Abstract
Since early 2020, COVID-19 has wreaked havoc in many societies around the world. As of the present, the SARS-CoV-2-borne disease is propagating in almost all countries, affecting hundreds of thousands of people in an unprecedented way. As the name suggests, the novel coronavirus, widely known as SARS-CoV-2, is a new emerging human pathogen. A novel disease of relatively unknown origin, COVID-19 does not seem to be amenable to the currently available medicines since there is no specific cure for the disease. In the absence of any vaccine or effective antiviral medication, we have no tools at our disposal, but the method of quarantine, be it domestic or institutional, to hinder any further progression of this outbreak. However, there is a record of physicians in the past who practiced convalescent blood transfusion. To their awe, the method seemed to be useful. It is anticipated that these contemporary methods will outdo any other vaccination process in the time being, as blood transfusion is instead a cost-effective and time-friendly technique. Following a successful trial, this new approach of contemporary nature to a viral disease may serve as an emergency intervention to intercept infectious outbreaks and prevent an impending epidemic/pandemic. In this review, we document the most recent evidence regarding the efficiency of convalescent plasma and serum therapy on SARS, MERS, and particularly COVID-19, while discussing potential advantages and possible risks of such practice.
Keywords: SARS-CoV-2/2019-nCoV/COVID-19, Therapy, Convalescent blood transfusion, Plasma, Serum (sera)
Introduction
Following the identification of several patients with an affliction that resembled viral pneumonia, the world faced a downward spiral centered on a local seafood wholesale market in Wuhan, China [1]. The pathogen responsible for the instigation of this strange outbreak rapidly rose to global prominence [2], and therefore was named 2019-nCoV by the World Health Organization (WHO) [3]. After further investigations which revealed the true nature of this novel coronavirus, it was unanimously agreed to be named “SARS-CoV-2,” which is short for “severe acute respiratory syndrome coronavirus 2.” Accordingly, the disease was called “coronavirus disease 2019,” further to be simplified to “COVID-19” [4]. As of October 23, over 41,570,883 million confirmed COVID-19 cases and 1,134,940 confirmed deaths were reported to the WHO [5]. The recent outbreak of COVID-19 in China was announced as a public health emergency of international concern by the WHO [4]. On March 11, 2020, the WHO officially proclaimed that the COVID-19 epidemic had become a pandemic [6].
Near 2 decades ago, >8,000 patients developed severe acute respiratory syndrome (SARS), a pneumonia-like disease similarly caused by a coronavirus [7]. This was followed by another series of respiratory afflictions about a decade later, which was ultimately revealed to be also due to a coronavirus. The latter, known as Middle East respiratory syndrome (MERS), has been confirmed in 2,494 patients since September 2012 [8, 9]. Interestingly, both SARS and MERS epidemics were caused by virions belonging to the family of β-coronaviruses [4].
Like its predecessors, SARS-CoV-2 was also recognized as a new member of β-coronaviruses [2]. This has been confirmed with next-generation sequencing conducted on virions extracted from the respiratory epithelial cells of patients with COVID-19 [10]. The disease has an incubation period spanning 3–7 days [11]. Hallmark symptoms are fever, cough, and fatigue; however, some patients also develop nasal congestion, rhinorrhea, and diarrhea [12]. In severe cases, patients may rapidly deteriorate and develop life-threatening conditions, for example, acute respiratory distress syndrome, septic shock, metabolic acidosis, arrhythmia, and bleeding disorders [13, 14]. An infection of the lower respiratory tract, SARS-CoV-2, manifests with clinical symptoms closest to that of SARS and MERS, albeit milder at the beginning [3]. This is a remarkable distinction, as COVID-19 is demonstrated with mild initial symptoms, even in patients who later develop a critical illness. On computed tomography of the chest, COVID-19 is mostly recognized by ground-glass opacities and bilateral patchy infiltrations [15]. From a laboratory point of view, lymphopenia and elevated serum levels of C-reactive protein can be tracked in some patients, but not all of them [16]. Despite being decisive, the clinical symptoms and laboratory findings mentioned here are not distinctive since they cannot be easily distinguished from pneumonia caused by pathogens other than SARS-CoV-2 [6].
At the time of writing this article, there is no vaccine for the prevention of SARS-CoV-2 infection [17]. Several countries have already initiated programs for developing an effective vaccine that most likely targets spike glycoprotein as an antigen. Still, given the ample diversity of antigenic variants, it is virtually impossible to provide cross-protection by vaccination, even when all pathogenic strains belong to the same phylogenetic subcluster [18]. That is why the treatment of COVID-19 has been limited to palliative care and respiratory support, that is, oxygen therapy in mild and extracorporeal membrane oxygenation in severe cases [17]. Apart from palliative care, certain therapeutic agents are currently being investigated as potential therapies for COVID-19 [19].
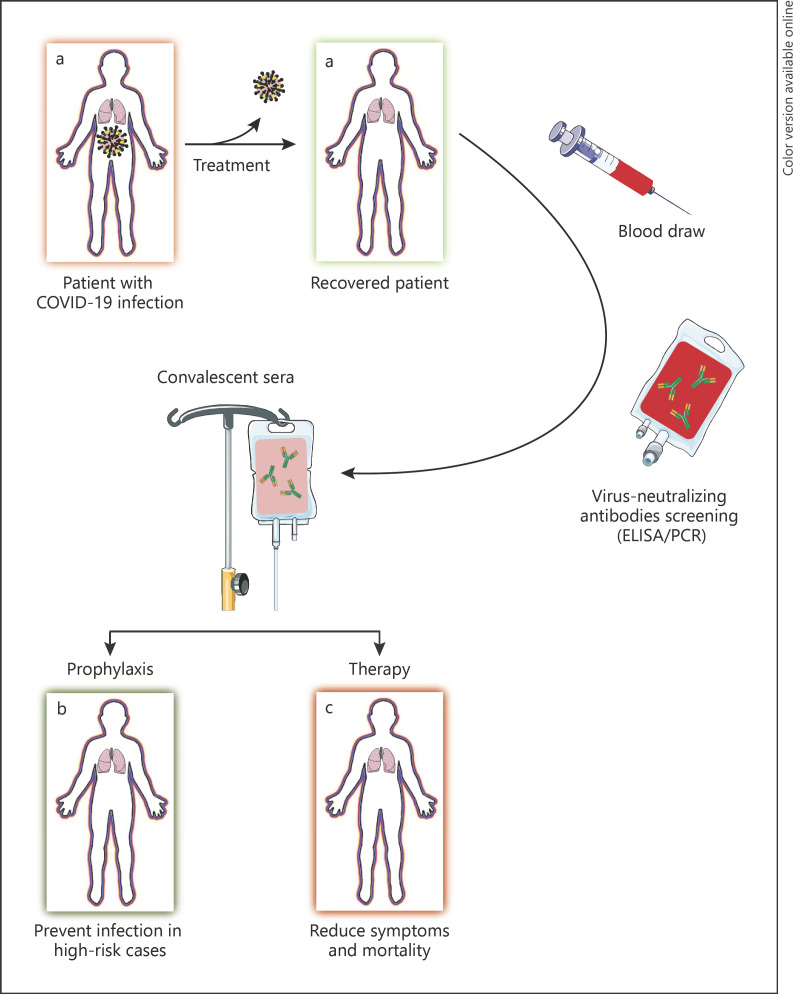
A legacy method, convalescent blood transfusion, might be of value in prevention and even treatment of COVID-19. The biggest challenge here is finding an adequate number of volunteer donors with a history of COVID-19 [20, 21]. Despite all concerns regarding the safety of blood transfusion in terms of SARS-CoV-2 infection, there were no reports of blood-borne SARS-CoV infection back in 2003 [22]. A great portion of therapeutic approaches currently being investigated for their efficacy against COVID-19 are inspired by our experience in treating SARS, MERS, and other viral epidemics [23]. Figure 1 illustrates the convalescent serum therapy procedure (modified from [20]). In this review, we document the most recent evidence regarding the efficiency of convalescent plasma and serum therapy on SARS, MERS, and particularly COVID-19, while discussing potential advantages and possible risks of such practice.
