodeficiency. Curr Allergy Asthma Rep 16, 2 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Manthiram K et al. Common genetic susceptibility loci link PFAPA syndrome, Behcet’s disease, and recurrent aphthous stomatitis. Proc Natl Acad Sci U S A 117, 14405–14411 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.TerHaar NM et al. Clinical characteristics and genetic analyses of 187 patients with undefined auto inflammatory diseases. Ann Rheum Dis 78, 1405–1411 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Renko M et al. Arandomized, controlled trial of tonsillectomy in periodic fever, aphthous stomatitis, pharyngitis, and adenitis syndrome. J Pediatr 151, 289–92 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Tasher D, Somekh E & Dalal I PFAPA syndrome: new clinical aspects disclosed. Arch Dis Child 91, 981–4 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butbul Aviel Y, Tatour S, Gershoni Baruch R & Brik R Colchicine as a therapeutic option in periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA) syndrome. Semin Arthritis Rheum 45, 471–4 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Gunes M, Cekic S & Kilic SS Is colchicine more effective to prevent periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis episodes in Mediterranean fever gene variants? Pediatr Int 59, 655–660 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Soylu A, Yildiz G, Torun Bayram M & Kavukcu S IL-1beta blockade in periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome: case-based review. Rheumatol Int (2019). [DOI] [PubMed]
- 35.Pehlivan E et al. PFAPA Syndrome in a Population with Endemic Familial Mediterranean Fever. J Pediatr 192, 253–255 (2018). [DOI] [PubMed] [Google Scholar]