Fig. 1.
The convalescent serum therapy procedure (modified from [20]).
Passive Antibody Therapy
With a history dating back to the 1890s, passive antibody therapy was the cornerstone of therapeutic interventions for treating some viral diseases before the advent of antimicrobial therapy in the 1940s [20]. Generally, passive antibody therapy is a more effective method used as a preventive intervention for prophylaxis against diseases, rather than a therapeutic approach for treatment of that illness. In this technique, individuals suspected to be at risk for developing certain ailments are injected with specific antibodies. The antibody incites a relevant immune response that may hinder the occurrence of any given disease in the future or can further accelerate the function of the immune system when used as a therapeutic approach. Thus, passive antibody administration is the only means of providing immediate immunity to susceptible persons [24, 25]. In order to be effective, the antibody should be amply injected. When dispensed to a vulnerable individual, this antibody will disseminate in general circulation and reach small capillary beds in different tissues, ultimately inciting a provisional immune response against a particular infection. Protection conferred by the antibody largely depends on its composition and dosage and can last from several weeks to many months [20].
According to the experience with other types of coronavirus from the previous outbreaks, such as SARS-CoV, the blood plasma or serum of patients who have surmounted the disease, or are said to be in convalescence, contains neutralizing antibodies against the causative virus [26]. Regarding SARS-CoV-2, it is thought that “viral neutralization” is the primary underlying mechanism through which passive antibody therapy yields protection against the virus. The human convalescent serum is one potential source of antibodies against SARS-CoV-2 extracted from patients who have recovered from COVID-19 [27]. Today, this is the only source that gives immediate access to antibodies against the novel coronavirus. In terms of therapy, antibodies are most effective when injected only shortly after the emergence of clinical symptoms. To our knowledge, this is because passive antibody therapy acts through neutralizing the initial inoculum, that is, an aggregate of pathogens limited to an area in the body, which is vastly disseminated once the disease has taken place [28].
History of Blood Therapy
A legacy method, convalescent serum therapy, held a remarkable place in the early 20th century, when influenza reigned. Despite the variable efficacy depending on the type of the virus, the old-time consensus was that serum therapy worked as a therapeutic intervention [20]. That very same consensus held true almost a century later when clinicians resorted to convalescent serum antibodies to fight against the 2009–2010 H1N1 influenza pandemic. These antibodies, obtained from sera of patients who had recovered, were used for the treatment of severe H1N1 influenza in patients under intensive care [29]. As of the new millennium, a lack of effective therapies for the 2 relatively menacing coronavirus epidemics, that is, SARS and MERS, led to the practice of serum therapy on certain patients [30].
According to the anecdotal evidence, all patients who had received human convalescent serum survived, suggesting that the legacy method was a successful approach. Although one must not overlook subtle dissimilarities in different viral infections, our recent memory reminds us of the promising results achieved with serum therapy, making it a powerful method of approach when COVID-19 is wreaking havoc in a myriad of societies [20].
Convalescent Plasma
Similar to previous viral epidemics, passive immunotherapy by transfusion of convalescent plasma is yet considered a promising approach toward the treatment of severely ill patients with COVID-19 [31]. This arguably old-fashioned method becomes even more apposite as one subtilizes the egregious lack of vaccines and medicines required for successful treatment of COVID-19 [32]. Given the route of administration, the efficacy of convalescent plasma therapy can be explained by the immediate access of antibody molecules to virions disseminated in general circulation. Immunoglobulin-rich convalescent plasma was once regarded as a last resort in treating SARS, mostly indicated for critically ill patients who did not respond well to methylprednisolone. Several studies have reported that recipients of convalescent plasma not only presented a lower mortality rate but also had a shorter hospital stay compared to patients who did not receive such care [19].
Convalescent Serum
Blood serum is obtained when plasma is stripped of its fibrinogen, a coagulation factor that readily aggregates upon clot formation. Normally, fibrinogen has a concentration of <4 g/L in the blood [33]. Fibrinogen concentration can further rise in the blood of elderly COVID-19 patients, who also have underlying health conditions in many instances. This concentration can be as high as 8–12 g per 400 mL, duly evidenced with the blood obtained from elderly donors for transfusion to severely ill COVID-19 patients. Despite being incapable of inciting a graft versus host reaction, the donor's fibrinogen is still considered a foreign antigen by the recipient's immune system, especially when received in large amounts. The excess foreign material is thus removed from the recipient's blood at the expense of macrophages and T cells that would otherwise mediate an immune response against SARS-CoV-2. Therefore, a fibrinogen-laden plasma not only does not improve the condition of ill patients but also incapacitates their immune system. Accordingly, the foreign fibrinogen macromolecules must be removed to avoid the development of allergy or any undesirable effect on treatment. This revised form of passive immunotherapy has been named “serum therapy” due to its refined nature [34] and can be safely utilized for the treatment of COVID-19 patients in intensive care units [31].
A large number of patients who recover from COVID-19 produce specific antibodies against the causative agent [34]. This makes their serum a valuable asset for hindering reinfection with SARS-CoV-2. While serving as preventive agents, antibodies can also decelerate the reproduction of the virus in the acute phase of COVID-19, pruning the twisted course of the disease [29].
Studies on Convalescent Plasma and Serum Therapy
Table 1 explains details of the studies about convalescent plasma and serum therapy on patients with CoV.
Table 1.
Plasma therapy in the SARS epidemic
| Therapy | Location/date | Donors | Recipient patients, n/sex/age | Previous therapies | Treat groups | Results | Side effects | Discharge criteria/follow-up | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Convalescent plasma (200 mL) | Prince of Wales Hospital/March 2003 | 200 mL of plasma donated by SARS patients | A 57-yr-old woman | Cefotaxime, levofloxacin, oseltamivir, ribavirin, prednisolone, methylprednisolone | A 57-yr-old woman | Fever subsided, CT: further resolution of basal lung infiltrates, uneventful recovery | None | Probable therapeutic benefits of ribavirin, convalescent plasma, and corticosteroids | |
| Convalescent plasma (200–400 mL) with continuing high-dose methylprednisolone treatment | China 10 March–10 April 2003 | SARS-recovered patients, seropositive for coronavirus (titer 160–2,560), seronegative for hepatitis B/C, HIV, and syphilis/600–900 mL | 40 SARS patients with progressive disease; G1: 38.7±12.39 G2: 47.9±19.60 | Ribavirin, methylprednisolone | G1 (n = 19): convalescent plasma (optional pulsed steroids) G2 (n = 21): further pulsed methylprednisolone |
G1: discharged rate (p 0.001): 73.4% (n = 14) versus 19% (n = 4) lower mortality (p 0.049) Death rate (0.049); G1: 0% G2: 23.8% (n = 5) Comorbidities (0.05); G1: 1 (DM, old TB) G2: 1 SLE, 2 DM with old TB, 4 hypertension, and 1 atrial fibrillation |
None | Afebrile for 4 consecutive days; improvement of previous tests (WBC counts, platelet counts, CPK, LDH, liver function tests, and CRP); radiographic improvement; a period of at least 21 days following the onset of illness (detectable coronavirus in the stools of recovered patients for up to 3 weeks) | Soo et al. [35] |
| Convalescent plasma (500 mL) | Taiwan hospital | 500 mL convalescent plasma from each of 3 SARS patients/blood type: O seronegative for hepatitis B/C, HIV, syphilis, and HTLV-I and -II, serum IgG titer: >640/RT-PCR | 3 infected HCWs/antigen microarray | Ribavirin, methylprednisolone |
3 infected HCWs | One day after transfusion, viral load dropped from 495×103, 76×103, or 650×103 copies/mL to 0 or 1 copy/mL, anti-SARS-CoV IgM and IgG: increased in a time-dependent manner | None | All 3 patients survived. One HCW became pregnant subsequently, delivering 13 months after discharge with positive anti-SARS-CoV IgG in the newborn. Passive transfer of antibody from the mother is possible | |
| Convalescent plasma 200–400 mL (4–5 mL/kg) of ABO-compatible convalescent plasma around day 14 (range, 7–30 days) following the onset of symptoms | Hong Kong/20 March–26 May 2003 | SARS-recovered patients: an afebrile status for at least 7 days, CT improvement of 25%, no need an oxygen supplement, seronegative for hepatitis B/C, HIV, and syphilis, seropositive for coronavirus/600–900 mL plasma | 80 patients/43 females; 37 males/median age: 45 (21–82) | Starting in mid-March: cefotaxime, levofloxacin, clarithromycin, ribavirin, prednisolone, methylprednisolone | 279.3±127.1 mL (range, 160–640 mL); G1 (n = 48): therapy before day 14 of illness, G2 (n = 32): therapy after day 14 of illness | G1: discharge rate: 58.3 versus 15.6% (p < 0.001) 33 patients with good outcome, 30 patients with PCR positive/seronegative at the time of plasma infusion (66.7 vs. 20%; p = 0.001) Mortality rates; overall: 12.5%, between groups: (p = 0.08): G1: 6.3%; G2: 21.9% |
None | (i) Afebrile status for 4 consecutive days; (ii) improved leukocyte counts, platelet counts, CPK, LDH, liver function tests, and CRP; (iii) improved radiographic; (iv) at least 21 days following the onset of illness Age: a poor prognostic factor |
Cheng et al. [30] |
Outcome or good clinical outcome is defined as discharge by day 22 following the onset of symptoms; poor response is defined as death or hospitalization beyond 22 days. SARS, severe acute respiratory syndrome; CT, computed tomography; G1, group 1; G2, group 2; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; CRP, C-reactive protein; PCR, polymerase chain reaction; WBC, white blood cell; SLE, systemic lupus erythematosus; TB, tuberculosis; HTLV-I and -II, human T-cell lymphotropic virus types I and II; IgG, immunoglobulin G; IgM, immunoglobulin M; HCWs, healthcare workers.
SARS and MERS
A clinical trial on 80 patients with SARS amid the outbreak in 2002–2003, Hong Kong, found that patients who received human convalescent serum within 2 weeks from the onset of their symptoms were more likely to be discharged from the hospital than patients who were not treated as such [30]. A year following the SARS outbreak, a retrospective analysis concluded that convalescent plasma therapy had a more desirable impact on both patient survival rate and duration of hospital stay, compared to the outcomes achieved with high doses of methylprednisolone [35]. Another investigation conducted on convalescent plasma obtained from SARS patients in 2005 reported that 87 out of 99 samples contained neutralizing antibodies. A geometric mean titer of 1:61 was also measured for this antibody [26]. These findings suggested that, in reality, few patients retain a high titer of neutralizing antibodies, as these molecules are gradually worn out over time. The study also implied that there might be a good chance that these patients also produced nonneutralizing antibodies, which further helped their immune system to keep the infection contained [20]. Table 1 lists studies on SARS therapy through convalescent plasma.
Published in 2014, a meta-analysis on clinical outcomes of convalescent plasma therapy in the treatment of SARS and MERS further emphasized the desirable effects of this method on mortality rate and viral load of such respiratory infections. This study reported promising clinical outcomes in terms of mortality among patients with different kinds of severe acute respiratory infection who had received variable doses of convalescent plasma [32]. The following year, scientists in Guinea reported an inconclusive attempt to evaluate convalescent plasma therapy in treating patients with MERS. This investigation could not bring reliable data on the table since it failed to look for high levels of neutralizing antibodies in patients. It was speculated that the study could lead to more conclusive findings had it been conducted on patients who were at earlier stages of their disease, rather than individuals who had already been fighting MERS for an extended period [36]. Further progress was made in the same year, as an official protocol was published to apply convalescent plasma in the treatment of MERS in 2015 [37]. Lastly, a more recent study in 2016 corroborated the efficacy of convalescent plasma therapy when applied within a short period following the onset of clinical symptoms. The study also asserted that not all recovering MERS patients displayed high titers of neutralizing antibodies in their blood, regarding it as a challenge in the clinical practice of this approach [38].
COVID-19
As for the ongoing SARS-CoV-2 pandemic, there have been reports that China, as the country of origin with a staggering number of patients, approved convalescent serum to be used for treating several patients. For this matter, an interventional study on 10 COVID-19 patients confirmed the safety of convalescent plasma transfusion and reported significant improvement in clinical conditions and lab findings of these patients. The study concluded that convalescent plasma therapy was very well tolerated by the patients and thus could be considered a prospective approach for neutralizing viremia in patients with severe disease [39]. Soon after, 2 separate trials reported similar results with convalescent serum therapy on 4 [40] and 5 [41] critically ill patients who had been diagnosed with COVID-19. The beneficial effects of convalescent plasma were not only restricted to younger patients, as scientists in China were able to successfully treat an elderly patient who was over 100 years old. After every single attempt resulted in failure, the centenarian patient was administered with convalescent plasma, which led to a decrease in the serum viral load [42]. Such clinical improvements were also observed with the radiological findings in patients with COVID-19, who had opted to receive convalescent plasma [43]. Shortly, Korea published its very first report on the successful application of convalescent blood products along with corticosteroids for treating severe pneumonia associated with COVID-19 [44]. However, further investigations did not find this technique useful.
One particular randomized clinical trial, published in June 2020, sought to investigate the potential effects of convalescent plasma therapy on the time of clinical improvement, only to find out that this treatment modality did not result in any statistically significant difference in the clinical condition of patients, compared to the standard treatment regimen. It was argued that maybe the early termination of the study after only 28 days was the reason for such unfavorable results [45]. Salazar et al. [46], in their study on patients (n = 25) with severe and/or life-threatening COVID-19 disease, reported safety as the primary study outcome and clinical status as the secondary outcome at day 14 posttransfusion. Comparably, another study conducted on 6 patients with respiratory failure concluded that transfusion of convalescent plasma might reduce the shedding of the virus. Still, it could not improve the survival of end-stage patients with COVID-19. The eventual death of 5 patients in this study indicated that such therapeutic measures should have been taken well before the progression of the disease to respiratory failure [47].
From that day forward, several other reports have been published on the promising results achieved with plasma therapy in the treatment of COVID-19. For instance, a group of physicians in the USA saved a 30-year-old pregnant patient with simultaneous administration of convalescent plasma and remdesivir [48]. Another patient with COVID-19 in China substantially improved after being treated with plasma containing high titers of IgM and IgG. According to this report, the patient no longer required mechanical ventilation on the eleventh day following plasma transfusion [49]. A recently published systematic review deemed convalescent plasma therapy a safe and effective treatment modality [50]. The technique has also been suggested to be a life-saving approach in treating COVID-19 patients with liver dysfunction or diabetes [51]. Table 2 lists the studies on COVID-19 therapy through convalescent plasma.
Table 2.
Plasma therapy in the COVID-19 pandemic
| CPT dosage | Location/date | Donors | Recipient patients, n/sex/age | Results | Side effects Discharged criteria/follow-up | Ref. | |
|---|---|---|---|---|---|---|---|
| One dose of 200 mL of CP within 4h in third week | China/23 January–19 February 2020 | Recovered donors with the neutralizing antibody titers above 1:640 | 10 severe patients with supportive care and antiviral agents/6 M:4F/age: mean 52.5 yr | Increased neutralizing antibody increased rapidly up to 1:640, increase of oxyhemoglobin saturation, increased lymphocyte counts (0.65×109/L vs. 0.76×109/L), and decreased CRP (55.98 vs. 18.13 mg/L) CT showed varying degrees of absorption of lung lesions The undetectable viral load after transfusion |
None | CP group: 3 discharged, 7 much improved and ready for discharge Control group: 3 deaths, 6 cases in stabilized status, 1 case in improvement |
Duan et al. [39] |
| 400 mL CP between 10 and 22 days after admission | China/20 January–25 March 2020 |
5 patients recovered from COVID-19/IgG-binding titer >1:1,000, a neutralization titer >40 (end-point dilution titer) | 5 critically ill patients with COVID-19 and ARDS with antiviral treatment and mechanical ventilation/age: 36–65 yr/2 women | Normalized body temperature, decreased SOFA score, and increased PaO2/FiO2 from 172–276 to 284–366, decreased viral loads, increased neutralizing antibody titers from 40–60 to 80–320 | None | 4 patients with resolved ARDS, 3 patients weaned from mechanical ventilation, 3 patients discharged, and 2 are in stable condition | Shen et al. [41] |
| CP in 2 dose administration (200 mL at first week of hospitalization and 100 mL in second week) | China/February 2020 | Recovered patient from COVID-19 with SARS-CoV-2 S-RBD-specific IgG titer of >1:640 | A 100-yr-old male with 2 months of persistent cough, difficulty expectorating, and dyspnea | Significant improvement in laboratory indicators (relatively minor lung changes) and clinical symptoms (cough and dyspnea). Sharp decrease in viral load after the first transfusion (from 2.55×104 to 1.39×103 copies/mL) and undetectable after the second transfusion |
Patient discharge with the stable vital signs, CP efficiency in the management of the elderly | ||
| CP (in first and second weeks, 500 mL of 2 doses at 12-h interval in) | Korea/February 2020 | A recovered male donor in his 20s with IgG OD ratio: 0.586, a recovered male donor in his 20s with IgG OD: 0.532 | Two cases of COVID-19 (71/M and 67/F) with severe pneumonia and ARDS treated with systemic corticosteroid and antiviral therapy | Decrease in fever subsided and oxygen demand, decreased CRP and IL-6 (5.7 mg/L and <1.5 pg/mL), increased PaO2/FiO2 up to 230 and 300, further resolution and improved the density of bilateral infiltration of both lung infiltrates in CT, Ct changed from 24.98 to 33.96 and from 20.51 to 36.33, immediately recovered leukocytosis and lymphopenia | None | Successfully weaned from the mechanical ventilator, discharged from the hospital | Ahn et al. [44] |
| ABO-compatible CP at least one cycle of transfusion (200 mL for each cycle) over a 30-min period (patients 1 & 3: 600 mL in 3 doses, patient 2: 400 mL in 2 doses, patients 4–6: 200 mL) | China/February–March 2020 | Recovered patient from COVID-19 with an afebrile status for at least 3 days, seronegative for anti-HBV, HCV, and HIV, and seropositive for anti-SARS-CoV-2 | 6 laboratory-confirmed COVID-19 patients (69/M, 75/F, 56/M, 63/F, 28/F, 57/M) treated with antiviral drug arbidol | GGOs resolved Respiratory distress alleviation, increase in IgM/IgG titers 2-folds, reduced consolidation gradually, scattered GGOs Complete resolution consolidation, GGOs resolution, increased IgM/IgG titers, reduced GGOs, increased 2 IgM/IgG, improved symptoms, GGOs resolved |
None | Promising state-of-art therapy, discharge based on a relief of symptoms, 4 patients discharged, 1 ready to discharge, 1 under further clinical monitoring | Ye et al. [43] |
| 900 mL in 3 doses, 200 mL, 2,400 mL in 8 doses, 300 mL | China/16 February–15 March |
Recovered patients | 4 critically ill patients with SARS-CoV-2 infection including 3 men (69, 55, 73 yrs old) and a 31-yr-old pregnant woman (35 weeks and 2 days) with supportive care and antiviral treatment |
Extubated and noninvasion ventilation, CT persistent absorption of consolidation, viral load dropping from 55×105 to 3.9×104 to 180 copies/mL. Increased pO2 to 97 mm Hg with OI of 198 mm Hg, CT showed absorption of interstitial pneumonia. Positive IgG, CT absorbed infiltrative lesions but pneumothorax, decreased IgM to normal range, transferred to unfenced ICU, MOF. Removed CRRT and ECMO, IgM changed from positive to weakly positive to negative, persistently positive IgG, CT with near-complete absorption of opacities, trachea cannula removed |
Discharged on 44th, 19th, 15th, and 46th d |
Zhang et al. [40] | |
| CPT | China/14 February–1 April | Recovered patients | 52 laboratory-confirmed COVID-19 with ARDS and/or hypoxemia or life-threatening (shock, organ failure, or requiring mechanical ventilation) with standard treatment (median age: 70 yr, 60 [58.3%] male) | Clinical improvement in 51.9% (27/52) of patients, 87.2% patients with negative conversion rate of viral PCR at 72 h, adverse events which improved with supportive care | Discharged alive/reduction of 2 points on a 6-point disease severity scale in 91.3% (21/23) of patients, and in 20.7% (6/29) of patients with life-threatening disease | Li et al. [64] |
|
| 600 mL plasma divided into 2300 mL units | USA/28 March–14 April |
Recovered SARS-CoV-2 infective patients asymptomatic for 14 or more days | 25 patients with severe and/or life-threatening COVID-19 | At day 7 after CPT, 9 patients with at least a 1-point improvement in clinical scale, 7 discharged patients. By day 14 posttransfusion, 19 (76%) patients with at least a 1-point improvement in clinical status. 11 discharged patients | None | Primary outcome: safety. Secondary outcome: clinical status 20/25 patients discharged | Salazar et al. [46] |
| 200–400 mL plasma | China/until 1 April | Young adult individuals recovered from COVID-19, negative at SARS-CoV-2 RNA and IgM testing and positive at IgG testing, seronegative for hepatitis B/C, AIDS, and syphilis | 6 patients with COVID-19 and respiratory failure, 5 male, median age: 61.5 | Negative for SARS-CoV-2 RNA | None | 1 recovered patient, 5 death, longer survival in the treatment group than control | Zeng et al. [47] |
| CPT and then remdesivir as a late addition | USA/2020 | A critically ill obstetric patient with COVID-19, 35-yr-old woman |
Decreased oxygen requirements, normalized liver enzymes, no renal dysfunction, no episodes of cardiac arrhythmia, stable vital signs | None | Patient improved and extubated 5 days after initiation of remdesivir and discharged | Anderson et al. [48] | |
| 200 mL CP | China 2020 | 6 recovered donors with 2 consecutively negative SARS-CoV-2 PCR assays and serologic negative for hepatitis B/C virus, HIV, and syphilis, high IgG titers (≥1:320) | A 64-yr-old female with progression of the clinical condition transferred to ICU | Lymphocyte count: below 0.5×109/L for 1 week, not require mechanical ventilation | None | The patient was transferred to a general ward | Zhang and Liu [31] |
COVID-19, corona virus disease 2019; CP, convalescent plasma; CRP, C-reactive protein; GGO, ground-glass opacities; CT, computed tomography; OD, optical density; Ct, cycle threshold; CPT, convalescent plasma therapy; ARDS, acute respiratory distress syndrome; SOFA, Sequential Organ Failure Assessment; MOF, multiple organ failure; ICU, intensive care unit; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; AIDS, acquired immunodeficiency syndrome.
Being the most populated country in the world, it should not come as a surprise that China could secure ample plasma samples from donors, most of whom were volunteers who had recovered from COVID-19. The country also put great effort into the detection and extraction of viral antigens and antibodies to each of them, thus accelerating the manufacture of diagnostic kits for timely detection of the infection [34].
It is thought that specific antibodies should bind the SARS-CoV-2 spike protein and neutralize the virions, preventing further infection of intact cells [21]. However, an appropriate titer of such antibody is yet to be determined [52]. Given the theoretically high efficacy of the method, it remains unclear whether an adequate pool of volunteer donors can be sustained. Past work on MERS-CoV appalled scientists on this very ground, as the verdict was that human convalescent serum did not contain the minimal therapeutic titer of antibody required for treatment of patients [37]. Viremia, the presence of virions in blood, usually occurs in the first week following a viral infection. Since this statement holds true for many viral infections, convalescent plasma therapy would be most effective at the early stage of such diseases [53].
The US Food and Drug Administration
Clinical application of plasma collected from recovered patients has recently been approved by the US Food and Drug Administration (FDA) to treat COVID-19, albeit on certain conditions. Despite being a less commonly used method, convalescent plasma therapy has been adopted to treat ailments, such as the 1918 Spanish flu, polio, measles, mumps, and several respiratory infections, throughout history. A long-studied last resort, plasma therapy was an entirely plausible option that could not possibly be overlooked by the New York officials, as they permitted clinical trial of this technique on severely ill patients in the state. This, in turn, prompted the FDA to issue their official approval regarding the clinical application of convalescent plasma that requires several conditions to be met [54]. Table 3 lists the FDA requirements for the donation of convalescent plasma for COVID-19 [55].
Table 3.
FDA requirements for donation of CPT for COVID-19
| All standard donors | ||
|---|---|---|
| Code of Federal Regulations | 21 CFR 630.10 21 CFR 630.15 21 CFR 630.30 21 CFR 630.40 |
|
| The required tests | Positive for SARS-CoV-2 | A diagnostic test during illness |
| A serologic test after recovery | ||
| Completely resolved symptoms | 14 days prior to donation | |
| Female donors | Have never been pregnant | |
| HLA antibodies negative since their most recent pregnancy | ||
| Recommended testing for neutralizing antibody | Titers ≥160 | |
| In unavailable ABO-compatible unit: titers ≥80 is acceptable | ||
| In not available titers: consider storing a retention sample for future testing | ||
FDA, Food and Drug Administration; CPT, convalescent plasma therapy; COVID-19, corona virus disease 2019.
Required Conditions for Blood Product Therapy
The official large-scale practice of convalescent blood product therapy on COVID-19 patients is only approved, provided that specific prerequisites are met [20]: (1) access to a sufficiently large community of donors who have recently recovered from COVID-19; (2) convenience of special facilities for storing and processing of the collected blood; (3) accessibility of both serological and virological assays for precise detection and measurement of SARS-CoV-2 virions in the blood; (4) a reliable platform for priming of all lab tests; (5) authentic protocols for prophylaxis and treatment that are established based on findings reported by clinical trials; and (6) regulatory compliance that may vary by institution or location. Hence, the proper application of this technique requires a multitude of different centers, working unanimously on delivering a well-grounded treatment for patients under intensive care [56].
Benefits
Convalescent plasma/serum can mutually be used for prophylaxis against SARS-CoV-2 infection and treatment of COVID-19 [20]. When taken as a prophylactic measure, this method can be of great benefit to individuals who are more susceptible to this infection. If hesitant, convalescent sera can also be administered for symptomatic patients in hopes of palliating the most obstinate symptoms, concomitantly alleviating the ultimate risk of death. Based on our knowledge of antibody-containing serum therapy, it is strongly recommended to utilize this method as a preventive action in due time, rather than resort to it as a remedial potion when it is not as effective [57].
Potential Risks
Treatment with human immunoglobulin has conferred a nonnegligible increased risk of thrombotic events [58]. For this reason, potential benefits of immunoglobulin therapy should be carefully assayed, and intervention must only take place when the benefits outweigh possible risks. Since the entire biology of the novel coronavirus is unbeknownst to us, it would be a wise strategy to locally collect convalescent plasma and administer it only to patients who have contracted COVID-19 from the same community. This could be the most valid option yet, as it minimizes the risk of incompatibility between the antibody and causative strain [21].
Convalescent serum therapy spawns an array of risks that fall into 2 major categories: known and theoretical [20]. Known risks are well-studied adverse events associated with transfusion of blood products, on top of which stands the risk of inadvertent blood-borne infection. Hypersensitivity and immune reactions to blood constituents is another menacing risk. Following the advent of modern and precise techniques for screening of blood-borne pathogens and cross-matching procedures, such risks have been rendered virtually nonexistent. Nevertheless, known risks are not only limited to common events, as some patients with pulmonary diseases may develop rare conditions such as transfusion-related acute lung injury shortly after the transfusion. Since COVID-19 is a potentially life-threatening pulmonary disease, any convalescent serum must be rigorously screened and cross-matched before it is administered to patients [59].
As for theoretical risks, antibody-dependent enhancement of infection (ADE) is one such incident. Caused by exposure to specific antibodies, ADE is a condition in which the underlying disease is aggravated following parenteral treatment with an antibody. In the case of coronaviruses, it is speculated that exposure to antibodies specific to one strain might provoke infection with another strain [60]. However, ADE is unlikely to occur in this scenario, as convalescent serum preparations are exclusively produced against the same strain responsible for the pandemic. Pieces of evidence on the previous application of serum therapy in the treatment of SARS and MERS [61] strongly support the safety of this modality.
It is also possible that treatment with exogenous antibodies would incapacitate the immune system, leading to catastrophic consequences in individuals who had previous exposure to SARS-CoV-2. In this context, several studies have indicated that passive immunotherapy via antibodies before vaccination against the respiratory syncytial virus might leave a negative impact on humoral immunity, but not cellular [62].
Ethics
Issues to be considered in this context are the lack of reliable and comprehensive studies on this subject and the far-fetched convenience of adequate volunteer donors with a high titer of neutralizing antibodies in their blood [58]. Since humans are the source of antibodies in this therapeutic modality, all processes involved in serum preparation must be carefully monitored with due attention to the ethical protocols issued by the institutes [63].
Conclusions
Based on the guidelines by the WHO, primary prevention stands on the pinnacle of strategies adopted for the management of the COVID-19 outbreak, with monitoring, screening for suspected cases, and supportive care following. Lack of reliable evidence is the primary reason behind the absence of specific treatments. To overcome this disastrous situation, research and development programs should urgently be initiated to find effective therapies to halt the ever-growing chain of the infection efficiently.
Convalescent plasma/serum has been confirmed to be of therapeutic value by several studies, without leaving any major adverse effects on patients with viral diseases. Immunotherapy through transfusion of neutralizing antibodies might be an effective method in the prevention of new infections. Based on our experience with devastating outbreaks such as influenza, SARS, and MERS, immunotherapy through transfusion of convalescent plasma/serum obtained from recovered patients with high titers of neutralizing antibodies in their circulation might be helpful to reduce viral load and mortality rate. Therefore, it is sensible to allocate enough manpower for efficacy and safety assessment of convalescent plasma therapy in treating COVID-19.
Future Perspectives
In every case that human convalescent plasma/serum therapy is indicated, expected benefits and potential risks must be weighed against each other to make a conclusive decision for treatment of patients.
With more safety and higher potency, purified anti-SARS-CoV-2 immunoglobulin-rich preparations are considered better options in terms of therapy, as opposed to convalescent sera collected from recovered patients. Nonetheless, numerous challenges must be overcome for the ultimate consideration of convalescent plasma as a good therapeutic option. To name a few, on-demand access to a well-stocked supply of donor samples, a careful review of clinical conditions specific to each patient, having a basic understanding of viral kinetics, and host immune response to SARS-CoV-2 are all major challenges that need to be confronted.
Caution and discretion are strongly advised in clinical trials on convalescent sera, as the slightest likelihood of risk occurrence, be it known or theoretical, must be identified and addressed.
Other Recent Developments in the Management of COVID-19
In the meanwhile, there has also been a number of other recent developments, aside from immunotherapy, aimed at treatment of COVID-19 [2, 64, 65, 66, 67, 68, 69].
Admission to the Intensive Care Unit and Subsequent Isolation and Quarantine Measures
It is strongly advised that suspected cases with equivocal findings be isolated from other individuals and subsequently be quarantined in a single room. Patients with a definitive diagnosis of COVID-19 can be admitted to the same isolated ward and treated accordingly. Patients with severe disease, on the other hand, need to be hospitalized in the intensive care unit and undergo immediate treatment [64].
General Treatments
As the term might suggest, general treatment strategies encompass a broad array of inpatient and outpatient interventions that are not solely aimed at patients who meet certain criteria, but rather are recommended to all patients with symptomatic disease. These strategies include rest at home, intravenous administration of methylprednisolone and/or adrenaline, adequate energy intake, maintenance of homeostasis (balance between the level of water and electrolytes in the body), and monitoring of vital signs (pulse rate, respiratory rate, blood pressure, body temperature, oxygen saturation, and pain) [64, 66].
Drugs
Antiviral Therapies
A diverse number of antiviral medications have been considered in the treatment of COVID-19. The most notable examples are IFN-α (induction of resistance to viral infection in prospective host cells) [70], lopinavir/ritonavir and darunavir (HIV-1 protease inhibitors) [67, 71], ribavirin (viral nucleosidase inhibitor) [72], umifenovir/arbidol and favipiravir (inhibition of viral entry to the target cell) [73], remdesivir (termination of viral replication) [74], and oseltamivir (neuraminidase inhibitor) [66]. The results of recent clinical trials have supported the potential clinical efficacy of these antiviral medications to some extent, especially remdesivir, lopinavir/ritonavir, and favipiravir [69].
Immunosuppressive Therapy
Tocilizumab and sarilumab are 2 humanized and human monoclonal antibodies, respectively, that inhibit IL-6 receptors. These antibodies have shown promise in the treatment of COVID-19 in patients with certain conditions [67, 75].
Antibiotic Therapy
Intramuscular and/or intravenous administration of antibiotics such as moxifloxacin may be an effective method for prevention of secondary bacterial infection in patients with COVID-19, as some studies suggest [66].
Cellular Therapy
With their evidence-based therapeutic implications in acute lung injury and acute respiratory distress syndrome, mesenchymal stem cells can potentially reverse the process of fibrosis and accelerate tissue repair in the lungs by alleviating the excess secretion of proinflammatory cytokines and inhibiting the infiltration of immune cells to the pulmonary tissue [76, 77]. On a more factual and idiosyncratic level, there are human neutral killer cells as part of the cell-mediated immunity that can recognize antibody-coated virus-infected cells and digest them during an elaborate process called antibody-dependent cellular cytotoxicity [78].
Traditional Medicine
An apropos representative of traditional medicine, Chinese herbal medicine, has long recognized certain compounds with specific characteristics that might be of therapeutic value in the case of COVID-19. Herbal compounds with potential indications in the treatment of COVID-19 may include glycyrrhizin and hesperetin, potential inhibitors of viral entry associated with molecular pathways pertaining to membrane lipid rafts [79] and transmembrane ACE2 [80]; quercetin, another compound with inhibitory effects on viral entry through the blocking of 3CLpro of SARS-CoV [81, 82]; and baicalin, known for its potential antiviral activity [83].
Nanobiotechnology
An insidious pathology of viral origin, HIV infection, has been reported to be responsive to specific nanoparticles conjugated with antiviral agents, especially nucleoside analogs. Today, there is an ample number of nanotechnology-based drug delivery platforms that can be experimentally adopted for the treatment of COVID-19 with specific therapeutic compounds as conjugates, in hopes of alleviating the symptoms of COVID-19 and shortening the natural course of this notorious malady [2].
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
Funding Sources
This review was financially supported by the Zahedan University of Medical Sciences and the Iran University of Medical Sciences (Grant No. IR.ZAUMS.REC.1399.197).
Author Contributions
All authors contributed to this project. Roghayeh Sheervalilou, Milad Shirvaliloo, and Sakine Shirvalilou designed this study. Soraiya Bahari, Ziba Nazarlou, and Zinat Shams conducted a literature search. Dr. Jafar Shahraki and Omolbanin Shahraki validated and interpreted the results. Roghayeh Sheervalilou, Milad Shirvaliloo, and Saman Sargazi drafted the manuscript. Dr. Habib Ghaznavi and Ramin Saravani reviewed and edited. Dr. Habib Ghaznavi, Roghayeh Sheervalilou, Milad Shirvaliloo, and Saman Sargazi revised the manuscript. All the authors read through the manuscript and agreed to the submission of the final version.
Acknowledgements
This review was conducted under the supervision of the Zahedan University of Medical Sciences and the Iran University of Medical Sciences.
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 Mar 26;382((13)):1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheervalilou R, Shirvaliloo M, Dadashzadeh N, Shirvalilou S, Shahraki O, Pilehvar-Soltanahmadi Y, et al. COVID-19 under spotlight: a close look at the origin, transmission, diagnosis, and treatment of the 2019-nCoV disease. J Cell Physiol. 2020 Dec;235((12)):8873–924. doi: 10.1002/jcp.29735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020 Apr;34((2)):75–80. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Available from: https://www.who.int/data#reports.
- 6.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 Nov 19;71((16)):2027–34. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saif L. Animal coronaviruses: What can they teach us about the severe acute respiratory syndrome? Rev Sci Tech. 2004;23((2)):643–60. doi: 10.20506/rst.23.2.1513. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. 2020. Accessed 2020 May 2. Available from: https://www.who.int/csr/sars/country/table2004_04_21/en/; 2004.
- 9.World Health Organization Middle East respiratory syndrome coronavirus (MERSCoV) 2020. Accessed 2020 May 2. Available from: https://www.who.int/emergencies/mers-cov/en/2013.
- 10.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382((8)):727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020:10. [Google Scholar]
- 12.Wang M, Wu Q, Xu W, Qiao B, Wang J, Zheng H, et al. Clinical diagnosis of 8274 samples with 2019-novel coronavirus in Wuhan. medRxiv. 2020 [Google Scholar]
- 13.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395((10223)):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C-I, Postema PG, Arbelo E, Behr ER, Bezzina CR, Napolitano C, et al. SARS-CoV-2, COVID-19 and inherited arrhythmia syndromes. Heart Rhythm. 2020 Sep;17((9)):1456–62. doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323((11)):1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) Medrxiv. 2020 [Google Scholar]
- 17.L-s W, Wang Y-r, Ye D-w, Liu Q-q. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. Int J Antimicrob Agents. 2020 Sep;56((3)):106137. doi: 10.1016/j.ijantimicag.2020.106137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11((12)):836–48. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020 Apr;20((4)):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casadevall A, Pirofski L-a. The convalescent sera option for containing COVID-19. J Clin invest. 2020 Apr 1;130((4)):1545–48. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: old tricks for new challenges. Springer; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization WHO recommendations on SARS and blood safety. 2020. Accessed 2020 May 2. Available from: https://www.who.int/csr/sars/guidelines/bloodsafety/en/; 2003.
- 23.Dhama K, Sharun K, Tiwari R, Sircar S, Bhat S, Malik YS, et al. Coronavirus disease 2019–COVID-19. 2020 doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casadevall A, Scharff MD. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21((1)):150–61. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casadevall A, Dadachova E, Pirofski L-a. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2((9)):695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JS, Chen JT, Liu YX, Zhang ZS, Gao H, Liu Y, et al. A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol. 2005;77((2)):147–50. doi: 10.1002/jmv.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beigel JH, Voell J, Kumar P, Raviprakash K, Wu H, Jiao JA, et al. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis. 2018;18((4)):410–8. doi: 10.1016/S1473-3099(18)30002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171((6)):1387–98. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 29.Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52((4)):447–56. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24((1)):44–6. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020 May;92((5)):479–90. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mair-Jenkins J, Saavedra-Campos M, Kenneth Baillie J, Cleary P, Khaw F-M, Lim WS. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. 2015 Jan 1;211((1)):80–90. doi: 10.1093/infdis/jiu396. [cited 2020 Feb 15]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McQuilten ZK, Wood EM, Bailey M, Cameron PA, Cooper DJ. Fibrinogen is an independent predictor of mortality in major trauma patients: a five-year statewide cohort study. Injury. 2017;48((5)):1074–81. doi: 10.1016/j.injury.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Law PK. Emergent serum therapy and antibody medicine to counteract sudden attacks of COVID-19 and other pathogenic epidemics. Scientific Research Publishing; 2020. [Google Scholar]
- 35.Soo YO, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KK, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10((7)):676–8. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 37.Arabi Y, Balkhy H, Hajeer AH, Bouchama A, Hayden FG, Al-Omari A, et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4((1)):709–8. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arabi YM, Hajeer AH, Luke T, Raviprakash K, Balkhy H, Johani S, et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerging Infect Dis. 2016;22((9)):1554. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117((17)):9490–6. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, et al. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. 2020 Jul;158((1)):e1–13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID19 with convalescent plasma. JAMA. 2020 Apr 28;323((16)):1582–9. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong Y, Cai C, Ling L, Zeng L, Wu M, Wu Y, et al. Successful treatment of a centenarian with coronavirus disease 2019 (COVID-19) using convalescent plasma. Transfus Apher Sci. 2020 Oct;59((5)):102820. doi: 10.1016/j.transci.2020.102820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020 Oct;92((10)):1890–1901. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020 Apr 13;35((14)):e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020 Aug 4;324((5)):460–70. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, Christensen PA, et al. Treatment of COVID-19 patients with convalescent plasma. Am J Pathol. 2020 Aug;190((8)):1680–90. doi: 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng Q-L, Yu Z-J, Gou J-J, Li G-M, Ma S-H, Zhang G-F, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020 Jun;222((1)):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson J, Schauer J, Bryant S, Graves CR. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Rep Womens Health. 2020;27:e00221. doi: 10.1016/j.crwh.2020.e00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, Pang R, Xue X, Bao J, Ye S, Dai Y, et al. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging. 2020;12((8)):6536. doi: 10.18632/aging.103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajendran K, Narayanasamy K, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J Med Virol. 2020 Sep;92((9)):1475–83. doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pawar AY, Hiray AP, Sonawane DD, Bhambar RS, Derle DV, Ahire YS. Convalescent plasma: a possible treatment protocol for COVID-19 patients suffering from diabetes or underlying liver diseases. Diabetes Metab Synd. 2020 Jul-Aug;14((4)):665–9. doi: 10.1016/j.dsx.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020 Apr 21;64((5)):e00399–20. doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.GR K. Immune defenses. In: S B, editor. Medical microbiology. 4th ed. Galveston, TX: University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- 54.Tanne JH. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;368:m1256. doi: 10.1136/bmj.m1256. [DOI] [PubMed] [Google Scholar]
- 55.Barone P, DeSimone RA. Convalescent plasma to treat coronavirus disease 2019 (COVID-19): considerations for clinical trial design. Transfusion. 2020 Jun;60((6)):1123–7. doi: 10.1111/trf.15843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.JS H. Drugmaker Takeda is Working on Coronavirus Drug. Accessed 2020 March 10. Available from: https://www.wsj.com/articles/drugmaker-takeda-is-working-on-coronavirus-drug-11583301660. Published March 4, 2020. Wall Street Journal. 2020.
- 57.Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med. 2010;38((4 Suppl)):e66–73. doi: 10.1097/CCM.0b013e3181d44c1e. [DOI] [PubMed] [Google Scholar]
- 58.Menis M, Sridhar G, Selvam N, Ovanesov MV, Divan HA, Liang Y, et al. Hyperimmune globulins and same-day thrombotic adverse events as recorded in a large healthcare database during 2008–2011. Am J Hematol. 2013;88((12)):1035–40. doi: 10.1002/ajh.23559. [DOI] [PubMed] [Google Scholar]
- 59.Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176((9)):886–91. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol. 2020 Feb 14;94((5)):e02015–19. doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211((1)):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crowe JE, Firestone CY, Murphy BR. Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol. 2001;167((7)):3910–8. doi: 10.4049/jimmunol.167.7.3910. [DOI] [PubMed] [Google Scholar]
- 63.Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14((2)):152. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Liu S-M, Yu X-H, Tang S-L, Tang C-K. Coronavirus disease 2019 (COVID-19): current status and future perspective. Int J Antimicrob Agents. 2020 May;55((5)):105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cevik M, Bamford CGG, Ho A. COVID-19 pandemic-a focused review for clinicians. Clin Microbiol Infect. 2020;26((7)):842–7. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alfonso J, Rodriguez-Morales D, Katterine B-A, Ruchi T, Ranjit S, et al. COVID-19, an emerging coronavirus infection: current scenario and recent developments: an overview. J Pure Appl Microbiol. 2020;14((1)):1–8. [Google Scholar]
- 67.Abd El-Aziza TM, Stockand JD. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2): an update on the status. Infect Genet Evol. 2020;83:104327. doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helena F F, Ron K, Daniella V-K, Rita C A, Barbara C, Eilam Y, et al. Immune-mediated approaches against COVID-19. Nature Nanotechnol. 2020;15:630–45. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drożdżal S, Rosik J, Lechowicz K, Machaj F, Kotfis K, Ghavami S, et al. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updat. 2020;53:100719. doi: 10.1016/j.drup.2020.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ströher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F, et al. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-alpha. J Infect Dis. 2004;189((7)):1164–7. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pulido F, Arribas JR, Delgado R, Cabrero E, González-García J, Pérez-Elias MJ, et al. Lopinavir-ritonavir monotherapy versus lopinavir-ritonavir and two nucleosides for maintenance therapy of HIV. AIDS. 2008;22((2)):F1–9. doi: 10.1097/QAD.0b013e3282f4243b. [DOI] [PubMed] [Google Scholar]
- 72.Jones BM, Ma ES, Peiris JS, Wong PC, Ho JC, Lam B, et al. Prolonged disturbances of in vitro cytokine production in patients with severe acute respiratory syndrome (SARS) treated with ribavirin and steroids. Clin Exp Immunol. 2004;135((3)):467–73. doi: 10.1111/j.1365-2249.2003.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khamitov RA, Loginova SI, Shchukina VN, Borisevich SV, Maksimov VA, Shuster AM. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol. 2008;53((4)):9–13. [PubMed] [Google Scholar]
- 74.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC-C, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020 Apr 27;:ciaa478. doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matthay MA, Goolaerts A, Howard JP, Lee JW. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit Care Med. 2010 Oct;38((10 Suppl)):S569–73. doi: 10.1097/CCM.0b013e3181f1ff1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El Agha E, Kramann R, Schneider RK, Li X, Seeger W, Humphreys BD, et al. Mesenchymal stem cells in fibrotic disease. Cell stem cell. 2017;21((2)):166–77. doi: 10.1016/j.stem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 78.Hammer Q, Rückert T, Romagnani C. Natural killer cell specificity for viral infections. Nat Immunol. 2018;19((8)):800–8. doi: 10.1038/s41590-018-0163-6. [DOI] [PubMed] [Google Scholar]
- 79.Chen H, Du Q. Potential natural compounds for preventing SARS-CoV-2 (2019-nCoV) infection. Preprints. 2020 [Google Scholar]
- 80.Lin CW, Tsai FJ, Tsai CH, Lai CC, Wan L, Ho TY, et al. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68((1)):36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L, Li J, Luo C, Liu H, Xu W, Chen G, et al. Binding interaction of quercetin-3-β-galactoside and its synthetic derivatives with SARS-CoV 3CLpro: structure-activity relationship studies reveal salient pharmacophore features. Bioorg Med Chem. 2006;14((24)):8295–306. doi: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yi L, Li Z, Yuan K, Qu X, Chen J, Wang G, et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78((20)):11334–9. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31((1)):69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]